Abstract
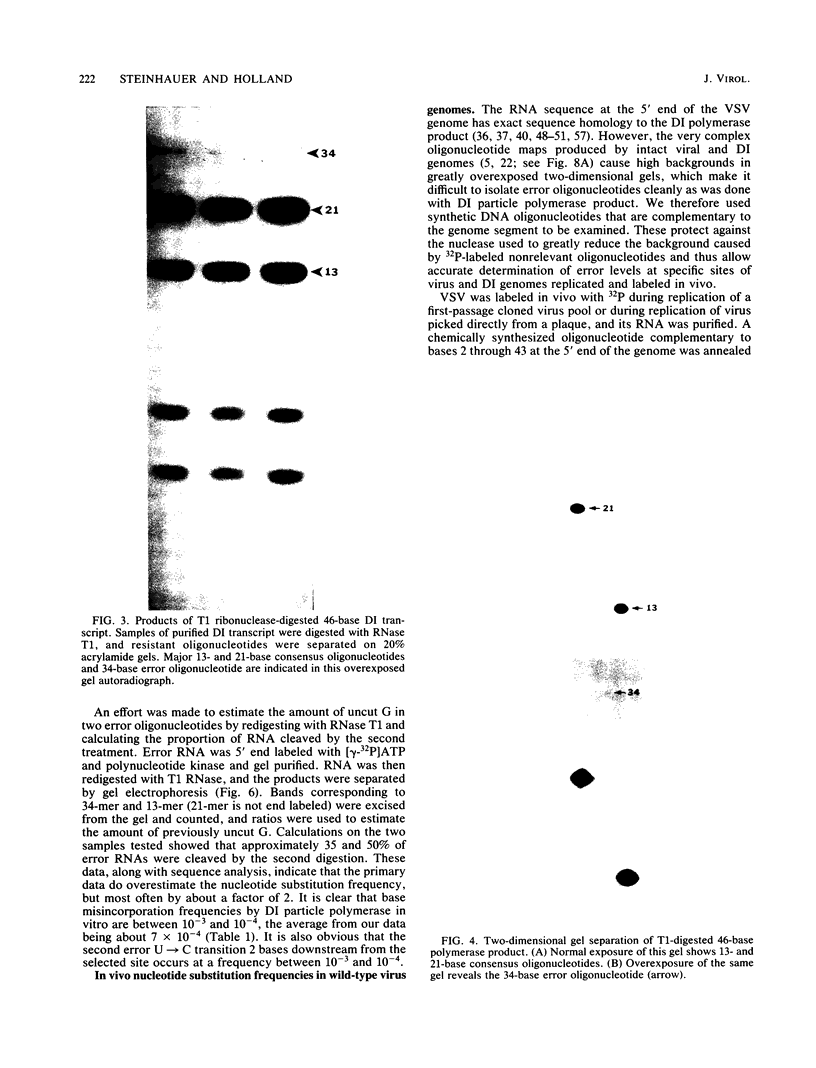
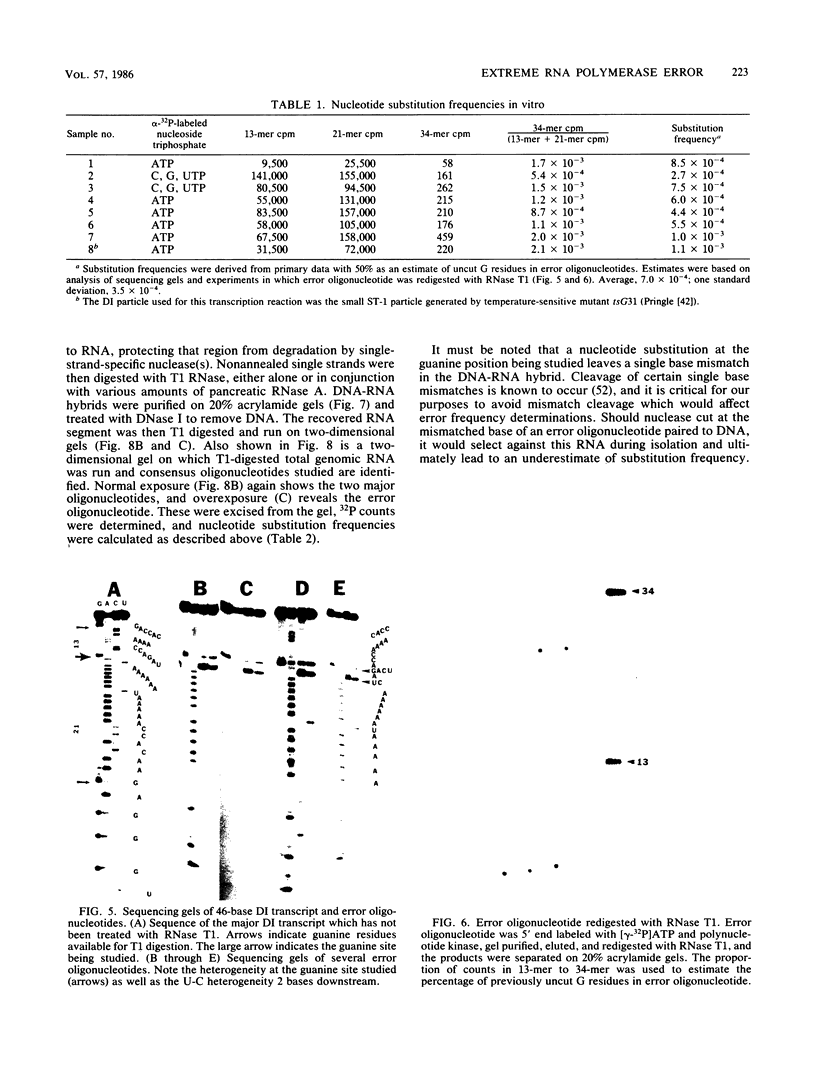
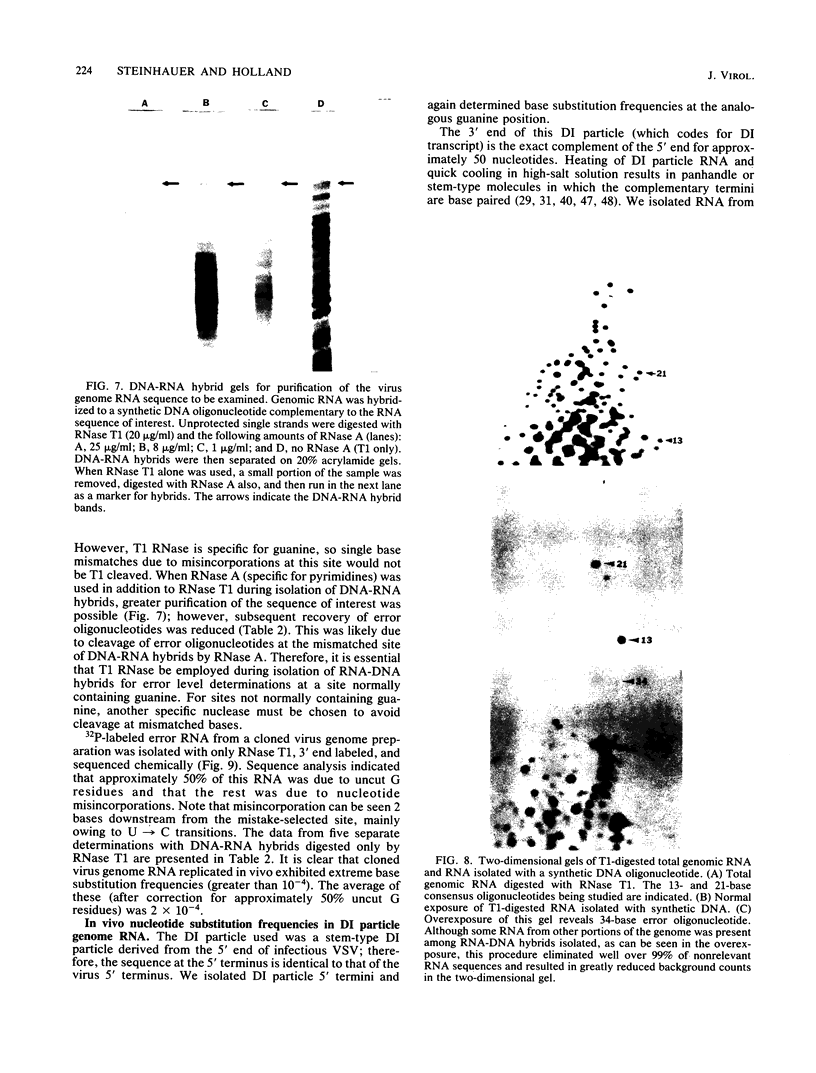
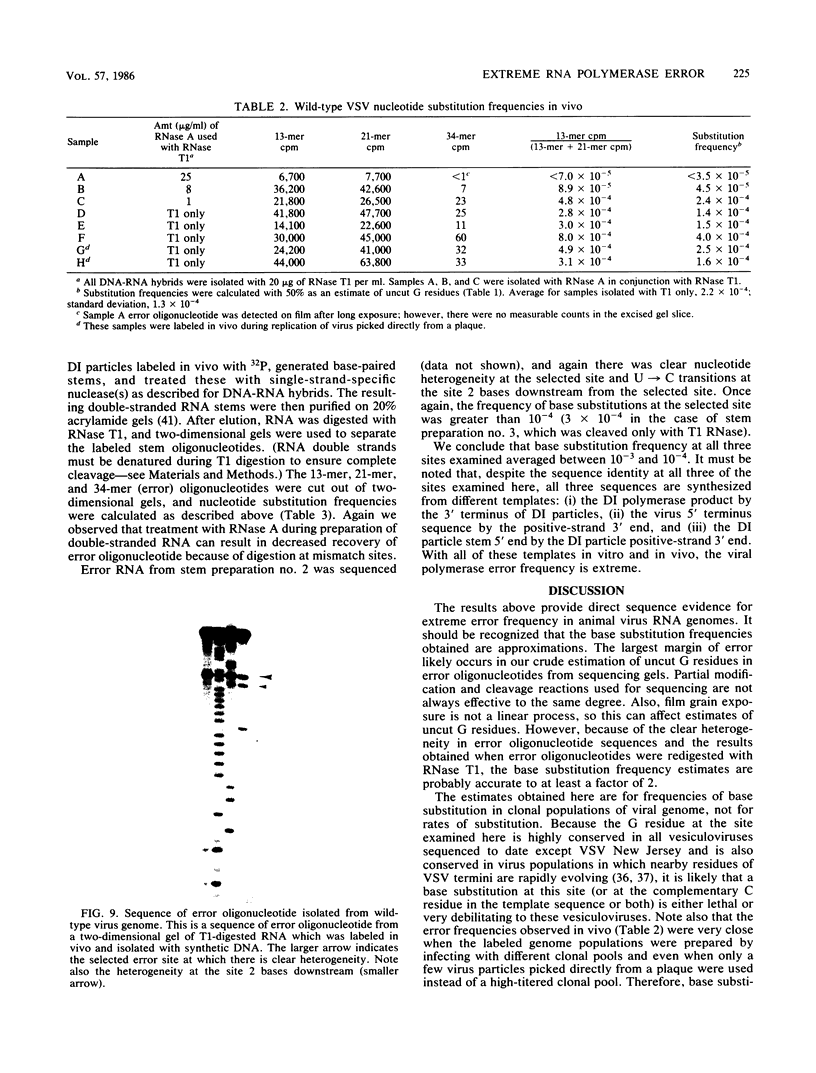
Methods are described which allow direct quantitation and sequence analysis of base substitution levels at predetermined single nucleotide positions in cloned pools of an RNA virus genome or in its RNA transcripts in vitro. Base substitution frequencies for vesicular stomatitis virus (VSV) at one highly conserved site examined were reproducible and extremely high, averaging between 10(-4) and 4 X 10(-4) substitutions per base incorporated at this single site. If polymerase error frequencies averaged as high at all other sites in the 11-kilobase VSV genome, then every member of a cloned VSV population would differ from most other genomes in that clone at a number of different nucleotide positions. The preservation of a consensus sequence in such variable RNA virus genomes then could only result from strong biological selection (in a single host or multihost environment) for the most fit and competitive representatives of extremely heterogeneous virus populations.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benzer S. ON THE TOPOGRAPHY OF THE GENETIC FINE STRUCTURE. Proc Natl Acad Sci U S A. 1961 Mar;47(3):403–415. doi: 10.1073/pnas.47.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand C., Palese P. Sequential passage of influenza virus in embryonated eggs or tissue culture: emergence of mutants. Virology. 1980 Dec;107(2):424–433. doi: 10.1016/0042-6822(80)90309-8. [DOI] [PubMed] [Google Scholar]
- Breindl M., Holland J. J. Coupled in vitro transcription and translation of vesicular stomatitis virus messenger RNA. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2545–2549. doi: 10.1073/pnas.72.7.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. P., Mak T. W. Fluidity of a retrovirus genome. J Virol. 1984 Jun;50(3):759–765. doi: 10.1128/jvi.50.3.759-765.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewley J. P., Bishop D. H., Kang C. Y., Coffin J., Schnitzlein W. M., Reichmann M. E., Shope R. E. Oligonucleotide fingerprints of RNA species obtained from rhabdoviruses belonging to the vesicular stomatitis virus subgroup. J Virol. 1977 Jul;23(1):152–166. doi: 10.1128/jvi.23.1.152-166.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M., Tsichlis P. N., Barker C. S., Voynow S., Robinson H. L. Variation in avian retrovirus genomes. Ann N Y Acad Sci. 1980;354:410–425. doi: 10.1111/j.1749-6632.1980.tb27982.x. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H., Farabaugh P. J., Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978 Aug 24;274(5673):775–780. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- Darlix J. L., Spahr P. F. High spontaneous mutation rate of Rous sarcoma virus demonstrated by direct sequencing of the RNA genome. Nucleic Acids Res. 1983 Sep 10;11(17):5953–5967. doi: 10.1093/nar/11.17.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo E., Dávila M., Ortín J. Nucleotide sequence heterogeneity of the RNA from a natural population of foot-and-mouth-disease virus. Gene. 1980 Nov;11(3-4):333–346. doi: 10.1016/0378-1119(80)90073-6. [DOI] [PubMed] [Google Scholar]
- Domingo E., Flavell R. A., Weissmann C. In vitro site-directed mutagenesis: generation and properties of an infectious extracistronic mutant of bacteriophage Qbeta. Gene. 1976;1(1):3–25. doi: 10.1016/0378-1119(76)90003-2. [DOI] [PubMed] [Google Scholar]
- Domingo E., Sabo D., Taniguchi T., Weissmann C. Nucleotide sequence heterogeneity of an RNA phage population. Cell. 1978 Apr;13(4):735–744. doi: 10.1016/0092-8674(78)90223-4. [DOI] [PubMed] [Google Scholar]
- Drake J. W., Allen E. F., Forsberg S. A., Preparata R. M., Greening E. O. Genetic control of mutation rates in bacteriophageT4. Nature. 1969 Mar 22;221(5186):1128–1132. [PubMed] [Google Scholar]
- Emerson S. U., Dierks P. M., Parsons J. T. In vitro synthesis of a unique RNA species by a T particle of vesicular stomatitis virus. J Virol. 1977 Sep;23(3):708–716. doi: 10.1128/jvi.23.3.708-716.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Fields B. N., Joklik W. K. Isolation and preliminary genetic and biochemical characterization of temperature-sensitive mutants of reovirus. Virology. 1969 Mar;37(3):335–342. doi: 10.1016/0042-6822(69)90217-7. [DOI] [PubMed] [Google Scholar]
- Fields S., Winter G. Nucleotide-sequence heterogeneity and sequence rearrangements in influenza virus cDNA. Gene. 1981 Nov;15(2-3):207–214. doi: 10.1016/0378-1119(81)90130-x. [DOI] [PubMed] [Google Scholar]
- GRANOFF A. Induction of Newcastle disease virus mutants with nitrous acid. Virology. 1961 Apr;13:402–408. doi: 10.1016/0042-6822(61)90270-7. [DOI] [PubMed] [Google Scholar]
- Hall J. D., Coen D. M., Fisher B. L., Weisslitz M., Randall S., Almy R. E., Gelep P. T., Schaffer P. A. Generation of genetic diversity in herpes simplex virus: an antimutator phenotype maps to the DNA polymerase locus. Virology. 1984 Jan 15;132(1):26–37. doi: 10.1016/0042-6822(84)90088-6. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Grabau E. A., Jones C. L., Semler B. L. Evolution of multiple genome mutations during long-term persistent infection by vesicular stomatitis virus. Cell. 1979 Mar;16(3):495–504. doi: 10.1016/0092-8674(79)90024-2. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Horodyski F. M., Nichol S. T., Spindler K. R., Holland J. J. Properties of DI particle resistant mutants of vesicular stomatitis virus isolated from persistent infections and from undiluted passages. Cell. 1983 Jul;33(3):801–810. doi: 10.1016/0092-8674(83)90022-3. [DOI] [PubMed] [Google Scholar]
- Keene J. D., Schubert M., Lazzarini R. A., Rosenberg M. Nucleotide sequence homology at the 3' termini of RNA from vesicular stomatitis virus and its defective interfering particles. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3225–3229. doi: 10.1073/pnas.75.7.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendal A. P., Noble G. R., Skehel J. J., Dowdle W. R. Antigenic similarity of influenza A (H1N1) viruses from epidemics in 1977--1978 to "Scandinavian" strains isolated in epidemics of 1950--1951. Virology. 1978 Sep;89(2):632–636. doi: 10.1016/0042-6822(78)90207-6. [DOI] [PubMed] [Google Scholar]
- Kew O. M., Nottay B. K., Hatch M. H., Nakano J. H., Obijeski J. F. Multiple genetic changes can occur in the oral poliovaccines upon replication in humans. J Gen Virol. 1981 Oct;56(Pt 2):337–347. doi: 10.1099/0022-1317-56-2-337. [DOI] [PubMed] [Google Scholar]
- King A. M., Underwood B. O., McCahon D., Newman J. W., Brown F. Biochemical identification of viruses causing the 1981 outbreaks of foot and mouth disease in the UK. Nature. 1981 Oct 8;293(5832):479–480. doi: 10.1038/293479a0. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D. Isolation and characterization of Sendai virus DI-RNAs. Cell. 1976 Aug;8(4):547–555. doi: 10.1016/0092-8674(76)90223-3. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Schaaper R. M., Beckman R. A., Loeb L. A. On the fidelity of DNA replication. Effect of the next nucleotide on proofreading. J Biol Chem. 1981 Oct 10;256(19):9883–9889. [PubMed] [Google Scholar]
- Leppert M., Kort L., Kolakofsky D. Further characterization of Sendai virus DI-RNAs: a model for their generation. Cell. 1977 Oct;12(2):539–552. doi: 10.1016/0092-8674(77)90130-1. [DOI] [PubMed] [Google Scholar]
- MUNDRY K. W., GIERER A. Die Erzeugung von Mutationen des Tabakmosaikvirus durch chemische Behandlung seiner Nucleinsäure in vitro. Z Vererbungsl. 1958;89(4):614–630. [PubMed] [Google Scholar]
- Mudd J. A., Summers D. F. Polysomal ribonucleic acid of vesicular stomatitis virus-infected HeLa cells. Virology. 1970 Dec;42(4):958–968. doi: 10.1016/0042-6822(70)90344-2. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Desselberger U., Palese P. Recent human influenza A (H1N1) viruses are closely related genetically to strains isolated in 1950. Nature. 1978 Jul 27;274(5669):334–339. doi: 10.1038/274334a0. [DOI] [PubMed] [Google Scholar]
- Nottay B. K., Kew O. M., Hatch M. H., Heyward J. T., Obijeski J. F. Molecular variation of type 1 vaccine-related and wild polioviruses during replication in humans. Virology. 1981 Jan 30;108(2):405–423. doi: 10.1016/0042-6822(81)90448-7. [DOI] [PubMed] [Google Scholar]
- O'Hara P. J., Horodyski F. M., Nichol S. T., Holland J. J. Vesicular stomatitis virus mutants resistant to defective-interfering particles accumulate stable 5'-terminal and fewer 3'-terminal mutations in a stepwise manner. J Virol. 1984 Mar;49(3):793–798. doi: 10.1128/jvi.49.3.793-798.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara P. J., Nichol S. T., Horodyski F. M., Holland J. J. Vesicular stomatitis virus defective interfering particles can contain extensive genomic sequence rearrangements and base substitutions. Cell. 1984 Apr;36(4):915–924. doi: 10.1016/0092-8674(84)90041-2. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault J., Leavitt R. W. Inverted complementary terminal sequences in single-stranded RNAs and snap-back RNAs from vesicular stomatitis defective interfering particles. J Gen Virol. 1978 Jan;38(1):35–50. doi: 10.1099/0022-1317-38-1-35. [DOI] [PubMed] [Google Scholar]
- Pringle C. R., Devine V., Wilkie M., Preston C. M., Dolan A., McGeoch D. J. Enhanced mutability associated with a temperature-sensitive mutant of vesicular stomatitis virus. J Virol. 1981 Aug;39(2):377–389. doi: 10.1128/jvi.39.2.377-389.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C. R. Genetic characteristics of conditional lethal mutants of vesicular stomatitis virus induced by 5-fluorouracil, 5-azacytidine, and ethyl methane sulfonate. J Virol. 1970 May;5(5):559–567. doi: 10.1128/jvi.5.5.559-567.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann M. E., Villarreal L. P., Kohne D., Lesnaw J., Holland J. J. RNA polymerase activity and poly(A) synthesizing activity in defective T particles of vesicular stomatitis virus. Virology. 1974 Mar;58(1):240–249. doi: 10.1016/0042-6822(74)90158-5. [DOI] [PubMed] [Google Scholar]
- Rowlands D. J., Clarke B. E., Carroll A. R., Brown F., Nicholson B. H., Bittle J. L., Houghten R. A., Lerner R. A. Chemical basis of antigenic variation in foot-and-mouth disease virus. Nature. 1983 Dec 15;306(5944):694–697. doi: 10.1038/306694a0. [DOI] [PubMed] [Google Scholar]
- Schubert M., Harmison G. G., Meier E. Primary structure of the vesicular stomatitis virus polymerase (L) gene: evidence for a high frequency of mutations. J Virol. 1984 Aug;51(2):505–514. doi: 10.1128/jvi.51.2.505-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Herman R. C., Lazzarini R. A. Site on the vesicular stomatitis virus genome specifying polyadenylation and the end of the L gene mRNA. J Virol. 1980 May;34(2):550–559. doi: 10.1128/jvi.34.2.550-559.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Lazzarini R. A. A specific internal RNA polymerase recognition site of VSV RNA is involved in the generation of DI particles. Cell. 1979 Nov;18(3):749–757. doi: 10.1016/0092-8674(79)90128-4. [DOI] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Lazzarini R. A., Emerson S. U. The complete sequence of a unique RNA species synthesized by a DI particle of VSV. Cell. 1978 Sep;15(1):103–112. doi: 10.1016/0092-8674(78)90086-7. [DOI] [PubMed] [Google Scholar]
- Semler B. L., Perrault J., Abelson J., Holland J. J. Sequence of a RNA templated by the 3'-OH RNA terminus of defective interfering particles of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4704–4708. doi: 10.1073/pnas.75.10.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semler B. L., Perrault J., Holland J. J. The nucleotide sequence of the 5' terminus of vesicular stomatitis virus RNA. Nucleic Acids Res. 1979 Aug 24;6(12):3923–3931. doi: 10.1093/nar/6.12.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. E., Rhodes C., Rigby P. W., Berg P. Biochemical method for mapping mutational alterations in DNA with S1 nuclease: the location of deletions and temperature-sensitive mutations in simian virus 40. Proc Natl Acad Sci U S A. 1975 Mar;72(3):989–993. doi: 10.1073/pnas.72.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrino F., Dávila M., Ortín J., Domingo E. Multiple genetic variants arise in the course of replication of foot-and-mouth disease virus in cell culture. Virology. 1983 Jul 30;128(2):310–318. doi: 10.1016/0042-6822(83)90258-1. [DOI] [PubMed] [Google Scholar]
- Spindler K. R., Horodyski F. M., Holland J. J. High multiplicities of infection favor rapid and random evolution of vesicular stomatitis virus. Virology. 1982 May;119(1):96–108. doi: 10.1016/0042-6822(82)90068-x. [DOI] [PubMed] [Google Scholar]
- Takeda N., Miyamura K., Ogino T., Natori K., Yamazaki S., Sakurai N., Nakazono N., Ishii K., Kono R. Evolution of enterovirus type 70: oligonucleotide mapping analysis of RNA genome. Virology. 1984 Apr 30;134(2):375–388. doi: 10.1016/0042-6822(84)90305-2. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G., Air G. M., Schild G. C. Molecular mechanisms of variation in influenza viruses. Nature. 1982 Mar 11;296(5853):115–121. doi: 10.1038/296115a0. [DOI] [PubMed] [Google Scholar]
- Yang F., Lazzarini R. A. Analysis of the recombination event generating a vesicular stomatitis virus deletion defective interfering particle. J Virol. 1983 Feb;45(2):766–772. doi: 10.1128/jvi.45.2.766-772.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. F., Desselberger U., Palese P. Evolution of human influenza A viruses in nature: sequential mutations in the genomes of new H1N1. Cell. 1979 Sep;18(1):73–83. doi: 10.1016/0092-8674(79)90355-6. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Jones E. V., Kelly M., Frielle D. W. Generation and amplification of temperature-sensitive mutants during serial undiluted passages of vesicular stomatitis virus. Virology. 1981 Jan 15;108(1):87–97. doi: 10.1016/0042-6822(81)90529-8. [DOI] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]