Abstract
Cocaine addicts are reported to have decreased numbers of striatal dopamine D2 receptors. However, in rodents, repeated cocaine administration consistently produces hypersensitivity to the psychomotor activating effects of both indirect dopamine agonists, such as cocaine itself, and importantly, to direct-acting D2 receptor agonists. The current study reports a possible resolution to this long-standing paradox. The dopamine D2 receptor exists in both a low and a high affinity state, and dopamine exerts its effects via the more functionally relevant high-affinity D2 receptor (D2High). We report here that cocaine self-administration experience produces a large (approximately 150%) increase in the proportion of D2High receptors in the striatum with no change in the total number of D2 receptors, and this effect is evident both 3 and 30 days after the discontinuation of cocaine self-administration. Changes in D2High receptors would not be evident with the probes used in human (and non-human primate) imaging studies. We suggest, therefore, that cocaine addicts and animals previously treated with cocaine may be hyper-responsive to dopaminergic drugs in part because an increase in D2High receptors results in dopamine supersensitivity. This may also help explain why stimuli that increase dopamine neurotransmission, including drugs themselves, are so effective in producing relapse in individuals with a history of exposure to cocaine.
Keywords: self-administration, cocaine, dopamine, dopamine D2 receptor, high affinity state of D2
Introduction
A consistent finding from imaging studies is that cocaine addicts show a small but very persistent decrease in dorsal striatal dopamine D2 receptors (Volkow, et al., 1990, Martinez, et al., 2004, Volkow, et al., 2004), and studies in non-human primates report similar decreases following cocaine self-administration, suggesting that this may be a consequence of cocaine use (Nader and Czoty, 2005, Nader, et al., 2006). However, a long-standing puzzle in this area is that preclinical studies have consistently found that rats treated with amphetamine or cocaine, and then withdrawn, are hypersensitive to the psychomotor activating and incentive motivational effects of these “indirect agonists” (Robinson and Berridge, 1993), and even more importantly, to the psychomotor effects of direct-acting D2 agonists (Ujike, et al., 1990, De Vries, et al., 2002, Edwards, et al., 2007). Studies examining the effects of cocaine treatment on D2 receptor binding in rodents are mixed, with reports of increases (Trulson and Ulissey, 1987, Peris, et al., 1990), decreases (Kleven, et al., 1990, Maggos, et al., 1998) or no change (Dwoskin, et al., 1988, Claye, et al., 1995). Obviously, the puzzle is: why are cocaine-treated rats functionally hypersensitive to D2 agonists if cocaine treatment decreases D2 receptor binding?
There are a number of possible explanations for this discrepancy, but an especially interesting explanation is raised by the finding that dopamine D2 receptors exist in two interconvertible affinity states for the agonist dopamine, a high-affinity state and a low-affinity state, and receptors can rapidly change between the two states. The transmitter dopamine binds primarily to the high-affinity state of the D2 receptor (D2High), making this the most functionally relevant state (Seeman, et al., 2005). Furthermore, many different treatments that produce a functional supersensitivity to dopamine (including repeated amphetamine administration) produce a large increase in the number of D2High receptors in the dorsal striatum, even if they produce no change or even a decrease in the total number of D2 receptors (Seeman, et al., 2004, Seeman, et al., 2005, Seeman, et al., 2007). Unfortunately, the ligands used in the human imaging and non-human primate studies do not discriminate between the low- and high-affinity states of the D2 receptor and are, therefore, insensitive to changes in the number of D2High receptors.
Although it has been reported that experimenter-administered amphetamine increases the number of D2High receptors (Seeman et al., 2002, 2007), no studies have examined the effects of self-administered cocaine on D2High receptors, which is more directly relevant to human imaging studies. Therefore, in the present study we asked whether cocaine self-administration experience increases the proportion of D2High receptors in the dorsal striatum, which could account for the well-documented behavioral hypersensitivity to D2 agonists seen in animals with a history of exposure to cocaine. In addition, we studied rats that had either limited or extended access to self-administered cocaine because the latter procedure is thought to be especially effective in producing a number of symptoms of addiction (Ahmed and Koob, 1998, Vanderschuren and Everitt, 2004, Ferrario, et al., 2005).
Materials and Methods
Subjects
Forty-eight male Wistar rats (Harlan, Indianapolis, IL) weighing 200–225g at the start of the experiment were individually housed in square plastic hanging cages (8 × 9 × 8 cm). The animals were housed in a temperature and humidity controlled room with a 14:10 light/dark cycle, with water available ad libitum. Animals were food restricted throughout the experiment to maintain at least 90% of their free feeding body weight. All procedures were approved by the University of Michigan Committee on the Use and Care of Animals (UCUCA).
Apparatus
Drug administration took place in 16 operant chambers measuring 22 × 18 × 13 cm (Med Associates, St. Albans, VT, USA) located inside larger sound-attenuating chambers. For the drug self-administration, each operant chamber had two nose-poke holes equipped with cue lights. A tone (2900 Hz) was also available inside of the chamber. The floor of the chamber consisted of 19 stainless steel rods (4 mm in diameter) spaced 1.5 cm apart (center-to-center). Med-PC for Windows software (v. 1.1, Med Associates) controlled all drug delivery, tone presentation and data collection in each system via a Pentium PC.
Surgical Procedures
Animals were anaesthetized with ketamine and xylazine anesthesia (77:1.5 mg/ml, intraperitoneal [IP], at 0.1 mL/100 gm of body weight) and a silicone catheter (Plastics One, Roanoke, VA) was inserted into the right jugular vein and passed subcutaneously to exit from the animals’ back (see Caine, et al., 1993). Animals were allowed to recover from surgery for a minimum of 3 days prior to drug administration. Catheters were flushed daily with 0.1mL of gentamicin (50 mg/kg, in 0.9% sterile bacteriostatic saline). Catheter patency was verified by intravenous infusion of sodium pentothal (2 mg/infusion).
Cocaine Self-Administration
Rats were transported from their home cage to an operant chamber 6 days a week for 4 weeks, where they were allowed to nose-poke for cocaine (0.4 mg/kg/infusion in 50 µL of saline administered over 1.6 s) on a continuous reinforcement schedule (FR1) with a time-out of 20 s. A training session commenced with the illumination of the “active” nose-poke hole stimulus light. Responding in this hole resulted in drug delivery. Responding in the other nose-poke hole, designated inactive, had no consequences. Rats were initially trained during daily one-hour sessions for a total of 6 days. Animals that did not acquire stable self-administration behavior (at least 5 infusions each day for 3 consecutive days) were removed from the study. After the initial 6-day training period animals were divided into two groups: an extended access group (ExtA) and a limited access group (LtdA). These groups were balanced according to the amount of drug they administered during the first week of training. Animals in the LtdA group continued to receive one-hour test sessions, whereas animals in the ExtA group received 6-hour test sessions for an additional 16 sessions. A third group of rats (no-drug control group; ND) received sham surgery and were transported each day to a novel test room where they were placed into Plexiglas chambers, similar to the operant chambers.
Tissue Collection
Either 3 or 30 days following their final self-administration session all animals were decapitated and their brains removed. The entire dorsal striatum (caudate-putamen) in the left and right hemispheres were dissected and stored at −70°C until used. The left and right striata were pooled and homogenized in buffer (4 mg frozen tissue per ml buffer), using a Teflon-glass homogenizer with the piston rotating at 500 rpm and 10 up and down strokes of the glass container. The buffer contained 50 mM Tris-HCl (pH 7.4 at 208C), 1 mM EDTA, 5 mM KCl, 1.5 mM CaCl2, 4 mM MgCl2, and 120 mM NaCl. The homogenate was washed three times by centrifugation at 10,000 rpm at 4 °C for 10min and resuspending the pellet in 15 ml of buffer, although it is known that some of the D2 receptors can be lost by this procedure (Seeman, et al., 1984).
[3H]domperidone/DA competition
[3H]Domperidone was custom synthesized as [phenyl-3H(N)]domperidone (42–68 Ci/mmol) by PerkinElmer Life Sciences Inc., Boston, MA, and used at a final concentration of 2 nM. Because the Kd of [3H]domperidone is 0.47 nM at D2 receptors in rat striatum, the final concentration of 2 nM occupied 81% of the D2 receptors, using the equation f = C/(C + Kd), where f is the fraction of receptors occupied by [3H]domperidone, and C is 2 nM (Seeman, et al., 2003).
The competition between dopamine and [3H]domperidone for binding at the receptors was done as follows. Each incubation tube (12 × 75 mm, glass) received, in the following order, 0.5 ml buffer (containing dopamine at various concentrations, and with or without a final concentration of 10 µM S-sulpiride to define nonspecific binding to the dopamine D2 receptors), 0.25 ml [3H]domperidone and 0.25 ml of tissue homogenate. The tubes, containing a total volume of 1 ml, were incubated for 2 h at room temperature (20°C), after which the incubates were filtered, using a 12-well cell harvester (Titertek, Skatron, Lier, Norway) and buffer-presoaked glass fiber filter mats (Whatman GF/C). After filtering the incubate, the filter mat was rinsed with buffer for 15 s (7.5 ml buffer). The filters were pushed out and placed in scintillation polystyrene minivials (7 ml, 16 × 54 mm; Valley Container Inc., Bridgeport, Conn.). The minivials received 4 ml each of scintillant (Research Products International Corp., Mount Prospect, IL), and were monitored 6 h later for tritium in a Beckman LS5000TA scintillation spectrometer at 55% efficiency. The specific binding of [3H]domperidone was defined as total binding minus that in the presence of 10 µM S-sulpiride.
The proportion of D2High receptors in the striata was measured by the competition of dopamine with [3H]domperidone, as previously described (Seeman et al., 2003; see Figure 1). Dopamine inhibited the binding of [3H]domperidone in two phases. At low concentrations, corresponding to the high-affinity state of the dopamine D2 receptor (D2High) dopamine inhibited the binding of [3H]domperidone between 1 nM and 100 nM dopamine. A second phase of inhibition occurred above 100 nM dopamine. The demarcation between the two phases were sharp and unambiguous, clearly and readily permitting the measurement of the D2High component as a percent of the total amount of specific [3H]domperidone binding, as defined by the presence of 10 µM S-sulpiride. No computer-assisted analysis was needed. Note that the currently available methods are not sufficiently sensitive for examination of D2High receptors in either small brain regions or brain regions with relatively few D2 receptors. Indeed, the technique requires a relatively large sample rich in D2 receptors. We were limited, therefore, to analysis of the entire dorsal striatum in the present study.
Figure 1.
Representative experiments for the method of competition between dopamine and [3H]domperidone. The proportion of D2 receptors in the high-affinity state for dopamine in the control tissue was 17.7%, while that for the extended access rat was 49%, a 2.8-fold increase.
Statistical Analyses
Cocaine Self-Administration
The number of infusions for the first hour of each session and for the entirety of the session was determined for each day of self-administration. Repeated measures two-way ANOVAs were performed with session (1–16) as the repeated variable, drug group (LtdA, ExtA) as the independent variable and the number of infusions (i.e. during the first hour or entire session) as the dependent variable. When significant main effects or interactions were revealed, Bonferroni corrected t-tests were conducted. For all analyses, alpha was set at 0.05.
D2 High Affinity Receptor Density
As a relative measure of D2 receptor density the total number of receptors occupied by [3H]domperidone was calculated (reflecting 81% of the total number of D2 receptors). A two-way ANOVA was performed with drug group (ND, LtdA, ExtA) and withdrawal time (3 or 30 days) as the independent variables and disintegrations per minute (DPM), a measure of [3H]domperidone binding, as the dependent variable. For the proportion of D2High receptors, a two-way ANOVA was performed with drug group and withdrawal time as the independent variables and the percentage of total binding representing the D2High receptors as the dependent variable. When significant main effects or interactions were revealed, Bonferroni corrected t-tests were conducted.
Results
Cocaine Self-administration
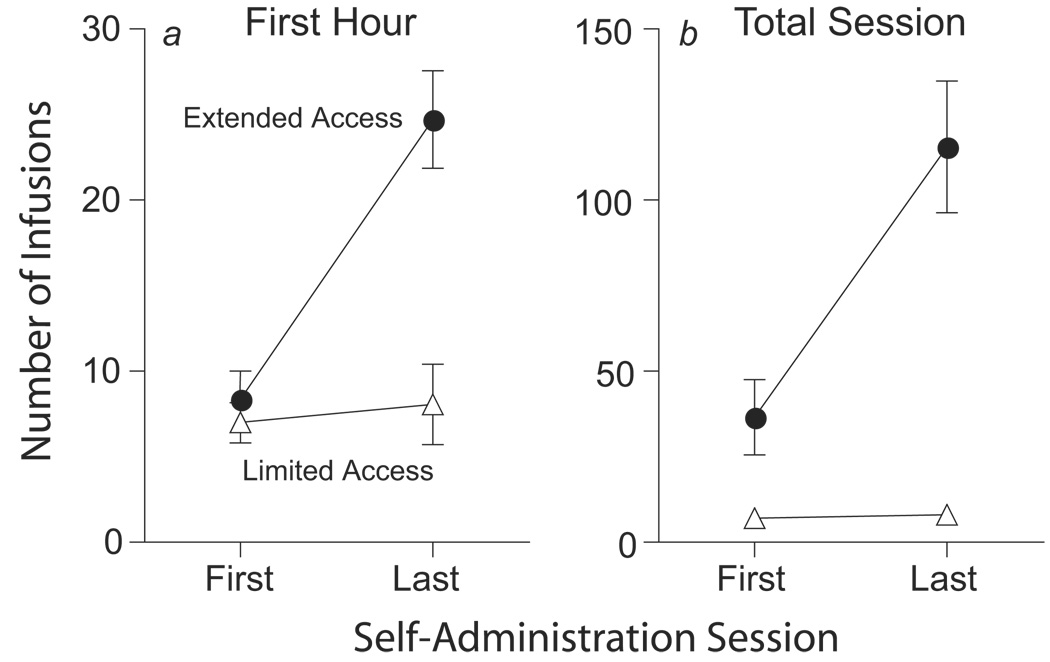
Figure 2 shows that limited access (1 hr/day) to self-administered cocaine produced a stable level of intake over time (approximately 2.8 mg/kg/session), but as expected, extended access to cocaine (6 hr/day) resulted in a pronounced escalation of intake between the first and last test session (by the last test session these animals were taking an average of 45 mg/kg/day).
Figure 2.
A. Mean (+/−SEM) number of cocaine infusions during the first hour of each self-administration session. By the 7th escalation session, animals allowed extended access to cocaine (6 hr session; ExtA) took significantly more infusions than animals allowed only limited access (1 hr sessions; LtdA) [main effect of group, F(1,476)=64.24, p<.0001, main effect of day, F(17,476)=6.61, p<0.0001, group by day interaction, F(17,476)=6.97, p<.0001]. B. The mean (+/−SEM) number of cocaine infusions over the entire session for ExtA (6 hr sessions) and LtdA (1 hr sessions) groups.
D2 Receptors
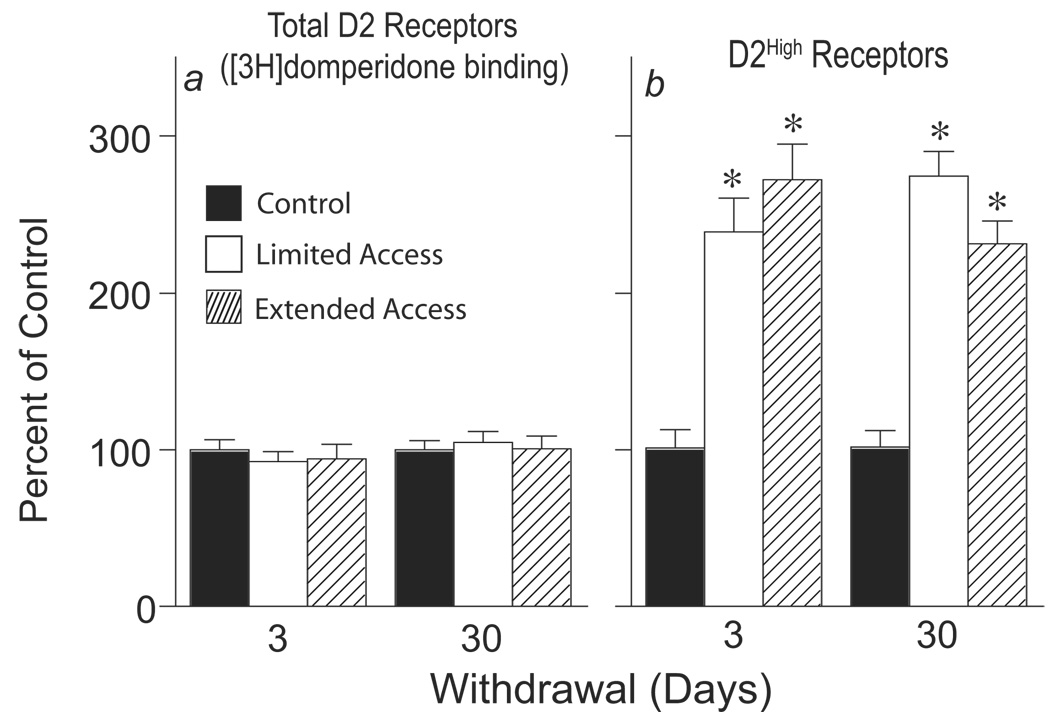
There were no group differences in the total number of receptors occupied by [3H]domperidone at either withdrawal time (Figure 3a). However, there was a large (approximately 150%) increase in the proportion of dorsal striatal D2High receptors in both the LtdA and ExtA groups, relative to the no drug control group (Figure 3b), and this was evident both 3 and 30 days after the discontinuation of cocaine self-administration. Given that there was no change in total D2 binding there was presumably a proportionate decrease in D2Low receptors.
Figure 3.
Total dopamine D2 and D2High receptors in the dorsal striatum of no-drug control animals and animals given limited or extended access to self-administered cocaine, either 3 or 30 days after the last self-administration session. Panel a shows the total number of D2 receptors ([3H]domperidone binding) in the cocaine-treated groups expressed as a percent of the control group. There were no group differences in the total number of D2 receptors (Overall ANOVA, group, F(2,40)=.03, p=.97). Panel b shows the proportion of D2High receptors, again with the cocaine-treated groups expressed as a percent of the control group. In control animals the proportion of D2 high affinity receptors was 18±1.4%. Cocaine self-administration experience greatly increased the proportion of D2High receptors at both 3 and 30 days of withdrawal [main effect of group, F(2,42)=68.89, p<.0001, main effect of withdrawal time, F(1,42)=.65, p=.43, group by time interaction, F(2,32)=2.8, p=.07, *, differs from the no drug group as determined by Bonferroni’s corrected t-tests].
Discussion
Cocaine self-administration experience had a profound effect on the proportion of D2High receptors in the dorsal striatum. Relative to drug-naïve controls, rats that had 4 weeks of either limited (1 hr/day) or extended (6 hr/day) access to cocaine had approximately a 150% increase in dorsal striatal D2High receptors, and this effect was evident both 3 and 30 days after the discontinuation of cocaine self-administration. There were no changes in the total number of D2 receptors, and therefore, there was presumably a proportionate decrease in the number of D2 receptors in the low-affinity state in cocaine-experienced animals.
It is interesting that there was no difference in the magnitude of the increase in D2High receptors in animals that had limited vs. extended access to cocaine, as these two procedures result in a number of different outcomes (Ahmed and Koob, 1998, Deroche-Gamonet, et al., 2004, Vanderschuren and Everitt, 2004, Ferrario, et al., 2005). It is not clear, therefore, which of the many behavioral and psychological consequences of cocaine self-administration are due to changes in dorsal striatal D2High receptors. Seeman and his colleagues (2002, 2007) have reported that sensitization to amphetamine is associated with an increase in D2High receptors, and both experimenter-administered cocaine and cocaine self-administration experience have been reported to produce behavioral supersensitivity to a challenge injection with a direct D2 receptor agonist (De Vries, et al., 2002, Edwards, et al., 2007). It has also been reported that both the limited and extended access cocaine self-administration procedures used in the current study produce psychomotor sensitization (Ferrario, et al., 2005, Knackstedt and Kalivas, 2007). In the Ferrario et al. (2005) study, however, animals given extended access showed more robust sensitization than those given only limited access. Therefore, the absence of any difference between the ExtA and LtdA groups in D2High receptors suggest this alone does not account for the differential sensitization, or, there may be a ceiling effect. Although the ExtA animals took significantly more cocaine than the LtdA animals, even the LtdA group took considerably more cocaine (nearly 3mg/kg/day i.v. for 24 days) than used in a typical sensitization study. On the other hand, (Ferrario, et al., 2005, Knackstedt and Kalivas, 2007) recently reported that ExtA and LtdA groups showed comparable psychomotor sensitization, although they used different behavioral measures than Ferrario et al. (2005). It is clear, therefore, that to determine the nature of the relationship between the increase in D2High receptors and any specific change in behavior or psychological process, including behavioral sensitization, will require examination of a greater range of cocaine doses, treatment regimens and conditions.
Nevertheless, it is important to contrast the increase in striatal D2High receptors produced by cocaine self-administration experience reported here with reports that there is a decrease in striatal D2 receptors in cocaine addicts (Volkow et al., 1990; 1993; Martinez et al., 2004) and in non-human primates allowed to self-administer cocaine (Volkow, et al., 1990, Volkow, et al., 1993, Martinez, et al., 2004, Nader and Czoty, 2005, Nader, et al., 2006). We did not find a decrease in the total number of D2 receptors, and therefore, there was presumably a decrease in the number of D2 receptors in the low-affinity state proportionate to the increase in D2High receptors. Previous studies examining the effects of cocaine administration on D2 receptor binding in rats are very mixed, with reports of increases (Trulson and Ulissey, 1987, Peris, et al., 1990), decreases (Kleven, et al., 1990, Maggos, et al., 1998) and no change (Dwoskin, et al., 1988, Claye, et al., 1995). Although it might be argued that the difference between the present study and the human and non-human primate studies is a result of species differences, the amount of cocaine used, etc., it is important to consider another possibility. The ligands used in the human and non-human primate imaging studies do not discriminate between the low- and high-affinity states of the D2 receptor and would be, therefore, insensitive to changes in the number of D2High receptors (Seeman et al., 2003; 2005). Furthermore, there are many examples where an increase in D2High receptors has been associated with dopamine supersensitivity, even when there was no change or even a decrease in total D2 receptors (Seeman et al., 2005). It is also worth mentioning that the human and non-human primate studies were performed in vivo and thus the apparent decrease in binding could be due to an increase in endogenous dopamine, which would interfere with ligand binding. Whatever the case, it will clearly be important to conduct studies in addicts using ligands being developed to quantify the D2High receptor (Graff-Guerrero, et al., 2007, Willeit, et al., 2008) to determine if the reported decrease in D2 receptors in addicts is more apparent than real.
The results reported here are also of some clinical importance because they suggest that rather than being in a hypo-dopaminergic state, addicts may actually be supersensitive to dopamine and dopaminergic drugs after the discontinuation of drug use, and may remain so for very long periods of time. This may help explain why stimuli that activate dopaminergic systems, which include addictive drugs themselves, direct D2 agonists, stress, and cues associated with drugs, are so effective in reinstating self-administration behavior (producing relapse) after extinction of the behavior (Shaham, et al., 2003). In thinking about the development of therapies for cocaine addiction it will obviously be critical to determine whether a hypo- or hyper-dopaminergic state best represents the situation in addiction, and under what conditions.
Acknowledgements
We thank James M. Dell’Orco, Rachel I. Imershein, Dr. Julia Garcia-Fuster and Dr. H.-C. Guan for their technical assistance.
Role of the Funding Source
This research was supported by grant R37 DA04294 from the National Institute on Drug Abuse to Terry Robinson and grant MOP74676 from the Canadian Institutes for Health Research to Philip Seeman. The funding bodies had no further role in study design, data collection, analysis, or interpretation, writing of the manuscript, or the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Claye LH, Akunne HC, Davis MD, DeMattos S, Soliman KF. Behavioral and neurochemical changes in the dopaminergic system after repeated cocaine administration. Mol Neurobiol. 1995;11:55–66. doi: 10.1007/BF02740684. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Raaso H, Vanderschuren LJ. Relapse to cocaine- and heroin-seeking behavior mediated by dopamine D2 receptors is time-dependent and associated with behavioral sensitization. Neuropsychopharmacology. 2002;26:18–26. doi: 10.1016/S0893-133X(01)00293-7. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [see comment]. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Peris J, Yasuda RP, Philpott K, Zahniser NR. Repeated cocaine administration results in supersensitivity of striatal D-2 dopamine autoreceptors to pergolide. Life Sci. 1988;42:255–262. doi: 10.1016/0024-3205(88)90634-0. [DOI] [PubMed] [Google Scholar]
- Edwards S, Whisler KN, Fuller DC, Orsulak PJ, Self DW. Addiction-related alterations in D1 and D2 dopamine receptor behavioral responses following chronic cocaine self-administration. Neuropsychopharmacology. 2007;32:354–366. doi: 10.1038/sj.npp.1301062. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Willeit M, Ginovart N, Mamo D, Mizrahi R, Rusjan P, Vitcu I, Seeman P, Wilson AA, Kapur S. Brain region binding of the D(2/3) agonist [(11)C]-(+)-PHNO and the D(2/3) antagonist [(11)C]raclopride in healthy humans. Hum Brain Mapp. 2007 doi: 10.1002/hbm.20392. [EPub Ahead of Print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleven MS, Perry BD, Woolverton WL, Seiden LS. Effects of repeated injections of cocaine on D1 and D2 dopamine receptors in rat brain. Brain Res. 1990;532:265–270. doi: 10.1016/0006-8993(90)91768-c. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J Pharmacol Exp Ther. 2007;322:1103–1109. doi: 10.1124/jpet.107.122861. [DOI] [PubMed] [Google Scholar]
- Maggos CE, Tsukada H, Kakiuchi T, Nishiyama S, Myers JE, Kreuter J, Schlussman SD, Unterwald EM, Ho A, Kreek MJ. Sustained withdrawal allows normalization of in vivo [11C]N-methylspiperone dopamine D2 receptor binding after chronic binge cocaine: a positron emission tomography study in rats. Neuropsychopharmacology. 1998;19:146–153. doi: 10.1016/S0893-133X(98)00009-8. [DOI] [PubMed] [Google Scholar]
- Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, Perez A, Frankle WG, Cooper T, Kleber HD, Fischman MW, Laruelle M. Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- Nader MA, Czoty PW. PET imaging of dopamine D2 receptors in monkey models of cocaine abuse: genetic predisposition versus environmental modulation. Am J Psychiatry. 2005;162:1473–1482. doi: 10.1176/appi.ajp.162.8.1473. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Peris J, Boyson SJ, Cass WA, Curella P, Dwoskin LP, Larson G, Lin LH, Yasuda RP, Zahniser NR. Persistence of neurochemical changes in dopamine systems after repeated cocaine administration. J Pharmacol Exp Ther. 1990;253:38–44. [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Seeman P, Tallerico T, Ko F. Dopamine displaces [3H]domperidone from high-affinity sites of the dopamine D2 receptor, but not [3H]raclopride or [3H]spiperone in isotonic medium: Implications for human positron emission tomography. Synapse. 2003;49:209–215. doi: 10.1002/syn.10232. [DOI] [PubMed] [Google Scholar]
- Seeman P, Tallerico T, Ko F. Alcohol-withdrawn animals have a prolonged increase in dopamine D2high receptors, reversed by general anesthesia: relation to relapse? Synapse. 2004;52:77–83. doi: 10.1002/syn.20005. [DOI] [PubMed] [Google Scholar]
- Seeman P, McCormick PN, Kapur S. Increased dopamine D2(High) receptors in amphetamine-sensitized rats, measured by the agonist [(3)H](+)PHNO. Synapse. 2007;61:263–267. doi: 10.1002/syn.20367. [DOI] [PubMed] [Google Scholar]
- Seeman P, Ulpian C, Wreggett KA, Wells JW. Dopamine receptor parameters detected by [3H]spiperone depend on tissue concentration: analysis and examples. J Neurochem. 1984;43:221–235. doi: 10.1111/j.1471-4159.1984.tb06700.x. [DOI] [PubMed] [Google Scholar]
- Seeman P, Tallerico T, Ko F, Tenn C, Kapur S. Amphetamine-sensitized animals show a marked increase in dopamine D2 high receptors occupied by endogenous dopamine, even in the absence of acute challenges. Synapse. 2002;46:235–239. doi: 10.1002/syn.10139. [DOI] [PubMed] [Google Scholar]
- Seeman P, Weinshenker D, Quirion R, Srivastava LK, Bhardwaj SK, Grandy DK, Premont RT, Sotnikova TD, Boksa P, El-Ghundi M, O'Dowd BF, George SR, Perreault ML, Mannisto PT, Robinson S, Palmiter RD, Tallerico T. Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc Natl Acad Sci U S A. 2005;102:3513–3518. doi: 10.1073/pnas.0409766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Ulissey MJ. Chronic cocaine administration decreases dopamine synthesis rate and increases [3H] spiroperidol binding in rat brain. Brain Res Bull. 1987;19:35–38. doi: 10.1016/0361-9230(87)90162-6. [DOI] [PubMed] [Google Scholar]
- Ujike H, Akiyama K, Otsuki S. D-2 but not D-1 dopamine agonists produce augmented behavioral response in rats after subchronic treatment with methamphetamine or cocaine. Psychopharmacology (Berl) 1990;102:459–464. doi: 10.1007/BF02247125. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R, Dewey SL, Logan J, Bendriem B, Christman D, et al. Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry. 1990;147:719–724. doi: 10.1176/ajp.147.6.719. [DOI] [PubMed] [Google Scholar]
- Willeit M, Ginovart N, Graff A, Rusjan P, Vitcu I, Houle S, Seeman P, Wilson AA, Kapur S. First human evidence of d-amphetamine induced displacement of a D2/3 agonist radioligand: A [11C]-(+)-PHNO positron emission tomography study. Neuropsychopharmacology. 200;33:279–289. doi: 10.1038/sj.npp.1301400. [DOI] [PubMed] [Google Scholar]