Abstract
Long range movement of the iron-sulfur protein (ISP) between the cytochrome b (cyt b) and cyt c1 redox centers plays a key role in electron transfer within the cyt bc1 complex. A series of 21 mutants in the cyt b ef loop of Rhodobacter sphaeroides cyt bc1 were prepared to examine the role of this loop in controlling the capture and release of the ISP from cyt b. Electron transfer in the cyt bc1 complex was studied using a ruthenium dimer to rapidly photooxidize cyt c1 within 1 μs and initiate the reaction. The rate constant for electron transfer from the iron-sulfur center [2Fe2S] center to cyt c1 was k1 = 60,000 s−1. Famoxadone binding to the Qo site decreases k1 to 5,400 s−1, indicating that a conformational change on the surface of cyt b decreases the rate of release of the ISP from cyt b. The mutation I292A on the surface of the ISP binding crater decreased k1 to 4,400 s−1, while addition of famoxadone further decreased it to 3,000 s−1. The mutation L286A at the tip of the ef loop decreased k1 to 33,000 s−1, but famoxadone binding caused no further decrease, suggesting that this mutation blocked the conformational change induced by famoxadone. Studies of all the mutants provide further evidence that the ef loop plays an important role in regulating the domain movement of the ISP to facilitate productive electron transfer and prevent short-circuit reactions.
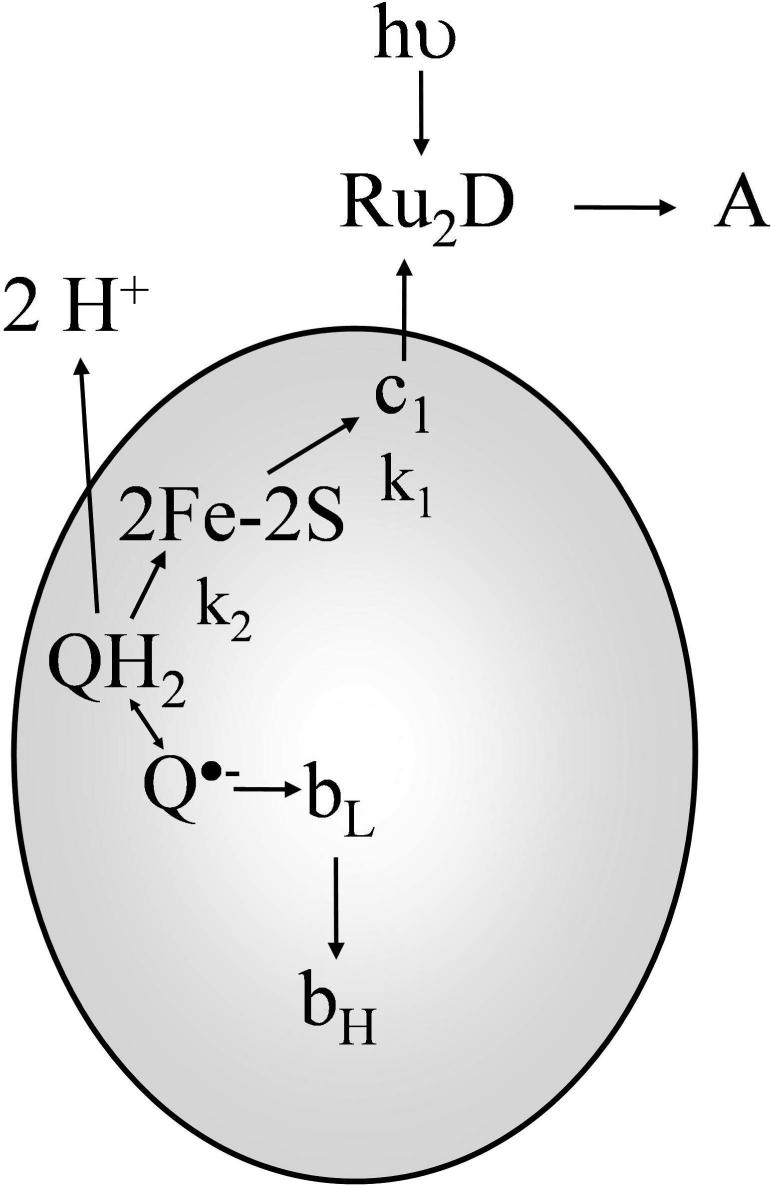
The cytochrome bc1 complex (ubiquinol:cytochrome c reductase) is an integral membrane protein in the electron transport chains of mitochondria and many respiratory and photosynthetic prokaryotes (1, 2). The complex contains the Rieske iron-sulfur protein (ISP), cyt c1, and two b-type hemes (bL and bH) in the cyt b subunit (1, 2). The complex translocates four protons to the positive side of the membrane as two electrons are transferred from ubiquinol (QH2) to cyt c using a widely-accepted Q-cycle mechanism (2). In a key bifurcated reaction, QH2 binds to the Qo site located near the outside of the membrane, and transfers its first electron to the Rieske iron-sulfur center ([2Fe2S]), and then to cyt c1 and cyt c (1,2). The second electron is transferred from semiquinone in the Qo site to cyt bL and then to cyt bH, leading to reduction of ubiquinone in the Qi site to the semiquinone. This cycle is repeated to reduce the semiquinone at the Qi site to QH2. A recent freeze-quench EPR study has shown that QH2 reduced [2Fe2S] and cyt bL simultaneously with a half-time of 250 μs, providing direct evidence for the bifurcation mechanism, and indicating that either the semiquinone is very short-lived or the reaction is concerted (3). X-ray crystallographic studies have shown that the conformation of the ISP depends on the presence of inhibitors in the Qo site as well as the crystal form (4-7). An anomalous signal for [2Fe2S] is found close to cyt bL in native I4122 bovine crystals, but its intensity is small, indicating that the ISP is conformationally mobile (4,7). In beef, chicken and yeast cyt bc1 crystals grown in the presence of stigmatellin, the ISP is in a conformation with [2Fe2S] proximal to the cyt bL heme, called the b state (5-8). However, in native chicken or beef P6522 crystals in the absence of Qo site inhibitors, the ISP is in a conformation with [2Fe2S] close to cyt c1, called the c1 state (5,6). A novel shuttle mechanism for the ISP has been proposed based on these structural studies (4-7). With the ISP initially in the b state, QH2 in the Qo site transfers an electron to the oxidized [2Fe2S] center. The ISP then rotates by 57o to the c1 state where reduced [2Fe2S] transfers an electron to cyt c1. The main features of this mobile shuttle mechanism have been supported by a variety of experimental studies using mutation and/or cross-linking to immobilize the ISP or alter the conformation of the neck region (9-20).
An important question regarding the Q-cycle mechanism is how QH2 at the Qo site can deliver two electrons sequentially to the high and low potential chains, even though thermodynamics would favor delivery of both electrons to the high potential chain (1,2). A number of mechanisms have been proposed in which the conformations of the ISP and/or the quinone substrates are controlled to favor the reversible oxidation of QH2 and minimize short circuit reactions (21-29). Studies of the effects of Qo site inhibitors have indicated that there is a linkage between the occupant of the Qo site and the conformation and dynamics of the ISP. Stigmatellinforms a hydrogen bond with the His-161 ligand of the [2Fe2S] center, thus increasing its redox potential by 200−250 mV and immobilizing the ISP in the b conformation (5-8, 30). Famoxadone binding to the Qo site leads to significant conformational changes on the surface of cyt b which trigger a long-range conformational change in the ISP from the mobile state to a state with [2Fe2S] proximal to cyt b (31). The ef loop plays an important role in relaying conformational changes in the Qo pocket to surface domains that control the binding of the ISP. Moreover, molecular dynamics simulations have shown that residues 263−268 of the ef loop are displaced by up to 2 Å as the ISP rotates from the b state to the c1 state (32). In this paper, the effects of mutations of ef loop residues on electron transfer from QH2 to the iron-sulfur center and then to cyt c1 are studied using the binuclear ruthenium complex, Ru2D, to rapidly photooxidize cyt c1 and initiate the reaction (9, 17). The ruthenium photooxidation method provides a unique way to measure the dynamics of the ISP domain movement from the b state to the c1 state (9, 17, 33).
Experimental Procedures
Materials
A modification of the method of Downard et al. (34) was used to prepare Ru2D. Succinate, p-benzoquinone and antimycin A were obtained from Sigma Chemical Co., stigmatellin was purchased from Fluka Chemical Co., and N-Dodecyl-β-D-maltoside was obtained from Anatrace. [Co(NH3)5Cl]2+ was synthesized as described in reference (35), and 2,3-dimethoxy-5-methyl-6-(10-bromodecyl-) 1,4-benzoquinol, Q0C10BrH2, was prepared as previously reported (36). Succinate cytochrome c reductase (SCR) was purified as previously described (37).
Generation of R. sphaeroides strains expressing the his6-tagged bc1 complexes
The Quickchange™ XL site-directed mutagenesis kit from Stratagene was employed for mutagenesis. Plasmid pGEM7Zf(+)-fbcB was used as a template and forward and reverse primers were used for PCR amplification. Template plasmid pGEM7Zf(+)-fbcB was constructed by ligating the fragment between NsiI and XbaI in the plasmid pRKDfbcFBC6HQ into NsiI and XbaI sites of the pGEM7ZF(+) plasmid. The primers used are listed in the Table 1.
Table 1.
Primers used during mutagenesis operations
| Mutant (in the cytochrome b) |
Primers |
|---|---|
|
H276A (D252) |
5′-GCCGAACTACCTCGGCGCCCCCGACAACTACATCG-3′ |
| 3′-CGGCTTGATGGAGCCGCGGGGGCTGTTGATGTAGC-5′ | |
|
P277A (P253) |
5′-CGAACTACCTCGGCCACGCCGACAACTACATCGAGG-3′ |
| 3′-GCTTGATGGAGCCGGTGCGGCTGTTGATGTAGCTCC-5′ | |
|
D278A (D254) |
5′-CTACCTCGGCCACCCCGCCAACTACATCGAGGCGAAC-3′ |
| 3′-GATGGAGCCGGTGGGGCGGTTGATGTAGCTCCGCTTG-5′ | |
|
D278N |
5′-CTACCTCGGCCACCCCAACAACTACATCGAGGCGAAC-3′ |
| 3′-GATGGAGCCGGTGGGGTTGTTGATGTAGCTCCGCTTG-5′ | |
|
D278E |
5′-CTACCTCGGCCACCCCGAAAACTACATCGAGGCGAAC-3′ |
| 3′-GATGGAGCCGGTGGGGCTTTTGATGTAGCTCCGCTTG-5′ | |
|
D278I |
5′-CTACCTCGGCCACCCCATCAACTACATCGAGGCGAAC-3′ |
| 3′-GATGGAGCCGGTGGGGTAGTTGATGTAGCTCCGCTTG-5′ | |
|
D278H |
5′-CTACCTCGGCCACCCCCACAACTACATCGAGGCGAAC-3′ |
| 3′-GATGGAGCCGGTGGGGGTGTTGATGTAGCTCCGCTTG-5′ | |
|
N279A (N255) |
5′-CTCGGCCACCCCGACGCCTACATCGAGGCGAACCC-3′ |
| 3′-GAGCCGGTGGGGCTGCGGATGTAGCTCCGCTTGGG-5′ | |
|
Y280A (Y256) |
5′-GGCCACCCCGACAACGCCATCGAGGCGAACCCGC-3′ |
| 3′-CCGGTGGGGCTGTTGCGGTAGCTCCGCTTGGGCG-5′ | |
|
L286A (L262) |
5′-CATCGAGGCGAACCCGGCCTCGACGCCCGCGCAC-3′ |
| 3′-GTAGCTCCGCTTGGGCCGGAGCTGCGGGCGCGTG-5′ | |
|
L286E |
5′-CATCGAGGCGAACCCGGAGTCGACGCCCGCGCAC-3′ |
| 3′-GTAGCTCCGCTTGGGCCTCAGCTGCGGGCGCGTG-5′ | |
|
L286I |
5′-CATCGAGGCGAACCCGATCTCGACGCCCGCGCAC-3′ |
| 3′-GTAGCTCCGCTTGGGCTAGAGCTGCGGGCGCGTG-5′ | |
|
S287A (N263) |
5′-CGAGGCGAACCCGCTCGCCACGCCCGCGCACATCG-3′ |
| 3′-GCTCCGCTTGGGCGAGCGGTGCGGGCGCGTGTAGC-5′ | |
|
A290S (A266) |
5′-CCGCTCTCGACGCCCTCGCACATCGTGCCGG-3′ |
| 3′-GGCGAGAGCTGCGGGAGCGTGTAGCACGGCC-5′ | |
|
H291A (H267) |
5′-GCTCTCGACGCCCGCGGCCATCGTGCCGGAATGG-3′ |
| 3′-CGAGAGCTGCGGGCGCCGGTAGCACGGCCTTACC-5′ | |
|
I292A (I268) |
5′-CTCGACGCCCGCGCACGCCGTGCCGGAATGGTACTTC-3′ |
| 3′-GAGCTGCGGGCGCGTGCGGCACGGCCTTACCATGAAG-5′ | |
|
I292L |
5′-CTCGACGCCCGCGCACCTGGTGCCGGAATGGTACTTC-3′ |
| 3′-GAGCTGCGGGCGCGTGGACCACGGCCTTACCATGAAG-5′ | |
|
I292M |
5′-CTCGACGCCCGCGCACATGGTGCCGGAATGGTACTTC-3′ |
| 3′-GAGCTGCGGGCGCGTGTACCACGGCCTTACCATGAAG-5′ | |
|
I292V |
5′-CTCGACGCCCGCGCACGTCGTGCCGGAATGGTACTTC-3′ |
| 3′-GAGCTGCGGGCGCGTGCAGCACGGCCTTACCATGAAG-5′ | |
|
I292E |
5′-CTCGACGCCCGCGCACGAGGTGCCGGAATGGTACTTC-3′ |
| 3′-GAGCTGCGGGCGCGTGCTCCACGGCCTTACCATGAAG-5′ | |
| I292R | 5′-CTCGACGCCCGCGCACCGCGTGCCGGAATGGTACTTC-3′ |
| 3′-GAGCTGCGGGCGCGTGGCGCACGGCCTTACCATGAAG-5′ |
After mutagenesis, NsiI-XbaI fragment from the pGEM7Zf(+)-fbcBm was ligated into NsiI and XbaI sites of the pRKDfbcFBbpKmC6H plasmids which contained Kanamycin-resistant gene in the BstI and pinA sites (38). Strains containing recombinant plasmid will be sensitive to antibiotics kanamycin. The pRKDfbcFBmC6HQ plasmid in E. coli S17−1 cells was mobilized into R. sphaeroides BC-17 through conjugation (38). The engineered mutations were confirmed by DNA sequencing before and after photosynthetic growth as previously reported (38), which was performed by the Recombinant DNA/Protein Core Facility at Oklahoma State University.
Mutant cytochrome bc1 was purified as described by Xiao et al.(39). The steady-state activity of bc1 complexes was determined as described by Liu et al.(40).
Flash Photolysis Experiments
Flash photolysis experiments were carried out on 300 μL solutions contained in a 1-cm glass semimicrocuvette using the detection system described by Heacock et al. (41). A Phase R model DL1400 flash lamp-pumped dye laser using coumarin LD 490 produced a 480 nm light flash of < 0.5 μs duration. Samples typically contained 5 μM cyt bc1, 20 μM Ru2D, 5 mM [Co(NH3)5Cl]2+, in 20 mM sodium borate, pH 9.0, 0.01% dodecylmaltoside. The [Co(NH3)5Cl]2+ was used as a sacrificial electron acceptor. The cyt bc1 was treated with 10 μM QoC10BrH2, 1 mM succinate, and 50 nM SCR to completely reduce [2Fe2S] and cyt c1, and reduce cyt bH by 30%. The photooxidation and reduction of cyt c1 was monitored at 552 nm, while the reduction of cyt bH was monitored at 561 − 569 nm. Under the conditions used the reoxidation of cyt bH by Q in the Qi site was much slower than reduction because of the low concentration of oxidized Q.
Results
The kinetics of electron transfer within R. sphaeroides cytochrome bc1 were studied using the ruthenium dimer Ru2D to photoinitiate the reaction (9). Laser flash photolysis of a solution containing Ru2D and cyt bc1 with cyt c1 and [2Fe2S] initially reduced resulted in rapid photooxidation of cyt c1, followed by electron transfer from from [2Fe2S] to cyt c1 with a rate constant of k1 = 60,000 s−1, monitored at 552 nm (Figure 1) (9). The oxidant-induced reduction of cyt bH monitored at 561 − 569 nm has a rate constant k2 = 2,300 s−1 (Figure 1), which is rate-limited by electron transfer from QH2 to [2Fe2S] with rate constant k2 followed by rapid electron transfer from [2Fe2S] to cyt c1, and from the semiquinone to cyt bL and cyt bH (Scheme 1) (9). Binding famoxadone to the Qo site decreases the rate constant k1 from 60,000 s−1 to 5,400 s−1, indicating that the dynamics of rotation of the ISP from the b state to the c1 state was significantly decreased (Figure 1) (33). Since famoxadone binding affected the conformation of the ef loop in cyt b, it was suggested that the ef loop might play a role in controlling the conformation of the ISP protein (31, 33). In order to examine this conformational link further, a series of mutants in which ef loop residues were substituted with Ala were prepared. These included the ef loop residues H276, P277, D278, N279, Y280, L286, S287, A290, H291, and I292 (Figures 2, 3). The steady-state activity and the values of the rate constants k1 and k2 were measured for each of these mutants, both in the presence and absence of famoxadone. A number of the mutants caused relatively small decreases in the rate constant k1, but the largest changes were seen for the mutants D278A, Y280A, L286A, and I292A (Table 2). Additional mutants at residues D278, L286 and I292 were prepared to further explore the roles of these residues.
Figure 1.
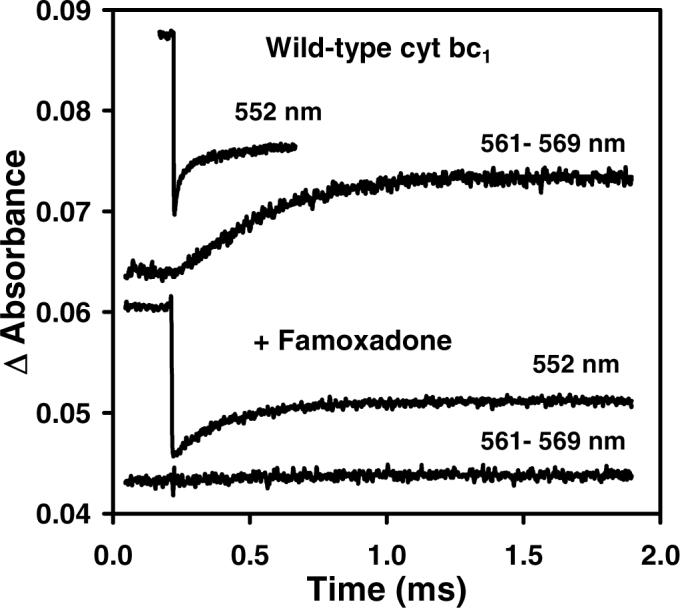
Electron transfer within wild-type R. sphaeroides cyt bc1 following photooxidation of cyt c1 (9). The 300 μL sample contained 5 μM cyt bc1, 20 μM Ru2D, 5 mM [Co(NH3)5Cl]2+, in 20 mM sodium borate, pH 9.0 with 0.01% dodecylmaltoside. The cyt bc1 was treated with 10 μM QoC10BrH2, 1 mM succinate, and 50 nM SCR to completely reduce [2Fe2S] and cyt c1, and reduce cyt bH by about 30%. The sample was then excited with a 480 nm laser flash to photooxidize cyt c1 within 1 μs. (Top two traces) The 552 nm transient indicates that cyt c1 was photooxidized within 1 μs, and then reduced in a biphasic reaction with rate constants of 60,000 s−1 and 2,000 s−1. The rate constant for the reduction of cyt bH measured at 561 − 569 nm was 2,300 s−1. (Bottom two traces) Addition of 30 μM famoxadone decreased the rate of reduction of cyt c1 to 5,400 s−1 and eliminated reduction of cyt bH.
Scheme 1.
Figure 2.
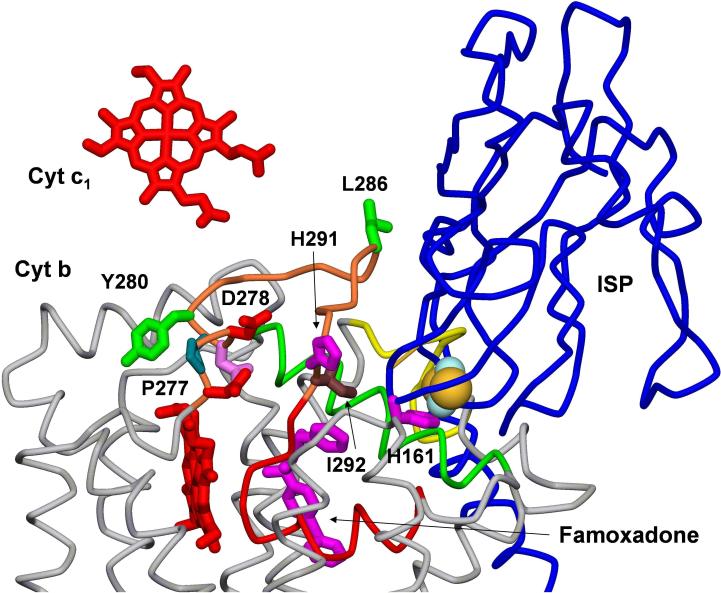
X-ray crystal structure of bovine cyt bc1 with bound famoxadone (31). The cyt c1 and cyt bL hemes are colored red, the [2Fe2S] center is represented by a CPK model, the ISP is blue, and cyt b is grey. Residues 252−268 in the ef loop are colored orange while residues 269−283 in the PEWY sequence and the ef helix are red. Residues 136−152 in the cd1 helix are green and residues 163−171 in the neck-contacting domain are colored yellow. The residues in cyt b that were mutated are shown as sticks and labeled with R. sphaeroides sequence numbering.
Figure 3.
Sequence alignment of residues near the Qo pocket of the cyt b subunit in cyt bc1 complexes from bovine (BT) and R. sphaeroides (RS). Residues with a high sequence similarity are in boldface. Helices determined crystallographically are indicated by gray rectangles. Residues in direct contact with the ISP are indicated with a black dot. Adopted from reference 21.
Table 2.
Effect of the mutations in the ef loop of cyt b on the kinetics of electron transfer within R. sphaeroides cyt bc1.
| Cyt bc1 Mutanta | Specific Activityb | k1c (s−1) | Famoxadone k1 (s−1) | k2d(s−1) | Δ CAe(Å) |
|---|---|---|---|---|---|
| WT | 2.35 | 60,000 | 5,400 | 2,300 | |
| H276A (D252) | 1.56 | 42,000 | 2,800 | 2,200 | 3.3 |
| P277A (P253) | 1.10 | 35,000 | 4,500 | 1,700 | 3.0 |
| D278A (D254) | 1.43 | 26,000 | 2,300 | 2,300 | 2.9 |
| D278N | 1.88 | 35,000 | 2,500 | 2,400 | |
| D278E | 1.60 | 51,000 | 6,700 | 2,900 | |
| D278I | 2.41 | 38,000 | 4,300 | 2,000 | |
| D278H | 1.49 | 36,000 | 4,100 | 1,500 | |
| N279A (N255) | 2.43 | 37,000 | 4,300 | 1,900 | 1.5 |
| Y280A (Y256) | 1.34 | 7,900 | 3,200 | 2,800 | 0.5 |
| L286A (L262) | 0.78 | 33,000 | 35,000 | 740 | |
| L286E | 0.88 | 17,000 | 12,000 | 1,900 | |
| L286I | 2.40 | 18,000 | 2,900 | 2,300 | |
| S287A (N263) | 2.16 | 55,000 | 5,700 | 1,000 | 0.7 |
| A290S (P266) | 2.65 | 49,000 | 5,600 | 1,900 | 0.9 |
| H291A (H267) | 2.54 | 51,000 | 4,100 | 2,000 | 1.4 |
| I292A (I268) | 0.81 | 4,400 | 3,000 | 350 | 1.4 |
| I292L | 1.30 | 10,000 | 4,700 | 1,500 | |
| I292M | 0.92 | 6,200 | 2,800 | 1,700 | |
| I292V | 0.92 | 33,000 | 3,500 | 1,300 | |
| I292E | 0.12 | - | - | - | |
| I292R | 0.56 | 10,000 | 2,500 | 1,700 |
The corresponding residues of bovine cyt b are given in parenthesis.
Specific activity is expressed as micromole of cyt c reduced/min/nmol cyt b at 25 °C using chromatophores. The error limits are ± 15%.
The rate constant k1 for electron transfer from [2Fe2S] to cyt c1 was measured at 552 nm in a solution containing 5 μM cyt bc1, 20 μM Ru2D, 5 mM [Co(NH3)5Cl]2+, in 20 mM sodium borate, pH 9.0, 0.01% dodecylmaltoside. The cyt bc1 was treated with 10 μM QoC10BrH2, 1 mM succinate, and 50 nM SCR to completely reduce [2Fe2S] and cyt c1, and reduce cyt bH by about 30%. Famoxadone (30 μM) was added where indicated. The error limits are ± 20%.
The rate constant k2 for electron transfer from QH2 to [2Fe2S] was measured from the rate of cyt bH reduction at 561−569 nm under the conditions described for k1 above. The error limits are ± 20%. Addition of famoxadone eliminated that 561−569 nm transients for all of the mutants, indicating that famoxadone bound to all the mutants in the Qo pocket.
ΔCA is the displacement of the α carbon of the indicated residue in bovine cyt bc1 induced by famoxadone binding (31).
The mutation I292A decreased the steady-state activity from 2.35 to 0.81, k1 from 60,000 s−1 to 4,400 s−1 and k2 from 2,300 s−1 to 350 s−1 (Figure 4A). Famoxadone binding had a relatively small additional effect on k1, decreasing it only to 3,000 s−1. All of the mutations at I292 led to significant decreases in k1 and k2 (Table 2). The I292L mutation decreased k1 to 10,000 s−1, while the I292M mutation decreased k1 to 6,200 s−1 (Figures 4B, 4C). Famoxadone binding had a rather small effect on these two mutants, decreasing k1 to 4,700 s−1 and 2,800 s−1, respectively. The largest effect was observed for the I292E mutation, which decreased the steady-state activity to 0.12, and eliminated all electron transfer transients in the ruthenium photooxidation experiment. Famoxadone binding eliminated the reduction of cyt bH observed at 561 nm for all of the mutants, indicating that famoxadone binding was not affected.
Figure 4.
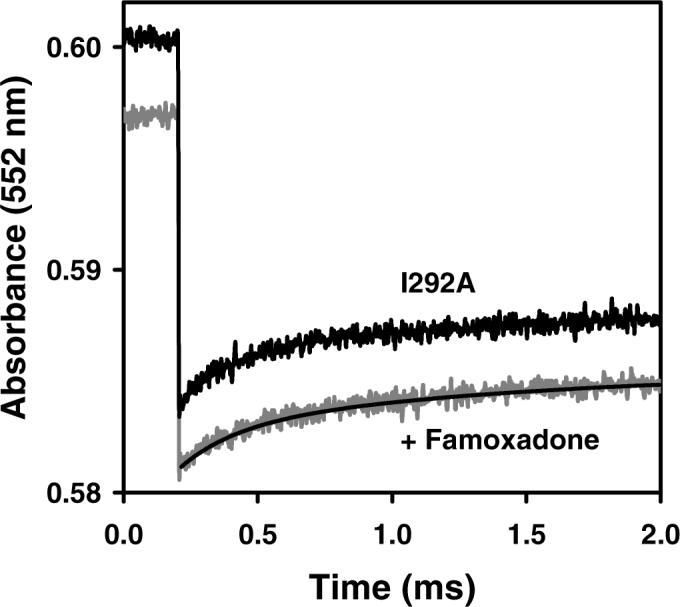

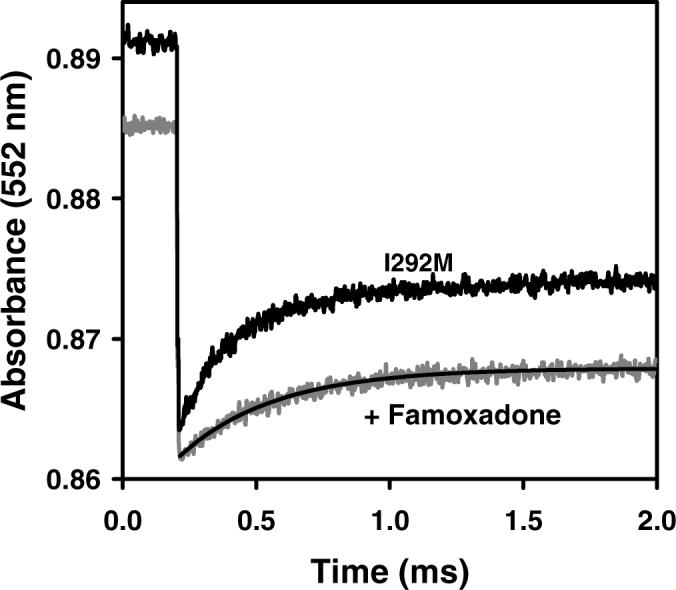
Electron transfer from [2Fe2S] to cyt c1 in R. sphaeroides cyt bc1 mutants following photooxidation of cyt c1. The conditions were the same as described in Figure 1. (A) I292A cyt bc1. The rate constant k1 is 4,400 s−1 in the absence of inhibitor, and 3000 s−1 in the presence of 30 μM famoxadone. (B) I292L cyt bc1. The rate constant k1 is 10,000 s−1 in the absence of inhibitor, and 4,700 s−1 in the presence of famoxadone. (C) I292M cyt bc1. The rate constant k1 is 6,200 s−1 in the absence of inhibitor, and 2,800 s−1 in the presence of famoxadone.
The mutation L286A decreased the steady-state rate by 3-fold, k1 to 33,000 s−1, and k2 to 740 s−1. Most surprisingly, famoxadone binding did not significantly affect the rate constant k1, but it did completely inhibit reduction of cyt bH. The L286E mutant decreased k1 to 17,000, while addition of famoxadone only decreased k1 to 12,000. The conservative mutation L286I decreased k1 to 18,000, but famoxadone binding decreased k1 by a larger factor, to 2,900.
The D278A mutation decreased the steady-state activity from 2.35 to 1.43, and the rate constant k1 from 60,000 s−1 to 26,000 s−1, but did not affect the rate constant k2. Famoxadone binding decreased the rate constant k1 to 2,300 s−1. The mutants D278N, D278E, D278I, and D278H did not have as large an effect on k1 or k2 as the D278A mutation. The Y280A mutation decreased the steady-state activity from 2.35 to 1.34, and the rate constant k1 from 60,000 s−1 to 7,900 s−1, without significantly affecting the rate constant k2. Famoxadone did bind well to this mutant, as indicated by the complete inhibition of reduction of cyt bH, but it only decreased the rate constant k1 from 7,900 s−1 to 3,200 s−1.
Discussion
Changes in the conformation of the ISP play an essential role in the mechanism of cyt bc1, and it is important to understand how the dynamics of these conformational changes control electron transfer within the complex. The ruthenium photooxidation technique provides a unique method to measure the rate constants for electron transfer from the [2Fe2S] center to cyt c1, as well as from QH2 to the [2Fe2S] center. A number of experiments have indicated that the measured rate constant k1 for electron transfer from [2Fe2S] to cyt c1 is rate-limited by a conformational gating mechanism (33). If k1 was rate-limited by true electron transfer from [2Fe2S] to cyt c1, then Marcus theory would predict a large dependence on the driving force of the reaction, which depends on the difference in redox potentials of [2Fe2S] and cyt c1. However, k1 is independent of the protonation state of His-161, which significantly affects the redox potential of [2Fe2S] (33). Moreover, the rate constant is not affected by ISP mutations which decrease the redox potential of [2Fe2S] by up to 160 mV (33). These studies indicate that the rate constant k1 provides a direct measure of the dynamics of the conformational change of the ISP from the b-state and/or the mobile state to the c1 state (33, 42).
A number of mechanisms have been proposed for the bifurcated electron transfer reaction at the Qo site in which the first electron is transferred from QH2 to [2Fe2S], while the second electron is transferred from semiquinone to cyt bL (21-29). An important requirement for these mechanisms is that they account for the reversibility of the reaction, as well as minimization of short circuit reactions, such as the delivery of both electrons from QH2 to [2Fe2S]. Double gating mechanisms have been proposed in which QH2 only reacts at the active Qo site when the ISP is in the b-state and both [2Fe2S] and cyt bL are oxidized (23, 25-29). In another proposal, the semiquinone anion moves from the distal site near [2Fe2S] to a position near oxidized cyt bL to favor electron transfer to the latter (22, 27). Concerted mechanisms have also been proposed in which QH2 transfers both electrons to [2Fe2S] and cyt bL simultaneously without formation of a semiquinone intermediate (23, 26, 28, 29). A recent freeze-quench EPR experiment has shown that QH2 reduces [2Fe2S] and cyt bL simultaneously with a half-time of 250 μs for a fast phase which accounts for about 10% reduction of each redox center (3). No semiquinone was detected at the Qo site during oxidation of QH2, consistent with earlier studies. It is apparent that either the semiquinone intermediate is very short-lived, or the bifurcation reaction involves a concerted mechanism.
It has been proposed that a structural linkage between the quinol substrate in the Qo binding site and the conformation of the ISP might play an important role in the mechanism of bifurcated electron transfer (21). Unfortunately, it has not been possible to experimentally detect QH2, semiquinone, or Q in the Qo binding pocket. Nevertheless, Qo site inhibitors have many similarities to the natural ubiquinol substrate, and it has been suggested that the effects of these inhibitors on the conformational linkage between cyt b and the ISP might provide insight into the catalytic mechanism of the bifurcation reaction (21). X-ray crystallographic studies have shown that Qo site inhibitors including stigmatellin, UHDBT, famoxadone, and JG-144 displace both the cd1 helix and the PEWY sequence in the ef helix outward to expand the Qo pocket and form a binding crater which captures the ISP in the b-state conformation (21, 43). Stigmatellin binding leads to the largest changes in the Qo pocket, and forms a hydrogen bond with the His-161 ligand of the reduced [2Fe2S] center, thus increasing its redox potential by 200 − 250 mV, and completely immobilizing the ISP in the b conformation (5-8, 30). It has been proposed that QH2 might bind in a conformation similar to that of stigmatellin, with a hydrogen bond to His-161 to facilitate proton-coupled electron transfer to [2Fe2S] (22).
Famoxadone binds somewhat deeper in the Qo pocket than stigmatellin, and does not form a hydrogen bond with His-161 on the ISP (31). Even though famoxadone does not contact the ISP, its binding leads to extensive conformational changes on the surface of cyt b which are correlated with the capture of the ISP from the loose state to the b state (31). These changes include one domain between residues 160−175 (bovine numbering) that contacts the neck region of the ISP, another domain between residues 262 and 268 at the end of the ef loop which forms the docking crater for the ISP, and the middle of the ef loop (residues 252−256) which connects the Qo pocket with the other two surface domains (Figures 2, 3) (31). Famoxadone binding to the Qo site decreases the rate constant k1 for electron transfer from [2Fe2S] to cyt c1 from 60,000 s−1 to 5,400 s−1 (33). Therefore, famoxadone does not completely lock the ISP in the b state, but instead significantly decreases the rate of release of the ISP from the b state to the c1 state.
In order to further explore the role of the ef loop in regulating the dynamics of the ISP, a series of mutants at residues in the ef loop were constructed. R. sphaeroides cyt b residues 276 − 280 correspond to bovine residues 252 − 256 which are in the middle of the ef loop on the surface of cyt b (Figures 2, 3). These residues undergo large displacements upon famoxadone binding, suggesting that they might relay conformational changes in the Qo pocket to the ISP docking crater. Surprisingly, mutation of residues H276, P277, D278, and N279 led to relatively small changes in k1 and k2, suggesting that if these residues do help relay conformational changes, that function is not sensitive to substitution of individual amino acid side changes. The D278A mutation led to the largest decrease in k1 for this group, from 60,000 s−1 to 26,000 s−1, but no significant change in rate constant k2. Famoxadone binding to this mutant decreased k1 to 2,300 s−1, which is similar to the percent reduction observed in wild-type cyt bc1, suggesting that this mutant does not modulate the effect of famoxadone. The mutation Y280A led to a substantial decrease in k1 to 7,900 s−1, while famoxadone binding only caused a further decrease to 3,200 s−1. This mutation might cause a conformational change similar to that of famoxadone, limiting the additional effect of famoxadone binding.
R. sphaeroides cyt b residues 286 − 292 correspond to bovine residues 262 − 268 in the ef loop which forms part of the ISP docking crater. Mutation of residues S287, A290, and H291 had relatively little effect on k1, which is surprising in the case of H291 since the histidine side chain moves 9.3 Å upon famoxadone binding (31). However, mutations at I292 located in the ISP docking crater near the [2Fe2S] center had a large effect on electron transfer activity (Table 2). The I292A mutation decreased k1 to 4,400 s−1 while famoxadone binding only further decreased it to 3,000 s−1. The mutations I292L and I292M also led to substantial decreases in k1, while famoxadone binding caused relatively small additional decreases in k1. It appears that these mutations significantly decrease the rate of release of the ISP from cyt b, but famoxadone binding leads to a relatively small additional decrease in the rate of release. The I292A mutation led to a decrease in the rate constant k2 for QH2 oxidation to 350 s−1, indicating that this mutation had an effect on the conformation of the QH2 reaction site. Substitution of I292 with a basic arginine residue was tolerated with a decrease in k1 to 10,000 s−1 and only a modest change in k2. In contrast, substitution of I292 with an acidic glutamate nearly completely eliminated both steady-state activity and electron transfer activity measured by the ruthenium photooxidation method. It is apparent that I292 plays an important role in controlling the conformation of the ISP.
The effects of mutation of L286 at the tip of the ef loop are particularly interesting. The mutation L286A decreases the rate constant k1 to 33,000 s−1 and k2 to 740 s−1. However, famoxadone binding does not lead to any further decrease in k1, (Table 2) suggesting that this mutation might block the famoxadone-induced conformational change in the wild-type protein which decreases the rate constant k1 by such a large factor. The L286E mutation also led to a significant decrease in the rate constant k1, to 17,000 s−1, while famoxadone binding did not cause much further decrease. The importance of L286 is also illustrated in experiments by Darrouzet and Daldal (14, 19). They found that insertion of one alanine at residue 46 in the neck region of the ISP decreased the rate of the domain movement from the b state to the c1 state to a half time of 10 ms, which was slow enough to measure in R. capsulatus chromatophores. A second revertant mutation substituting Leu for Phe at residue 286 restored rapid domain movement, which was too fast to measure in chromatophores (19). This finding is particularly remarkable given that cyt b residue 286 is more than 25 Å from the neck region of the ISP. Revertant mutations were also found in the ISP neck region for a primary mutation at T288 in the ef loop (24). A possible mechanism for this linkage between the ef loop and ISP neck region is suggested by the effect of famoxadone binding, which causes correlated conformational changes in both the ef loop region and the cyt b residues 179 −187 which contact the ISP neck region. It appears likely that the conformational linkage between the ISP neck region and the cyt b ef loop is mediated by the cyt b protein using structural linkages similar to those caused by famoxadone binding.
It has been proposed that the conformation of the ISP plays an important role in several different stages of the mechanism of cyt bc1 (21). First, it is proposed that binding QH2 to the active Qo pocket displaces the cd1 helix and the ef helix outwards to capture the oxidized ISP in the b state conformation. The formation of a hydrogen bond between QH2 and His-161 would stabilize the active site conformation, and allow proton-coupled electron transfer from QH2 to oxidized [2Fe2S]. After the second electron is transferred from semiquinone to cyt bL and cyt bH, oxidized Q would leave the Qo binding pocket and the cd1 helix and the ef helix would relax to their original positions, releasing the ISP and allowing it to rotate to the c1 position and transfer an electron to cyt c1 (21). An important question about this mechanism is whether ISP is released from the b state after the first electron is transferred to [2Fe2S] and the hydrogen bond to His-161 is broken, or remains locked in the b state until cyt bH is reduced. Famoxadone binding is informative in this regard, because it leads to many of the same conformational changes in the ISP binding crater as stigmatellin, but does not form a hydrogen bond to His-161. The rate of release of the ISP from the b-state is decreased from 60,000 s−1 to 5,000 s−1 by famoxadone binding, but the ISP is not completely locked in the b-state (33). Once the ISP is released from the b state and rotates to the cyt c1 state, there may be conformational linkages that prevent its return to the b state under conditions that would lead to short-circuit reactions. When cyt bH is reduced in the presence of antimycin, an electron is not transferred from cyt b to the ISP on a long time scale, suggesting that oxidized ISP does not return to the b state in the absence of QH2. The ef loop presents a barrier to the rotation of the ISP between the b and c1 states (19, 32), and this barrier might regulate the rotation in both directions to facilitate productive electron transfer and prevent short-circuit reactions.
Acknowledgments
This work was supported by NIH grants GM20488 (FM and BD), NCRR COBRE 1 P20 RR15569 (FM and BD), and GM30721 (C.- A. Yu).
Abbreviations used
- cyt
cytochrome
- ISP
Rieske iron-sulfur protein
- [2Fe2S]
Rieske iron-sulfur center
- Ru2D
[Ru(bpy)2]2(qpy)(PF6)4
- qpy
2,2’:4’,4”:2”,2”’–quaterpyridine
- Q0C10BrH2
2,3-dimethoxy-5-methyl-6-(10-bromodecyl-) 1,4-benzoquinol
- SCR
succinate cytochrome c reductase
References
- 1.Trumpower BL, Gennis RB. Energy transduction by cytochrome complexes in mitochondrial and bacterial respiration: the enzymology of coupling electron transfer reactions to transmembrane proton translocation. Annu. Rev. Biochem. 1994;63:675–716. doi: 10.1146/annurev.bi.63.070194.003331. [DOI] [PubMed] [Google Scholar]
- 2.Trumpower BL. The Protonmotive Q Cycle. Energy Transduction by Coupling of Proton Translocation to Electron Transfer by the Cytochrome bc1 Complex. J. Biol. Chem. 1990;265:11409–11412. [PubMed] [Google Scholar]
- 3.Zhu J, Egawa T, Yeh S-R, Yu L, Yu C-A. Simultaneous Reduction of Iron-sulfur Protein and Cytochorme bL during ubiquinol oxidation in Cytochrome bc1 Complex. Proc. Nat. Acad. Sci. USA. 2006 doi: 10.1073/pnas.0607812104. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia D, Yu C-A, Kim H, Xia J-Z, Kachurin AM, Zhang L, Yu L, Deisenhofer J. Crystal Structure of the Cytochrome bc1 Complex from Bovine Heart Mitochondria. Science. 1997;277:60–66. doi: 10.1126/science.277.5322.60. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Z, Huang L, Shulmeister VM, Chi Y-I, Kim KK, Hung L-W, Crofts AR, Berry EA, Kim S-H. Electron Transfer by Domain Movement in Cytochrome bc1. Nature. 1998;392:677–684. doi: 10.1038/33612. [DOI] [PubMed] [Google Scholar]
- 6.Iwata S, Lee JW, Okada K, Lee JK, Wata M, Rasmussen B, Link TA, Ramaswamy S, Jap BK. Complete Structure of the 11-Subunit Bovine Mitochondrial Cytochrome bc1 Complex. Science. 1998;281:64–71. doi: 10.1126/science.281.5373.64. [DOI] [PubMed] [Google Scholar]
- 7.Kim H, Xia D, Yu C-A, Xia J-Z, Kachurin AM, Zhang L, Yu L, Deisenhofer J. Inhibitor binding changes domain mobility in the iron-sulfur protein of the mitochondrial bc1 complex from bovine heart. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8026–8033. doi: 10.1073/pnas.95.14.8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunte C, Koepke J, Lange C, Rossmanith T, Michel H. Structure at 2.3 Å resolution of the cytochrome bc(1) complex from the yeast Saccharomyces cerevisiae co-crystallized with an antibody Fv fragment. Stucture Fold Des. 2000;8:669–84. doi: 10.1016/s0969-2126(00)00152-0. [DOI] [PubMed] [Google Scholar]
- 9.Sadoski RC, Engstrom G, Tian H, Zhang L, Yu C-A, Yu L, Durham B, Millett F. Use of a Photoactivated Ruthenium Dimer Complex To Measure Electron Transfer between the Rieske Iron-Sulfur Protein and Cytochrome c1 in the Cytochrome bc1 Complex. Biochemistry. 2000;39:4231–4236. doi: 10.1021/bi000003o. [DOI] [PubMed] [Google Scholar]
- 10.Tian H, Yu L, Mather M, Yu CA. Flexibility of the Neck Region of the Rieske Iron-Sulfur Protein is Functionally Important in the Cytochrome bc1 Complex. J. Biol. Chem. 1998;273:27953–27959. doi: 10.1074/jbc.273.43.27953. [DOI] [PubMed] [Google Scholar]
- 11.Xiao K, Yu L, Yu C-A. Confirmation of the involvement of protein domain movement during the catalytic cycle of the cytochrome bc1 complex by the formation of an intersubunit disulfide bond between cytochrome b and the iron-sulfur protein. J. Biol. Chem. 2000;275:38597–38604. doi: 10.1074/jbc.M007444200. [DOI] [PubMed] [Google Scholar]
- 12.Tian H, White S, Yu L, Yu C-A. Evidence for the head domain movement of the rieske iron-sulfur protein in electron transfer reaction of the cytochrome bc1 complex. J. Biol. Chem. 1999;274:7146–7152. doi: 10.1074/jbc.274.11.7146. [DOI] [PubMed] [Google Scholar]
- 13.Darrouzet E, Valkova-Valchanova M, Daldal F. Probing the role of the Fe-S subunit hinge region during Q(o) site catalysis in Rhodobacter capsulatus bc(1) complex. Biochemistry. 2000;39:15475–15483. doi: 10.1021/bi000750l. [DOI] [PubMed] [Google Scholar]
- 14.Darrouzet E, Valkova-Valchanova M, Moser CC, Dutton PL, Daldal F. Uncovering the [2Fe2S] domain movement in cytochrome bc1 and its implications for energy conversion. Proc. Natl. Acad. Sci. U.S.A. 2000;25:4567–72. doi: 10.1073/pnas.97.9.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nett JH, Hunte C, Trumpower BL. Changes to the length of the flexible linker region of the Rieske protein impair the interaction of QH2 with the cytochrome bc1 complex. Eur. J. Biochem. 2000;267:5777–5782. doi: 10.1046/j.1432-1327.2000.01650.x. [DOI] [PubMed] [Google Scholar]
- 16.Gosh M, Wang C, Ebert E, Vadlamuri S, Beattie DS. Substituting leucine for alanine-86 in the tether region of the iron-sulfur protein of the cytochrome bc1 complex affects the mobility of the [2Fe2S] domain. Biochemistry. 2001;40:327–335. doi: 10.1021/bi001708t. [DOI] [PubMed] [Google Scholar]
- 17.Engstrom G, Xiao K, Yu C-A, Yu L, Durham B, Millett F. Photoinduced electron transfer between the Rieske iron-sulfur protein and cytochrome c(1) in the Rhodobacter sphaeroides cytochrome bc(1) complex. Effects of pH, temperature, and driving force. J. Biol. Chem. 2002;277:31072–31078. doi: 10.1074/jbc.M202594200. [DOI] [PubMed] [Google Scholar]
- 18.Darrouzet E, Valkova-Valchanova M, Daldal F. The [2Fe-2S] cluster E(m) as an indicator of the iron-sulfur subunit position in the ubihydroquinone oxidation site of the cytochrome bc1 complex. J. Biol. Chem. 2002;277:3464–3470. doi: 10.1074/jbc.M107973200. [DOI] [PubMed] [Google Scholar]
- 19.Darrouzet E, Daldal F. Movement of the Iron-Sulfur Subunit beyond the ef Loop of Cytochrome b is required for Multiple Turnovers of the bc1 Complex but not for a Single Turnover Qo Site Catalysis. J. Biol. Chem. 2002;277:3471–3476. doi: 10.1074/jbc.M107974200. [DOI] [PubMed] [Google Scholar]
- 20.Brugna M, Rodgers S, Schricker A, Montoya G, Kazmeier M, Nitschike W, Sinning I. A spectroscopic method for observing the domain movement of the Rieske iron-sulfur protein. Proc. Nat. Acad. Sc. US. 2000;97:2069–2074. doi: 10.1073/pnas.030539897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esser L, Gong X, Yang S, Yu L, Yu C-A, Xia D. Surface-modulated motion switch: Capture and release of iron-sulfur protein in the cytochrome bc1 complex. Proc. Nat. Acad. Sci. U.S.A. 2006;103:13045–13050. doi: 10.1073/pnas.0601149103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong S, Ugulava N, Guergova-Kuras M, Crofts AR. The Energy Landscape for Ubihydroquinone Oxidation at the Qo Site of the bc1 Complex in R. sphaeroides. J. Biol. Chem. 1999;274:33931–33944. doi: 10.1074/jbc.274.48.33931. [DOI] [PubMed] [Google Scholar]
- 23.Osyczka A, Moser CC, Daldal F, Dutton PL. Reversible redox energy coupling in electron transfer chains. Nature. 2004;427:607–12. doi: 10.1038/nature02242. [DOI] [PubMed] [Google Scholar]
- 24.Darrouzet E, Daldal F. Protein-protein interactions between cytochrome b and the Fe-S protein subunits during QH2 oxidation and large-scale domain movement in the bc1 complex. Biochemistry. 2003;42:1499–507. doi: 10.1021/bi026656h. [DOI] [PubMed] [Google Scholar]
- 25.Rich P. The quinine chemistry of bc complexes. Biochim. Biochphys. Acta. 2004;1658:165–171. doi: 10.1016/j.bbabio.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Osyczka A, Moser CC, Dutton PL. Fixing the Q cycle. Trends Biochem. Sci. 2005;30:176–182. doi: 10.1016/j.tibs.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Crofts AR, Lhee S, Crofts SB, Cheng J, Rose S. Proton pumping in the bc(1) complex: A new gating mechanism that prevents short circuits. Biochim Biophys Acta. 2006 doi: 10.1016/j.bbabio.2006.02.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Mulkidjanian AY. QH2 oxidation in the cytochrome bc1 complex: Reaction mechanism and prevention of short-circuiting. Biochim. Biophys. Acta. 2005;1709:5–34. doi: 10.1016/j.bbabio.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Osyczka A, Zhang H, Mathe C, Rich PR, Moser CC, Dutton PL. Role of the PEWY Glutamate in Hydroquinone-Quinone Oxidation-Reduction Catalysis in the Q(o) Site of Cytochrome bc(1) Biochemistry. 2006;45:10492–503. doi: 10.1021/bi060013a. [DOI] [PubMed] [Google Scholar]
- 30.von Jagow G, Ohnishi T. The chromone inhibitor stigmatellin--binding to the QH2 oxidation center at the C-side of the mitochondrial membrane. FEBS Lett. 1985;185:311–315. doi: 10.1016/0014-5793(85)80929-7. [DOI] [PubMed] [Google Scholar]
- 31.Gao X, Wen X, Yu C-A, Esser L, Tsao S, Quinn B, Zhang L, Yu L, Xia D. The crystal structure of mitochondrial cytochrome bc1 in complex with famoxadone: the role of aromatic-aromatic interaction in inhibition. Biochemistry. 2002;41:11692–11702. doi: 10.1021/bi026252p. [DOI] [PubMed] [Google Scholar]
- 32.Izrailev S, Crofts AR, Berry EA, Schulten K. Steered molecular dynamics simulation of the Rieske subunit motion in the cytochrome bc(1) complex. Biophys J. 1999;77:1753–68. doi: 10.1016/S0006-3495(99)77022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao K, Engstrom G, Rajagukguk S, Yu C-A, Yu L, Durham B, Millett F. Effect of Famoxadone on Photoinduced Electron Transfer between the Iron-Sulfur Center and Cytochrome c1 in the Cytochrome bc1 Complex. J. Biol. Chem. 2003;278:11419–11426. doi: 10.1074/jbc.M211620200. [DOI] [PubMed] [Google Scholar]
- 34.Downard AJ, Honey GE, Phillips LF, Steel PJ. Inorg. Chem. 1991;30:2259–2260. [Google Scholar]
- 35.Moeller T, editor. Inorganic Synthesis. V. McGraw Hill Book Company, Inc.; New York: 1957. p. 185. [Google Scholar]
- 36.Yu CA, Yu L. Syntheses of biologically active ubiquinone derivatives. Biochemistry. 1982;21:4096–4101. doi: 10.1021/bi00260a028. [DOI] [PubMed] [Google Scholar]
- 37.Yu L, Yu CA. Quantitative resolution of succinate-cytochrome c reductase into succinate-ubiquinone and QH2-cytochrome c reductases. J. Biol. Chem. 1982;257:2016–2021. [PubMed] [Google Scholar]
- 38.Mather MW, Yu L, Yu C-A. The involvement of threonine 160 of cytochrome b of Rhodobacter sphaeroides cytochrome bc1 complex in quinone binding and interaction with subunit IV. J. Biol. Chem. 1995;270:28668–28675. doi: 10.1074/jbc.270.48.28668. [DOI] [PubMed] [Google Scholar]
- 39.Xiao K, Yu L, Yu C-A. Confirmation of the involvement of protein domain movement during the catalytic cycle of the cytochrome bc1 complex by the formation of an intersubunit disulfide bond between cytochrome b and the iron-sulfur protein. J. Biol. Chem. 2000;275:38597–38604. doi: 10.1074/jbc.M007444200. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Yu CA, Yu L. The role of extra fragment at the C-terminal of cytochrome b (Residues 421−445) in the cytochrome bc1 complex from Rhodobacter sphaeroides. J. Biol. Chem. 2004;279:47363–47371. doi: 10.1074/jbc.M406497200. [DOI] [PubMed] [Google Scholar]
- 41.Heacock C, Liu R, Yu C-A, Yu L, Durham B, Millett F. Intracomplex electron transfer between ruthenium-cytochrome c derivatives and cytochrome c1. J. Biol. Chem. 1993;268:27171–27175. [PubMed] [Google Scholar]
- 42.Yu CA, Wen X, Xiao K, Xia D, Yu L. Inter- and intra-molecular electron transfer in the cytochrome bc(1) complex. Biochim. Biophys. Acta. 2002;1555:65–70. doi: 10.1016/s0005-2728(02)00256-6. [DOI] [PubMed] [Google Scholar]
- 43.Esser L, Quinn B, Li YF, Zhang M, Elberry M, Yu L, Yu CA, Xia D. Crystallographic studies of quinol oxidation site inhibitors: a modified classification of inhibitors for the cytochrome bc(1) complex. J Mol Biol. 2004;341:281–302. doi: 10.1016/j.jmb.2004.05.065. [DOI] [PubMed] [Google Scholar]