Abstract
Previous studies have shown large decreases in cochlear-implant psychophysical detection thresholds during the weeks following the onset of electrical testing. The current study sought to determine the variables underlying these threshold decreases by examining the effects of four deafening and implantation procedures on detection thresholds and implant impedances. Thirty-two guinea pigs were divided into four matched groups. Group I was deafened and implanted Day 0 and began electrical testing Day 1. Group II was deafened and implanted Day 0 and began electrical testing Day 45. Group III was deafened Day 0, implanted Day 45 and began electrical testing Day 46. Group IV was not predeafened but was implanted Day 0 and began electrical testing Day 1. All groups showed threshold decreases over time but the magnitude of change, time course and final stable threshold levels depended on the type and time course of treatment. Impedances increased over the first two weeks following the onset of electrical testing except in Group II. Results suggest that multiple mechanisms underlie the observed threshold shifts including (1) recovery of the cochlea from a temporary pathology caused by the deafening and/or implantation procedures, (2) effects of electrical stimulation on the auditory pathway, (3) tissue growth in the implanted cochlea. They also suggest that surviving hair cells influence electrical-threshold levels.
Keywords: Cochlear implant, Electrical detection threshold, Neomycin deafening, Animal psychophysics, Impedance, Guinea pig
1. Introduction
Previous studies involving chemical deafening and implantation of the cochlea in animal research on cochlear implants showed large fluctuations in psychophysical detection thresholds to electrical stimuli for a period of time following surgery. In nonhuman primates, thresholds increased within days after surgery, then decreased over a period of one to several months, eventually stabilizing at or below the originally measured levels (Pfingst et al., 1979; Pfingst, 1990). In guinea pigs, threshold decreases were observed several days after surgery culminating in stable, relatively low thresholds after 30 – 40 days (Miller et al., 2000). It is important to understand the functional condition of the auditory system during these periods of change, particularly if that condition is changing rapidly over time, and to understand its relationship to the longer term, more stable periods that characterize patients with cochlear implants. This study aims to define variables responsible for these postimplantation fluctuations and considers several possible hypotheses for mechanisms underlying the observed changes.
One hypothesis is that postimplantation threshold changes may reflect a temporary pathology and subsequent recovery of the cochlea from trauma caused by the deafening agent and/or the physical insertion of the implant. In our cochlear implant animal models, the cochlea is deafened through the administration of ototoxic drugs (aminoglycosides) intended to destroy hair cells and produce a cochlea similar to that found in human cochlear implant candidates. Aminoglycoside infusion into guinea pig cochleae has been shown to produce histological changes in the auditory nerve within hours (Leake-Jones et al., 1980; Dodson, 1997). Some of these changes, such as the swelling of neurons, might be reversible over time resulting in the observed early increases and later decreases in thresholds. Threshold decreases have been observed in implanted humans with long-term deafness as well (Michelson, 1971; Eddington et al., 1978), implying that implantation alone may also result in the observed changes. However, reported observations are sparse because implants in humans are usually not activated until roughly one month after implantation. Thus most postimplantation changes might be over before testing of these subjects begins. Further studies are required to determine the relative effects of the deafening agent versus effects of implantation alone. To test the effects of these procedures we formed groups of animals that differed in the timing of the implantation and/or deafening procedures, as detailed in the Methods section.
Effects of electrical stimulation on the auditory nerve or central auditory neurons comprise additional mechanisms that could be hypothesized to underlie the threshold changes observed. Many studies have shown that both peripheral and central changes in the auditory pathway, such as improvements in the neurochemical environment and expansion of the central representation of the stimulated region, occur as a result of postdeafening electrical stimulation (reviewed by Miller, 2001). These physiological changes or other effects of electrical stimulation could result in an increase in sensitivity to electrical stimuli over time. To test this hypothesis, we compared thresholds of animals in which electrical stimulation began shortly after deafening and implantation with those in which the onset of electrical stimulation was delayed in order to determine if thresholds would decrease over time in the absence of electrical stimulation.
The process of learning to listen to an electrical signal may also play a roll in the threshold changes observed. Since electrical stimuli undoubtedly sound different from their acoustic counterparts, some practice in listening to the electrical signal might be necessary before subjects begin responding optimally. The possibility that learning plays a role in the observed threshold decreases will be discussed in the context of results from these and previous experiments.
Finally, we considered that tissue growth in the scala tympani might affect detection threshold levels. Tissue growth including fibrous tissue and new bone formation is commonly observed in the implanted cochlea (Pfingst et al., 1981; Duckert, 1983; Kawano et al., 1998). These alterations could affect current pathways from the electrodes to the neurons, resulting in changes in the amount of current that reaches the sites of action-potential initiation. These changes also affect the impedances of the current passed between electrodes (Newbold et al., 2004) so we used impedance to estimate the time-course of tissue growth around the implant.
2. Materials and Methods
In this study four distinct subject groups, comprised of eight animals each, were tested. Psychophysical detection thresholds and implant impedances were measured as a function of time. The four groups allowed us to examine the effects of (a) the deafening procedure, (b) the implantation procedure, and (c) postimplantation electrical stimulation and psychophysical testing.
2.1. Subjects
Subjects tested in this experiment were adult pigmented guinea pigs born in a specific pathogen free (SPF) environment and then maintained in the laboratory under a modified SPF protocol. At the time of their arrival from the Elm Hill breeding facility, animals weighed approximately 250 – 300 g. They were gradually acclimated to a sound attenuating chamber and a restraint harness that kept them oriented towards the front of the test chamber. Their diet remained unrestricted until they weighed approximately 400 g. At this time their food was rationed to 25 g per day, which they would receive after each testing session. This regime ensured the animals motivation in the positively reinforced task while maintaining a healthy weight. Also at this time, hearing was assessed physiologically at 2, 8 and 16 kHz using auditory brainstem responses (ABRs) in order to minimize preimplant variation in the condition of the implanted ear. Only animals with ABR thresholds that fell within the normal range in at least one ear were included in the study. Normal ranges were established using data from over 200 previously tested ears. After the animals reached 600 g in weight, the food allowance was increased to 30 g per day. On this diet, weights stabilized around 800 to 1200 g. Animals were always allowed free access to water.
This study was performed in accordance with National Institutes of Health Guidelines (Guide for the Care and Use of Laboratory Animals, 1996). The University Committee on the Use and Care of Animals at the University of Michigan approved the animal protocols. Veterinary care and animal husbandry were provided by the Unit for Laboratory Animal Medicine, in facilities certified by the Association for Assessment and Accreditation of Laboratory Animal Care, International (AAALAC, Intl.). After completing the current experiment, the animals were used in other psychophysical and/or neurophysiological experiments before they were euthanized.
2.2. Psychophysical Procedures
Subjects were trained in increments using positive reinforcement procedures. They were trained to perform a psychophysical task in which they initiated a trial by pressing a button (the observing response) and reported detection of an acoustic signal by releasing the button. They learned to press and hold down the button through a randomly-variable foreperiod (1 to 6 seconds) and then to release it within one second of the auditory stimulus onset. Button releases within one second of stimulus onset (the detection response) were rewarded by delivery of a food pellet (Noyes/Research Diets). Once the animals responded reliably at moderate sound pressure levels, they were trained to respond to very soft sounds. Animals were considered trained when they could reliably detect and respond to various types of stimuli at threshold and suprathreshold levels. The entire training process took 3 to 6 months.
For both acoustic and electrical detection threshold determinations, the method of constant stimuli was used. Stimulus tables consisted of six to eight levels of attenuation at a step size of 5 dB for acoustic and 2 dB for electrical stimuli. Stimuli were arranged in descending order from the most to the least audible stimulus, but presented in random order. Stimulus levels were selected to maintain a relatively constant rate of reinforcement across conditions in order to avoid situations that might lead to a change in response strategy. The percentage of correct responses as a function of attenuation level was assessed and threshold was defined as the level at which the animal responded correctly 50% of the time, as determined by linear interpolation from the psychometric function. Guess rates (releases of the response button during a one second unmarked observation period on trials where no stimulus was presented) were measured during all sessions. Thresholds were considered valid if subjects completed at least 15 trials at each stimulus level, generated a smooth psychometric function and had a guess rate that was no greater than 20%. Daily testing sessions lasted from one to three hours.
2.3. Experimental Groups
Trained animals were assigned to one of four groups. Animals were matched across groups in regards to sex, age, and implant type. That is, for each animal in Group I, there were sex, age, and implant matched counterparts in Groups II, III, and IV.
Procedures and time courses for the four groups are summarized in Table 1. Animals in Group I were chemically (neomycin) deafened and implanted in the same surgery and electrical threshold testing was initiated the next day. Animals in Group II were chemically deafened and implanted in the same surgery, but electrical threshold testing was not initiated until 45 days after implantation (similar to the time of activation relative to implantation in clinical practice). Animals in Group III were chemically deafened and then implanted 45 days later. Electrical threshold testing was initiated the day after implantation. For Groups II and III, behavior in the psychophysical task, during the period between deafening and the initiation of electrical threshold testing, was maintained with daily stimulus detection testing using acoustic stimuli to the non-deafened ear. Group IV animals were implanted but not chemically deafened. Electrical threshold testing for this group began the day after implantation.
Table 1.
Summary of the time courses followed for the four treatment groups.
| Group | Day of neomycin infusion | Day of cochlear implantation | Day of electrical threshold testing initiation |
|---|---|---|---|
| I | 0 | 0 | 1 |
| II | 0 | 0 | 45 |
| III | 0 | 45 | 46 |
| IV | NA | 0 | 1 |
2.4. Surgical Procedures
Both the chemical deafening (if applicable) and the implantation were performed using sterile surgical procedures. For deafening and implantation surgeries, animals were anesthetized with an intramuscular injection of ketamine (40 mg/kg) and xylazine (10 mg/kg). Maintenance doses of ketamine were given subcutaneously as needed. Atropine (0.05 mg/kg) was given subcutaneously to help decrease salivation and improve respiration.
The unilateral chemical deafening procedure for Groups I, II and III consisted of a 60 µl intracochlear injection through the round window of a 10% neomycin sulfate solution in sterile water. Previous studies have shown that neomycin injections into the cochlea result in loss of all auditory responses within about 10 minutes (Nuttall et al., 1977; Middlebrooks and Snyder, 2007) and destroy hair cells and create a flat, uniform epithelium in the organ of Corti within 4 days (Duckert, 1983; Kim and Raphael, 2007). For the cochlear implant surgery, a percutaneous connector attached to the implant was mounted on the skull with methyl methacrylate. The implant was led under the postauricular muscle into an opening made in the bulla and inserted gently into the scala tympani through a cochleostomy made near the round window. Implant insertion depths were estimated at the time of implantation based on the number of electrode rings observed outside the cochleostomy. These depths varied from 3.7 – 4.7 mm inside the scala tympani (4.2 mm on average). No electrode was implanted to a depth beyond the basal turn of the scala tympani. Once the implant reached the maximum depth allowed by the dimensions of the scala tympani, the implant was secured at the bulla defect with a silk tie and Durelon cement. The ground electrode was tucked into the muscle overlying the temporal bone and then the skin incision was closed.
The default ear for implantation was the left ear. However, in cases where this was not appropriate due to hearing loss in the left ear as determined by ABRs (two subjects), the right ear was used.
2.5. Implants
For these experiments we used two types of implants that were representative of implants typically used in animal experiments. Half of the animals in each group received a two-electrode ball and helix scala tympani implant previously described by Miller et al. (1995). The approximate center to center distance between electrodes was 1.3 mm. The other half of the animals received a six-electrode scala tympani implant, manufactured by Cochlear Corporation (Nucleus, Ltd., Lane Cove, Australia), resembling the apical end of the Nucleus CI 22 human implant (Patrick et al., 1985). The approximate center to center distance between electrodes in this implant was 0.75 mm. Animals that received the six-electrode implants were put into other studies following the completion of this study. No systematic differences in psychophysical detection thresholds were found between the two implant types. Therefore, the data and results presented here will not distinguish between them.
2.6. Impedance Measurements
Impedances were measured using a custom built impedance meter with a 1 kHz sinusoidal test current at 1 µA rms. Since impedances can increase following a period of non-stimulation and then decrease following electrical stimulation, we always measured impedances following the psychophysical test sessions. This allowed us to assess long term changes in impedance independent of these short-term fluctuations that depended on electrical stimulation. The impedances were used to monitor the electrical integrity of the implant and to monitor changes over time in conditions near the electrodes, such as tissue growth, that might affect current flow to the neural tissue.
2.7. Experimental Parameters
Psychophysical electrical threshold changes over time were monitored using a 100 Hz sinusoidal electrical stimulus, presented in trains of 200 msec bursts separated by 100 msec gaps, at multiple stimulation sites. The initial phase of the stimulus was negative and began and ended at 0 phase, gated on and off by a 15 msec cosine gating function. This study concentrates on the data (i.e., electrical detection thresholds and impedances) of only one stimulation site per subject; either the deepest or second deepest intracochlear electrode served as the main stimulation site with the extracochlear electrode serving as the return. The location of the intracochlear electrode tested (deepest or second deepest) did not produce a systematic difference in thresholds across subjects. Hence, the results reported do not distinguish between the data from these two sites. The deepest (most apical) sites were chosen because they are more reliably positioned in the scala tympani. Previous dissections have shown that the tip of the implant fills the scala tympani and thus is always located near the modiolus whereas the medial-lateral location of more basal sites within the scala tympani is variable. Monopolar stimulation was chosen because of its typically broad excitation pattern, which would be likely to reflect the condition of a large portion of the cochlea in the region of the implant. The data from other stimulation sites were not consistently collected within the time of the initial threshold decreases and thus could not be reliably assessed for changes over time with sufficient temporal resolution.
2.8. Equipment and Test Environment
Subjects were tested in custom built wire-mesh test cages located inside sound-attenuating chambers produced by Acoustic Systems (Model RF Shielded Animal Test Chambers), Industrial Acoustics Corporation (Model 1201-A) and Tracoustics (Models 240-B and 240-C). Stimulus generation and delivery for psychophysical testing were controlled by a locally written program running on personal computers. Stimuli were output via a Tucker Davis Technologies (TDT) digital to analog converter (DA3-2), and attenuated by a TDT programmable attenuator (PA4). Acoustic signals were amplified (Parasounds Zap/Zone, HCA-500, HCA-600 or HCA-750A), and presented by a speaker mounted above the test cage. Electrical stimuli were delivered by a custom built voltage controlled constant current source.
2.9. Statistical Analysis
An analysis of variance (SigmaStat) was applied to each of the six measures to determine if any of them showed a statistically significant effect of group. The factor of interest was treatment group (Groups I, II, III, or IV). A post hoc pairwise multiple comparison procedure using the Student-Newman-Keuls method (SigmaStat) was then performed to determine which groups were significantly different from each other.
3. Results
3.1. Psychophysical Thresholds
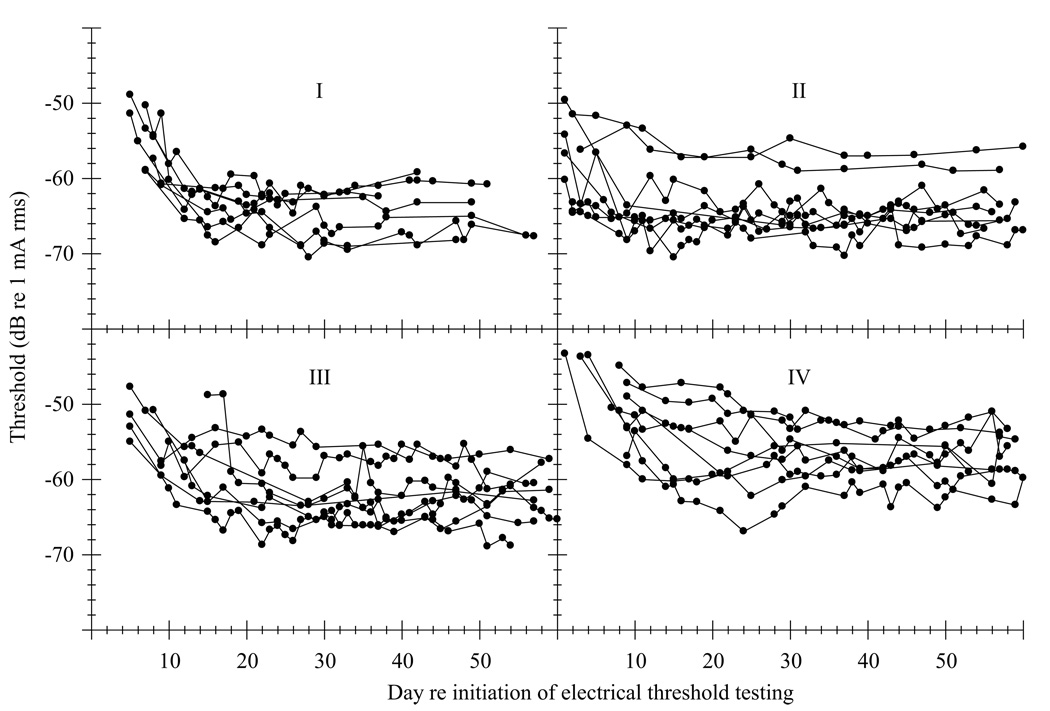
Figure 1 shows thresholds as a function of time after onset of electrical threshold testing for all subjects. The figure is divided into four separate panels, each of which corresponds to a single group (Group I, II, III or IV). These data were quantified and analyzed using the following specific measures: (A) time to the first threshold, (B) stabilization time, (C) level of the first threshold, (D) stable threshold level, (E) magnitude of threshold change from the first threshold to the stable threshold level, (F) magnitude of change from Day 9 to the stable threshold level.
Fig. 1.
Electrical detection thresholds (100 Hz sinusoids, 200 msec stimulus duration) for all subjects as a function of time after initiation of electrical threshold testing. Each panel corresponds to the data from one group, each function represents one subject and each point represents one electrical detection threshold measurement. Group I subjects were chemically deafened and then implanted on the same day (during the same surgery) and began electrical threshold testing the day after surgery. Group II subjects were chemically deafened and then implanted on the same day (during the same surgery) and began electrical threshold testing 45 days after surgery. Group III subjects were chemically deafened in one surgery, implanted in a separate surgery 45 days later, and began electrical threshold testing the day after implantation. Group IV subjects were implanted only (they were not chemically deafened) and began electrical threshold testing the day after surgery.
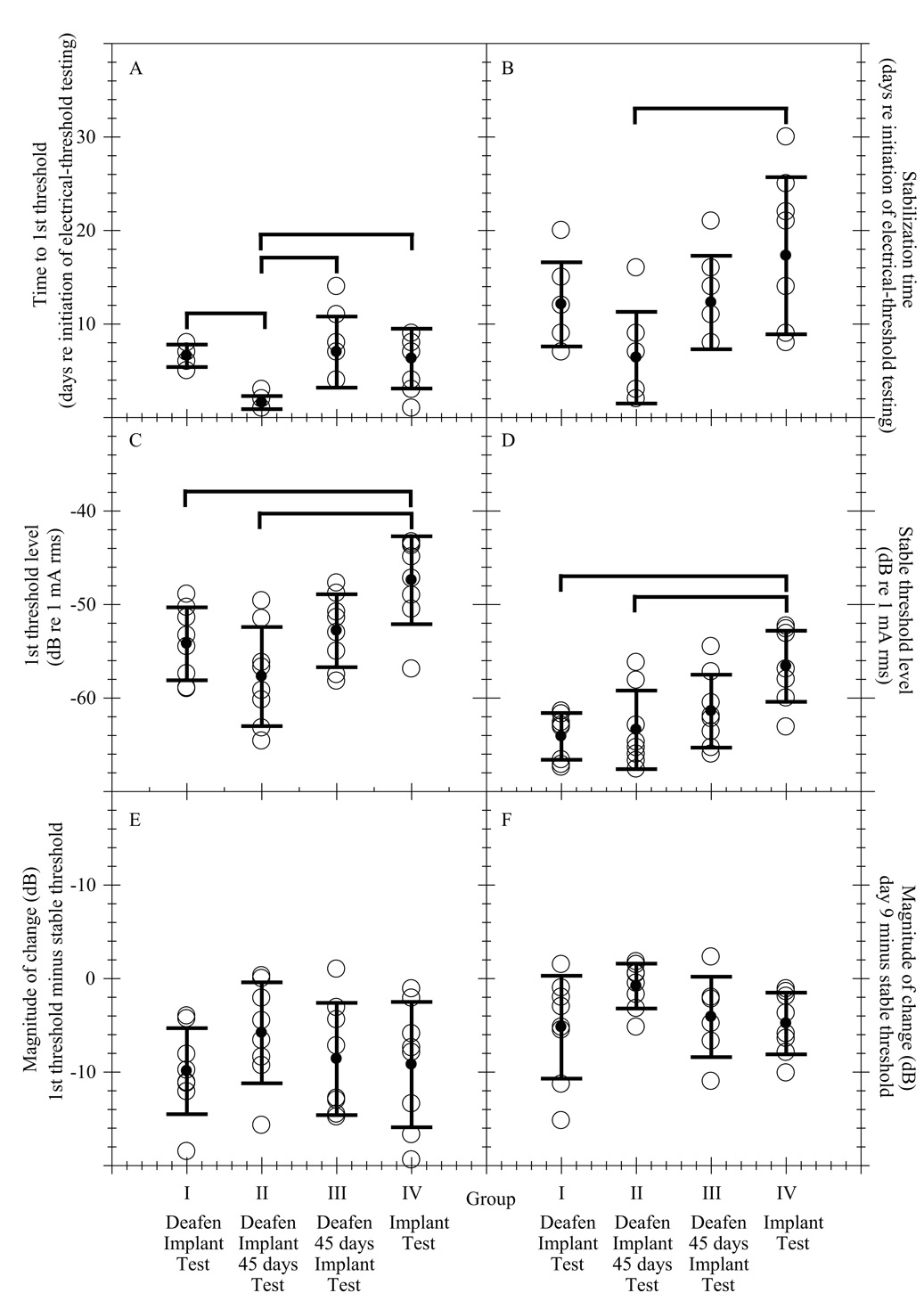
Means and standard deviations per group for measures A through F are summarized in Table 2. These data, along with indicators of significant differences across groups, are plotted in Figure 2. A one-factor analysis of variance (SigmaStat) was applied to each of the six measures to determine if any of them showed a statistically significant effect of group. The factor of interest was treatment group (Groups I, II, III, or IV). A post hoc pairwise multiple comparison procedure using the Student-Newman-Keuls method (SigmaStat) was then performed to determine which groups were significantly different from each other.
Table 2.
Summary of the means and standard deviations of the six dependent measures presented in Figure 2 for each group.
| Group |
||||
|---|---|---|---|---|
| I | II | III | IV | |
| Time to 1st threshold (days re initiation of electrical threshold testing) |
6.6 ± 1.2 | 1.6 ± 0.7 | 7.0 ± 3.8 | 6.3 ± 3.2 |
| Stabilization time (days re initiation of electrical threshold testing) |
12.1 ± 4.5 | 6.4 ± 4.9 | 12.3 ± 5.0 | 17.3 ± 8.4 |
| 1st threshold level (dB re 1 mA rms) |
−54.2 ± 3.9 | −57.7 ± 5.3 | −52.8 ± 3.9 | −47.4 ± 4.7 |
| Stable threshold level (dB re 1 mA rms) |
−64.1 ± 2.5 | −63.4 ± 4.2 | −61.4 ± 3.9 | −56.6 ± 3.8 |
| Magnitude of change (dB) 1st threshold - stable threshold |
−9.9 ± 4.6 | −5.8 ± 5.4 | −8.6 ± 6.0 | −9.2 ± 6.7 |
| Magnitude of change (dB) day 9 threshold - stable threshold |
−5.2 ± 5.5 | −0.8 ± 2.4 | −4.1 ± 4.6 | −4.8 ± 3.3 |
Fig. 2.
A comparison of the average group values for dependent measures 1–6. Open circles denote the average values for individual subjects. In some cases panels appear to have less than 8 open circles per group. This is because some subjects have identical data and overlap. The filled circles denote the group averages and their error bars denote ± one standard deviation. The thick solid lines above the plots indicate significant differences between groups. These data are presented numerically in Table 2.
A. Time to the First Threshold
The first day of electrical testing was designated as Day 1. Subjects began working reliably and producing valid thresholds (see Psychophysical Procedures) at varying times after the initiation of electrical threshold testing. The number of days from the first day of electrical-threshold testing (Day 1) until subjects produced their first valid threshold was called the time to first threshold. All subjects produced their first valid threshold sometime between Day 1 and Day 14. For this measure, an effect of group was found (F = 7.44, df = 3, p = 0.0014). The significant differences in means were between Groups II and I (t = 3.83, df = 14, p < 0.05), Groups II and III (t = −5.38, df = 14, p < 0.05) and between Groups II and IV (t = −3.54, df = 14, p < 0.05). Group II animals obtained their first valid threshold 5 days sooner, on average, than animals in each of the other groups (Fig. 2 A).
B. Stabilization Time
The stabilization time was the time in days from Day 1 until the first of the nine thresholds meeting the stabilization criteria (see measure D, Stable Threshold Level) was obtained. For this measure, an effect of group was found (F = 4.20, df = 3, p = 0.0186). The significant difference in means was between Groups II and IV (t = −3.55, df = 14, p < 0.05), with Group IV taking, on average, 10.9 days longer to reach stable detection thresholds than Group II (Fig. 2 B).
C. Level of the First Threshold
The level of the first threshold was the level in dB re 1 mA rms of the first threshold for each subject that was considered valid according to the criteria explained previously in Psychophysical Procedures. For this measure, an effect of group was found (F = 7.29, df = 3, p = 0.0016). The significant differences in means were between Groups IV and I (t = 3.06, df = 14, p < 0.05) and Groups IV and II (t = −4.59, df = 14, p < 0.05) with thresholds for Group IV being, on average, 6.8 and 10.3 dB higher than those for Group I and Group II respectively (Fig. 2 C). Mean first threshold levels for animals in Group II were lower than the means of any other group. However, the within-group variability in this measure was large and the mean for Group II was not statistically significantly different from that of either Group I or III.
D. Stable Threshold Level
A running standard deviation of five consecutive thresholds was monitored until the standard deviation reached a value of ≤ 2 dB. The five values that reached this criterion plus the next four values were then analyzed by simple linear regression using Sigma Stat software (Jandel). If the slope of that regression line was not significantly different from zero (p > 0.05) and the standard deviation of the nine values was ≤ 2 dB, the thresholds were considered stable over time. The mean of the nine stable thresholds defined the stable threshold level. For this measure, an effect of group was found (F = 7.12, df = 3, p = 0.0018). The significant differences in means were between Groups IV and I (t = −4.17, df = 14, p < 0.05), and Groups IV and II (t = −3.81, df = 14, p < 0.05). Group IV stable thresholds were, on average, 7.5 and 6.8 dB higher than those of Group I and Group II respectively (Fig. 2 D).
E. Magnitude of Change: First Threshold minus Stable Threshold
The magnitude of change from the first threshold was defined as the difference between the stable threshold level and the first threshold level. There was no statistically significant effect of group on this measure (F = 0.904, df = 3, p = 0.4588). All groups showed an average magnitude of change (decrease in threshold) of greater than 4 dB which was two times the standard deviation that we used as a criterion for threshold stability (Fig. 2 E). Across groups these mean threshold changes ranged from −5.8 to −9.9 dB (Table 2) and across individual subjects threshold changes ranged from 0 to −19.4 dB.
F. Magnitude of Change: Day 9 Threshold minus Stable Threshold
Subjects did not produce their first threshold at the same time, a result most likely due to the varying degrees of trauma, both acutely (in the ear) and holistically, sustained by each individual animal as a result of the surgical procedure(s) they underwent. Therefore, a standardized day by which 30 out of 32 subjects had produced at least their first valid threshold was selected as the point from which a normalized magnitude of change was calculated. This point was Day 9 re the initiation of electrical threshold testing. Threshold levels were derived through linear interpolation when there was no valid data point exactly on Day 9. The two subjects that did not produce valid thresholds prior to Day 9 were excluded from this analysis. There was no statistically significant effect of group on this measure (F = 2.40, df = 3, p = 0.0966). All groups except Group II showed an average decrease in threshold of greater than 4 dB (Fig. 2 F) with average group threshold changes for Groups I, III and IV that ranged from −4.1 to −5.2 dB (Table 2) and individual subject threshold changes that ranged from −1 to −15.2 dB. Magnitude of change from Day 9 was small for all of the animals in Group II (1.8 to −5.8 dB), which is consistent with the low first thresholds and the relatively short stabilization times for this group. However, the variability in this measure was sufficiently large for most of the groups that the differences among groups were not statistically significant.
3.2. Impedances
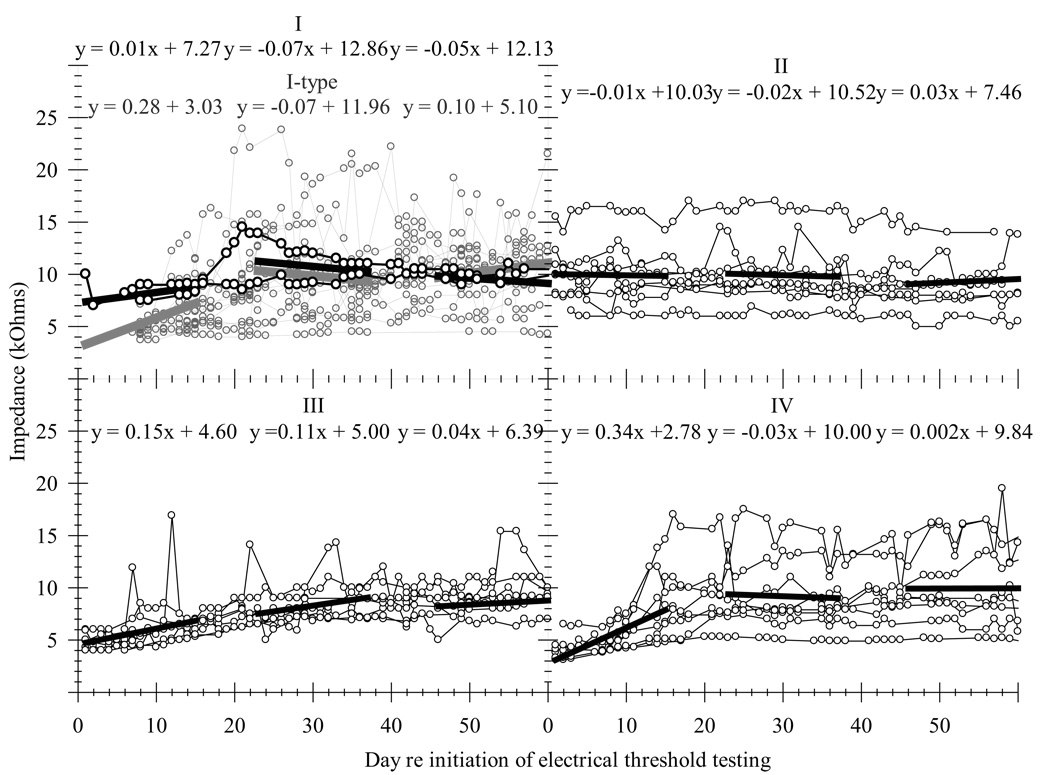
Impedances for each animal's implant in all four groups were followed over time to monitor implant integrity. These impedances should also reflect tissue growth in the scala tympani near the electrodes (Newbold et al., 2004) which in turn could affect implant function. Figure 3 plots impedance in kOhms for all four groups against the day re initiation of electrical threshold testing (Days 0 through 60). In each group, the functions of impedances over time for each individual subject were divided into an early (Days 1–15), a middle (Days 23–37) and a late (Days 46–60) time period. The slopes of the best fit lines for each function during each time period were then averaged to obtain a mean slope for all three time periods for each group. The average slopes of the functions during the early, middle and late time periods are presented in Table 3. Unfortunately only two animals in Group I had a sufficient amount of reliable impedance data with which to compare to the other groups. This is because impedances were not tested regularly after each behavioral testing session when this study first began, which was the time when most Group I animals were participating in the experiment. However, more recent studies conducted in this laboratory have utilized the same deafening and implantation protocol on subjects that was applied to Group I animals (chemically deafened and implanted on the same day and began electrical threshold testing the day after surgery). For the purposes of this study, 14 of these subjects were selected and designated as “Group-I-type”. Psychophysical data for Group I and Group-I-type subjects followed similar trends. Because more impedance data were obtained for Group-I-type animals, we used these data to provide an estimate of Group I impedance trends.
Fig. 3.
Impedance measured with a 1 kHz sinusoidal signal at 1 µA rms for all groups as a function of time re initiation of electrical threshold testing. Each panel corresponds to the data from one group, each function represents one subject and each point represents one impedance measurement. Group I (black) and “Group-I-type” (gray) subjects were chemically deafened and then implanted on the same day (during the same surgery) and began electrical threshold testing the day after surgery. Group II subjects were chemically deafened and then implanted on the same day (during the same surgery) and began electrical threshold testing 45 days after surgery. Group III subjects were chemically deafened in one surgery, implanted in a separate surgery 45 days later and began electrical threshold testing the day after implantation. Group IV subjects were implanted only (they were not chemically deafened) and began electrical threshold testing the day after surgery. Each solid thick line represents linear regressions for the collective data from each group for the early (days 1 – 15), middle (days 23 – 37) and late (days 46 – 60) time periods and reported above each regression is its corresponding equation.
Table 3.
Summary of the average group slopes (kOhms/day) for the best fit lines of the impedance data between Days 1–15, Days 23–37 and Days 46–60 re initiation of electrical threshold testing.
| Group | Days 1–15 | Days 23–37 | Days 46–60 |
|---|---|---|---|
| I | 0.1 | −0.07 | −0.05 |
| I–type | 0.28 | −0.07 | 0.10 |
| II | −0.01 | −0.02 | 0.03 |
| III | 0.15 | 0.11 | 0.04 |
| IV | 0.34 | −0.03 | 0.002 |
Groups I (as estimated using the data from Group-I-type animals), Group III and Group IV animals all showed increases in impedance over time after the onset of electrical testing with impedances reaching relatively stable levels by Day 45. Impedances for these three groups started at around 4 kOhms and increased to about 10 kOhms by Day 45. In contrast, implants of animals in Group II started at an average impedance of about 10 kOhms on the first day after electrical testing and remained relatively stable thereafter. Note that Groups I, III and IV all began electrical testing one day after implantation, i.e., for these three groups, Day 1 following implantation is synonymous with Day 1 re initiation of electrical threshold testing. However, for Group II subjects, Day 46 following implantation is synonymous with Day 1 re initiation of electrical threshold testing. So in terms of days from implantation the early time period for Group II (Day 1–15 re initiation of electrical threshold testing) is comparable to the late time periods for Groups I, III and IV (Day 46–60 re initiation of electrical threshold testing). The slopes and y-intercepts for the impedance-versus-time functions of Groups I, III and IV during the late period resemble closely those of Group II during the early period. That is, for all Groups, impedances 45 days following implantation were relatively constant despite differences in the amount of electrical stimulation they received.
3.3. Summary of Results
The mean values for the time to the first threshold (A) and stabilization time (B) for Group II were significantly lower than those of Groups I, III and IV for the first measure but only significantly lower than Group IV for the second measure. This suggests that by waiting 45 days after the deafening and implantation surgery before initiating electrical threshold testing, animals will begin to work more quickly and their thresholds will stabilize more quickly. Both the levels of the first threshold (C) and the stable threshold levels (D) for Group IV tended to be higher than the other groups, significantly higher than Groups I and II. Group IV animals took significantly more time to stabilize than Group II animals, and on average (although not statistically significant), they needed more time to stabilize than Groups I and III as well. This suggests that implantation alone without neomycin deafening results in thresholds that remain unstable for a longer period of time following surgery. Group III was not significantly different from Group I for measures A–D which suggests that allowing 45 days for recovery between deafening and implantation does not significantly change the effects on thresholds observed postimplantation. There was no statistically significant difference in magnitudes of change either from the first threshold (E) or the Day 9 threshold (F) across groups suggesting that the variables leading to changes over time were deafening and/or implantation and that the effects of these variables dissipated over time. However, the average magnitude of change seen in Group II was smaller than Groups I, III and IV. The overall trend seen in the impedance data of Groups I, III and IV was a gradual increase over time after implantation and the onset of electrical testing, reaching relatively stable levels within 46 days. Impedances for Group II on the first day of electrical testing (46 days after implantation) were equivalent to those reached by the other groups at 46 days after implantation.
4. Discussion
4.1. Potential Mechanisms
We begin our discussion of the results with an interpretation based on the "recovery from temporary pathology" model as described in the introduction. This model assumes that the magnitude of threshold decrease over time reflects the magnitude of the pathology, minus any recovery that occurred before the first valid threshold was obtained. This model is based, in part, on our previous observations of postimplantation threshold fluctuations in nonhuman primates (Pfingst et al., 1979; Pfingst, 1990). Electrical detection thresholds obtained from nonhuman primates within the first few days following deafening and implantation were at levels near the stable threshold level. Thresholds then increased (became less sensitive) over a period of a few days and then decreased over a period of several weeks, eventually returning to their original level. These observations suggest that threshold changes resulted from a temporary alteration (pathology) in the auditory pathway due to the deafening and/or implantation procedure and/or the onset of electrical stimulation, and that the system gradually recovered from this alteration, returning to its original sensitivity. The temporary pathology assumed in this model could be a simple peripheral event, such as swelling of the auditory nerve fibers (Juiz et al., 1998), resulting in decreased sensitivity to electrical stimulation, or it could include changes in central auditory pathways, which have been observed to occur following disruption of the cochlea in previous anatomical and biochemical studies (Leake-Jones et al., 1980; Irvine and Rajan, 1993; Dodson, 1997; Miller, 2001).
Our observations in guinea pigs are similar to those from the previous studies in nonhuman primates, but they differ in two details. First, the time to reach a stable threshold level was shorter in the guinea pig. Guinea pigs stabilized within 2 to 40 days whereas the monkeys took several months. Second, we rarely observed an initial threshold increase in the guinea pigs prior to the threshold decreases. We believe that the observations in guinea pigs and monkeys reflect the same underlying mechanisms but that the time course is faster in the guinea pig. Thus, in guinea pigs, the initial decrease in sensitivity may have occurred before we could observe it behaviorally. There are other means of assessment that could be applied closer to the time of deafening and implantation such as electrically-evoked auditory-brainstem responses. However, these measures are based on different underlying mechanisms than behavioral thresholds and thus may reflect different aspects of post-deafening and implantation pathology.
Assuming the temporary-pathology model is correct, an important question to address is whether the temporary pathology is due to the neomycin deafening, the implantation, or both. The magnitude and time course of threshold decreases for Group III which was implanted 45 days after chemical deafening were similar to those for Group I which was implanted on the day of deafening (Fig. 2). This suggests that implantation in a predeafened ear produces a similar effect to that produced by deafening and implantation at the same time. This raises the possibility that implantation alone, not neomycin deafening produced the temporary pathology that affected detection thresholds. Another possibility is that neomycin deafening in Group III produced a temporary pathology and that the nerve recovered from that pathology during the 45 day waiting period only to be affected again to a similar degree by the implantation. The current experiments do not allow us to distinguish between these two alternatives. Group IV gives another view of the effects of implantation alone. However, effects of the presence of hair cells in some of the animals from this group, as discussed below, complicates comparison to the other groups with regard to effects of implantation.
Another question to address is whether the observed decreases in thresholds occurred spontaneously or whether electrical stimulation of the auditory system affected this process. Repeated stimulation of one site in a cochlear implant is known to expand the range of auditory neurons activated in the central auditory nervous system (Snyder et al., 1990), which could increase its sensitivity to electrical stimuli. On average, Group II started working reliably earlier, had lower initial thresholds and took less time to stabilize than any other group (Fig. 2 A and B). The earlier starting day was most likely due to the recovery of the animal from the effects of the deafening and implantation surgery during the 45 day waiting period before electrical threshold testing was initiated. Whether this general recovery led as well to lower initial thresholds and more rapid stabilization is not known. Interestingly, despite the 45 days allowed for recovery, Group II thresholds still decreased over time (Fig. 1 and 2 C). Although the average decrease observed for Group II animals was less than that for the other groups, Group II threshold shifts were variable from subject to subject and the average amount of change observed in this group was not significantly different from that of the other groups. The observed decrease in thresholds over time after onset of electrical stimulation for Group II suggests that although the ear did recover from the trauma of deafening and implantation within 45 days, its recovery was not complete. The onset of electrical testing after day 45 may have contributed to the further recovery of the ears and/or the auditory central nervous system, resulting in the threshold decreases observed in Group II. Evidence of this has been seen in some animals from recent experiments that did not begin electrical threshold testing until much later than 45 days after surgery (for example 55, 62 and 92 days following surgery) and yet still showed threshold decreases over time.
There are other factors that might have contributed to the decreases in thresholds observed. For example, learning to listen to the electrical stimulus may improve (decrease) thresholds. However, it seems highly unlikely that learning was the sole mechanism underlying this change because the decreases were often equal to a significant percentage of the dynamic range for electrical hearing, which is typically less than 15 dB. Thus, assuming no change in sensitivity or stimulus loudness over time, animals with high initial thresholds would be failing to respond initially to stimuli that were very loud. Also, in previous experiments, we have found that even animals with experience listening to electrical signals with an implant in one ear, showed threshold decreases to electrical signals following implantation of the other ear. We examined data from 6 animals implanted in the contralateral ear after data had been collected from the ipsilateral ear. Five of the 6 showed decreases in threshold over time for stimulation of the second ear and in three of these cases the decreases were larger than those seen in the first ear. If decreases in threshold were a result of the animal learning to listen to electrical stimuli, one would expect to see little or no decrease over time in detection thresholds for the second implant. Finally, the previously mentioned studies in nonhuman primates also argue against learning as a major contributor to threshold decreases because thresholds for these animals started out low and then increased over time before threshold decreases similar to those observed in the guinea pigs began.
Another potential contributor to post surgical threshold shifts is lingering effects of the surgical anesthesia. If the animals were producing data, despite feeling ill or groggy from surgery, the thresholds obtained might not be accurate reflections of the true sensitivity to the electrical signal. However, if this were the case, it would be expected that acoustic thresholds of animals that were tested in the untreated (contralateral) ear the day after the deafening and/or implantation of the ipsilateral ear and for 45 days thereafter, would show decreases equivalent to those observed for the electrical thresholds. In fact however, these acoustic thresholds showed only minor day to day variation with no consistent decrease in threshold over time.
Notably, the first threshold and stable threshold levels of Group IV (subjects that were implanted but not chemically deafened) were higher on average than those of the other groups (significantly higher than those for Groups I and II; Fig. 2 B). We believe that the higher thresholds seen in Group IV may have been a result of inner hair cell induced auditory nerve activity. Hu et al. (2003) demonstrated that the blocking of inner hair cell transduction with furosemide resulted in several changes in electrically evoked compound action potentials suggestive of changes in across-fiber synchronization of the responses of auditory nerve fibers to electrical stimulation. These data suggest that the psychophysical sensitivity of the auditory nerve to electrical stimulation could be affected by spontaneous activity generated in the nerve by surviving hair cells. For example, at any given instant, spontaneous activity in the auditory nerve could reduce psychophysical sensitivity due to refractory effects following spontaneous discharges in a subpopulation of fibers.
Impedances for Groups I (Group-I-type), III and IV increased over the first weeks after electrical testing began from a starting value of about 4 kOhms to a stable high of about 10 kOhms. In contrast, impedances for Group II started at about 10 kOhms on the first day of electrical testing and remained stable after that. The first day of impedance testing was the day of implantation for Groups I, III and IV but 46 days after implantation for Group II because the experiment design did not allow electrical stimulation for Group II during the first 45 days after implantation. Therefore, we do not know the timecourse of the impedances during the first 45 days after implantation for Group II. However, since the impedances at 46 days after implantation were as high as those of the other groups 46 days after implantation, it is reasonable to assume that the thresholds for Group II followed a similar time course after implantation as that observed for the other groups. These data suggest that it was the presence of the implant in the scala tympani rather than the electrical stimulation that caused the increase in impedances over time. Furthermore, comparison of the impedance data from Groups III and IV with those for Group I suggests that the impedance changes were not dependent on the neomycin deafening.
The likely mechanism underlying the increases in impedances over time after implantation was tissue growth around the implant (Newbold et al., 2004). This tissue growth could have increased the efficiency of the path from the electrodes to the sites of action potential initiation, resulting in lower thresholds for neural activation. However, two observations suggest that this mechanism was not the only contributor to the observed threshold decreases over time. One observation, from the current experiment, is that, in contrast to Groups I, III and IV, impedances for implants in Group II animals were stable or even decreased slightly as electrical thresholds were decreasing over time. A second piece of evidence, from the previous experiments in nonhuman primates, is that thresholds showed a nonmonotonic pattern over time, increasing and then decreasing, which would not be predicted assuming a steady growth of fibrous tissue around the implants resulted in improved current paths to the neurons.
In summary, the results of this study indicate that multiple factors may contribute to observed threshold changes over time after deafening and/or implantation. Recovery of the auditory pathway from the trauma caused by deafening and implantation is a likely candidate for a mechanism underlying these changes. The apparent effect that electrical stimulation has on psychophysical detection threshold changes over time indicates that it should also be considered a factor. Tissue growth around the electrode resulting in increases in impedance might result in improved efficiency of the conductive paths from the electrodes and the sites of action potential initiation, resulting in decreasing thresholds over time. It was also observed that the presence of viable inner hair cells may be associated with higher threshold levels for 100 Hz sinusoidal electrical stimulation. We assume that the condition of residual hair cells in an implanted ear can change over time resulting in increased intersubject and intrasubject variability in the detection of electrical stimuli.
4.2. Significance
The primary significance of these functional changes that occur in the first month following deafening and/or implantation is in regard to the use of animal models for the study of cochlear implants. Typically, experiments using these animal models are focused within a period of only a few hours, days or weeks. During this short period, the animals are deafened and/or implanted, experimental manipulations are carried out, and data are collected. For example, studies focusing on the effects of chronic stimulation on spiral ganglion cell survival might last for a few weeks with stimulation occurring during a period when fluctuations in the sensitivity to electrical stimulation are large. Thus, it is important to understand the functional condition of the auditory system during these short-term periods, particularly if that condition is changing rapidly over time, and to understand its relationship to the longer term, more stable conditions that characterize patients with cochlear implants.
The significance of these studies for implanted humans is less direct but still important. Since cochlear implants in human subjects are typically not activated until approximately one month after surgery, functional changes that occur during the early period after implantation in most cases will go unnoticed. However, these studies hold significance for patients for several reasons. First, they have implications for experimental design and interpretation of data obtained from animal models, which can ultimately affect the development of cochlear implants for human use. Second, because threshold fluctuations do occur in human subjects (Skinner et al., 1995), a better understanding of the mechanisms underlying fluctuations in electrical thresholds is needed. More generally, information about mechanisms affecting threshold levels might aid in interpreting the large threshold differences that are commonly observed across subjects and across stimulation sites within subjects. Finally, whatever changes occur during the first month following implantation, whether observed or not, might result from conditions in the cochlea and auditory pathway that could ultimately affect the long-term performance of the implant.
Acknowledgements
Supported by NIH grants R01-DC03389, R01-DC007634 and R01-DC04312. We appreciate the technical assistance of Nadia Tayeh, Jennifer Benson, the University of Michigan Center for Statistical Consultation and Research and the KHRI Histology, Shops and Computing cores (supported by NIH grant P30 DC05188) for their contributions to this work.
Abbreviations
- ABR
auditory brainstem response
- dB
decibel
- mA
milliamperes
- rms
root-mean-square
- SPF
specific pathogen free
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Dodson HC. Loss and survival of spiral ganglion neurons in the guinea pig after intracochlear perfusion with aminoglycosides. J. Neurocytol. 1997;26:541–556. doi: 10.1023/a:1015434524040. [DOI] [PubMed] [Google Scholar]
- Duckert LG. Morphological changes in the normal and neomycin-Perfused guinea pig cochlea following chronic prosthetic implantation. Laryngoscope. 1983;93:841–855. doi: 10.1288/00005537-198307000-00001. [DOI] [PubMed] [Google Scholar]
- Eddington DK, Dobelle WH, Brackmann DE, Mladejovsky MG, Parkin JL. Auditory prosthesis research with multiple channel intracochlear stimulation in man. Ann. Otol. Rhinol. Laryngol. (St. Louis) 1978;87:1–39. [PubMed] [Google Scholar]
- Hu N, Abbas PJ, Miller CA, Robinson BK, Nourski KV, Jeng F, Abkes BA, Nichols JM. Auditory response to intracochlear electric stimuli following furosemide treatment. Hear. Res. 2003;185:77–89. doi: 10.1016/s0378-5955(03)00261-2. [DOI] [PubMed] [Google Scholar]
- Irvine DRF, Rajan R. Plasticity in the frequency organization of auditory cortex of adult mammals with restricted cochlear lesions. Biomed. Res. 1993;14 Suppl. 4:55–59. [Google Scholar]
- Juiz J, Rueda J, Merchan JA. Reversible damage to the nerve fibres in the organ of Corti after surgical opening of the cochlea in the rat. Acta Otolaryngol. (Stockh) 1988;106:29–33. doi: 10.3109/00016488809107367. [DOI] [PubMed] [Google Scholar]
- Kawano A, Seldon HL, Clark GM, Ramsden RT, Raine CH. Intracochlear factors contributing to psychophysical percepts following cochlear implantation. Acta OtoLaryngol. (Stockh) 1998;118:313–326. doi: 10.1080/00016489850183386. [DOI] [PubMed] [Google Scholar]
- Kim YH, Raphael Y. Cell division and maintenance of epithelial integrity in the deafened auditory epithelium. Cell Cycle. 2007;6:612–619. doi: 10.4161/cc.6.5.3929. [DOI] [PubMed] [Google Scholar]
- Leake-Jones PA, O'Reilly BF, Vivion MC. Neomycin ototoxicity: Ultrastructural surface pathology of the organ of corti. Scanning Microsc. 1980;3:426–434. [PubMed] [Google Scholar]
- Michelson RP. Electrical stimulation of the human cochlea: A preliminary report. Arch. Otolaryngol. Head Neck. Surg. 1971;93:317–323. doi: 10.1001/archotol.1971.00770060455016. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Snyder RL. Auditory prosthesis with a penetrating nerve array. J. Assoc. Res. Otolaryngol. 2007;8:258–279. doi: 10.1007/s10162-007-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL. Effects of chronic stimulation on auditory nerve survival in ototoxically deafened animals. Hear. Res. 2001;151:1–14. doi: 10.1016/s0378-5955(00)00226-4. [DOI] [PubMed] [Google Scholar]
- Miller AL, Morris DJ, Pfingst BE. Effects of time after deafening and implantation on guinea pig electrical detection thresholds. Hear. Res. 2000;144:175–186. doi: 10.1016/s0378-5955(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Miller CA, Woodruff KE, Pfingst BE. Functional responses from guinea pigs with cochlear implants. I. Electrophysiological and psychophysical measures. Hear. Res. 1995;92:89–99. doi: 10.1016/0378-5955(95)00204-9. [DOI] [PubMed] [Google Scholar]
- Newbold C, Richardson R, Huang CQ, Milojevic D, Cowan R, Shepherd R. An in vitro model for investigating impedance changes with cell growth and electrical stimulation: implications for cochlear implants. J. Neural. Eng. 2004;1:218–227. doi: 10.1088/1741-2560/1/4/005. [DOI] [PubMed] [Google Scholar]
- Nuttall AL, Marques DM, Lawrence M. Effects of perilymphatic perfusion with neomycint on the cochlear microphonic potential in the guinea pig. Acta Otolaryngol. (Stockh) 1977;83:393–400. doi: 10.3109/00016487709128863. [DOI] [PubMed] [Google Scholar]
- Patrick JF, Crosby PA, Hirshorn MS, Kuzma JA, Money DK, Ridler J, Seligman PM. Australian multi-channel implantable hearing prosthesis. In: Schindler RA, Merzenich MM, editors. Cochlear Implants. New York: Raven Press; 1985. pp. 93–100. [Google Scholar]
- Pfingst BE. Changes over time in thresholds for electrical stimulation of the cochlea. Hear. Res. 1990;50:225–236. doi: 10.1016/0378-5955(90)90047-s. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Donaldson JA, Miller JM, Spelman FA. Psychophysical evaluation of cochlear prostheses in a monkey model. Ann. Otol. Rhinol. Laryngol. (St. Louis) 1979;88:613–625. doi: 10.1177/000348947908800505. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Sutton D, Miller JM, Bohne BA. Relation of psychophysical data to histopathology in monkeys with cochlear implants. Acta Otolaryngol. (Stockh) 1981;92:1–13. doi: 10.3109/00016488109133232. [DOI] [PubMed] [Google Scholar]
- Skinner MW, Binzer SM, Holden LK, Holden TA. Hearing changes in adults with cochlear implants. Semin. Hear. 1995:228–238. [Google Scholar]
- Snyder RL, Rebscher SJ, Cao K, Leake PA, Kelly K. Chronic intracochlear electrical stimulation in the neonatally deafened cat. I. Expansion of central representation. Hear. Res. 1990;50:7–33. doi: 10.1016/0378-5955(90)90030-s. [DOI] [PubMed] [Google Scholar]