Abstract
We have previously developed a novel technique for isolation of cDNAs encoding M phase phosphoproteins (MPPs). In the work described herein, we further characterize MPP10, one of 10 novel proteins that we identified, with regard to its potential nucleolar function. We show that by cell fractionation, almost all MPP10 was found in isolated nucleoli. By immunofluorescence, MPP10 colocalized with nucleolar fibrillarin and other known nucleolar proteins in interphase cells but was not detected in the coiled bodies stained for either fibrillarin or p80 coilin, a protein found only in the coiled body. When nucleoli were separated into fibrillar and granular domains by treatment with actinomycin D, almost all the MPP10 was found in the fibrillar caps, which contain proteins involved in rRNA processing. In early to middle M phase of the cell cycle, MPP10 colocalized with fibrillarin to chromosome surfaces. At telophase, MPP10 was found in cellular structures that resembled nucleolus-derived bodies and prenucleolar bodies. Some of these bodies lacked fibrillarin, a previously described component of nucleolus-derived bodies and prenucleolar bodies, however, and the bulk of MPP10 arrived at the nucleolus later than fibrillarin. To further examine the properties of MPP10, we immunoprecipitated it from cell sonicates. The resulting precipitates contained U3 small nucleolar RNA (snoRNA) but no significant amounts of other box C/D snoRNAs. This association of MPP10 with U3 snoRNA was stable to 400 mM salt and suggested that MPP10 is a component of the human U3 small nucleolar ribonucleoprotein.
INTRODUCTION
During M phase of the cell cycle of higher eukaryotes, most cell structures undergo massive rearrangements to allow appropriate division of cellular components to both daughter cells. Often these structural changes involve breakdown of interphase structures into smaller subunits. For instance, when cells enter M phase, the nuclear envelope breaks down into vesicles, and the nuclear lamins, which form a stable lining on the nucleoplasmic surface of the nuclear envelope in interphase, disassemble (Gerace and Blobel, 1980; Ottaviano and Gerace, 1985). These events are regulated by M phase promoting factor, a kinase consisting of the p34cdc2 catalytic subunit and a cyclin B regulatory subunit (Dunphy et al., 1988; Gautier et al., 1988, 1990; Draetta et al., 1989; Labbéet al., 1989; Meijer et al., 1989; Heald and McKeon, 1990; Peter et al., 1990; Ward and Kirschner, 1990). Consequently, lamins become phosphorylated and remain phosphorylated during M phase until telophase, when the nuclear lamina structure reforms. With the breakdown of the nucleus, many nuclear components become exposed to cytoplasmic enzymes that they do not encounter in interphase. This mixing of the nucleus with the cytoplasm could lead to some undesirable interactions that cells may work to avoid. Phosphorylation of cellular proteins could provide a means of shutting down interphase nuclear functions and disassembling nuclear structures.
Recently, we have developed a technique for identifying proteins that are phosphorylated during M phase and may be involved in some of the structural modifications that occur upon entry into M phase (Westendorf et al., 1994). Our technique resulted in the identification of 10 novel proteins that are phosphorylated during M phase (Matsumoto-Taniura et al., 1996). By immunofluorescence, each protein is found in its own characteristic locations within the cell. One of these proteins, M phase phosphoprotein 10 (MPP10), localized strongly to the nucleolus in interphase and to the chromosomes in M phase. This pattern of localization was similar to that exhibited by fibrillarin and several other nucleolar proteins (Yasuda and Maul, 1990; Gautier et al., 1992, 1994; Medina et al., 1995) and suggested that MPP10 might be involved in ribosome synthesis, assembly, or transport as are various other nucleolar proteins.
Autoantibodies to the protein fibrillarin have identified the U3 small nucleolar ribonucleoprotein (snoRNP), in vertebrates the most abundant of the snoRNPs (Lischwe et al., 1985; Reimer et al., 1987). In humans, it consists of an RNA component 217 nucleotides long complexed with at least six proteins, one of which is fibrillarin (Parker and Steitz, 1987; Lubben et al., 1993; reviewed in Maxwell and Fournier, 1995; Venema and Tollervey, 1995), a protein found in a wide variety of organisms. Studies in both vertebrates and the yeast Saccharomyces cerevisiae have demonstrated a requirement for the U3 snoRNP in processing of precursors to the mature 18S rRNA (Savino and Gerbi, 1990; Hughes and Ares, 1991; Beltrame and Tollervey, 1992; Beltrame et al., 1994; Beltrame and Tollervey, 1995; Hughes, 1996). It is likely that the U3 snoRNP acts as a rRNA chaperone, folding or presenting the precursor rRNA to the cleavage enzymes through direct base pairing between the small nucleolar RNA (snoRNA) and the pre-rRNA (Beltrame and Tollervey, 1995; Elela et al., 1996; Hughes, 1996). Although the RNA–RNA interactions are fairly well characterized, the nature and role of the protein components remains unknown. Specifically, other than fibrillarin, no protein component of any vertebrate U3 snoRNP has been cloned and sequenced.
In this article, we describe the full-length coding sequence of MPP10 and further examine its role in nucleolar structure and function. Our results indicate that MPP10 colocalizes with fibrillarin under most cellular conditions and that, like fibrillarin, it is a component of the U3 snoRNP, which is involved in rRNA processing. Nevertheless, in contrast to fibrillarin, MPP10 is not a major component of ribonuclear particles containing snoRNAs other than U3, and MPP10 relocalizes to the nucleolus at the end of mitosis by a course distinct from that of fibrillarin.
MATERIALS AND METHODS
Cloning the 5′ cDNA Sequences of MPP10
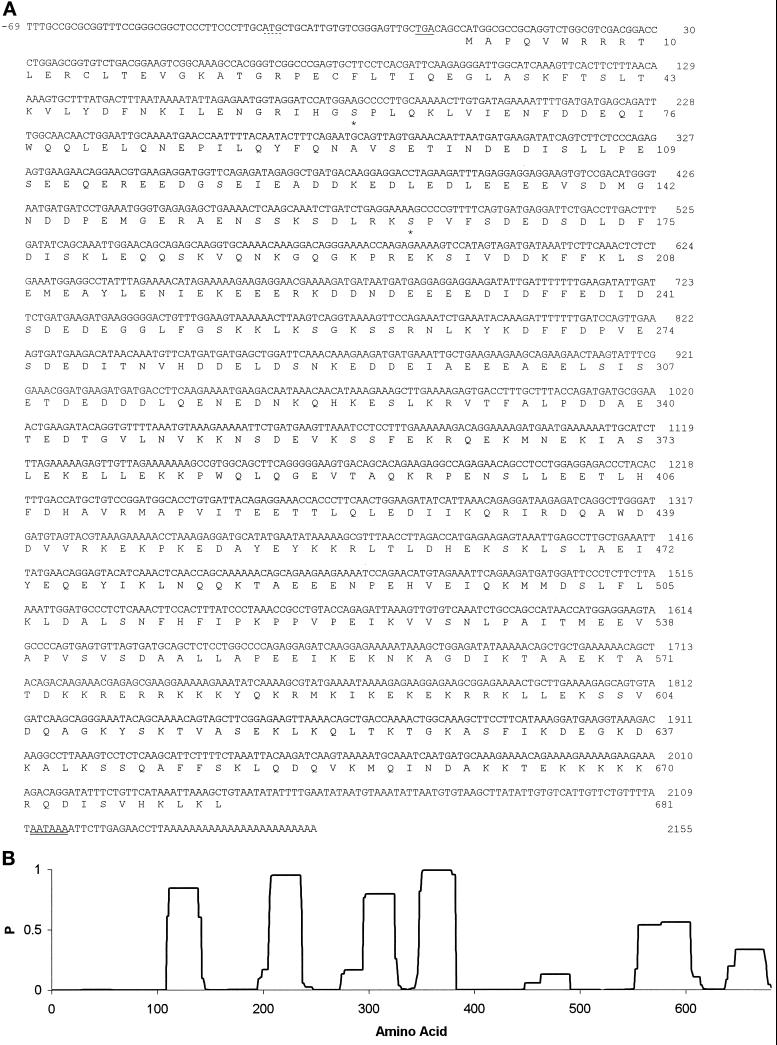
To obtain the complete coding sequence for MPP10, several complementary procedures were used. In one method, the 284-bp 5′ end EcoRI–EcoRV restriction fragment from MPP10 clone 2 (Matsumoto-Taniura et al., 1996) was labeled with [α-32P]dCTP by the random-primer method and used to screen a total of 2 × 106 clones from two HeLa cDNA-containing λZAPII libraries, one from Stratagene and the other a gift from Dr. P. Chambon (Institut de Chimie Biologique, Strasbourg, France). In this way one cDNA, clone 10c8 containing positions from −17 to +217 (Figure 1A) was isolated from the library obtained from Dr. Chambon.
Figure 1.
MPP10 cDNA and predicted amino acid sequence. (A) The cDNA and predicted amino acid sequences of MPP10 were determined as described in MATERIALS AND METHODS. The dotted underline indicates an upstream ATG in the 5′ noncoding sequence, the single underline indicates a stop codon in the same reading frame as the upstream ATG, the double underline indicates the poly(A) addition signal, and the stars indicate probable sites of MPM2-reactive phosphorylation (EMBL database accession number, X98494). (B) Probability of coiled-coil α-helix formation as determined by the program of Lupas et al. (1991).
In the second method, 5′ rapid amplification of cDNA ends was performed on a λgt11 human placental cDNA library by using one oligonucleotide complementary to the vector and one complementary to MPP10 sequences near the 5′ end (Frohman, 1994). The vector primer (λgt11R) was 5′-TTGACACAGACCAACTGGTAATG and the MPP10 primer (MPP10R-2) was 5′-GTCCTCCTTGTCATCAGCCTCTATC. After a 7-min denaturation at 94°C, the polymerase chain reaction (PCR) was performed for 30 cycles (94°C, 45 s; 55°C, 45 s; 72°C, 45 s; on a Perkin Elmer–Cetus 2400 thermocycler. The 1.1-kb PCR fragment was cloned by TA cloning into the pCR2.1 vector (Invitrogen, San Diego, CA).
cDNAs were sequenced by the Sanger dideoxynucleotide method using custom primers and 35S-labeled dATP or automated sequencing, which was performed with an Applied Biosystems 373A sequencer at the core facility at The Scripps Research Institute or an Applied Biosystems 373 Stretch sequencer at the W.M. Keck Foundation Biotechnology Resource Laboratory at Yale University. Sequences were analyzed with GCG and Intelligenetics programs and database searches were performed with the Blast program provided by the National Center for Biotechnology Information/National Institutes of Health.
In Vitro Translation of Full-Length MPP10 Protein
To make a clone containing a full-length MPP10 coding sequence, the 2000-bp BamHI–EcoRI fragment from clone 2 was isolated and cloned into BamHI/EcoRI-digested pBluescriptSK−. The resulting construct was then digested with NotI, and the ends were filled in with the Klenow fragment in the presence of dGTP. Then clone 10c8 was digested with PspAI, and the ends were filled in with the Klenow fragment in the presence of dCTP. After cutting both the clone 2 and the clone 10c8 derivatives with BamHI, the small fragment of 10c8 was cloned into the large fragment of the clone 2 derivative. The resulting plasmid containing bases −17 to +170 (see Figure 1A) of clone 10c8 and bases 171 to 2155 (see Figure 1A) of clone 2 were transcribed and translated in vitro with T3 RNA polymerase and the TNT system (Promega, Madison, WI) in the presence of [35S]methionine.
Antibodies
Monoclonal antibody 72B9 against fibrillarin was a gift from Michael Pollard (Reimer et al., 1987), the rabbit polyclonal antibody against recombinant p80-coilin was a gift from Ed Chan (Andrade et al., 1991), the monoclonal antibody against the trimethylguanosine (TMG) cap was obtained from Oncogene Science (Manhassett, NY), and the Y-12 monoclonal antibody against Sm antigens was a gift from Joan Steitz (Lerner and Steitz, 1979). For antibodies to MPP10, guinea pigs were injected with a fusion protein consisting of T7 protein 10 fused to amino acids 58–681, and the resulting antisera were affinity purified as previously described (Matsumoto-Taniura et al., 1996). Normal human serum was obtained from a young male volunteer.
Immunofluorescence by Light and Confocal Laser Scanning Microscopy
For immunofluorescence, HEp-2 cells were grown directly on glass coverslips (22 mm2; Corning, Corning, NY) in six-well plates, rinsed briefly with phosphate-buffered saline (PBS; 10 mM phosphate), and then fixed at −20°C with methanol for 5 min followed by acetone for 2 min. Commercially available prefixed HEp-2 cell substrates (Bion, Park Ridge, IL) were also used. Primary antibodies were diluted in PBS and incubated with cells at 25°C for 1 h. For double labeling, purified guinea pig antibodies against MPP10 and murine monoclonal antibodies, characterized human serum, or rabbit polyclonal antibodies against other antigens were mixed together before incubation with cells. After three washes with PBS, the cells were incubated with fluorescein isothiocyanate-conjugated anti-guinea pig and rhodamine-conjugated anti-mouse, anti-human, or anti-rabbit (Caltag Laboratories, San Francisco; and Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 h at 25°C, washed, and mounted on slides with an antifade mounting medium (Vectashield, Vector Laboratories, Burlingame, CA). Conventional epifluorescence microscopy was performed with an Olympus fluorescence microscope, and specimens were photographed with Kodak Ektachrome 400 film.
Microscopy was also performed with an MRC-600 confocal laser scanning instrument (Bio-Rad Laboratories, Cambridge, MA) fitted to a Zeiss Axiovert epifluorescence microscope with a ×63/1.4 numerical aperature oil-immersion lens. In most experiments, images were the product of 30 scans, each collected from a single focal plane (about 0.4 μm) and together averaged by the Kalman method with the Bio-Rad COMOS program. In the actinomycin D experiment, sequential optical planes were acquired along the Z-axis through the cultured cells, and the stored graphic files were collapsed to a single virtual image (Z-series projection) by using software provided by Bio-Rad Laboratories. Fluorescence images were collected simultaneously from the fluorescein and rhodamine channels; differential interference contrast images were collected subsequently. The images were digitized and imported into Adobe Photoshop 4.0 (Adobe Systems, Mountain View, CA) for further image processing.
Immunoprecipitation from HeLa Cells and In Vitro Translations
To precipitate in vitro-translated MPP10, a 20-μl TNT translation reaction of full-length MPP10 was diluted with 0.25 ml of high salt RIPA buffer [50 mM Tris-HCl, pH 8.0, 500 mM NaCl, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate, 2 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 10 μg/ml pepstatin, 10 mM EDTA, 10 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 10 mM benzamidine, 10 mM tosyl arginylmethyl ester], and MPP10 was precipitated with 5 μl of MPP10 antiserum bound to 20 μl of protein A-Sepharose 6 MB. For precipitation of HeLa cell MPP10 protein, 4 × 107 exponentially growing HeLa cells were lysed with high salt RIPA and centrifuged at 100,000 × g, and MPP10 was precipitated with 10 μl of MPP10 antiserum bound to 40 μl of protein A-Sepharose 6 MB beads. After an overnight incubation, immunoprecipitates were washed three times with high salt RIPA, and bound proteins were eluted with SDS sample buffer. Samples were separated by SDS-PAGE and analyzed by immunoblot with anti-MPP10 and autoradiography.
Subcellular Fractionation
Fractionation of HeLa cells was performed according to Warner (1979) with the following modifications. The fractionation was performed at 4°C and all centrifugations were carried out in a JA-20 rotor (Beckman, Fullerton, CA) except where noted. HeLa cells (1 × 108 cells) were collected by centrifugation and washed two times with PBS. The cells were resuspended in 5 ml of buffer A (10 mM HEPES-KOH, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, and 0.5 mM dithiothreitol), swollen for 10 min on ice, then broken with 12–15 strokes of a tight fitting Dounce homogenizer, and centrifuged to pellet nuclei. The supernatant from this centrifugation was considered to be the cytoplasmic fraction and the pellet was termed “nuclei I.”
To further purify the nuclei, the pellet was resuspended in 10 ml of buffer containing 10 mM Tris-Cl, pH 7.5, 3.3 mM MgCl2, and 0.25 M sucrose and centrifuged for 5 min at 1000 rpm. The resulting pellet was resuspended in 2.5 ml of 10 mM MgCl2 and 0.25 M sucrose, layered over 2.5 ml of 0.5 mM MgCl2 and 0.35 M sucrose, and centrifuged for 10 min at 2500 rpm. The resulting pellet of purified nuclei was resuspended in 2.5 ml of 0.5 mM MgCl2 and 0.35 M sucrose and sonicated (four or five times; 15 s) with a Branson sonifier (setting 1) to disrupt the nuclei. When disruption was complete, as determined by microscopy of a sample stained with Azure C (Muramatsu et al., 1963), the sonicate (nuclei II) was layered over 2.5 ml of 0.5 mM MgCl2 and 0.88 M sucrose and centrifuged for 15 min at 4000 rpm in an SW41 rotor (Beckman). The resulting pellet, which contained purified nucleoli, was resuspended in NET-2 (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.05% Nonidet P-40 [NP-40]), sonicated (five times; 30 s) at setting 3 of a Branson sonifier and centrifuged for 10 min at 10,000 rpm (12,000 × g). The supernatant was analyzed by SDS gel electrophoresis and immunoblot with anti-MPP10.
Immunoblotting
Proteins separated by SDS-PAGE were transferred to Immobilon-P or Hybond-N (Amersham, Arlington Heights, IL) and reacted with MPP10 antiserum at a dilution of 1:2000 to 1:10,000 in Tris-buffered saline containing Tween (50 mM Tris-HCl, pH 7.9, 150 mM NaCl, 0.1% Tween-20) with or without 5% nonfat dried milk. Bound antibodies were detected with horseradish peroxidase-conjugated anti-guinea pig antibody (Boehringer Mannheim, Indianapolis, IN, or Jackson ImmunoResearch Laboratories, West Grove, PA) at a dilution of 1:5000 and the ECL system (Amersham).
Small Nuclear Ribonucleoprotein (snRNP) Immunoprecipitations
Immunoprecipitations for detection of associated RNAs were performed essentially as described (Lerner et al, 1981). For direct RNA labeling, 10 μl of normal human serum, 40 μl of anti-fibrillarin culture supernatant (72B9) and 2.5 or 10 μl of affinity-purified MPP10 were bound to 4 mg of protein A-Sepharose. For Northern blot analysis of RNAs, the following antibodies were bound to 2.5 mg of protein A-Sepharose CL-4B for 16 h at 4°C: 10 μl of crude or affinity-purified anti-MPP10 polyclonal guinea pig serum, 100 μl of cell culture supernatant containing mouse monoclonal anti-fibrillarin (72B9), 10 μl of mouse ascites containing monoclonal anti-TMG plus 10 μl of rabbit anti-mouse IgG, or no added antibody (mock). The antibody–protein A-Sepharose CL-4B pellets were washed three times with NET-2 (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.05% NP-40). HeLa cells or mouse L cells (1 × 108 cells) were resuspended in NET-2 or NET-400 (20 mM Tris-HCl, pH 7.5, 400 mM NaCl, 0,05% NP-40), as indicated, and sonicated (five times; 30 s) at setting of 3 with a Branson sonifier. The sonicate was centrifuged for 10 min at 10,000 rpm (12,000 × g). The supernatant from this centrifugation was added to the washed antibody–protein A-Sepharose CL-4B pellets, mixed for 1 h at 4°C, and the pellets were washed with the buffer in which the extract was made.
RNA was recovered from the pellets by extraction with PCA (phenol:chloroform:isoamyl alcohol, 25:24:1) and ethanol precipitation. The recovered RNA was analyzed either by 3′-end-labeling with 32P-labeled pCp and T4 RNA ligase (England et al., 1980) or by Northern blotting with antisense RNA probes. In both cases, RNA was analyzed by denaturing gel electrophoresis.
Northern Blots
For Northern blots, RNAs purified from precipitates were electrophoresed on an 8% denaturing polyacrylamide gel and blotted to a Zeta-Probe membrane (Bio-Rad Laboratories; Kass et al., 1987). Blots were hybridized with antisense RNAs complementary to U3, U2, and U1 or U8, produced as previously described (Black and Pinto, 1989; Baserga et al., 1991).
RESULTS
Characterization of a Full-Length Coding Sequence for MPP10
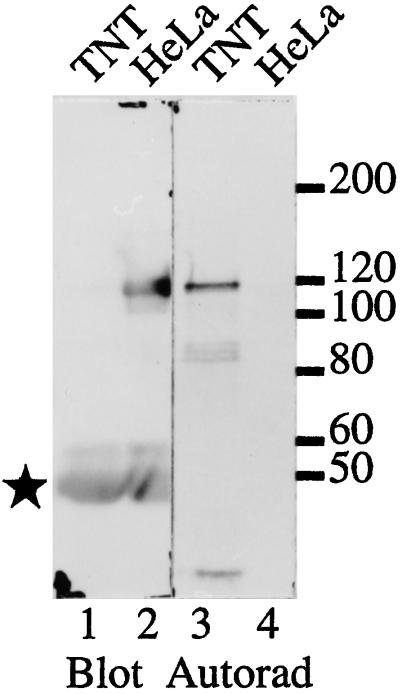
cDNA clone 2 encoding the C-terminal portion of MPP10 was isolated by expression cloning of MPPs that are reactive with the phosphoepitope-specific monoclonal antibody MPM2 (Davis et al., 1983; Westendorf et al., 1994; Matsumoto-Taniura et al., 1996). When clone 2 cDNA was transcribed into RNA and translated in vitro, the translation product migrated at 85 kDa (our unpublished observations), considerably faster than HeLa cell MPP10 protein, which migrates at 120 kDa (Matsumoto-Taniura et al., 1996). To obtain sequences encoding the amino terminus of MPP10, we isolated cDNAs overlapping with clone 2 by screening cDNA libraries with labeled cDNA fragments and by PCR on cDNA libraries. Figure 1A presents a full-length coding sequence for MPP10, which consists of 69 nucleotides of 5′ noncoding sequence, a start codon that is surrounded by nucleotides found to be conducive to start of translation (Kozak, 1996), an in-frame stop codon, and 109 nucleotides of 3′ noncoding sequence. There is an additional ATG, which is in a poor context for start of translation, 5′ of the proposed start codon, but it is followed eight codons later by a stop codon in the same reading frame. When RNA transcribed from cDNA containing MPP10 cDNA sequences from positions −17 through the poly(A) tail is translated, the protein product comigrates with MPP10 immunoprecipitated from HeLa cells (Figure 2). This indicates that the full-length MPP10 protein is encoded by the long open reading frame of 681 codons shown in Figure 1A.
Figure 2.
Comigration of MPP10 translated in vitro and MPP10 from Hela cells. MPP10 transcribed and translated in the presence of [35S]methionine from a full-length cDNA coding sequence and MPP10 from HeLa cells were precipitated with anti-MPP10 serum, fractionated by SDS-PAGE, and immunoblotted with anti-MPP10 serum. Bound antibodies were detected by ECL (lanes 1 and 2), and radioactive MPP10 was detected by autoradiography (lanes 3 and 4). Positions of migration of molecular mass markers in kDa are indicated on the right; the star indicates the position of migration of IgG heavy chain.
The sequence of full-length MPP10 predicts a protein of 78,868 Da, considerably less mass than the 120,000 Da indicated by its migration on SDS gels. T7 protein 10-MPP10 fusion protein also migrates anomalously slowly on gels (Matsumoto-Taniura et al., 1996). This characteristic could be due to the extreme acidity of the protein; the predicted pI is 4.52. MPP10 contains acidic and basic stretches of amino acids, which are concentrated in portions of the protein that are predicted to have a high probability of being in coiled-coil α-helices (Lupas et al., 1991; Figure 1B). The first stretch is 28 amino acids long, has a 0.84 predicted probability of forming a coiled-coil α-helix, and contains 18 acidic and 2 basic residues; the second is 29 long, has a 0.94 probability, and contains 16 acidic and 3 basic residues; the third is 28 long, has a 0.79 probability, and contains 14 acidic and 1 basic residues; the fourth is 35 long, has a 0.99 probability, and contains 8 acidic and 10 basic residues; the fifth is 50 long, has a 0.55 probability, and contains 8 acidic and 23 basic residues; and the sixth is 28 long, has a 0.33 probability, and contains 4 acidic and 10 basic residues. Short stretches of probable coiled-coil α-helix such as these could be involved in intramolecular and/or intermolecular interactions (Lupas, 1996), and the charged amino acids found in these domains may also be involved in formation of structures. Acidic and basic stretches are found in many other nucleolar proteins also (Shaw and Jordan, 1995).
Although the sequence of MPP10 is not highly homologous to any known protein, database searches indicate weak relationships between MPP10 and the nucleolar protein nucleolin (22% identity), acid-rich proteins, and proteins containing coiled-coil α-helices. Interestingly, the sequence most similar to MPP10 found in GenBank is a yeast open reading frame identified by sequencing of the yeast genome. Experiments in yeast indicate that this gene, which is 30% identical to hMPP10, is likely to be the yeast homologue (Dunbar et al., 1997). By comparison with a database of protein motifs, we find that MPP10 contains basic amino acid stretches that could represent nuclear localization sequences at amino acid positions 251–256, 574–579, 583–588, 592–597, and 666–671. The last four sequences are all within portions of the protein that have substantial potential to form coiled-coil α-helices. MPP10 contains two potential MPM2-reactive phosphorylation sites at amino acids 61 and 163 (Westendorf et al., 1994; Matsumoto-Taniura et al., 1996).
Characterization of Nucleolar MPP10
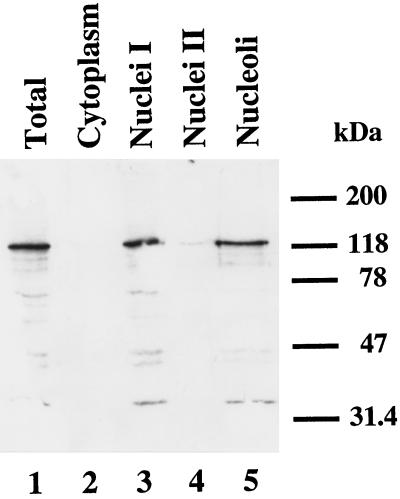
The nucleolar localization of MPP10 suggests that MPP10 is likely to be involved in generation of ribosomes. Possible functions include a role in rRNA processing, assembly of ribosomal subunits, and nucleo-cytoplamic transport of ribosomal components. To learn more about a possible role of MPP10 in these processes, the intracellular localization of MPP10 was examined in more detail. First, HeLa cells were fractionated into cytoplasmic and nuclear fractions after hypotonic lysis. Crude nuclei (nuclei I) were further purified and then subfractionated into nucleoplasm (nuclei II) and nucleoli (see MATERIALS AND METHODS). When fractions representing an equal number of cells were separated by SDS-PAGE and immunoblotted with antibodies to MPP10 (Figure 3), MPP10 was found in total cellular lysate (Figure 3, lane 1) and the nuclear (Figure 3, lane 3) and nucleolar (Figure 3, lane 5), but not the cytoplasmic (Figure 3, lane 2) and nucleoplasmic (Figure 3, lane 4) fractions.
Figure 3.
MPP10 in subcellular fractions of HeLa cells. First, HeLa cells were fractionated into cytoplasmic and nuclear fractions. Then, the crude nuclear fraction (Nuclei I) was further fractionated into nucleoplasm (Nuclei II) and nucleoli. Fractions representing an equal number of cells were separated by SDS-PAGE, transferred to Hybond-N, and reacted with anti-MPP10 serum. Bound antibodies were subsequently reacted with horseradish peroxidase-coupled secondary antibodies and detected with ECL. Positions of migration of molecular mass markers are indicated on the right.
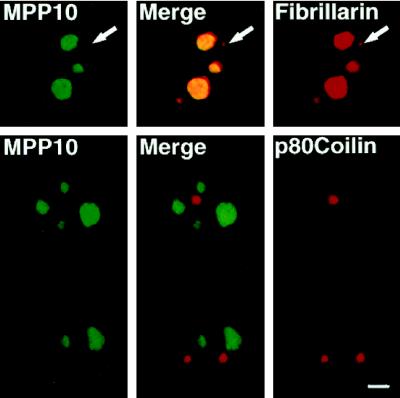
Second, colocalization of MPP10 and a number of other nucleolar proteins in cultured cells was examined. In interphase cells, MPP10 staining overlapped with the nucleolar staining of fibrillarin, an abundant antigen of the fibrillar component of the nucleolus (Figure 4). Likewise, MPP10 colocalized to the nucleoli stained by antibodies to RNA polymerase I, the 90-kDa nucleolar organizer protein/upstream binding factor, and the PmScl and Th-To autoimmune antigens. To learn whether fibrillarin and MPP10 colocalize to a similar portion of the nucleolus, we treated cells with actinomycin D, which arrests rRNA transcription and causes the separation of the nucleolus into fibrillar caps containing proteins involved in rRNA processing and a granular region containing proteins involved in ribosome assembly and transport. In cells containing optimally segregated nucleoli, MPP10 primarily localized to the fibrillar caps as did fibrillarin, a protein known to be involved in rRNA processing (Figure 5). Further, merging of the confocal images of anti-MPP10 and anti-fibrillarin immunofluorescence indicated that portions of MPP10 and fibrillarin colocalize. Nevertheless, anti-MPP10 and anti-fibrillarin staining did not cover exactly the same parts of segregated nucleolus; some fibrillarin localized outside areas containing MPP10 and some MPP10 was found outside areas containing fibrillarin. Thus, the localization of MPP10 to the fibrillar caps of drug-segregated nucleoli indicates that MPP10 is likely to be involved in rRNA processing. Because some of the MPP10 colocalizes with fibrillarin, MPP10 may be involved in one or more of the processing steps that involves fibrillarin.
Figure 4.
MPP10 colocalization with fibrillarin in the interphase nucleolus but not in the coiled body. Fixed human HEp-2 cells were stained with affinity-purified guinea pig anti-MPP10 and mouse anti-fibrillarin or anti-p80 coilin, and staining was detected with FITC-labeled anti-guinea pig and rhodamine-labeled anti-mouse or rabbit. Images were obtained by confocal microscopy. Bar, 4 μm.
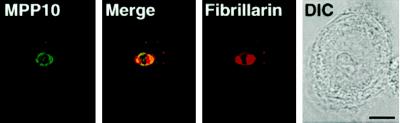
Figure 5.
Localization of MPP10 and fibrillarin in cells treated with actinomycin D. HEp-2 cells were incubated with 0.1 μg/ml actinomycin D mannitol for 4 h. After drug treatment, cells were washed, fixed, and stained with affinity-purified guinea pig anti-MPP10 and mouse anti-fibrillarin, and staining was detected with FITC-labeled anti-guinea pig and rhodamine-labeled anti-mouse. Images were obtained by confocal microscopy. DIC, differential interference contrast microscopy. Bar, 5 μm.
To further define the localization of MPP10, we examined coiled bodies, which contain numerous nucleolar proteins, including fibrillarin, as well as proteins involved in mRNA processing. Although the function of the coiled body is unknown, it can be stained specifically by antibodies to coilin, a protein found only in the coiled body. We never detected MPP10 staining in coiled bodies stained for either coilin or fibrillarin (Figure 4). This suggests that MPP10 does not participate in the functions of the coiled body.
Dynamics of MPP10 during the Cell Cycle
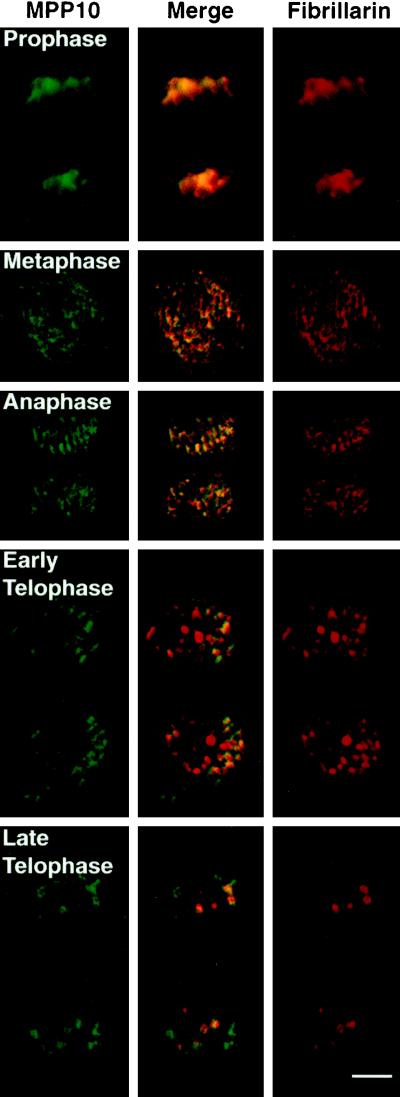
At the start of M phase, nucleoli break down, and only proteins involved in rRNA transcription remain associated with the rRNA genes. We have shown previously that after dissolution of the nucleolus in early M phase, MPP10, like fibrillarin, becomes associated with chromosomes (Matsumoto-Taniura et al., 1996, see also Figure 6). MPP10 and fibrillarin continue to be near the chromosomes throughout metaphase and anaphase. In telophase, when the nuclear envelope reforms, MPP10 and fibrillarin leave the chromosomal surfaces and are found concentrated in small cellular bodies (Figure 6). Two types of telophase bodies containing nucleolar constituents such as fibrillarin have been previously described. They are nucleolus-derived foci, which are present in the cytoplasm of late anaphase and early telophase cells, and prenucleolar bodies, which are present in the nucleus of telophase and early G1 cells (Jiménez-García et al., 1989, 1994; Azum-Gélade et al., 1994; Medina et al., 1995; Dundr et al., 1997). We find, however, that the bodies containing MPP10 in telophase are distinct from most of the fibrillarin-containing bodies (Figure 6). At a time in telophase when the fibrillarin bodies are all within the nucleus, many of the MPP10 bodies are within the cytoplasm. Later, when fibrillarin is almost completely localized to the nucleoli, MPP10 is primarily found in nuclear structures resembling prenucleolar bodies. Thus, the reassociation of MPP10 with nucleoli is a distinct event, which is preceded by the arrival of fibrillarin.
Figure 6.
Localization of MPP10 and fibrillarin during M phase of the cell cycle. Fixed HEp-2 cells were stained with affinity-purified guinea pig anti-MPP10 and mouse anti-fibrillarin, and staining was detected with fluorescein-labeled anti-guinea pig and rhodamine-labeled anti-mouse. Images were obtained by confocal microscopy. Bar, 5 μm.
Characterization of RNAs Found in MPP10 Immunoprecipitates
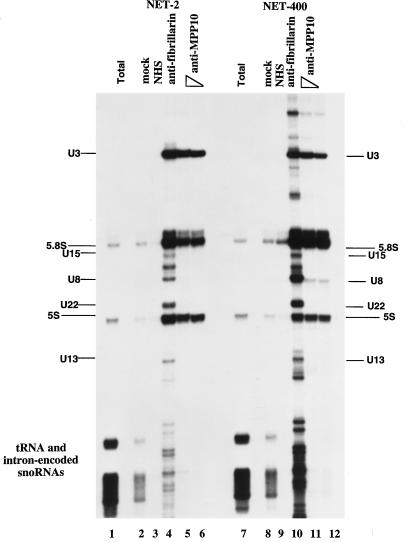
Localization of MPP10 to actinomycin D-segregated nucleolar fibrillar caps, where fibrillarin is found, suggests that MPP10 may be associated with a snoRNA involved in rRNA processing. To test this possibility, MPP10-containing complexes were immunoprecipitated from HeLa cell sonicates, and coprecipitated RNAs were purified and labeled at their 3′ ends with 32P-labeled pCp (Figure 7). In the MPP10 immunoprecipitates, U3 was the only snoRNA found (Figure 7, lanes 5 and 6). Control immunoprecipitates with anti-fibrillarin contained large amounts of U3, U8, U22, U13 and many smaller box C/D snoRNAs (Figure 7, lane 4). The association of MPP10 with the U3 snoRNP remains stable in high salt (Figure 7, lanes 10–12). Nevertheless, there are a number of nonbox C/D snoRNAs that are visualized in the fibrillarin immunoprecipitation, indicating that the immunoprecipitations performed in high salt (400 mM) are less specific than those performed in low salt (150 mM). For this reason, we also consider the new RNA bands visualized in the MPP10 immunoprecipitations performed in high salt to be nonspecific. Thus, these findings suggest that MPP10 is a stably associated protein component of human U3 snoRNPs.
Figure 7.
Immunoprecipitation of U3 snoRNA by anti-MPP10. Protein A beads were incubated with no antibodies (mock), normal human serum (NHS), mouse monoclonal antibodies (72B9) to fibrillarin (anti-fibrillarin), or affinity-purified guinea pig anti-MPP10 (anti-MPP10; 10 μl, lanes 5 and 11; 2.5 μl, lanes 6 and 12). The resulting beads were washed and incubated with HeLa cell sonicates. RNA from the resulting immunoprecipitates was purified, end-labeled with 32P-labeled pCp, fractionated on 8% denaturing polyacrylamide gels, and autoradiographed. Lane 1 contains total RNA purified from 1% the number of cells used in the immunoprecipitations. The indicated positions of major RNAs were determined by comparison with the migration of pBR322 digested with MspI, end-labeled, and denatured. We do not consider the presence of 5S and 5.8S rRNAs in the precipitates to be specific because these RNAs are found in precipitates of splicing snRNPs also.
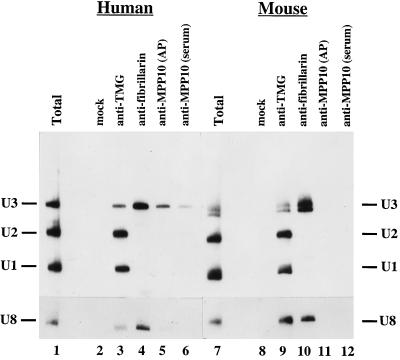
To further analyze the specificity of MPP10 association with U3 snoRNA, anti-MPP10, anti-TMG, and anti-fibrillarin antibodies were added to sonicates of HeLa cells, and the RNAs purified from the resulting immunoprecipitates were separated, blotted, and hybridized with probes for U1, U2, and U3 RNAs. RNA derived from the anti-MPP10 and anti-fibrillarin precipitates hybridized with only the U3 probe (Figure 8, lanes 4–6). In contrast, precipitates with anti-TMG, which recognizes the TMG cap of several small nuclear RNAs including the abundant U1 and U2 spliceosomal snRNAs and the U3 snoRNA, precipitated RNA that hybridized with all three probes (Figure 8, lane 3). When a U8 probe was used for hybridization, the U8 snoRNA was apparent in only the immunoprecipitations performed with anti-fibrillarin and anti-TMG cap antibodies. These results indicate further that MPP10 is found in U3 snoRNPs and, therefore, is likely to be involved in rRNA processing.
Figure 8.
Human and mouse anti-MPP10 immunoprecipitations analyzed by Northern blot analysis. Protein A beads were incubated with no antibodies (mock), mouse monoclonal antibodies to the TMG cap (anti-TMG), mouse monoclonal antibodies (72B9) to fibrillarin (anti-fibrillarin), affinity-purified guinea pig anti-MPP10 (anti-MPP10; affinity purified, AP), or anti-MPP10 guinea pig serum (anti-MPP10 [serum]), washed, and incubated with HeLa (lanes 2–6) or mouse L (lanes 8–12) cell sonicates. RNA from immunoprecipitates was isolated, fractionated on 8% denaturing polyacrylamide gels, blotted to Zeta-Probe, and hybridized with U1, U2, and U3, or U8 probes. Lanes 1 and 7 contain total RNA from 10% the number of cells used in the immunoprecipitations.
Conservation of MPP10 across Various Vertebrate Species
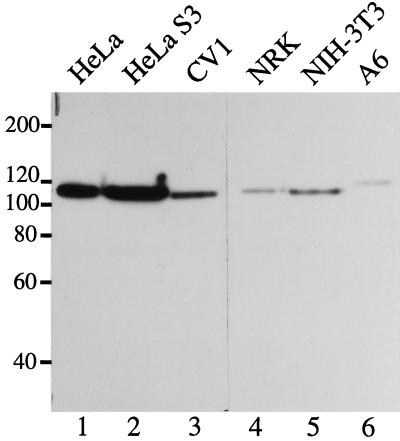
If MPP10 is involved in rRNA processing, an operation common to all cells, we would expect it to be conserved across various species. To learn whether there is a protein similar to MPP10 in cells other than human, we immunoblotted proteins from various animal cell lines with antibodies directed against amino acids 58 to 681. We found immunoreactive proteins approximately the same size as MPP10 in monkey, rat, mouse, and toad cells (Figure 9). From this, we conclude that an MPP10-like protein is present in diverse vertebrate species. In spite of the immuno-cross-reactivity we see by immunoblot, we find that the antibodies against human MPP10 do not recognize MPP10 in rat cells by immunofluorescence (our unpublished data) and are not able to immunoprecipitate U3 snoRNA from mouse cell sonicates (Figure 8, lanes 11 and 12). It is likely that most of the epitopes recognized by our antibodies are only exposed in denatured protein.
Figure 9.
MPP10 in various vertebrate cells. Samples of cells lysed directly in SDS sample buffer were separated by SDS-PAGE and immunoblotted with guinea pig anti-MPP10 serum. Reactive proteins were detected with horseradish peroxidase-conjugated anti-guinea pig and ECL reagents. Lane 1, human HeLa cervical carcinoma cell line growing attached to culture dishes; lane 2, human HeLa cervical carcinoma cell line growing in suspension; lane 3, African green monkey CV1 kidney fibroblast cell line; lane 4, rat NRK kidney epithelial cell line; lane 5, mouse NIH 3T3 embryo fibroblastic cell line; lane 6, South African clawed toad A6 kidney epithelial cell line.
DISCUSSION
We have shown that MPP10, a protein whose cDNA was isolated on the basis of its ability to be phosphorylated by M phase kinases, is a nucleolar protein of unique properties. In our experiments, the majority of MPP10 colocalized with fibrillarin, a well-characterized nucleolar protein, under most conditions. Nevertheless, even though a portion of fibrillarin is found in the coiled body, a nuclear structure of unknown function, MPP10 was never detected in the coiled body stained with either anti-fibrillarin or anti-p80 coilin. Again, like fibrillarin, MPP10 localized to small cellular bodies similar to nucleolus-derived foci and prenucleolar bodies in early telophase of mitosis. Nonetheless, many of the nucleolus-derived foci and prenucleolar bodies in which fibrillarin is found did not contain MPP10, which arrived at the newly forming nucleolus later than fibrillarin. Finally, like those of fibrillarin, antibodies against MPP10 immunoprecipitated the U3 RNA. In contrast to immunoprecipitates of fibrillarin, however, those of MPP10 did not contain substantial amounts of any other snoRNA.
The properties of MPP10 suggest that it is involved in rRNA processing. This potential function for MPP10 was first indicated by its localization to the fibrillar caps of actinomycin D-segregated nucleoli, which contain proteins involved in rRNA processing (reviewed in Ochs, 1997). Much stronger evidence for MPP10 involvement in rRNA processing comes from our immunoprecipitation experiments; anti-MPP10 precipitates U3 snoRNA, which is necessary for processing of 18S rRNA (Savino and Gerbi, 1990; Hughes and Ares, 1991; Beltrame and Tollervey, 1992; Beltrame et al., 1994; Beltrame and Tollervey, 1995; Hughes, 1996). The final proof of MPP10 involvement in this event in vertebrates, however, is still lacking. We will need to deplete MPP10 from cell extracts and show that some rRNA processing step is lost. To date, we have not been able to do this because our antibodies do not immunoprecipitate U3 from mouse L cell sonicates. This experiment will require development of other antibodies to MPP10. Interestingly, depletion of the MPP10 homologue in yeast causes an 18S rRNA processing defect similar to that obtained on depletion of two other U3 snoRNP components (Dunbar et al., 1997). Furthermore, mutations in yeast MPP10 suggest that it may be more directly involved in cleavage at the pre-rRNA A1 and A2 sites (Lee and Baserga, in press).
Because MPP10 is not detected in the coiled body, the functions of MPP10 that are similar to those of fibrillarin are likely to occur in the nucleolus, not the coiled body. As yet, the activities that are carried out in the coiled body and its relationship to the nucleolus are not understood. Nevertheless, overexpression of p80 coilin, the marker protein for the coiled body, disrupts nucleolar structure (Bohmann et al., 1995), and U3 snoRNP components including fibrillarin and U3 snoRNA have been localized to the coiled body (Figure 4; Raska et al., 1991; Jiménez-García et al., 1994). The localization of U3 snoRNA to coiled bodies is, however, controversial; in other experiments using different probes, the U3 snoRNA was not observed in coiled bodies (Carmo-Fonseca et al., 1993; Matera et al., 1994). Therefore, although we do not observe any MPP10 in the coiled body, we cannot be certain that it is not present in that structure. If the presence of U3 RNA and absence of MPP10 in coiled bodies prove to be correct, then the U3 snoRNPs that are found in that structure must lack MPP10.
Because MPP10 was isolated on the basis of its ability to be phosphorylated by M phase kinases, and we have shown that MPP10 is phosphorylated in M phase, we suspect that phosphorylation may play a role in determining the M phase function, M phase distribution, or stability of MPP10. We note that the potential MPM2-reactive phosphorylation site at amino acid 163 is in a PEST sequence, a motif that regulates protein turnover (Rechsteiner and Rogers, 1996). Although we have found U3 associated with MPP10 immunoprecipitated from M phase cells (our unpublished results), we cannot be certain that these complexes are not formed after dephosphorylation of MPP10 in cell lysates. As yet, we have not found cell lysis conditions capable of maintaining both U3 snoRNP structure and MPP10 phosphorylation. Our ability to determine the role of phosphorylation in MPP10 function in M phase will be aided by identification of the kinases and phosphatases that act on MPP10.
Throughout M phase, nucleolar proteins involved in rRNA transcription remain with the nucleolar organizer (Scheer and Rose, 1984; Jiménez-García et al., 1989; Chan et al., 1991). In contrast, many other nucleolar proteins localize to chromosome surfaces in prophase through anaphase. In telophase and G1, they follow a pathway involving cytoplasmic nucleolus-derived foci and, subsequently, nuclear structures termed prenucleolar bodies, and, finally, reassociate with forming nucleoli when rRNA transcription resumes (Benavente et al., 1987; Jiménez-García et al., 1989, 1994; Oakes et al., 1993; Azum-Gélade et al., 1994; Hernandez-Verdun and Gautier, 1994; Medina et al., 1995; Dundr et al., 1997). Although both MPP10 and fibrillarin follow this general pattern of reassembly into nucleoli, we were surprised to find that the small bodies in which MPP10 was found in telophase did not always correspond to the bodies that contain fibrillarin. Our finding that MPP10 and fibrillarin do not colocalize to all the same telophase structures suggests a further layer of complexity to the process of nucleolar reformation. It is possible that nucleolus-derived foci and prenucleolar bodies process nucleolar components for relocalization to nucleoli and that, during nucleolar reformation, MPP10 passes through these structures at a different time from other nucleolar components.
To date, MPP10 is the only characterized vertebrate protein that has been shown to coimmunoprecipitate with U3 RNA but no significant amounts of other snoRNAs. Fibrillarin, the best-characterized protein of the U3 snoRNP, is also found in snoRNPs containing various other snoRNAs that are involved in processing 5.8S and 28S rRNA and rRNA methylation (Peculis and Steitz, 1993; Kiss-Lászlóet al., 1996; Nicoloso et al., 1996). We do not yet know whether fibrillarin and MPP10 are complexed together in the same U3 RNP, and we have not determined whether there is a pool of MPP10 that is not complexed with the U3 snoRNP. Furthermore, although MPP10 is tightly bound to U3 snoRNP, we have not been able to demonstrate direct binding of MPP10 to the U3 snoRNA (our unpublished observations). It may be that the association of MPP10 with the U3 snoRNA occurs via protein–protein interactions. The short stretches of potential coiled-coil α-helix found in MPP10 might mediate these intermolecular associations. As more protein components of nucleolar RNPs are characterized, it will be interesting to sort out which proteins are found in the same multi-protein complex.
MPP10 is one of what are likely to be many protein components of the vertebrate U3 snoRNP. [35S]methionine and [32P]orthophosphate labeling of HeLa cells followed by immunoprecipitation with anti-fibrillarin antisera indicates that there may be at least six protein components of the U3 snoRNP, including the protein fibrillarin (Parker and Steitz, 1987). It is possible, however, that not all of these proteins are specific for the U3 RNP. For instance, we now know that fibrillarin is a component of many snoRNPs. By contrast, purification of the U3 snoRNP from hamster cells with anti-TMG and Mono Q anion-exchange chromatography yielded only three proteins, none of which were fibrillarin (Lubben et al., 1993). Both these isolation protocols identified a 55-kDa protein that may be the mammalian homologue of the 56-kDa yeast U3 snoRNP Sof1 (Jansen et al., 1993). The methods that have been used thus far for isolation of U3 snoRNPs may not, however, be adequate for identifying all its components. Glycerol gradients of HeLa extract indicate a size for the U3 monomeric snoRNP similar to that of the U1 mono-snRNP (12 S; Tyc and Steitz, 1989). Given that the vertebrate U1 snRNP has already been found to have 11 components (Fabrizio et al., 1994), the U3 snoRNP may contain many more proteins that have not yet been identified.
ACKNOWLEDGMENTS
J.M.W. and F.P. are thankful for the support of Dr. Didier Job (Institut National de la Santé et de la Recherche Médicale U366, Commissariat à l’Energie Atomique, Grenoble), in whose laboratory part of the work reported herein was done. We thank the following individuals for materials: Dr. Pierre Chambon for the HeLa λZap cDNA library, Drs. Ludger Hengst and Steven Reed for the HeLa UniZap cDNA library, Drs. Michael Pollard and Eng Tan for the 72B9 anti-fibrillarin monoclonal, Dr. Ed Chan for the polyclonal anti-coilin, and Dr. Joan Steitz for the Y12 anti-Sm monoclonal. This work was supported by a grant from the National Institutes of Health to L.G. J.M.W. was supported by fellowships from the Association pour la Recherche sur la Cancer and the Fondation pour la Recherche Médicale. S.J.B and S.W. were supported by National Institutes of Health grant R29 GM5281. S.J.B. is a member of the Yale Cancer Center.
REFERENCES
- Andrade LEC, Chan EKL, Raska I, Peebles CL, Roos G, Tan EM. Human autoantibody to a novel protein of the nuclear coiled body: immunological characterization and cDNA cloning of p80 coilin. J Exp Med. 1991;173:1407–1419. doi: 10.1084/jem.173.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azum-Gélade M-C, Noaillac-Depeyre J, Caizergues-Ferrer M, Gas N. Cell cycle redistribution of U3 snRNA and fibrillarin. Presence in the cytoplasmic nucleolus remnant and in the prenucleolar bodies in telophase. J Cell Sci. 1994;107:463–475. doi: 10.1242/jcs.107.2.463. [DOI] [PubMed] [Google Scholar]
- Baserga SJ, Yang XW, Steitz JA. An intact box C sequence is required for binding of fibrillarin, the protein common to the major family of nucleolar snRNPs. EMBO J. 1991;10:2645–2651. doi: 10.1002/j.1460-2075.1991.tb07807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DL, Pinto AL. U5 small nuclear ribonucleoprotein: RNA structure analysis and ATP-dependent interaction with U4/U6. Mol Cell Biol. 1989;9:3350–3359. doi: 10.1128/mcb.9.8.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrame M, Henry Y, Tollervey D. Mutational analysis of an essential binding site for the U3 snoRNA in the 5′ external transcribed spacer of yeast pre-rRNA. Nucleic Acids Res. 1994;22:4057–4065. doi: 10.1093/nar/22.20.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrame M, Tollervey D. Identification and functional analysis of two U3 binding sites on yeast pre-ribosomal RNA. EMBO J. 1992;11:1531–1542. doi: 10.1002/j.1460-2075.1992.tb05198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrame M, Tollervey D. Base pairing between U3 and the pre-ribosomal RNA is required for 18S rRNA synthesis. EMBO J. 1995;14:4350–4356. doi: 10.1002/j.1460-2075.1995.tb00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavente R, Rose KM, Reimer G, Hugle-Dorr B, Scheer U. Inhibition of nucleolar reformation after microinjection of antibodies to RNA polymerase I into mitotic cells. J Cell Biol. 1987;105:1483–1491. doi: 10.1083/jcb.105.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmann K, Ferreira JA, Lamond AI. Mutational analysis of p80 coilin indicates a functional interaction between coiled bodies and the nucleolus. J Cell Biol. 1995;131:817–831. doi: 10.1083/jcb.131.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca M, Ferreira J, Lamond AI. Assembly of snRNP-containing coiled bodies is regulated in interphase and mitosis–evidence that the coiled body is a kinetic nuclear structure. J Cell Biol. 1993;120:841–852. doi: 10.1083/jcb.120.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan EKL, Imai H, Hamel JC, Tan EM. Human autoantibody to RNA polymerase I transcription factor hUBF. Molecular identity of nucleolus organizer region autoantigen NOR-90 and ribosomal RNA upstream binding factor. J Exp Med. 1991;174:1239–1244. doi: 10.1084/jem.174.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FM, Tsao TY, Fowler SK, Rao PN. Monoclonal antibodies to mitotic cells. Proc Natl Acad Sci USA. 1983;80:2926–2930. doi: 10.1073/pnas.80.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draetta G, Luca F, Westendorf J, Brizuela L, Ruderman J, Beach D. Cdc2 protein kinase is complexed with both cyclin A and B: evidence for proteolytic inactivation of MPF. Cell. 1989;56:829–838. doi: 10.1016/0092-8674(89)90687-9. [DOI] [PubMed] [Google Scholar]
- Dunbar DA, Wormsley S, Agentis TM, Baserga SJ. Mpp10p, a U3 small nucleolar ribonucleoprotein component required for pre-18S rRNA processing in yeast. Mol Cell Biol. 1997;17:5803–5812. doi: 10.1128/mcb.17.10.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundr M, Meier UT, Lewis N, Rekosh D, Hammarskjold ML, Olson MOJ. A class of nonribosomal nucleolar components is located in chromosome periphery and in nucleolus-derived foci during anaphase and telophase. Chromosoma. 1997;105:407–417. doi: 10.1007/BF02510477. [DOI] [PubMed] [Google Scholar]
- Dunphy WG, Brizuela L, Beach D, Newport J. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell. 1988;54:423–431. doi: 10.1016/0092-8674(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Elela SA, Igel H, Ares M., Jr RNase III cleaves eukaryotic preribosomal RNA at a U3 snoRNP-dependent site. Cell. 1996;85:115–124. doi: 10.1016/s0092-8674(00)81087-9. [DOI] [PubMed] [Google Scholar]
- England TE, Bruce AG, Uhlenbeck OC. Specific labeling of 3′ termini of RNA with T4 RNA ligase. Methods Enzymol. 1980;65:65–74. doi: 10.1016/s0076-6879(80)65011-3. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Esser S, Kastner B, Luhrmann R. Isolation of S. cerevisiae snRNPs: comparison of U1 and U4/U6.U5 to their human counterparts. Science. 1994;264:261–265. doi: 10.1126/science.8146658. [DOI] [PubMed] [Google Scholar]
- Fritzler MJ. Autoantibodies: diagnostic fingerprints and etiologic perplexities. Clin Invest Med. 1997;20:50–66. [PubMed] [Google Scholar]
- Frohman MA. On beyond classic RACE. PCR Methods Appl. 1994;4:s40–s58. doi: 10.1101/gr.4.1.s40. [DOI] [PubMed] [Google Scholar]
- Gautier T, Fomproix N, Masson C, Azum-Gélade M-C, Gas N, Hernandez-Verdun D. Fate of specific nucleolar perichromosomal proteins during mitosis: cellular distribution and association with U3 snoRNA. Biol Cell. 1994;82:81–93. doi: 10.1016/s0248-4900(94)80010-3. [DOI] [PubMed] [Google Scholar]
- Gautier J, Minshull J, Lokha M, Glotzer M, Hunt T, Maller JL. Cyclin is a component of MPF from Xenopus. Cell. 1990;60:487–494. doi: 10.1016/0092-8674(90)90599-a. [DOI] [PubMed] [Google Scholar]
- Gautier J, Norbury C, Lokha M, Nurse P, Maller J. Purified maturation promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle control gene cdc2+ Cell. 1988;54:433–439. doi: 10.1016/0092-8674(88)90206-1. [DOI] [PubMed] [Google Scholar]
- Gautier T, Robert-Nicoud M, Guilly M-N, Hernandez-Verdun D. Relocation of nucleolar proteins around chromosomes at mitosis. A study by confocal laser scanning microscopy. J Cell Sci. 1992;102:729–737. doi: 10.1242/jcs.102.4.729. [DOI] [PubMed] [Google Scholar]
- Gerace L, Blobel G. The nuclear envelope is reversibly depolymerized during mitosis. Cell. 1980;19:277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- Heald R, McKeon F. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell. 1990;61:579–589. doi: 10.1016/0092-8674(90)90470-y. [DOI] [PubMed] [Google Scholar]
- Hernandez-Verdun D, Gautier T. The chromosome periphery during mitosis. Bioessays. 1994;16:179–185. doi: 10.1002/bies.950160308. [DOI] [PubMed] [Google Scholar]
- Hughes JMX. Functional base-pairing interaction between highly conserved elements of U3 small nucleolar RNA and the small ribosomal subunit RNA. J Mol Biol. 1996;259:645–654. doi: 10.1006/jmbi.1996.0346. [DOI] [PubMed] [Google Scholar]
- Hughes JMX, Ares M., Jr Depletion of U3 small nucleolar RNA inhibits cleavage in the 5′ external transcribed spacer of yeast pre-ribosomal RNA and prevents formation of 18S ribosomal RNA. EMBO J. 1991;10:4231–4239. doi: 10.1002/j.1460-2075.1991.tb05001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R, Tollervey D, Hurt EC. A U3 snoRNP protein with homology to splicing factor PRP4 and G beta domains is required for ribosomal RNA processing. EMBO J. 1993;12:2549–2558. doi: 10.1002/j.1460-2075.1993.tb05910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-García LF, Rothblum LI, Busch H, Ochs RL. Nucleologenesis: use of non-isotopic in situ hybridization and immunocytochemistry to compare the localization of rDNA and nucleolar proteins during mitosis. Biol Cell. 1989;65:239–246. doi: 10.1111/j.1768-322x.1989.tb00795.x. [DOI] [PubMed] [Google Scholar]
- Jiménez-García LF, Segura-Valdez MdL, Ochs RL, Rothblum LI, Hannan R, Spector DL. Nucleologenesis: U3 snRNA-containing prenucleolar bodies move to sites of active pre-rRNA transcription after mitosis. Mol Biol Cell. 1994;5:955–966. doi: 10.1091/mbc.5.9.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass S, Craig N, Sollner-Webb B. Primary processing of mammalian rRNA involves two adjacent cleavages and is not species specific. Mol Cell Biol. 1987;7:2891–2898. doi: 10.1128/mcb.7.8.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss-László Z, Henry Y, Bachellerie J-P, Caizergues-Ferrer M, Kiss T. Site-specific ribose methylation of preribosomal RNA: a novel function for small nucleolar RNAs. Cell. 1996;85:1077–1088. doi: 10.1016/s0092-8674(00)81308-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. Interpreting cDNA sequences: some insights from studies on translation. Mamm Genome. 1996;7:563–574. doi: 10.1007/s003359900171. [DOI] [PubMed] [Google Scholar]
- Labbé J-C, Capony JP, Caput D, Cavadore J-C, Derancourt J, Kaghdad M, Lelias J-M, Picard A, Dorée M. MPF from starfish oocytes at first meiotic metaphase is a heterodimer containing one molecule of cdc2 and one molecule of cyclin B. EMBO J. 1989;8:3053–3058. doi: 10.1002/j.1460-2075.1989.tb08456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner MR, Boyle JA, Hardin JA, Steitz JA. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupas erythematosus. Science. 1981;211:400–402. doi: 10.1126/science.6164096. [DOI] [PubMed] [Google Scholar]
- Lerner MR, Steitz JA. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci USA. 1979;76:5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lischwe MA, Ochs RL, Reddy R, Cook RG, Yeoman LC, Tan EM, Reichlin M, Busch H. Purification and partial characterization of a nucleolar scleroderma antigen (Mr = 34,000; pI, 8.5) rich in NG,NG-dimethylarginine. J Biol Chem. 1985;260:14304–14310. [PubMed] [Google Scholar]
- Lubben B, Marshallsay C, Rottman N, Luhrmann R. Isolation of U3 snoRNP form CHO cells: a novel 55-kDa protein binds to the central part of U3 snoRNA. Nucleic Acids Res. 1993;21:5377–5385. doi: 10.1093/nar/21.23.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A. Coiled coils: new structures and new functions. Trends Biochem Sci. 1996;21:375–382. [PubMed] [Google Scholar]
- Lupas A, VanDyke M, Stock J. Predicting coiled-coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Matera AG, Tycowski KT, Steitz JA, Ward DC. Organization of small nucleolar ribonucleoproteins (snornps) by fluorescence in situ hybridization and immunocytochemistry. Mol Biol Cell. 1994;5:1289–1299. doi: 10.1091/mbc.5.12.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto-Taniura N, Pirollet F, Monroe R, Gerace L, Westendorf JM. Identification of novel M phase phosphoproteins by expression cloning. Mol Biol Cell. 1996;7:1455–1469. doi: 10.1091/mbc.7.9.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell ES, Fournier MJ. The small nucleolar RNAs. Annu Rev Biochem. 1995;64:897–934. doi: 10.1146/annurev.bi.64.070195.004341. [DOI] [PubMed] [Google Scholar]
- Medina FJ, Cerdido A, Fernández-Gómez ME. Exp. Cell Res. 1995;221:111–125. doi: 10.1006/excr.1995.1358. [DOI] [PubMed] [Google Scholar]
- Meijer L, Arion D, Golsteyn R, Pines J, Brizuela L, Hunt T, Beach D. Cyclin is a component of the sea urchin egg M-phase specific histone H1 kinase. EMBO J. 1989;8:2275–2282. doi: 10.1002/j.1460-2075.1989.tb08353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu M, Smetana K, Busch H. Quantitative aspects of isolation of nucleoli of the Walker carcinosarcoma and liver of the rat. Cancer Res. 1963;25:693–697. [PubMed] [Google Scholar]
- Nicoloso M, Qu L-H, Michot B, Bachellerie J-P. Intron-encoded, antisense small nucleolar RNAs: the characterization of nine novel species points their direct role as guides for the 2′-O-ribose methylation of rRNAs. J Mol Biol. 1996;160:178–195. doi: 10.1006/jmbi.1996.0391. [DOI] [PubMed] [Google Scholar]
- Oakes M, Nogi Y, Clark MW, Nomura M. Structural alterations of the nucleolus in mutants of Saccharomyces cerevisiae defective in RNA polymerase I. Mol Cell Biol. 1993;13:2441–2455. doi: 10.1128/mcb.13.4.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs, R.L. (1997). Methods used to study structure and function of the nucleolus. Methods Cell Biol. (in press). [DOI] [PubMed]
- Ottaviano Y, Gerace L. Phosphorylation of the nuclear lamins during interphase and mitosis. J Biol Chem. 1985;260:624–632. [PubMed] [Google Scholar]
- Parker KA, Steitz JA. Structural analyses of the human U3 ribonucleoprotein particle reveal a conserved sequence available for base-pairing with pre-rRNA. Mol Cell Biol. 1987;7:2899–2913. doi: 10.1128/mcb.7.8.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peculis BA, Steitz JA. Disruption of U8 nucleolar snRNA inhibits 5.8S and 28S rRNA processing in the Xenopus oocyte. Cell. 1993;73:1233–1245. doi: 10.1016/0092-8674(93)90651-6. [DOI] [PubMed] [Google Scholar]
- Peter M, Nakagawa J, Doreé M, Labbé JC, Nigg EA. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell. 1990;61:591–602. doi: 10.1016/0092-8674(90)90471-p. [DOI] [PubMed] [Google Scholar]
- Raska I, Andrade LE C, Ochs RL, Chan EK L, Chang C-M, Roos G, Tan EM. Immunological and ultrastructural studies of the nuclear coiled body with autoimmune antibodies. Exp Cell Res. 1991;195:27–37. doi: 10.1016/0014-4827(91)90496-h. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- Reimer G, Pollard KM, Penning CA, Ochs RL, Lischwe MA, Busch H, Tan EM. Monoclonal antibody from a (New Zealand black × New Zealand white) F1 mouse and some human scleroderma sera target an Mr 34,000 nucleolar protein of the U3 RNP particle. Arthritis Rheum. 1987;30:793–800. doi: 10.1002/art.1780300709. [DOI] [PubMed] [Google Scholar]
- Savino R, Gerbi SA. In vivo disruption of Xenopus U3 snRNA affects ribosomal RNA processing. EMBO J. 1990;9:2299–2308. doi: 10.1002/j.1460-2075.1990.tb07401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer U, Rose KM. Localization of RNA polymerase I in interphase cells and mitotic chromosomes by light and electron microscopic immunocytochemistry. Proc Natl Acad Sci USA. 1984;81:1431–1435. doi: 10.1073/pnas.81.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Jordan EG. The nucleolus. Annu Rev Cell Dev Biol. 1995;11:93–121. doi: 10.1146/annurev.cb.11.110195.000521. [DOI] [PubMed] [Google Scholar]
- Tyc K, Steitz JA. U3, U8 and U13 comprise a new class of mammalian snRNPs localized to the cell nucleolus. EMBO J. 1989;8:3113–3119. doi: 10.1002/j.1460-2075.1989.tb08463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema J, Tollervey D. Processing of pre-ribosomal RNA in Saccharomyces cerevisiae. Yeast. 1995;11:1629–1650. doi: 10.1002/yea.320111607. [DOI] [PubMed] [Google Scholar]
- Ward GE, Kirschner MW. Identification of cell cycle-regulated phosphorylation sites on nuclear lamin C. Cell. 1990;61:561–577. doi: 10.1016/0092-8674(90)90469-u. [DOI] [PubMed] [Google Scholar]
- Warner JR. Distribution of newly formed ribosomal proteins in HeLa cell fractions. J Cell Biol. 1979;80:767–772. doi: 10.1083/jcb.80.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorf JM, Rao PN, Gerace L. Cloning of cDNAs for M phase phosphoproteins recognized by the MPM2 monoclonal antibody and determination of the phosphorylated epitope. Proc Natl Acad Sci USA. 1994;91:714–718. doi: 10.1073/pnas.91.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda Y, Maul GG. A nucleolar auto-antigen is part of a major chromosomal surface component. Chromosoma. 1990;99:152–160. doi: 10.1007/BF01735332. [DOI] [PubMed] [Google Scholar]