Abstract
When given in a warm environment MDMA (3,4-methylenedioxymethamphetamine, ecstasy) causes hyperthermia by increasing interscapular brown adipose tissue (iBAT) heat production and decreasing heat loss via cutaneous vasoconstriction. When given in a cold environment, however, MDMA causes hypothermia by an unknown mechanism. This paper addresses these mechanisms and in addition examines whether antagonists at 5-HT1A and D2 receptors reduce the hypothermic action of MDMA. Male Sprague-Dawley rats instrumented with a Doppler probe for measuring tail blood flow, and probes for measuring core and iBAT temperatures, were placed in a temperature controlled chamber. The chamber temperature was reduced to 10°C and vehicle (0.5 ml Ringer), the 5-HT1A antagonist WAY 100635 (0.5 mg/kg), the D2 antagonist spiperone (20 μg/kg), or their combination were injected s.c.. Thirty minutes later the antagonists were injected again along with MDMA (10 mg/kg) or the animal received vehicle. MDMA reduced core body temperature by preventing cold-elicited iBAT thermogenesis and by transiently reversing cold-elicited cutaneous vasoconstriction. Pretreatment with WAY 100635 prevented MDMA induced increases in tail blood flow, and briefly attenuated MDMA’s effects on iBAT and core temperature. While spiperone alone failed to affect any of the parameters, the combination of spiperone and WAY 100635 decreased MDMA-mediated hypothermia by attenuating both the effects on tail blood flow and iBAT thermogenesis. MDMA’s prevention of cold-induced iBAT thermogenesis appears to have a central origin as it rapidly reverses cold-induced increases in iBAT sympathetic nerve discharge in anesthetized rats. Our results demonstrate that MDMA in a cold environment reduces core body temperature by inhibiting iBAT thermogenesis and tail artery vasoconstriction and suggest that mechanisms by which this occurs include the activation of 5-HT1A and dopamine D2 receptors.
Keywords: MDMA, Thermoregulation, Ambient Temperature
Introduction
The substituted amphetamine 3,4-methylenedioxymethamphetamine, MDMA or ecstasy, has complex effects on thermoregulation. When administered to rats housed in warm environments, MDMA causes hyperthermia (Dafters, 1994; Green et al., 2005; Malberg et al., 1998) by uncoupling oxidative phosphorylation in interscapular brown adipose tissue (iBAT) and decreasing heat dissipation through constriction of cutaneous beds (Blessing et al., 2003, Blessing et al., 2006). Similar to core temperature, brain hyperthermia mediated by MDMA is exacerbated at elevated ambient temperatures (Brown and Kiyatkin, 2004). In contrast to its well-established hyperthermic effects, MDMA reduces body temperature when administered in a cold environment (Dafters, 1994, Malberg and Seiden, 1998, Green et al., 2005). Mechanisms underlying this hypothermia are unknown, but must reflect reduced heat production and/or reduced heat conservation. Heat production occurs through metabolism associated with muscle contraction (including shivering), from non-shivering thermogenesis in sympathetically-controlled iBAT (Kanosue et al., 1998, Cannon and Nedergaard, 2004), and by heat conservation via sympathetically-mediated constriction of cutaneous beds - in rats primarily the tail artery (Ootsuka et al., 2004a, Ootsuka et al., 2004b, Ootsuka and McAllen, 2005). Therefore, any drug which decreases iBAT metabolism and/or increases cutaneous blood flow should cause hypothermia - particularly if it is administered in the cold. Notable examples include the 5-HT1A agonist 8-hydroxy-2(di-n-propylamino) tetralin (8-OH-DPAT) (Ootsuka and Blessing, 2003, Blessing, 2004, Ootsuka and Blessing, 2006) and the dopamine D2-preferring drugs 7-hydroxy-2-(di-propylamino) tetraline (7-OH-DPAT) and quinpirole (Blessing and Ootsuka, 2007, Ootsuka et al., 2007). Agonists at 5-HT1A receptors inhibit both iBAT metabolism and cutaneous vasoconstriction (Ootsuka and Blessing, 2006), whereas agonists at dopamine D2 receptors reverse cold-initiated iBAT thermogenesis without effecting cutaneous blood flow (Ootsuka et al., 2007). Since MDMA, via actions on the neurotransmitter uptake transporter proteins, increases the active transmitter pool of both 5-hydroxytryptamine (5-HT) and dopamine (Gudelsky and Yamamoto, 2007), it is reasonable to consider actions at these receptors in studying the hypothermic effects of MDMA.
In the present study, using conscious rats with chronically implanted probes to measure tail artery blood flow and iBAT and core temperatures, we examined the thermoregulatory effects induced by MDMA when administered in a cold environment, and determined whether activation of 5-HT1A or D2 receptors contribute to these effects.
Experimental Procedures
Experiments were conducted in accordance with the European Community Council Directive of 24 November 1986 (86/609/EEC), and were approved by the Flinders University Animal Welfare Committee. All efforts were made to minimize the number of animals used and their suffering.
Surgical preparation in conscious rats
Male Sprague-Dawley rats (250-350 g) were anesthetized with isoflurane (Veterinary Companies of Australia Pty. Ltd., Kings Park, NSW, Australia). A Doppler ultrasonic flow probe (Iowa Doppler Products, Iowa City, IA) was implanted around the proximal portion of the tail artery about 3 cms from the base. Wires from the Doppler probe were passed s.c. and attached to a head socket. To measure iBAT, the tip of a telemetry temperature probe (TA-F40 W/TP, Data Sciences International, Transoma Medical, St. Paul, MN) was positioned under the right lobe of the iBAT and the transmitter body of the probe was placed in the peritoneal cavity (Ootsuka and Blessing, 2006). Core temperature was measured by placing a Subcue temperature probe (Subcue Dataloggers, Calgary, AB) in the peritoneal cavity (Ootsuka and Blessing, 2006) and insulated wires from the two-pin connector on the Subcue probe were attached to pins on the head socket, so that the probe could be reset after downloading the data in each experiment without removal. Animals recovered from surgery and were returned to the animal house for at least 1 week before experimentation. As previously described, the correct position of the iBAT probe for each rat used in the study was confirmed in a preliminary procedure where animals placed for 60 minutes in a cold (5-10°C) environment showed an increase of at least 0.5°C in iBAT temperature (Ootsuka and Blessing, 2006).
Experimental design in conscious rats
On the day of the experiment between the hours of 10:00 am and 2:00 p.m., animals were transferred from the animal house to the experimental room. The rat was placed in a cage fitted with an electronic swivel connected to the animals head socket by a flexible cable. The cage was placed inside an environmental chamber consisting of a commercial chest freezer modified with an inbuilt heating unit. The temperature of the chamber was controlled from outside using a locally built temperature regulator (Apple Macintosh computer programmed with IgorPro (Wavemetrics, Lake Oswego, OR) which alternated heating and cooling to maintain the ambient temperature within 1°C of a preset value. Tubing which circulated room air and a fluorescent light assured adequate light and ventilation for the animal.
After the rat was placed in the experimental cage, tail blood flow, iBAT and core body temperature were continuously recorded. The animal was left undisturbed for 60 minutes at an ambient temperature of 26°C at which time the chamber temperature was reduced, over 30-40 minutes, to 10°C. The animal was again left undisturbed for 60 minutes after which animals received drug treatments based on their experimental group (see Table 1). For subcutaneous (s.c.) injections of drug or vehicle, the chamber door was opened and the rat gently restrained by hand. As the durations of action WAY 100635 of and spiperone are unknown, but may be a short as 60 minutes (based on personal laboratory observations), an additional dose of either vehicle, WAY 100635, spiperone or both WAY 100635 and spiperone was given at the time of the MDMA injection. (see Table 1 and Fig. 1). After the final injection, animals were monitored for 60 minutes, removed from the cold environment and returned to the animal house.
Table 1.
Experimental groups
| Group | Pretreatment | Treatment |
|---|---|---|
| A | Vehicle | Vehicle |
| B | Vehicle | MDMA+vehicle |
| C | WAY | MDMA+WAY |
| D | Spip | MDMA+Spip |
| E | WAY+Spip | MDMA+WAY+Spip |
Vehicle = 0.5 ml of Ringer, WAY = WAY 100635 (0.5 mg/kg), Spip = spiperone (20 μg/kg).
Fig 1.
Original records in individual rats of iBAT and core temperature (unbroken and broken lines respectively in top panels of A and B), and tail artery blood flow (bottom panels of A and B). At the time indicated by the double arrows environmental temperature was reduced from room temperature to 10°C. Either vehicle followed by vehicle (A, ie a Group A animal) or vehicle followed by MDMA (10 mg/kg, s.c.) (B, ie a Group B animal) was administered at the times indicated by single arrows. Cold exposure induced iBAT thermogenesis and reductions in tail blood flow that were unaffected by treatment with vehicle (A). MDMA transiently increased tail artery blood flow, and caused long-lasting reductions iBAT thermogenesis and core body temperature (B). In other experimental conditions (Groups C to E) WAY 100635, spiperone, or a combination of both drugs was injected at the time points indicated by the first and second single arrows. In these groups, MDMA was also injected at the time indicated by the second single arrow.
Each rat underwent all five experimental conditions with at least 3 days elapsing between experiments. For a given rat, each particular drug condition was administered in a rotating order to control for serial effects. If one of the measuring devices (Doppler probes, DSI or Subcue probes) failed, the data for the remaining functioning probes was kept for the final analysis.
Surgical preparation in anesthetized rats
Rats were anesthetized with isoflurane, shaved, and an endotracheal tube was inserted via a tracheotomy. The right femoral artery and vein were cannulated for measurement of systemic arterial pressure and intravenous drug administration, respectively. Isoflurane anesthesia was then replaced with a cocktail of urethane (400-800 mg/kg i.v.) and α-chloralose (40-80 mg/kg i.v.) (50 mg of α-chloralose dissolved in 10 ml of 10% 2-hydroxypropyl-β-cyclodextrin (Sigma-Aldrich Chemie GmbH, Switzerland) (Storer et al., 1997). The level of anesthesia was maintained at a depth sufficient to abolish withdrawal reflex. Brown adipose tissue (iBAT) sympathetic nerves were dissected as previously described (Ootsuka and McAllen, 2006). Once nerve activity was confirmed, rats were paralyzed with pancuronium bromide (1 mg/kg i.v.), artificially ventilated with 100% O2, and end expiratory CO2 concentration (Normocap, Datex, Finland) maintained between 3.5% and 4.5% (resting condition). The temperature of the contralateral iBAT, abdominal skin and colon was measured with thermocouples. The skin of the shaved rat’s thorax and abdomen was cooled by perfusing ice cold water (5-10°C, for 1-6 min) through a water jacket, with an increase in iBAT sympathetic nerve discharge confirming that recording was from nerves supplying iBAT. At the end of the experiment, ganglionic blockade with chlorisondamine chloride (10 mg/kg i.v.) was administered with loss of nerve activity confirming the nerve’s identity as a sympathetic axon.
Drugs
All drugs were freshly prepared and injected s.c. in a total volume of 0.5 ml. Spiperone, WAY 100635 (Sigma Castle Hill, NSW, Australia) and racemic MDMA (Australian Government Analytical Laboratories, Sydney, Australia) were dissolved in Ringer’s solution. Drug doses were determined from previously published studies.
Data recording
Blood flow signals from the Doppler ultrasonic flow probes and temperature signals from DSI telemetry probes were recorded with MacLab and Chart (ADInstruments, Castle Hill, NSW, Australia). Flow and BAT temperature were digitized at 40 Hz. Temperature measurements (every 1 min) were stored in the memory of the Subcue probe during experiments, and downloaded after each experiment. In anesthetized rats, all data were digitized with MacLab and captured into a computer with Chart. iBAT sympathetic nerve activity was recorded (NL100 pre-amplifier, Digitimer, UK) with bipolar silver electrodes (band pass filter 1-1,000 Hz, gain 20,000, NL104 amplifier, Digitimer), and digitized at 400 Hz after passing through the 200 Hz built-in low pass filter of MacLab. Skin, iBAT, and rectal (body) temperatures were digitized at 10Hz. Arterial pressure was recorded (NT218, DC amplifier, Digitimer) and digitized at 100Hz. Heart rate was calculated by measuring R-R interval of ECG which was recorded (NT114, AC amplifier, Digitimer) and was digitized at 400Hz. The amplitude of iBAT sympathetic nerve activity was expressed as log total power spectral density between 0-20 Hz from the autospectra of sequential 5.12-s segments of iBAT sympathetic nerve activity.
Statistical analysis
Data were analyzed with Chart, Igor Pro (WaveMetrics, Lake Oswega, OR, USA), Statview 5 (SAS, Cary, NC) and SPSS 15.0 for Windows (Chicago, Illinois, USA). Grouped data are reported as changes from baseline (mean±S.E.M.) for the time points shown. For experiments comparing pretreatments, the change from baseline (5 minute time period prior to pretreatment) was compared between groups at each time point. For iBAT and Core temperature, the data analysis was conducted for the 60 minute time period after injection of MDMA. As tail flow effects were transient, the data analysis was conducted only for the 30 minute time period after injection of MDMA. Data were analyzed using a two way full factorial repeated measures analysis of variance including in the model main effects for study group, time, and their interaction. We performed post hoc comparisons between study groups at each time point shown with Pair-wise comparisons adjusted using an LSD procedure. For Anesthetized data pre and post-MDMA treatment was compared using a repeat measures ANOVA.
Results
Effects of treatment with vehicle on cold-induced changes in tail blood flow, iBAT temperature and core body temperature
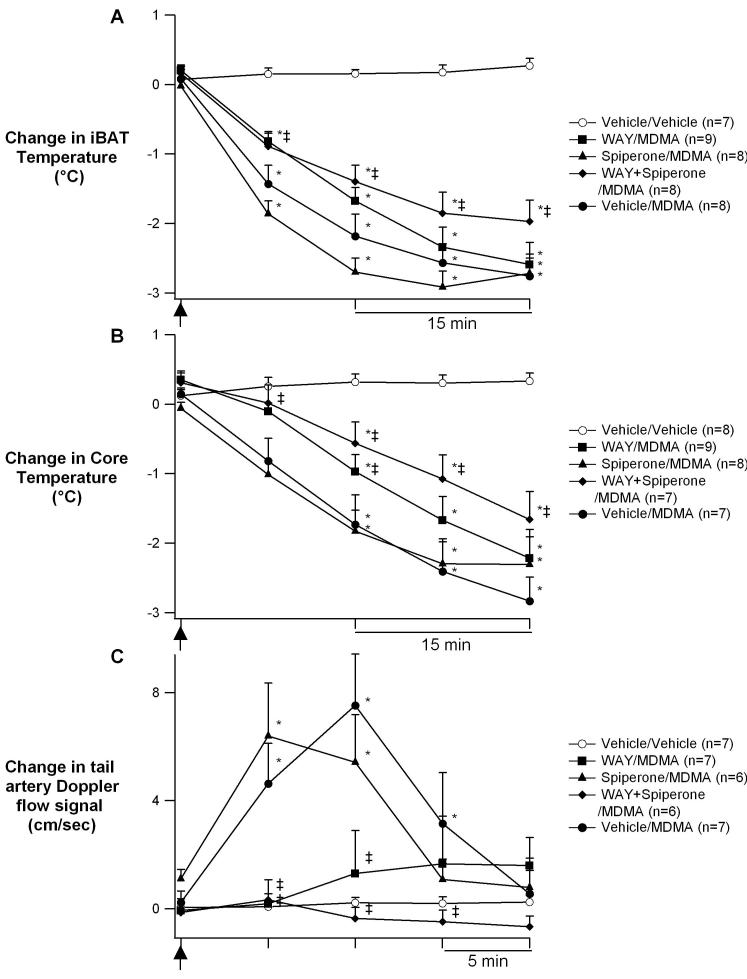
As previously reported from our laboratory, transferring the rat from its cage to the environmental chamber and connecting the headpiece causes a mild stress response (Blessing et al., 2006, Ootsuka et al., 2007). This is reflected as an initial increase in initial iBAT and core body temperature that gradually declined to the pre-transfer baseline level (Figs 1A and B). Exposure to the progressively decreasing environmental temperature induces iBAT thermogenesis, and reduces tail artery blood flow to near zero levels, with no substantial change in core temperature (Fig 1A) as we have also previously reported (Blessing et al., 2006, Ootsuka et al., 2007). Subsequent treatment with WAY 100635, spiperone, or the combination of WAY 100635 and spiperone (before administration of MDMA) did not significantly alter any of these parameters in comparison with vehicle (Figure 2, P>0.05 for all comparisons).
Fig 2.
Change from baseline (means±S.E.M) in iBAT temperature (A), core temperature (B), and tail artery blood flow (C) after injection of vehicle, or of MDMA (10 mg/kg s.c.) at the time point indicated by the arrow, following pretreatment with either vehicle, WAY 100635 (0.5 mg/kg s.c.), spiperone (20 μg/kg s.c.), or WAY and spiperone, thirty minutes prior to and again at the time of MDMA administration. Note the 15 min time scale in A and B, and the 5 min time scale in C. * Significantly different from vehicle control (p<0.05), ‡ Significantly different from MDMA without active drug pretreatment (p<0.05).
Effects of MDMA on cold-induced changes in tail blood flow, iBAT and core temperatures
In vehicle pre-treated rats, the administration of MDMA (10 mg/kg s.c.) during cold exposure increased tail artery blood flow and decreased both iBAT and core temperature (Figs 1B, 2A and B). iBAT temperature continued to fall throughout the entire period of cold exposure while increases in tail artery blood flow lasted only about 20 min (Figs. 1B, 2C) returning to the cold-induced, near zero, levels for the remainder of the experiment.
Effects of pretreatment with the 5-HT 1A receptor antagonist WAY 100635 on MDMA-elicited increases in tail blood flow, and decreases in iBAT and core body temperatures
Pretreatment with WAY 100635 alone (0.5 mg/kg s.c.), thirty minutes prior to and again at the time of MDMA administration, prevented the MDMA-elicited increase in tail artery blood flow (Fig 2C). In addition, WAY attenuates the MDMA-induced reduction of iBAT temperature at 15 minutes without effecting subsequent time points (Fig. 2A). Together these effects result in attenuation of MDMA-mediated hypothermia at early (30 minutes), but not later, time points (Fig. 2C).
Effects of pretreatment with the dopamine D2 receptor antagonist spiperone on MDMA-elicited increases in tail blood flow and decreases in iBAT and core body temperatures
Pretreatment with spiperone alone (20 μg/kg, s.c.), thirty minutes prior to and again at the time of MDMA administration, had no affect on MDMA-elicited changes in tail blood flow, iBAT temperature or core body temperature (Fig. 2).
Effects of combined pretreatment with WAY 100635 and spiperone on MDMA-elicited increases in tail blood flow and decreases in iBAT and core body temperatures
Pretreatment with the combination of WAY 100635 and spiperone, thirty minutes prior to and again at the time of MDMA administration, abolished the MDMA-elicited increase in tail artery blood flow and significantly reduced the MDMA-elicited decreases in iBAT temperatures (Fig. 2A and C). Together these effects significantly attenuated MDMA-mediated reductions in core temperature (Fig. 2B).
Effect of MDMA on cold-induced iBAT sympathetic nerve discharge in anesthetized rats
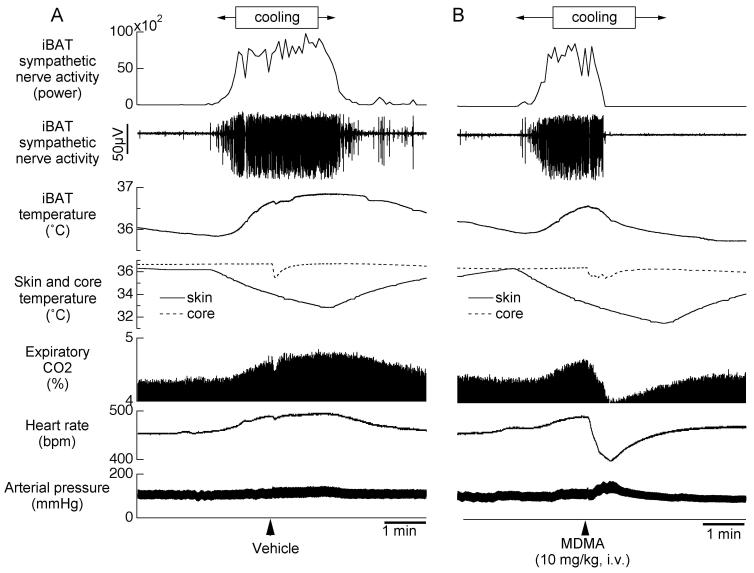
Under resting conditions in anesthetized rats (n=4) with a core temperature of 37.4±0.2 and skin temperature of 37.1±0.3°C, iBAT sympathetic nerve activity was absent or low. Perfusion of cold water (duration 139±13 sec, n=4) through a jacket around the rat’s trunk decreased skin temperature by 3.4±1.0°C. Cooling the skin increased iBAT SNA to 334±85 % of pre-cooling level (P<0.05). The cooling also increased iBAT temperature by 1.2±0.2 °C (from 36.6±0.3 to 37.2±0.2 °C, P<0.01), expired CO2 by 1.0±0.3% (from 4.3±0.3 to 5.3±0.2%, P<0.05) and heart rate by 64±18 bpm (from 435±12 to 499±11 bpm, P<0.05) (Fig. 3A). Ringer vehicle administered during skin cooling did not affect cooling-elicited responses in iBAT sympathetic nerve activity, iBAT temperature, expiratory CO2 or heart rate (n=3) (Fig. 3A). In contrast, MDMA (10 mg/kg i.v.) inhibited cooling-elicited increases in iBAT SNA (SNA was inhibited to 23±13 % of pre-MDMA level, P<0.05, n=4). iBAT temperature slightly decreased by 0.3±0.2 °C (from 36.6±0.3 to 36.3±0.3 °C, P>0.05, n=4) (Fig. 3B). Immediately after its administration, MDMA caused a transient decrease in heart rate and expiratory CO2, which rapidly returned to pre-cooling levels despite continual decreases in skin temperature. As previously reported (Blessing et al., 2003), MDMA increased arterial pressure (from 109±7 to 137±5 mmHg, P<0.01, n=4).
Fig 3.
Original records from different individual anesthetized rats showing the effect of Ringer vehicle (A) or MDMA (B) on directly recorded iBAT sympathetic nerve activity and other physiological parameters (see below) during a period when iBAT thermogenesis was increased by circulating cold water through a jacket around the rat’s body. From top panel to bottom panel; original recording of iBAT sympathetic nerve activity (SNA), iBAT SNA total power spectral density between 0 and 20Hz from autospectra of sequential 5.12-s segments of iBAT SNA, iBAT temperature, skin and core body temperature, expiratory CO2 concentration, heart rate (HR) and arterial pressure (AP). MDMA profoundly inhibits the cooling-induced increase in iBAT sympathetic nerve discharge, indicating an action within the CNS rather than in the periphery.
Discussion
This study is the first to demonstrate that MDMA, administered in a cold environment, causes hypothermia by inhibiting iBAT thermogenesis and thermoregulatory vascular constriction. These sympathetically mediated effects are opposite to those seen during the hyperthermia that results when MDMA is administered in a warm environment (Blessing et al., 2006). Our finding that shortly after its administration, MDMA rapidly reduces sympathetic nerve discharges to near zero levels provides strong evidence that MDMA’s thermoregulatory site of action is within the CNS.
Role of 5-HT1A receptors in hypothermic action of MDMA
Agonists at 5-HT1A receptors robustly lower body temperature in rats by reducing heat production via inhibition of sympathetic outflow to iBAT and by increasing heat loss via inhibition of sympathetic outflow to the tail thermoregulatory bed (Ootsuka and Blessing, 2006). In the present study WAY 100635, a specific and potent antagonist at 5-HT1A receptors, substantially abolished the MDMA-elicited increase in tail artery blood flow and attenuated early inhibition of iBAT. The principal actions of MDMA are thought to reflect increases in transmitter availability via effects on the presynaptic neurotransmitter uptake transporter systems (Gudelsky and Yamamoto, 2007). Since 5-HT has >1000-fold affinity for 5-HT1A receptors compared with 5-HT2A receptors (Peroutka et al., 1981), MDMA-mediated increases in 5-HT levels likely contribute to the inhibition of sympathetic outflow to thermoregulatory vascular beds via activation of inhibitory 5-HT1A receptors. Our present data supports this by showing an inhibitory thermoregulatory role for endogenous 5-HT when levels of the natural transmitter are pharmacologically increased by MDMA. MDMA’s effects on tail artery sympathetic outflow lasted approximately 20 minutes which is in agreement with our previous demonstration of a transient action following direct stimulation of 5-HT1A receptors via administration of 8-OH-DPAT (Ootsuka and Blessing, 2006). Presumably other 5-HT1A-independent neurotransmitter systems reactivate the cold-induced sympathetic outflow to the tail bed.
Inhibition of sympathetic outflow to iBAT is the principal hypothermia mechanism following 5-HT1A receptor activation (Ootsuka and Blessing, 2006). In the present study, WAY 100635, administered alone, reduced the inhibitory effect of MDMA on cold-induced iBAT thermogenesis 15 minutes after the injection of MDMA, but not at subsequent time points. Together, WAY’s effects on tail flow and iBAT temperature result in an early, yet unsustained, attenuation in MDMA-mediated hypothermia. We have similarly reported that, at normal ambient temperatures (25°C), WAY 100635 (0.5 mg/kg i.p.) prevents the initial transient hypothermia from MDMA (Rusyniak et al., 2007). This suggests that hypothermia mediated by MDMA at normal temperatures is, similar to effects in the cold, mediated at least in part by increased heat dissipation (tail vasodilation) and prevention of iBAT thermogenesis.
In vitro binding studies have shown that WAY 100635 has affinity at alpha-1 adrenoreceptors, but the relevant Ki was 45 nM compared with 0.24 nM for the corresponding affinity at 5-HT1A receptors (Johansson et al., 1997). Previous work has demonstrated that prazosin, an established alpha-1 adrenergic receptor antagonist, neither prevents cold induced thermogenesis nor affect core body temperature (Carlisle and Stock, 1995). Furthermore WAY 100635 (0.5 mg/kg, as used in the present study) does not lower arterial blood pressure, as would be expected if it possesses physiologically significant alpha-1 adrenergic activity. Thus it is unlikely that thermoregulatory effects of WAY 100635 are due to alpha-1 adrenoceptor antagonism in our studies.
At doses used in this study, WAY 100635 entirely prevents inhibition cold-induced tail vasoconstriction and cold-induced iBAT thermogenesis mediated by 8-hydoxy-DPAT (Ootsuka and Blessing, 2006).
Role of dopamine D2 receptors, or combinations of receptor actions, in hypothermic action of MDMA
Dopamine receptor agonists, when centrally injected, reduce core body temperature by stimulating D2 receptors (Faunt and Crocker, 1987). The mechanism by which D2 receptor stimulation causes hypothermia is dependent on the ambient temperature. At a temperature of 26-28°C, dopamine D2 receptor stimulation inhibits sympathetic outflow to the rat tail artery increasing tail blood flow and heat loss (Blessing and Ootsuka, 2007). Dopamine D2 receptor stimulation also inhibits stress-induced tail artery vasoconstriction (Blessing and Ootsuka, 2007). When administered in a cold environment, D2 receptor stimulation causes hypothermia not by reversing cold-induced thermoregulatory tail vasoconstriction but by reversing cold-induced iBAT thermogenesis (Ootsuka et al., 2007).
Since, via actions on the dopamine transporter, MDMA increases synaptic availability of dopamine, we were interested to determine the effect of dopamine D2 receptor blockade on MDMA-elicited thermoeffector actions. We found that Spiperone did not alter MDMA-elicited thermoregulatory actions on tail blood flow, iBAT thermogenesis or core body temperature.
We chose spiperone as a D2 antagonist based on previous thermoregulatory studies showing its effective D2 antagonism at the low dose used (Blessing and Ootsuka, 2007, Ootsuka et al., 2007). Although spiperone is a reasonably high affinity 5-HT2A receptor antagonist, we have shown that the doses required to show 5-HT2A-antagonist-like effects on cutaneous blood flow are 5-10 times greater then the 20 μg/kg dose used in this study (Blessing and Ootsuka, 2007). Likewise, we have previously shown that the dose of spiperone used in this study completely prevents decreases in iBAT temperature elicited by the specific D2 antagonist quinpirole (Ootsuka et al., 2007) and abolishes the vasodilating properties of the D2 agonists apomorphine, quinpirole, and 7-hydoxy-DPAT (Blessing and Ootsuka, 2007).
Our findings, that a D2 antagonist does not prevent MDMA-mediated hypothermia, are in contrast to previous studies where the dopamine antagonist remoxipride prevented hypothermia induced by MDMA in a cold (15°C) environment (Green et al., 2005). One possible explanation for the differences in these studies is MDMA’s effects at sigma receptors. Sigma agonists evoke hypothermia (Rawls et al., 2002), and recently it was demonstrated that MDMA’s affinity for sigma receptors was comparable to its affinity for both serotonin and dopamine transporters (Brammer et al., 2006). Likewise, motor effects induced by MDMA can be attenuated using a sigma-1 antagonist (Brammer et al., 2006). Although Green et al. attribute remoxipride-prevention of MDMA-elicited hypothermia to the antagonism of dopamine D2 receptors (Green et al., 2005) remoxipride has an affinity for sigma receptors 15 times greater then that for D2 receptors (Largent et al., 1988). This is in contrast to spiperone which has a very low affinity for sigma receptors (Weber et al., 1986).
Along with effects at their specific receptors, an interaction between dopamine and serotonin may also contribute to alterations in effects mediated by MDMA (Balcioglu and Wurtman, 1998, Bankson and Cunningham, 2001). A serotonin-dopamine interaction may explain why, in our study, the greatest attenuation of MDMA-induced hypothermia resulted from the combination of WAY 10035 and spiperone.
In summary hypothermia induced by MDMA in a cold environment involves in part the combined activation of D2 and 5-HT1A receptors. Results from this study have begun to unravel the possible identity of the central receptors involved in MDMA-mediated hypothermia. While these results do not bear on the neuroanatomical location of these neural pathways, potential sites would included the preoptic area, the dorsomedial hypothalamus, the rostral medullary raphe/parapyramidal region, and possibly spinal thermoregulatory centres (Morrison, 2004, Ootsuka et al., 2004b, Ootsuka and Blessing, 2005, Dimicco and Zaretsky, 2007, McAllen, 2007).
Conclusion
The present study demonstrates that the hypothermia elicited by MDMA administered in a cold environment reflects MDMA-mediated inhibition of sympathetic outflow to iBAT and cutaneous blood vessels reducing cold-initiated heat production and increasing heat loss. The complex pharmacological mechanisms underlying these effects include stimulatory actions on 5-HT1A and dopamine D2 receptors.
Acknowledgments
This study was supported by a USPHS grant DA020484 (DER) and by the Australian Government National Health and Medical Research Council (WWB). We thank Melissa Quinlan, Candice Morgan and Robyn Flook for technical assistance.
Abbreviations
- MDMA
3,4-methylenedioxymethamphetmaine
- iBAT
interscapular brown adipose tissue
- 8-OH-DPAT
8-hydroxy-2(di-n-propylamino) tetralin
- 7-OH-DPAT
7-hydroxy-2-(di-propylamino) tetraline
- s.c.
subcutaneous
- SNA
sympathetic nerve activity
- 5-HT
serotonin
- D
Dopamine
- CNS
Central Nervous System
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of conflicts of interest
None
Section Editor: Dr. Miles Herkenham
References
- Balcioglu A, Wurtman RJ. Dexfenfluramine enhances striatal dopamine release in conscious rats via a serotoninergic mechanism. J Pharmacol Exp Ther. 1998;284:991–997. [PubMed] [Google Scholar]
- Bankson MG, Cunningham KA. 3,4-Methylenedioxymethamphetamine (MDMA) as a unique model of serotonin receptor function and serotonin-dopamine interactions. J Pharmacol Exp Ther. 2001;297:846–852. [PubMed] [Google Scholar]
- Blessing WW. 5-hydroxytryptamine 1A receptor activation reduces cutaneous vasoconstriction and fever associated with the acute inflammatory response in rabbits. Neuroscience. 2004;123:1–4. doi: 10.1016/j.neuroscience.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Blessing WW, Ootsuka Y. Activation of dopamine D2 receptors in the CNS inhibits sympathetic cutaneous vasomotor alerting responses (SCVARs), contributing to clozapine’s SCVAR-inhibiting action. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:328–336. doi: 10.1016/j.pnpbp.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Blessing WW, Seaman B, Pedersen NP, Ootsuka Y. Clozapine reverses hyperthermia and sympathetically mediated cutaneous vasoconstriction induced by 3,4-methylenedioxymethamphetamine (ecstasy) in rabbits and rats. Journal of Neuroscience. 2003;23:6385–6391. doi: 10.1523/JNEUROSCI.23-15-06385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing WW, Zilm A, Ootsuka Y. Clozapine reverses increased brown adipose tissue thermogenesis induced by 3,4-methylenedioxymethamphetamine and by cold exposure in conscious rats. Neuroscience. 2006;141:2067–2073. doi: 10.1016/j.neuroscience.2006.05.050. [DOI] [PubMed] [Google Scholar]
- Brammer MK, Gilmore DL, Matsumoto RR. Interactions between 3,4-methylenedioxymethamphetamine and sigma1 receptors. Eur J Pharmacol. 2006;553:141–145. doi: 10.1016/j.ejphar.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PL, Kiyatkin EA. Brain hyperthermia induced by MDMA (ecstasy): modulation by environmental conditions. Eur J Neurosci. 2004;20:51–58. doi: 10.1111/j.0953-816X.2004.03453.x. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Carlisle HJ, Stock MJ. Temperature-dependent effects of [alpha]-adrenergic agonists and antagonists in the cold. Pharmacology Biochemistry and Behavior. 1995;51:263–270. doi: 10.1016/0091-3057(94)00374-r. [DOI] [PubMed] [Google Scholar]
- Dafters RI. Effect of ambient temperature on hyperthermia and hyperkinesis induced by 3,4-methylenedioxymethamphetamine (MDMA or “ecstasy”) in rats. Psychopharmacology. 1994;114:505–508. doi: 10.1007/BF02249342. [DOI] [PubMed] [Google Scholar]
- Dimicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R47–63. doi: 10.1152/ajpregu.00498.2006. [DOI] [PubMed] [Google Scholar]
- Faunt JE, Crocker AD. The effects of selective dopamine receptor agonists and antagonists on body temperature in rats. Eur J Pharmacol. 1987;133:243–247. doi: 10.1016/0014-2999(87)90019-7. [DOI] [PubMed] [Google Scholar]
- Green AR, O’Shea E, Saadat KS, Elliott JM, Colado MI. Studies on the effect of MDMA (‘ecstasy’) on the body temperature of rats housed at different ambient room temperatures. British Journal of Pharmacology. 2005;146:306–312. doi: 10.1038/sj.bjp.0706318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudelsky GA, Yamamoto BK. Actions of 3,4-methylenedioxymethamphetamine (MDMA) on cerebral dopaminergic, serotonergic and cholinergic neurons. Pharmacol Biochem Behav. 2007 Oct 16; doi: 10.1016/j.pbb.2007.10.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson L, Sohn D, Thorberg SO, Jackson DM, Kelder D, Larsson LG, Renyi L, Ross SB, Wallsten C, Eriksson H, Hu PS, Jerning E, Mohell N, Westlind-Danielsson A. The pharmacological characterization of a novel selective 5-hydroxytryptamine1A receptor antagonist, NAD-299. J Pharmacol Exp Ther. 1997;283:216–225. [PubMed] [Google Scholar]
- Kanosue K, Hosono T, Zhang YH, Chen XM. Neuronal networks controlling thermoregulatory effectors. Prog Brain Res. 1998;115:49–62. doi: 10.1016/s0079-6123(08)62029-4. [DOI] [PubMed] [Google Scholar]
- Largent BL, Wikstrom H, Snowman AM, Snyder SH. Novel antipsychotic drugs share high affinity for sigma receptors. Eur J Pharmacol. 1988;155:345–347. doi: 10.1016/0014-2999(88)90527-4. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Seiden LS. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. Journal of Neuroscience. 1998;18:5086–5094. doi: 10.1523/JNEUROSCI.18-13-05086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen RM. The cold path to BAT. Am J Physiol Regul Integr Comp Physiol. 2007;292:R124–126. doi: 10.1152/ajpregu.00651.2006. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Activation of 5-HT1A receptors in raphe pallidus inhibits leptin-evoked increases in brown adipose tissue thermogenesis. Am J Physiol Regul Integr Comp Physiol. 2004;286:R832–837. doi: 10.1152/ajpregu.00678.2003. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, Blessing WW. 5-Hydroxytryptamine 1A receptors inhibit cold-induced sympathetically mediated cutaneous vasoconstriction in rabbits. J Physiol. 2003;552:303–314. doi: 10.1113/jphysiol.2003.048041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ootsuka Y, Blessing WW. Activation of slowly conducting medullary raphe-spinal neurons, including serotonergic neurons, increases cutaneous sympathetic vasomotor discharge in rabbit. Am J Physiol Regul Integr Comp Physiol. 2005;288:R909–918. doi: 10.1152/ajpregu.00564.2004. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, Blessing WW. Thermogenesis in brown adipose tissue: increase by 5-HT2A receptor activation and decrease by 5-HT1A receptor activation in conscious rats. Neuroscience Letters. 2006;395:170–174. doi: 10.1016/j.neulet.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, Blessing WW, McAllen RM. Inhibition of rostral medullary raphe neurons prevents cold-induced activity in sympathetic nerves to rat tail and rabbit ear arteries. Neurosci Lett. 2004a;357:58–62. doi: 10.1016/j.neulet.2003.11.067. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, Heidbreder CA, Hagan JJ, Blessing WW. Dopamine D(2) receptor stimulation inhibits cold-initiated thermogenesis in brown adipose tissue in conscious rats. Neuroscience. 2007;147:127–135. doi: 10.1016/j.neuroscience.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, McAllen RM. Interactive drives from two brain stem premotor nuclei are essential to support rat tail sympathetic activity. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1107–1115. doi: 10.1152/ajpregu.00005.2005. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, McAllen RM. Comparison between two rat sympathetic pathways activated in cold-defense. Am J Physiol. 2006;291:R589–595. doi: 10.1152/ajpregu.00850.2005. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, Nalivaiko E, Blessing WW. Spinal 5-HT2A receptors regulate cutaneous sympathetic vasomotor outflow in rabbits and rats; relevance for cutaneous vasoconstriction elicited by MDMA (3,4-methylenedioxymethamphetamine, “Ecstasy”) and its reversal by clozapine. Brain Research. 2004b;1014:34–44. doi: 10.1016/j.brainres.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ, Lebovitz RM, Snyder SH. Two distinct central serotonin receptors with different physiological functions. Science. 1981;212:827–829. doi: 10.1126/science.7221567. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Baron DA, Geller EB, Adler MW. Sigma sites mediate DTG-evoked hypothermia in rats. Pharmacol Biochem Behav. 2002;73:779–786. doi: 10.1016/s0091-3057(02)00903-6. [DOI] [PubMed] [Google Scholar]
- Rusyniak DE, Zaretskaia MV, Zaretsky DV, DiMicco JA. 3,4-Methylenedioxymethamphetamine- and 8-hydroxy-2-di-n-propylamino-tetralin-induced hypothermia: role and location of 5-hydroxytryptamine 1A receptors. J Pharmacol Exp Ther. 2007;323:477–487. doi: 10.1124/jpet.107.126169. [DOI] [PubMed] [Google Scholar]
- Storer RJ, Butler P, Hoskin KL, Goadsby PJ. A simple method, using 2-hydroxypropyl-beta-cyclodextrin, of administering alpha-chloralose at room temperature. J Neurosci Methods. 1997;77:49–53. doi: 10.1016/s0165-0270(97)00110-6. [DOI] [PubMed] [Google Scholar]
- Weber E, Sonders M, Quarum M, McLean S, Pou S, Keana JF. 1,3-Di(2-[5-3H]tolyl)guanidine: a selective ligand that labels sigma-type receptors for psychotomimetic opiates and antipsychotic drugs. Proc Natl Acad Sci U S A. 1986;83:8784–8788. doi: 10.1073/pnas.83.22.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]