Abstract
The hippocampal formation receives extensive noradrenergic projections and expresses high levels of mineralocorticoid (MR) and glucocorticoid (GR) receptors. Considerable evidence suggests that the noradrenergic system influences hippocampal corticosteroid receptors. However, there is relatively little data describing the influence of glucocorticoids on noradrenergic receptors in the hippocampal formation. α1d adrenergic receptor (ADR) mRNA is expressed at high levels in the hippocampal formation, within cells that express MR or GR. In order to determine whether expression of α1d ADR mRNA is influenced by circulating glucocorticoids, male rats underwent bilateral adrenalectomy (ADX) or sham surgery, and were killed after 1, 3, 7 or 14 days. Levels of α1d ADR mRNA were profoundly decreased in hippocampal subfields CA1, CA2 and CA3 and the medial and lateral blades of the dentate gyrus, as early as 1 day after ADX, as determined by in situ hybridization. The effect was specific for the hippocampal formation, with levels of α1d mRNA unaltered by ADX in the lateral amygdala, reticular thalamic nucleus, retrosplenial cortex or primary somatosensory cortex. Additional rats underwent ADX or sham surgery and received a corticosterone pellet (10 or 50 mg) or placebo for 7 days. Corticosterone replacement prevented the ADX-induced decrease in hippocampal α1d ADR mRNA, with the magnitude of effect depending on corticosterone dose and hippocampal subregion. These data indicate that α1d ADR mRNA expression in the hippocampal formation is highly sensitive to circulating levels of corticosterone, and provides further evidence for a close interaction between glucocorticoids and the noradrenergic system in the hippocampus.
Keywords: adrenergic, adrenalectomy, corticosterone, hippocampus, dentate gyrus, in situ hybridization
1. Introduction
The hippocampal formation is the target of extensive noradrenergic innervation in both rats and humans (Loy et al., 1980; Powers et al., 1988). It is also an area that expresses high levels of both mineralocorticoid (MR) and glucocorticoid (GR) receptors (Reul and de Kloet, 1985; Herman et al., 1989), and is strongly influenced by circulating glucocorticoids (McEwen, 2007). Considerable evidence supports a close interaction between glucocorticoids and the noradrenergic system in the hippocampus. For example, lesions of the ascending noradrenergic pathways result in increased MR binding in the hippocampus and attenuated corticosterone responses to stress, suggestive of an indirect role for the noradrenergic system in glucocorticoid negative feedback (Maccari et al., 1990; Maccari et al., 1992). In addition, activation of α1 adrenergic receptors (ADRs) leads to a down-regulation of both MR and GR in the hippocampal formation (Kabbaj et al., 1995). More recently, it has been shown that chronic prazosin treatment, a non-selective α1 ADR antagonist, differentially alters hippocampal MR and GR binding in rats that are categorized as “high responder” or “low responder”, according to their differential stress response profiles (Kabbaj et al., 2007). However, there is relatively little data on the influence of glucocorticoids on the noradrenergic system within the hippocampus.
To date, nine adrenergic receptors have been cloned, of which 3 are in the α1 ADR family (Strosberg, 1993). Levels of α1a and α1b ADR mRNA expression are relatively low in the hippocampus (McCune et al., 1993; Pieribone et al., 1994; Day et al., 1997). αIn contrast, α1d ADR mRNA is expressed at high levels in all subfields of the rat hippocampus and dentate gyrus (McCune et al., 1993; Pieribone et al., 1994; Day et al., 1997), and this receptor is expressed in the human hippocampus also (Szot et al., 2005). There is extensive colocalization of α1d ADR mRNA with both MR and GR within the rat hippocampal formation (Williams et al., 1997), and thus α1D ADRs are in an excellent position to both modulate glucocorticoid sensitive hippocampal neurons, and in turn be modulated by glucocorticoids. In vitro experiments have shown that glucocorticoids can upregulate α1d ADR mRNA expression (Rouppe van der Voort et al., 1999). In contrast, early ex-vivo binding experiments using [3H]-prazosin did not demonstrate a change in α1 ADRs in the hippocampus after adrenalectomy (Biegon et al., 1985; Jhanwar-Uniyal and Leibowitz, 1986). However, data from α1d ADR knock-out mice have shown that very large changes in α1D ADR receptor levels are needed to detect a change in [3H]-prazosin binding in the hippocampus, with levels of hippocampal binding reduced by only 25% in this extreme case (Sadalge et al., 2003). Given the relatively low levels of either α1a and α1b ADR mRNA in hippocampal cells, these data suggest that [3H]-prazosin binding in the hippocampus may reflect binding to receptors with cell bodies extrinsic to the hippocampus. Hence, it is not clear whether glucocorticoids can influence expression of hippocampal α1D ADR in vivo. In the current experiments, bilateral adrenalectomy, with and without corticosterone replacement, was performed in rats to determine whether levels of α1d ADR mRNA in the hippocampal formation are regulated by circulating glucocorticoids. Our data showed that as early as 1 day after adrenalectomy, levels of α1d ADR mRNA were decreased throughout the hippocampus and dentate gyrus. Levels of α1d ADR mRNA were restored (CA1, CA3, dentate gyrus) or even enhanced (CA2) by corticosterone replacement. These data are consistent with the idea that there is a close relationship between the noradrenergic and glucocorticoid systems in the hippocampal formation, and may have further implications for their interactions in hippocampal functions such as cognition and stress responsiveness.
The general experimental details have been described previously in a study reporting changes in α1b ADR mRNA expression in the paraventricular nucleus of the hypothalamus (Day et al., 1999). This study reported the basic observations on weight gain and corticosterone levels, which are briefly included here for easy reference.
2. Results
Experiment 1: Adrenalectomy Time Course
Levels of corticosterone for all ADX animals were undetectable, whereas naive, unoperated, and sham controls had levels between 4 and 6 μg/dl at the time of killing. (P.M. Sample: Naïve, n = 4: 5.53 ± 0.98 μg/dl; UNOP, n = 3: 5.94 ± 0.13 μg/dl; SHAM 1d, n = 3: 4.40 ± 0.95 μg/dl; SHAM 3d, n = 3: 4.35 ± 0.43 μg/dl; SHAM 7d, n = 3: 5.86 ± 1.79 μg/dl; SHAM 14d, n = 3: 5.95 ± 0.78 μg/dl). Despite the addition of dextrose in the water, prolonged ADX resulted in significantly lower weight gain as compared with sham controls. At 7 d the percentage weight gain was not significantly different between sham and ADX animals (SHAM 7d, 14.0 ± 0.6%; ADX 7d, 8.9 ± 1.1%; p = 0.182). In contrast, after 14 d, the percentage weight gain of ADX animals was significantly lower than sham controls (SHAM 14d, 18.9 ± 4.2%; ADX 14d, 0.2 ± 2.7%; p < 0.001).
The effect of bilateral adrenalectomy on the expression of α1d ADR mRNA over time was studied. Two-way ANOVA revealed a significant effect of surgery (p < 0.001) across all hippocampal subfields studied (CA1, CA2, CA3, dentate gyrus: medial and lateral blades; see Figure 1 for specific regions analyzed). There were no significant effects of time, or a time by surgery interaction, for any of the hippocampal subfields. Post hoc comparisons (Least Significant Difference; LSD) showed that there were no significant differences in levels of α1d ADR mRNA expression in the hippocampal subfields between naive, unoperated, and sham controls, with the exception of the medial blade of the dentate gyrus, where unoperated and naïve animals expressed significantly higher levels of α1d ADR mRNA compared to SHAM operated animals (p = 0.030; p = 0.037 respectively). Levels of α1d ADR mRNA expression were significantly reduced in ADX rats compared with naïve, unoperated and sham controls (p < 0.001 for ADX compared to all control groups and for all hippocampal subfields; Figure 2). The magnitude of the effect of adrenalectomy appeared to be greater for the CA3 and dentate gyrus subfields than for the CA1 and CA2 subfields. There was no significant effect of time, or a time by surgery interaction, with the decrease in α1d ADR mRNA expression apparent as early as 1 day (22 - 24 hours) after ADX. The decrease appeared to be specific to the hippocampus, because no significant changes in α1d ADR mRNA expression were observed for the cortex (retrosplenial or primary somatosensory regions), lateral amygdala or the reticular nucleus of the thalamus (Table 1).
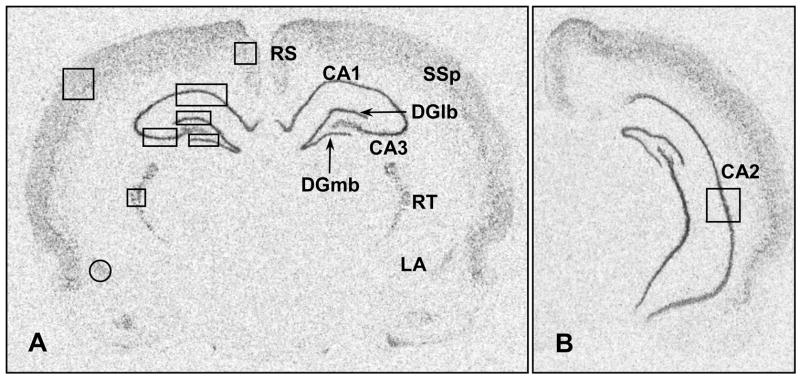
Figure 1.
Representative sections from a naïve animal showing the placement, size and shape of the templates used to analyze α1d ADR mRNA expression in the retrosplenial cortex (RS), primary somatosensory cortex (SSp), reticular thalamic nucleus (RT), lateral amygdala (LA), CA1, CA2 and CA3 regions of the hippocampus, and the medial (DGmb) and lateral (DGlb) blades of the dentate gyrus.
Figure 2.
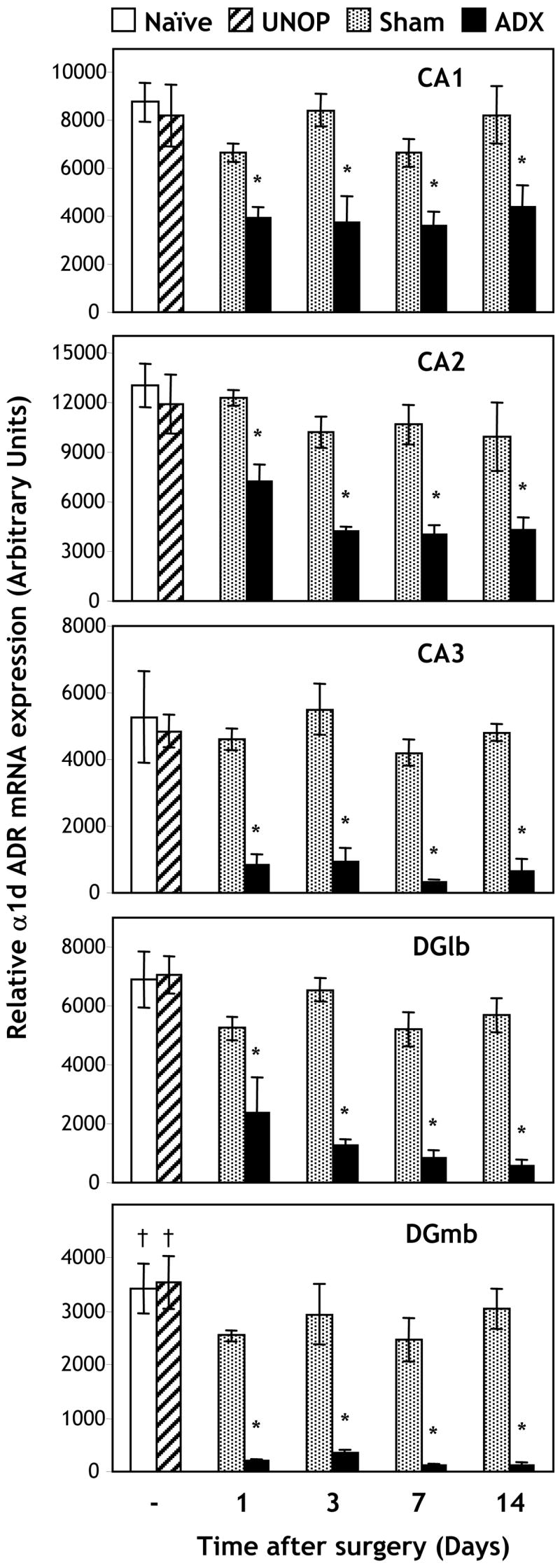
Experiment 1: Expression of α1d ADR mRNA in the CA1, CA2 and CA3 hippocampal subfields, and lateral (DGlb) and medial (DGmb) blades of the dentate gyrus, 1, 3, 7 or 14 days after bilateral adrenalectomy (ADX; n = 4/group) or sham operation (Sham; n = 3/group; Experiment 1). Additional animals remained untreated (naïve; n = 4) or were given the saline-dextrose solution to drink, but did not undergo surgery (UNOP; n = 3). * p < 0.001 for ADX versus Naïve, UNOP and Sham groups; † p < 0.05 for UNOP and Sham groups versus Naïve. Two way ANOVA indicated that there were no significant effects of time or a time by surgery interaction.
Table 1.
Relative levels of α1d ADR mRNA expression in the retrosplenial cortex (RS), primary somatosensory cortex (SSp), lateral amygdala and reticular thalamic nucleus
| Cortex(RS) | Cortex(SSp) | Amygdala(Lateral) | Thalamus(Reticular) | n | |
|---|---|---|---|---|---|
| Naïve | 576 ± 138 | 1658 ± 381 | 662 ± 36 | 1789 ± 228 | 4 |
| Unop. | 453 ± 26 | 1671 ± 484 | 641 ± 187 | 1853 ± 362 | 3 |
| Sham 1d | 381 ± 65 | 1725 ± 223 | 651 ± 146 | 1391 ± 205 | 3 |
| ADX 1d | 493 ± 66 | 1481 ± 322 | 691 ± 164 | 1791 + 333 | 4 |
| Sham 3d | 364 ± 35 | 1141 ± 176 | 463 ± 22 | 1710 ± 388 | 3 |
| ADX 3d | 517 ± 126 | 1261 ± 243 | 541 ± 182 | 1828 ± 630 | 4 |
| Sham 7d | 411 ± 25 | 1086 ± 36 | 454 ± 182 | 1285 ± 172 | 3 |
| ADX 7d | 402 ± 18 | 1192 ± 217 | 432 ± 37 | 1526 ± 148 | 4 |
| Sham 14d | 360 ± 67 | 1383 ± 293 | 543 ± 284 | 1404 ± 652 | 3 |
| ADX 14d | 430 ± 79 | 1826 ± 297 | 597 ± 111 | 1757 ± 411 | 4 |
Table 1 shows the relative levels of α1d ADR mRNA expression (arbitrary units) in the retrosplenial cortex (RS), primary somatosensory cortex (SSp), lateral amygdala and reticular thalamic nucleus 1, 3, 7 or 14 days after bilateral adrenalectomy (ADX; n = 4/group) or sham operation (Sham; n = 3/group; Experiment 1). Additional animals remained untreated (naïve; n = 4) or were given the saline-dextrose solution to drink, but did not undergo surgery (UNOP; n = 3). There were no significant differences in expression levels of α1d ADR mRNA in any of these brain regions.
Experiment 2: Adrenalectomy and Corticosterone Replacement
Levels of corticosterone were undetectable in 5 of the 6 animals in the ADX plus placebo group at either 3 d (A.M. sample) or 7 d (P.M. sample). The sixth animal had barely detectable levels of corticosterone (A.M.: 0.02 μg/dl; P.M.: 0.2 μg/dl). Data from this animal did not appear significantly different from the rest of the ADX + placebo group and therefore were included in the analysis. The naive and sham groups had comparable levels of corticosterone for both A.M. and P.M. samples. (NAÏVE, n = 6: A.M.: 0.11 ± 0.04 μg/dl; P.M.: 5.74 ± 1.19 μg/dl; SHAM, n = 6: A.M.: 0.27 ± 0.20 μg/dl; P.M.: 7.30 ± 1.11 μg/dl). For the ADX plus 10 mg of corticosterone group, levels of corticosterone were 2.42 ± 0.20 μg/dl after 3 d (A.M. sample) and 1.19 ± 0.29 μg/dl after 7 d (P.M. sample). For the ADX plus 50 mg of corticosterone group, levels were 7.47 ± 0.88 μg/dl after 3 d (A.M. sample) and 6.83 ± 0.82 μg/dl after 7 d (P.M. sample).
Weight gain for the animals varied across groups. Naive and sham groups exhibited a similar weight gain (NAÏVE, n = 6: 14.1 ± 0.8 %; SHAM, n = 6: 12.1 ± 1.2 %). ADX plus placebo gained significantly less weight than sham or naive animals (2.8 ± 2.2 %; n = 6; p < 0.001 with respect to both sham and naïve groups). In contrast, a low dose of corticosterone reversed this effect, so that ADX plus 10 mg group exhibited a similar weight gain as the naive and sham animals (11.5 ± 1.2 %). Animals treated with a high dose of corticosterone also demonstrated significantly lower weight gain than naive, sham, or ADX plus 10 mg groups (2.3 ± 1.9 %; p < 0.001 with respect to Naïve, Sham and ADX + 10 mg groups).
The effect of bilateral adrenalectomy with placebo, 10 mg or 50 mg corticosterone replacement on expression of α1d ADR mRNA was analyzed by one-factor ANOVAs (for “group”). There were significant differences between groups for all hippocampal subfields (CA1: p = 0.048; CA2: p = 0.002; CA3: p < 0.001; DGm: p < 0.001; DGl: p < 0.001; Figures 3 and 4). Post hoc comparisons (LSD) showed that there were no significant differences between naive and sham controls for any hippocampal region. In contrast, levels of α1d ADR mRNA were significantly decreased in the ADX + placebo group, compared with Sham controls across all subfields, with greater relative decreases in the medial and lateral blades of the dentate gyrus and CA3 subfield, than the CA1 and CA2 subfields (CA1: p = 0.028; CA2: p = 0.026; CA3: p < 0.001; DGm: p < 0.001; DGl: p < 0.001).
Figure 3.
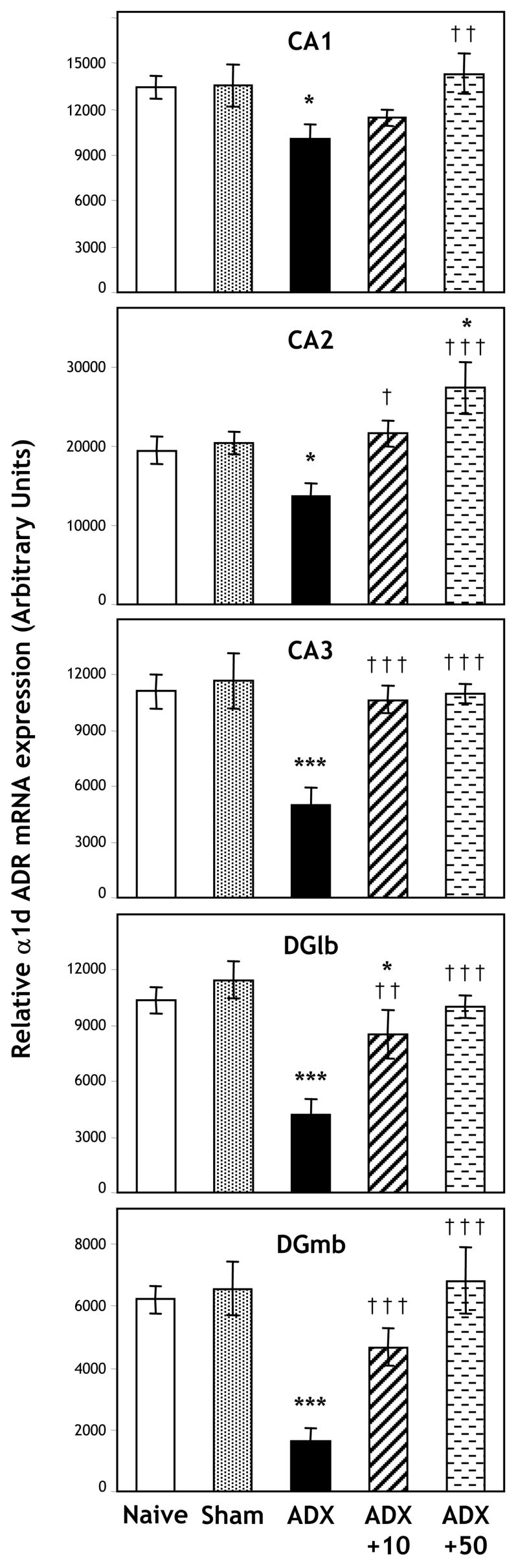
Experiment 2: Expression of α1d ADR mRNA in the CA1, CA2 and CA3 hippocampal subfields, and lateral (DGlb) and medial (DGmb) blades of the dentate gyrus, 7 days after bilateral adrenalectomy with placebo pellet replacement (ADX; n = 6), adrenalectomy with 10 mg corticosterone pellet replacement (ADX + 10; n = 6), adrenalectomy with 50 mg corticosterone pellet replacement (ADX + 50; n = 6) or sham operation (Sham; n = 6). Additional animals remained untreated (naïve; n = 6). * p < 0.05, ** p < 0.01, *** p < 0.001 relative to Sham control group; † p < 0.05, †† p < 0.01, ††† p < 0.001 relative to adrenalectomy plus placebo (ADX) group.
Figure 4.
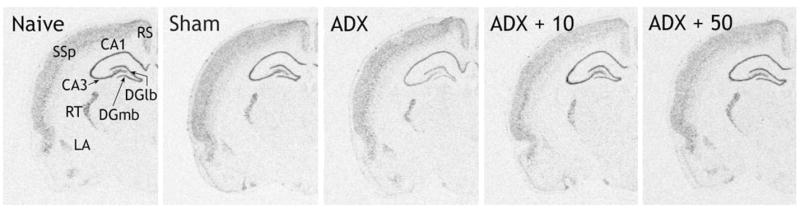
Experiment 2: Photographs of x-ray films showing the expression of α1d ADR mRNA in the rat brain at the level of the dorsal hippocampus, 7 days after bilateral adrenalectomy with placebo pellet replacement (ADX), adrenalectomy with 10 mg corticosterone pellet replacement (ADX + 10), adrenalectomy with 50 mg corticosterone pellet replacement (ADX + 50) or sham operation (Sham). Additional animals remained untreated (Naïve). Abbreviations: RS: retrosplenial cortex; SSp: primary somatosensory cortex: RT: reticular thalamic nucleus; LA: lateral amygdala; CA1, CA3: subfields of the hippocampus; DGmb, DGlb: medial and lateral blades of the dentate gyrus respectively.
A low dose of corticosterone mostly restored the expression of α1d ADR mRNA in the hippocampus, with levels of expression in the ADX + 10 group not significantly different from Sham controls, and significantly different from the ADX + placebo group for the CA2 subfield (p = 0.689 with respect to sham and p = 0.010 with respect to ADX + placebo), CA3 subfield (p = 0.481 with respect to sham and p < 0.001 with respect to ADX + placebo) and DG medial blade (p = 0.081 with respect to sham and p = 0.006 with respect to ADX + placebo). Partial restoration of expression was observed in the DG lateral blade, with a significantly increased level of expression compared with the ADX + placebo group (p = 0.003), but also a significantly decreased level of expression compared with Sham controls (p = 0.036). The effect of a low dose of corticosterone on levels of α1d ADR mRNA expression in the CA1 subfield was less clear with expression levels of the ADX + 10 group not significantly different from either Sham or ADX + placebo groups.
A high dose of corticosterone (ADX + 50 group) for the most part restored levels of α1d ADR mRNA expression to basal levels, but no higher, with no significant differences observed between the ADX + 50, naïve and sham groups for either the CA1, CA3, DGm or DGl. For these 4 subregions, levels of α1d ADR mRNA in the ADX + 50 groups were significantly higher than the ADX + placebo group (CA1: p = 0.008; CA3: p < 0.001; DGm: p < 0.001; DGl: p < 0.001). For the DGm levels in the ADX + 50 group were also significantly higher than the ADX + 10 group (p = 0.047). A significant difference between these two groups was not observed for the other hippocampal subfields (CA1: p = 0.064; CA2: p = 0.060; CA3: p = 0.824; DGl: p = 0.270). In the CA2 subfield, levels of α1d ADR mRNA expression in the ADX + 50 group were significantly increased compared with the ADX + placebo group (p < 0.001), but also compared with the naïve (p = 0.011) and sham (p = 0.026) controls.
3. Discussion
These data strongly suggest that expression of α1d ADR mRNA in the hippocampal formation is dependent on circulating levels of corticosterone, with a lack of corticosterone leading to a quick and profound decrease in α1d ADR mRNA expression in all hippocampal subfields and the dentate gyrus. This decrease was partially (DGl) or fully (CA2, CA3, DGm) restored with replacement of low levels of corticosterone. Higher levels of corticosterone led to full restoration of α1d mRNA expression levels in most subregions of the hippocampal formation (CA1, CA3, DGl, DGm), and enhanced expression levels in the CA2 hippocampal subfield.
One of the caveats that must be considered when discussing data obtained following adrenalectomy is the neuronal degeneration that adrenalectomy causes over time (Sloviter et al., 1989; MacLennan et al., 1998). In particular, the dentate gyrus is a region that is particularly susceptible to apoptosis following adrenalectomy (without corticosterone replacement). However, the fact that decreased α1d ADR mRNA expression was also observed in CA1, CA2 and CA3 hippocampal subfields after adrenalectomy suggests that the regulation observed is not simply a result of neuronal loss. This is because other hippocampal subfields are generally resistant to these neurodegenerative changes, even several months after adrenalectomy (Sloviter et al., 1989; Gould et al., 1990; Woolley et al., 1991). In addition, the speed at which levels of α1d ADR mRNA expression decreased in the dentate gyrus after adrenalectomy suggests that the changes were not due simply to a paucity of neurons. Neuronal degeneration is not apparent 24 hours after adrenalectomy, as determined by silver impregnation and glial fibrillary acidic protein immunohistochemistry (Krugers et al., 1994), and elimination of apoptotic granule cells takes 72 hours (Hu et al., 1997), yet α1d ADR mRNA was decreased in both the medial and lateral blades of the dentate gyrus within 24 hours of adrenalectomy. Hence it seems unlikely that the decrease in α1d mRNA observed in the hippocampal formation, and in particular the dentate gyrus, is simply due to neuronal cell loss. However, it is possible that decreased α1d ADR mRNA expression in the dentate gyrus is in some way related to or reflective of the apoptotic process, rather than a loss of cells per se.
Three α1 ADRs have been cloned, and while α1d ADR mRNA is the most highly expressed of the 3 receptors in the hippocampal formation, α1a and α1b mRNAs are also expressed at low levels in these regions (Day et al., 1997). In contrast to the data presented here, previous studies have not demonstrated any changes in α1 ADR binding in the hippocampus of adrenalectomized rats (Biegon et al., 1985; Jhanwar-Uniyal and Leibowitz, 1986). These studies utilized [3H]-prazosin which binds non-selectively to the three different alpha 1 ADRs with similar affinities (Hancock, 1996), and therefore theoretically should pick up changes in α1D ADR protein expression. It is of course possible that the changes in α1d mRNA that occur following adrenalectomy are not translated into changes in protein. However, the changes in hippocampal α1d mRNA levels are so global, and so profound, particularly in the dentate gyrus and CA3 subfield, it seems unlikely that there would be no decrease in protein levels at all. Alternatively, it is possible that there are compensatory increases in expression of α1a or α1b ADR protein, although adrenalectomy does not increase expression of α1b ADR mRNA in the hippocampus (Day et al., 1999). It is possible that prazosin binding in the hippocampus reflects binding to α1 receptors on neurons with cell bodies outside the hippocampus and it is worth noting that prazosin binding in the hippocampus of α1d ADR knockout mice is reduced by only 25% (Sadalge et al., 2003). This suggests that, at least in mice, very large changes in α1D ADR protein levels are required to detect significant changes in prazosin binding in the hippocampus. Thus changes due to adrenalectomy mayhave been below the detection threshold of the binding technique in the previously published studies (Biegon et al., 1985; Jhanwar-Uniyal and Leibowitz, 1986).
The function of α1D ADRs in the hippocampal formation is not well understood, and is hindered by the lack of selective receptor agonists and antagonists. Data from α1d ADR knockout mice, suggest that this receptor is important in switching from basal locomotor behavior to an activated state, in response to either environmental modulation or amphetamine administration, although the specific role of hippocampal α1D ADR in these behaviors has not been demonstrated (Sadalge et al., 2003). The hippocampus is recognized as an area important in the formation of episodic memories, and an extensive literature has investigated the role of glucocorticoids and cognition (Belanoff et al., 2001). α1 ADR activation has been associated with a novel form of long-term synaptic depression in rat hippocampus, which has been suggested to be involved in cognitive function (Scheiderer et al., 2004). In Alzheimer’s disease, the density of noradrenergic innervation is reduced and abnormal noradrenergic axons are observed (Powers et al., 1988). Furthermore, levels of α1d ADR mRNA expression, which in humans is expressed in CA1-3 hippocampal subfields (Szot et al., 2005), has been reported to be significantly reduced in the hippocampus of postmortem subjects with either Alzheimer’s disease or dementia with Lewy bodies (Szot et al., 2006).
The noradrenergic system has also been implicated extensively in stress and anxiety type responses (Bremner et al., 1996; Morilak et al., 2005). The hippocampus is well known to be influenced by stress, and increases in noradrenaline have been demonstrated consistently in the hippocampus in response to stress (Tanaka et al., 1983; Nisenbaum et al., 1991). In addition, the hippocampal formation expresses high levels of both MR (or type I corticosteroid receptor) and GR (or type II corticosteroid receptor) (Reul and de Kloet, 1985; Herman et al., 1989), making this a region that is highly susceptible to changes in circulating glucocorticoids (McEwen, 2007). The noradrenergic system appears to interact closely with corticosteroid receptors, with lesions of the ascending noradrenergic pathways leading to increased MR binding in the hippocampus and attenuated corticosterone responses to stress, suggestive of an indirect role for the noradrenergic system in glucocorticoid negative feedback (Maccari et al., 1990; Maccari et al., 1992). α1d ADR receptors are extensively colocalized with both MR and GR within the hippocampal formation (Williams et al., 1997) and activation of α1 ADR receptors led to a down-regulation of both MRs and GRs in the hippocampal formation (Kabbaj et al., 1995). Interestingly, it has been shown that chronic prazosin treatment differentially alters hippocampal MR and GR binding in “high responder” and “low responder” rats that have differential stress response profiles (Kabbaj et al., 2007). In addition to this α1 ADR modulation of hippocampal MR and GR, the present data indicate that hippocampal α1d ADR mRNA expression is modulated by glucocorticoids. Despite the fact that the 5′-flanking region (1.6 kb) of the rat α1d ADR gene has been sequenced (Xin et al., 1999), to our knowledge, a functional glucocorticoid response element (GRE) sequence has not been demonstrated in the rat α1d ADR gene. However, dexamethasone has been shown to upregulate α1d ADR mRNA expression in human monocytes in vitro (Rouppe van der Voort et al., 1999). Together with the data reported here, this suggests that the rat α1d ADR gene may contain a functional GRE that results in increased gene transcription when bound by the appropriate steroid-receptor complex. Alternatively, because levels of α1d ADR mRNA were not altered by adrenalectomy in other brain regions studied, it may be that glucocorticoids do not directly modulate transcription of α1d ADR, but rather modulate levels of transcription factors in the hippocampal formation that then modify transcription of the α1d ADR gene.
In an effort to determine whether MR or GR are involved in the regulation of α1d ADR mRNA, low and moderate doses of corticosterone were used in the replacement study. MR has a 6 to 10-fold higher affinity for corticosterone than GR, and this receptor is thought to be more important than GR in situations when circulating levels of corticosterone are low, as occurs at the nadir of the diurnal rhythm (light phase for rats). In contrast, when circulating levels of corticosterone rise, either due to the normal circadian rhythm, or following HPA activation, while the occupation of MR remains high, the relative importance of GR is increased (Reul and de Kloet, 1985; Dallman et al., 1991; De Kloet et al., 1998). The data presented here indicated that a relatively low dose of corticosterone (10 mg pellet) was sufficient to partially (DGl) or completely (CA2, CA3, DGm) reverse the decrease in expression levels of α1d ADR mRNA observed following bilateral adrenalectomy, which may indicate an involvement of MR. The fact that adrenalectomy plus the higher dose of corticosterone (50 mg pellet) led to a significant increase in α1d ADR mRNA relative to the 10 mg pellet in the medial dentate gyrus, and a significant increase over basal levels in the CA2 hippocampal subfield, suggests that GR may also play a role in α1d ADR mRNA regulation.
Although the data presented here do not address the specific mechanism of α1d ADR mRNA regulation by glucocorticoids, they nonetheless indicate that in all regions of the hippocampal formation, an area with important integrative functions, the absence of glucocorticoids, induced by adrenalectomy, dramatically decreases levels of α1d ADR mRNA expression, whereas corticosterone replacement restores or even enhances α1d ADR mRNA expression. These data provide further evidence for a close relationship between glucocorticoids and the noradrenergic system in the hippocampal formation, with possible implications for cognitive and stress-related processes.
4. Experimental Procedure
Animals
All procedures described were approved by UCUCA (University Committee on Use and Care of Animals, University of Michigan). Adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were used throughout. All animals were allowed to habituate to the housing conditions for at least 10 days prior to any experimental manipulation. Rats were housed 2 or 3 per cage under conditions of constant temperature and humidity, on a 12 h light/dark cycle (lights on 0700 h), with access to food and water ad libitum.
Experiment 1: Adrenalectomy Time Course
For the initial time course, 35 rats were used (mean weight 313 ± 4 g). Of these, 28 were anesthetized with pentobarbital and underwent bilateral adrenalectomy (ADX; 4 per group; N=16) or sham surgery (Sham; 3 per group; N=12). The adrenalectomy was performed via a dorsolateral approach, and the incision was closed with wound clips. Animals were housed 2 or 3 per cage, and drinking water was replaced with a solution containing 0.9% saline and 5% dextrose. Animals were killed 1 (22–24 h), 3, 7 or 14 days following surgery. In addition 3 unoperated (UNOP) animals were included, that did not undergo any surgical manipulation, but which were given the saline/dextrose solution to drink for 1, 3 or 7 days, plus 4 naïve animals that were not subjected to any experimental manipulation. Animals were killed 2 hours before lights off (17:00) so that there were detectable levels of plasma corticosterone in control animals against which to compare the effectiveness of the ADX surgery. Trunk blood was collected in chilled tubes containing EDTA, plasma separated and stored at −20°C for analysis of corticosterone. Brains were removed, frozen in isopentane cooled to −40 to −50°C, and stored at −80°C until processing for in situ hybridization
Experiment 2: Adrenalectomy and Corticosterone Replacement
For the second part of the study, 30 animals were used, divided into the following groups: NAÏVE (n=6), SHAM (n=6), ADX + placebo (n=6), ADX + 10 mg corticosterone (n=6), ADX + 50 mg corticosterone (n=6). The mean start weight was 291 ± 3 g. In addition to bilateral adrenalectomy, animals were implanted with a subcutaneous pellet (Innovative Research of America, Florida) within the dorsal neck region. Rats received placebo, 10 mg or 50 mg corticosterone in the form of 21 day release pellets. Surgery was performed over a 2 day period, n= 3 per group per day. Drinking water for SHAM and all ADX groups was replaced with an isotonic solution containing 0.45% saline and 2.5% dextrose. Lower concentrations of saline and dextrose were used in this experiment than for Experiment 1, so that the control animals were drinking a solution as similar to tap water as possible. A similar concentration of saline has been used successfully in other experiments involving adrenalectomized rats (Hanson et al., 1994; Bhatnagar et al., 2000), and appears to be sufficient for preventing adrenal insufficiency, at least over the short time course (1 week) of this experiment (Bjuro and Westling, 1964). Three days following surgery, a tail vein blood sample (75 μl) was taken from each animal 2 hours after lights on (09:00) to assess basal levels of corticosterone. For this procedure, animals were restrained lightly and a lateral tail vein punctured with the corner of a razor blade, and blood collected in a heparinized capillary tube. Animals were killed 7 days following surgery, 1–2 hours prior to lights off (17:00–18:00). Trunk blood and brains were collected as for Experiment 1.
Corticosterone Analysis
Levels of plasma corticosterone were analyzed as previously described (Day and Akil, 1996). Briefly, 10 μl duplicate samples of plasma were incubated overnight with an antibody against corticosterone (raised in our laboratory) and [3H]-corticosterone (Amersham). Bound versus free corticosterone was separated by charcoal extraction, and levels calculated by comparison with a standard curve.
In situ Hybridization
Sections (10 μm) were cut on a cryostat through the hippocampus, and stored at −80°C until processing for in situ hybridization (Day et al., 1997). Briefly, a cRNA probe to detect α1d mRNA (cDNA courtesy of Dr. D.M. Perez, Cleveland Clinic Research Institute, OH), labeled with 35S-UTP and 35S-CTP (Amersham, Arlington Heights, IL), was generated using standard transcription technology. Sections were fixed for 1 hour in 4% phosphate-buffered paraformaldehyde at room temperature. Following a series of washes in 2x standard saline citrate (SSC; 1× SSC = 150 mM sodium chloride and 15 mM sodium citrate), sections were incubated in 0.1 M triethanolamine with 0.25% acetic acid for 10 minutes. The tissue was rinsed in distilled water and dehydrated through a series of alcohols. Sections were incubated overnight at 55°C with diluted probe, in a 50% formamide hybridization buffer containing 10 mM dithiothreitol, to yield an approximate concentration of 2 × 106 c.p.m. per 65 μl. The following day sections were washed in 2× SSC and incubated in RNase A (200 μg/ml) at 37°C for 1 hour. Sections were then washed to a final stringency of 0.1× SSC at 70°C for 1 hour. Tissue was dehydrated through a series of alcohols and exposed to x-ray film (Kodak, Biomax-MR) for 4 days.
Semi-quantitative mRNA analysis
Levels of α1d ADR mRNA were analyzed by computer assisted optical densitometry. Brain section images from in situ hybridization experiments were captured digitally (CCD camera, model XC-77, Sony, Tokyo, Japan), and analyzed using Scion Image version 4.0.3 for Windows (Scion Corporation). A macro was written (Dr. Serge Campeau) which enabled signal above background to be automatically determined as follows. For each section, a background sample was taken over an area of tissue expressing background levels of α1d ADR mRNA (if possible over white matter), and the signal threshold calculated as mean gray value of background + 3.5 × standard deviation. The section was automatically density sliced at this value, so that only pixels with gray values exceeding these criteria were included in the analysis. The 3.5 standard deviations above the mean of background was chosen as a relatively stringent threshold criterion, which resulted in only a few pixels above threshold on the film background, on areas of white matter, or on areas of tissue previously shown to have undetectable α1d ADR mRNA expression, such as the habenula and parafascicular nucleus of the thalamus (Day et al., 1997). Despite the relatively stringent threshold conditions, the fact that a few pixels remained above threshold outside of the tissue section indicates that this method is suitable for detecting even relative low intensity mRNA expression. A template was used for each brain region, so that the same area was included in the analysis for each region (Figure 1). The number of pixels above background was multiplied by the signal above background, to give an integrated density value. This method has been shown to reflect both the number of cells expressing mRNA and the expression level per cell, as determined by cell and grain counts of emulsion dipped slides (Day et al., 2005). For each brain region 4 or 5 sections were analyzed, across at least 2 different slides, left and right sides separately, and the mean of these values was calculated to give a single value for each animal.
Statistical Analysis
Data were analyzed by two-way analysis of variance (ANOVA) for Experiment 1(with surgery and time as factors) and by one-way ANOVA (for group) for Experiment 2, followed by LSD (Least Significant Difference) post-hoc multiple comparisons tests, as indicated in the text. Significance was set at p < 0.05.
Acknowledgments
The authors thank Sharon Burke (University of Michigan) for her invaluable technical assistance and Dr. K. Itoi (Tohoku University School of Medicine, Japan) for his helpful protocol suggestions. This study was supported by NIDA Grant 5RO1 DA02265-18, NIMH Grant 2PO1 MH42251-11 (HA & SJW) and the Pritzker Network for the study of depression (HA & SJW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belanoff JK, Gross K, Yager A, Schatzberg AF. Corticosteroids and cognition. J Psychiatr Res. 2001;35:127–145. doi: 10.1016/s0022-3956(01)00018-8. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Bell ME, Liang J, Soriano L, Nagy TR, Dallman MF. Corticosterone facilitates saccharin intake in adrenalectomized rats: Does corticosterone increase stimulus salience? J Neuroendocrinol. 2000;12:453–460. doi: 10.1046/j.1365-2826.2000.00487.x. [DOI] [PubMed] [Google Scholar]
- Biegon A, Rainbow TC, McEwen BS. Corticosterone modulation of neurotransmitter receptors in rat hippocampus: A quantitative autoradiographic study. Brain Res. 1985;332:309–314. doi: 10.1016/0006-8993(85)90599-2. [DOI] [PubMed] [Google Scholar]
- Bjuro T, Westling H. The effect of sodium intake on the urinary histamine in adrenalectomized rats. Br J Pharmacol. 1964;22:453–462. doi: 10.1111/j.1476-5381.1964.tb01700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Scribner KA, Bradbury MJ, Dominique-Walker C, Strack AM, Cascio CS. Stress, feedback and facilitation in the hypothalmo-pituitary-adrenal axis. J Neuroendocinol. 1991;4:517–526. doi: 10.1111/j.1365-2826.1992.tb00200.x. [DOI] [PubMed] [Google Scholar]
- Day HE, Akil H. Differential pattern of c-fos mrna in rat brain following central and systemic administration of interleukin-1-beta: Implications for mechanism of action. Neuroendocrinology. 1996;63:207–218. doi: 10.1159/000126959. [DOI] [PubMed] [Google Scholar]
- Day HE, Campeau S, Watson SJ, Jr, Akil H. Distribution of alpha 1a-, alpha 1b- and alpha 1d-adrenergic receptor mrna in the rat brain and spinal cord. J Chem Neuroanat. 1997;13:115–139. doi: 10.1016/s0891-0618(97)00042-2. [DOI] [PubMed] [Google Scholar]
- Day HE, Campeau S, Watson SJ, Jr, Akil H. Expression of alpha(1b) adrenoceptor mrna in corticotropin-releasing hormone-containing cells of the rat hypothalamus and its regulation by corticosterone. J Neurosci. 1999;19:10098–10106. doi: 10.1523/JNEUROSCI.19-22-10098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Nebel S, Sasse S, Campeau S. Inhibition of the central extended amygdala by loud noise and restraint stress. Eur J Neurosci. 2005;21:441–454. doi: 10.1111/j.1460-9568.2005.03865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocrine Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, McEwen BS. Short-term glucocorticoid manipulations affect neuronal morphology and survival in the adult dentate gyrus. Neuroscience. 1990;37:367–375. doi: 10.1016/0306-4522(90)90407-u. [DOI] [PubMed] [Google Scholar]
- Hancock AA. Alpha1 adrenoceptor subtypes: A synopsis of their pharmacology and molecular biology. Drug Dev Res. 1996;39:54–107. [Google Scholar]
- Hanson ES, Bradbury MJ, Akana SF, Scribner KS, Strack AM, Dallman MF. The diurnal rhythm in adrenocorticotropin responses to restraint in adrenalectomized rats is determined by caloric intake. Endocrinology. 1994;134:2214–2220. doi: 10.1210/endo.134.5.8156924. [DOI] [PubMed] [Google Scholar]
- Herman JP, Patel PD, Akil H, Watson SJ. Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger rnas in the hippocampal formation of the rat. Mol Endocrinol. 1989;3:1886–1894. doi: 10.1210/mend-3-11-1886. [DOI] [PubMed] [Google Scholar]
- Hu Z, Yuri K, Ozawa H, Lu H, Kawata M. The in vivo time course for elimination of adrenalectomy-induced apoptotic profiles from the granule cell layer of the rat hippocampus. J Neurosci. 1997;17:3981–3989. doi: 10.1523/JNEUROSCI.17-11-03981.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhanwar-Uniyal M, Leibowitz SF. Impact of circulating corticosterone on alpha 1- and alpha 2-noradrenergic receptors in discrete brain areas. Brain Res. 1986;368:404–408. doi: 10.1016/0006-8993(86)90591-3. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Morley-Fletcher S, Le Moal M, Maccari S. Individual differences in the effects of chronic prazosin hydrochloride treatment on hippocampal mineralocorticoid and glucocorticoid receptors. Eur J Neurosci. 2007;25:3312–3318. doi: 10.1111/j.1460-9568.2007.05585.x. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Piazza PV, Simon H, Le Moal M, Maccari S. Opposite effects on hippocampal corticosteroid receptors induced by stimulation of beta and alpha-1 noradrenergic receptors. Neuroscience. 1995;66:539–545. doi: 10.1016/0306-4522(94)00620-k. [DOI] [PubMed] [Google Scholar]
- Krugers HJ, Medema RM, Postema F, Korf J. Induction of glial fibrillary acidic protein immunoreactivity in the rat dentate gyrus after adrenalectomy: Comparison with neurodegenerative changes using silver impregnation. Hippocampus. 1994;4:307–314. doi: 10.1002/hipo.450040314. [DOI] [PubMed] [Google Scholar]
- Loy R, Koziell DA, Lindsey JD, Moore RY. Noradrenergic innervation of the adult rat hippocampal formation. J Comp Neurol. 1980;189:699–710. doi: 10.1002/cne.901890406. [DOI] [PubMed] [Google Scholar]
- Maccari S, Le Moal M, Angelucci L, Mormede P. Influence of 6-ohda lesion of central noradrenergic systems on corticosteroid receptors and neuroendocrine responses to stress. Brain Res. 1990;533:60–65. doi: 10.1016/0006-8993(90)91795-i. [DOI] [PubMed] [Google Scholar]
- Maccari S, Mormede P, Piazza PV, Simon H, Angelucci L, Le Moal M. Hippocampal type i and type ii corticosteroid receptors are modulated by central noradrenergic systems. Psychoneuroendocrinology. 1992;17:103–112. doi: 10.1016/0306-4530(92)90049-d. [DOI] [PubMed] [Google Scholar]
- MacLennan KM, Smith PF, Darlington CL. Adrenalectomy-induced neuronal degeneration. Prog Neurobiol. 1998;54:481–498. doi: 10.1016/s0301-0082(97)00076-2. [DOI] [PubMed] [Google Scholar]
- McCune S, Voigt M, Hill J. Expression of multiple alpha adrenergic receptor subtype mrnas in the adult rat brain. Neuroscience. 1993;57:143–151. doi: 10.1016/0306-4522(93)90116-w. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Nisenbaum LK, Zigmond MJ, Sved AF, Abercrombie ED. Prior exposure to chronic stress results in enhanced synthesis and release of hippocampal norepinephrine in response to a novel stressor. J Neurosci. 1991;11:1478–1484. doi: 10.1523/JNEUROSCI.11-05-01478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieribone V, Nicholas A, Dagerlind A, Hokfelt T. Distribution of a-1 adrenoceptors in rat brain revealed by in situ hybridization experiments utilizing subtype-specific probes. J Neurosci. 1994;14:4252–4268. doi: 10.1523/JNEUROSCI.14-07-04252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RE, Struble RG, Casanova MF, O’Connor DT, Kitt CA, Price DL. Innervation of human hippocampus by noradrenergic systems: Normal anatomy and structural abnormalities in aging and in alzheimer’s disease. Neuroscience. 1988;25:401–417. doi: 10.1016/0306-4522(88)90248-5. [DOI] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: Microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Rouppe van der Voort C, Kavelaars A, van de Pol M, Heijnen CJ. Neuroendocrine mediators up-regulate alpha1b- and alpha1d-adrenergic receptor subtypes in human monocytes. J Neuroimmunol. 1999;95:165–173. doi: 10.1016/s0165-5728(99)00011-9. [DOI] [PubMed] [Google Scholar]
- Sadalge A, Coughlin L, Fu H, Wang B, Valladares O, Valentino R, Blendy JA. Alpha 1d adrenoceptor signaling is required for stimulus induced locomotor activity. Mol Psychiatry. 2003;8:664–672. doi: 10.1038/sj.mp.4001351. [DOI] [PubMed] [Google Scholar]
- Scheiderer CL, Dobrunz LE, McMahon LL. Novel form of long-term synaptic depression in rat hippocampus induced by activation of alpha 1 adrenergic receptors. J Neurophysiol. 2004;91:1071–1077. doi: 10.1152/jn.00420.2003. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Valiquette G, Abrams GM, Ronk EC, Sollas AL, Paul LA, Neubort S. Selective loss of hippocampal granule cells in the mature rat brain after adrenalectomy. Science. 1989;243:535–538. doi: 10.1126/science.2911756. [DOI] [PubMed] [Google Scholar]
- Strosberg AD. Structure, function, and regulation of adrenergic receptors. Protein Sci. 1993;2:1198–1209. doi: 10.1002/pro.5560020802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA. Alpha1-adrenoreceptor in human hippocampus: Binding and receptor subtype mrna expression. Brain Res Mol Brain Res. 2005;139:367–371. doi: 10.1016/j.molbrainres.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA. Compensatory changes in the noradrenergic nervous system in the locus ceruleus and hippocampus of postmortem subjects with alzheimer’s disease and dementia with lewy bodies. J Neurosci. 2006;26:467–478. doi: 10.1523/JNEUROSCI.4265-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Kohno Y, Nakagawa R, Ida Y, Takeda S, Nagasaki N, Noda Y. Regional characteristics of stress-induced increases in brain noradrenaline release in rats. Pharmacol Biochem Behav. 1983;19:543–547. doi: 10.1016/0091-3057(83)90132-6. [DOI] [PubMed] [Google Scholar]
- Williams A, Nguyen M, Morilak D. Co-localization of alpha-1d adrenergic receptor mrna with mineralocorticoid and glucocorticoid receptor mrna in hippocampus. J Neuroendocrinol. 1997;9:113–119. doi: 10.1046/j.1365-2826.1997.00522.x. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Sakai RR, Spencer RL, McEwen BS. Effects of aldosterone or ru28362 treatment on adrenalectomy-induced cell death in the dentate gyrus of the adult rat. Brain Res. 1991;554:312–315. doi: 10.1016/0006-8993(91)90207-c. [DOI] [PubMed] [Google Scholar]
- Xin X, Yang N, Faber JE. Platelet-derived growth factor-bb inhibits rat alpha1d-adrenergic receptor gene expression in vascular smooth muscle cells by inducing ap-2-like protein binding to alpha1d proximal promoter region. Mol Pharmacol. 1999;56:1152–1161. doi: 10.1124/mol.56.6.1152. [DOI] [PubMed] [Google Scholar]