Abstract
Apocynin (4-hydroxy-3-methoxyacetophenone) is a major active ingredient from the rhizomes of Picrorhiza kurroa, a botanical plant used as an herbal medicine for treatment of a number of inflammatory diseases. Recently, apocynin is regarded as a specific inhibitor for NADPH oxidase in cell and animal models. In vitro studies indicated conversion of apocynin to diapocynin in the presence of peroxidases, e.g., myloperoxidase, posing the possibility that diapocynin also contributes to the anti-oxidative action of apocynin. The objectives of this study are to examine the bioavailability of apocynin to plasma, liver and brain tissue after intraperitoneal (i.p.) injection, and to examine whether apocynin is converted to diapocynin in vivo. Diapocynin was chemically synthetized and characterized by NMR and IR. Apocynin (5 mg/kg body wt) was injected i.p. to adult male Sprague-Dawley rats and plasma, liver and brain were collected at different times (30 min, 1 h and 2 h) after injection. Samples were treated with β-glucuronidase to hydrolyze the glycosyl linkage and analyzed by HPLC/MS. At 30 min and 1 h after injection, approximately 50% of apocynin was converted to its glycosyl derivative and was distributed in plasma, liver and brain. No diapocynin was detected in any samples. These results indicate rapid glycosylation of apocynin and its transport to blood and other organs but no apparent conversion to diapocynin in vivo.
Keywords: Apocynin, NADPH oxidase inhibitor, bioavailability, glycoconjugates, serum, brain
Introduction
Reactive oxygen species (ROS), including superoxide anions, hydrogen peroxide, and hydroxyl radicals are produced by enzymic and non-enzymic mechanisms in mammalian cells. Although some of the ROS serve as signaling molecules in the cells, excessive production is detrimental and has been implicated to play an important role in the progression of many disease processes (Sun and Chen, 1998; Chan, 2001). In the past decade, substantial interest has been focused on developing novel phytocompounds that possess antioxidant properties to combat oxidative effects of ROS.
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase has recently been regarded as an important source of ROS in a number of cell systems including cells in the central nervous system (CNS) (Bedard and Krause, 2007). NADPH oxidase is comprised of multi-subunits and activation of this enzyme involves the translocation of the cytosolic subunits (p47/p67/p40 phox) to the membrane subunits (gp91 phox/p22 phox) and subsequent transfer of electrons from NADPH to molecular oxygen to produce superoxide anions. Recent studies have highlighted the mechanisms for activation of NADPH oxidase in the brain, which subsequently promotes oxidative injury, microglial activation, and neurodegeneration (Infanger et al., 2006).
Apocynin (4-hydroxy-3-methoxy-acetophenone) is discovered during activity-guided isolation of immunomodulatory constituents from the rhizome of Picrorhiza kurroa, a creeping plant native to the mountains of India, Nepal, Tibet and Pakistan (Picrorhiza kurroa, 2001). Picrorhiza kurroa is a medicinal herb containing a number of active ingredients, including acetophenone derivatives (which have anti-asthmatic properties), kutkin (kutkoside and iridioids, which are hepato-protective), and curcubitacins (which have anti-tumor effects) (Picrorhiza kurroa, 2001). In the traditional Chinese and Ayurvedic system of medicine, Picrorhiza has been used to treat liver diseases, upper respiratory tract disorders, chronic diarrhea, hemorrhoids, epilepsy, scorpion sting and fever (Picrorhiza kurroa, 2001).
In common with the polyphenolic compounds, apocynin has multiple biological actions (Barbieri et al., 2004). Particularly, it exhibits powerful anti-inflammatory and anti-oxidant effects in a variety of cell and animal models (Elmarakby et al., 2005; Hougee et al., 2006). Our recent study demonstrated the ability of apocynin to protect against oxidative damage induced by cerebral ischemia in gerbils (Wang et al., 2006). Since there is evidence that apocynin may be converted to diapocynin through peroxidases, e.g., myeloperoxidase (Ximenes et al., 2007), it is not clear whether the inhibitory effect for apocynin is due to its metabolite or both. Furthermore, information on bioavailability of apocynin and its kinetics in different organs has not been investigated in detail. In this study, we conducted a time course study to evaluate the bioavailability of apocynin in serum, liver and brain in rats. In addition, we chemically synthesized diapocynin from apocynin and used the purified compound to determine whether apocynin is converted to diapocynin in vivo after i.p. injection.
Materials and methods
Animals and sample preparation
Adult male Sprague-Dawley (250−300 g body wt) (Harlan, Indianapolis, IN) were housed in the Small Animal Facilities of the University of Missouri-Columbia. Rats were given free access to water and lab chow and maintained in a colony at 22 ± 2°C with a constant humidity under a 12:12 h light:dark cycle. The animal protocol was approved by the University of Missouri-Columbia Animal Care and Use Committee (Protocol #1741). Experiments were carried out in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals.
To assess the bioavailability of apocynin, rats (3 rats per group) were injected i.p. (5 mg/kg body wt) with apocynin (Sigma-Aldrich, St. Louis, MO) which was dissolved in normal saline. Animals were decapitated at 30 min, 1 h and 2 h after drug administration. Controls were injected similarly with saline. Blood was removed by cardiac puncture with heparin-moistened syringes and plasma was obtained by centrifugation at 2000 × g for 10 min. After collecting blood samples from the heart, animals were transcardially perfused with 200 ml of heparinized saline, and liver and brain were removed. The right lobe of liver and a portion of the frontal cortex of the brain (approx. 1 g) were removed and homogenized in 10 vol of 17 mM NH4OH (pH 8.0).
Extraction of apocynin from plasma and tissues
Aliquots of plasma and tissue homogenates were incubated at 37°C overnight in a system containing 1.0 M ammonium acetate buffer (pH 5.0) and ∼10 unit/ml of β-glucuronidase (Sigma). After incubation, samples were centrifuged at 14,000 rpm for 20 min. In order to remove lipids, CHCl3:CH3OH (3:1 v/v) was added to the supernatant and samples were mixed and centrifuged at 3000 rpm for 20 min. An aliquot of the upper aqueous phase was used for analysis.
Synthesis and characterization of diapocynin
For chemical synthesis of diapocynin, apocynin was recrystallized from water and dried in a desiccator before use. Diapocynin was synthesized by dissolving 2 g of recrystallized apocynin in 200 ml of deionized water with stirring and heating until the solution was boiling gently. After adding 0.15 g of ferrous sulfate heptahydrate and 1.6 g of sodium persulfate, a brown precipitate was formed. After cooling for 5 min, the solution filtered. The precipitate was dissolved in 3 N NH4OH and then re-precipitated by adding 6 N HCl. The precipitate was filtered and washed three times with 100 ml of boiling water. The diapocynin precipitate was further purified by washing three times with 100 ml of boiling methanol.
1H NMR and 1H-decoupled 13C-NMR spectra of diapocynin in DMSO-d6 were obtained using a Bruker ARX 500 MHz NMR. A 30° pulse width was used for the 1H NMR, with a 1 sec pulse delay. A 30° pulse width was used for the 13C-NMR spectra, with a 2 sec pulse delay. The hydrogen and carbon chemical shifts were referenced to the DMSO peaks, which were set to 2.50 ppm for hydrogen and 39.50 ppm for carbon respectively. The Attached Proton Test (APT) was used to distinguish between two groups of signals, namely, methyl/methine and methylene/quaternary. An FTIR spectrum was obtained using a Varian 800 FTIR (Palo Alto, CA) with an attenuated total reflectance (ATR) accessory, from PIKE Technologies (Madison, WI).
HPLC/MS analysis of apocynin and diapocynin
For detection, LC-MS analysis used selected ion monitoring (SIM) of ions with m/z = 163.5−164.5, corresponding to the [M-H]− ion produced by apocynin and m/z = 327.5 − 330.5 for diapocynin. An octadecyl silica (C18) column, 10 cm × 2.1 mm i.d., 100Å pore C18 Ace® analytical column and guard column, from MacMod Analytical, Inc (Chadds Ford, PA) was used for analysis. The LC mobile phase consisted of a gradient elution. Solvent A was 480:20:0.38 water:methanol:ammonium acetate (v/v/w) and solvent B was 20:480:0.38 water:methanol:ammonium acetate (v/v/w). The mobile phase started with 100% A for the first min, followed by a linear increase to 100% B from 1−16 min. This was followed by 100% B from 16−31 min, then a linear decrease to 100% A from 31− 40 min. The injection volume was 20 μl and the eluent flow rate was 0.25 ml/min.
Results
Chemical synthesis of diapocynin
In this study, we used a modified protocol for the synthesis of diapocynin from apocynin based on the synthesis of a similar compound, dehydrodivanillin (Elbs and Lerch, 1916). Structures of these compounds are shown in Figure 1. This is an oxidation-reduction reaction, requiring the in situ generation of sulfate radicals, which remove a single hydrogen from each molecule of apocynin, producing diapocynin. The reaction was conducted in boiling water for 5 min instead of the original 30 min in hot water (van den Worm, et al.; Elbs and Lerch, 1916). Using sodium salt of persulfate instead of the potassium salt produced higher yield. Also, the diapocynin was re-dissolved in 3 M NH4OH, instead of NaOH, because NaOH often is contaminated with chloride and carbonate (Weiss, 2004). Finally, this newer method of synthesis included washing the diapocynin three times each with boiling water and boiling methanol. Analysis of apocynin and diapocynin by HPLC showed retention times of 9.5 and 10.8 min respectively (Fig. 2). Both compounds showed linear relationship between concentrations and peak area (Fig. 2).
Fig. 1.
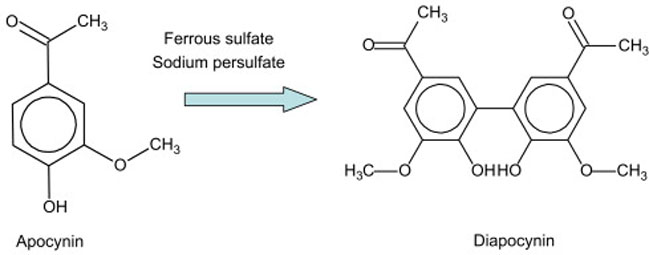
Reaction for conversion of apocynin to diapocynin.
Fig. 2.
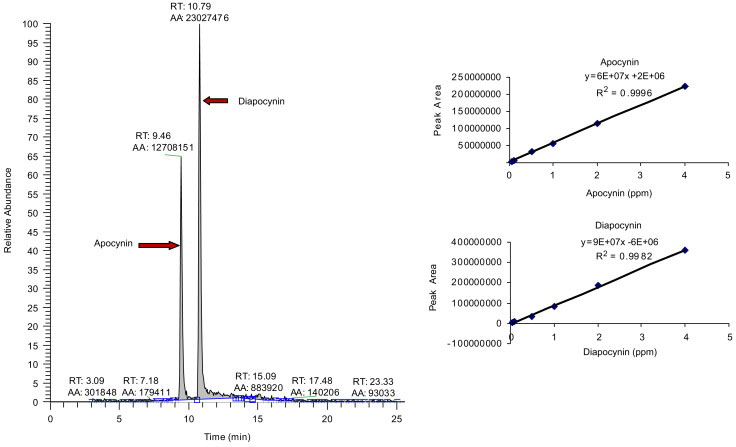
Analysis of apocynin and diapocynin standards by HPLC. See text for details of the analysis.
The 1H NMR spectrum (Dasari et al., 2008) of diapocynin contained peaks with the following chemical shifts, in ppm, and assignments, in parentheses: 2.494 (-CH3), 3.895 (-OCH3), 7.451 and 7.463 (aromatic CH) and 9.465 (-OH). The 13C-NMR spectrum (Fig. 3A) contained peaks with the following chemical shifts, in ppm, and assignments by comparing with the APT spectrum (Fig. 3B), in parentheses: 26.25 (-CH3), 55.98 (-CH3), 109.66 (aromatic C-2, CH), 124.46 (C-5, C), 125.25 (C-6, CH), 127.86 (C1, C), 147.44 (C-4, C), 149.11 (C-3, C) and 196.15 (C$#xFF1D;O). The FTIR spectrum (Dasari et al., 2008) contained the following peaks: λmax cm−1 3318 (OH), 1666 (C$#xFF1D;O), 1588 (aryl C$#xFF1D;C), 1286, 1204, 1127, 1083, 910. The negative ion LC-MS analysis of diapocynin (Fig. 4) indicated that diapocynin has a molecular weight of 330, since a [M-H−] ion was produced with m/z = 329. The UV spectrum indicated that diapocynin had a substituted benzene structure, similar to apocynin (Fig. 4).
Fig. 3.
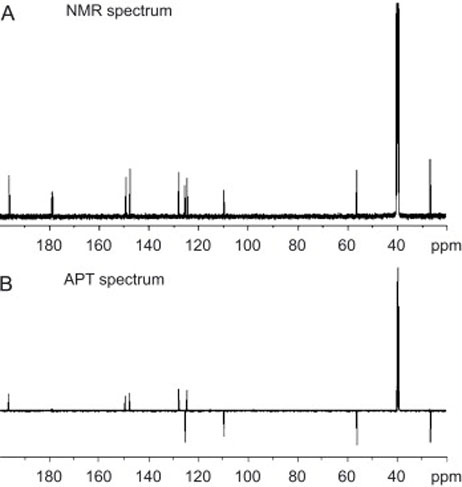
(A) Proton-Decoupled 13C-NMR Spectrum of Diapocynin in DMSO-d6. (B) APT Spectrum of Diapocynin in DMSO-d6. Positive peaks are due to C and CH2, negative peaks are due to CH and CH3.
Fig. 4.
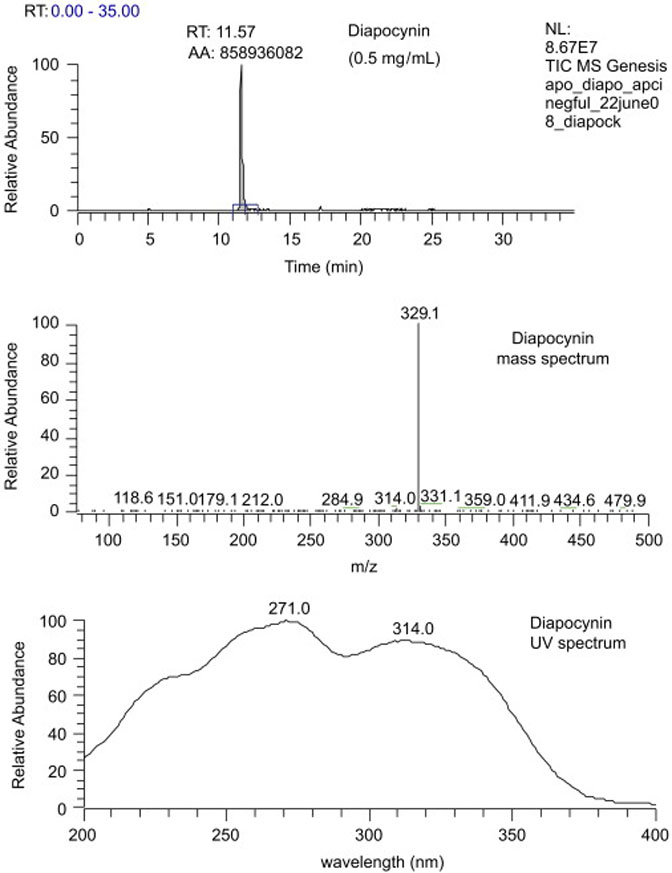
LC-MS and UV analysis of diapocynin.
Bioavailability of apocynin in plasma, liver and brain
An initial study was performed to analyze free apocynin in plasma at different times after i.p. injection (5 mg/kg). As shown in Figure 5, apocynin with a retention time of 9.6 min appeared in plasma as early as 30 min, peaked at 1 h and declined to low levels after 2 h. Spiking apocynin and diapocynin standards to plasma samples showed appearance of the respective standards at the expected retention time (data not shown). Although analysis of plasma samples showed a peak at 10.8 min, corresponding to that of diapocynin standard, this peak was observed in controls and did not change with time after apocynin injection (Fig 5). Since diapocynin is more non-polar than apocynin, analysis of other fractions of the extraction medium also showed no evidence of diapocynin in the samples. Therefore, these data demonstrate that apocynin was not actively converted to diapocynin in vivo.
Fig. 5.
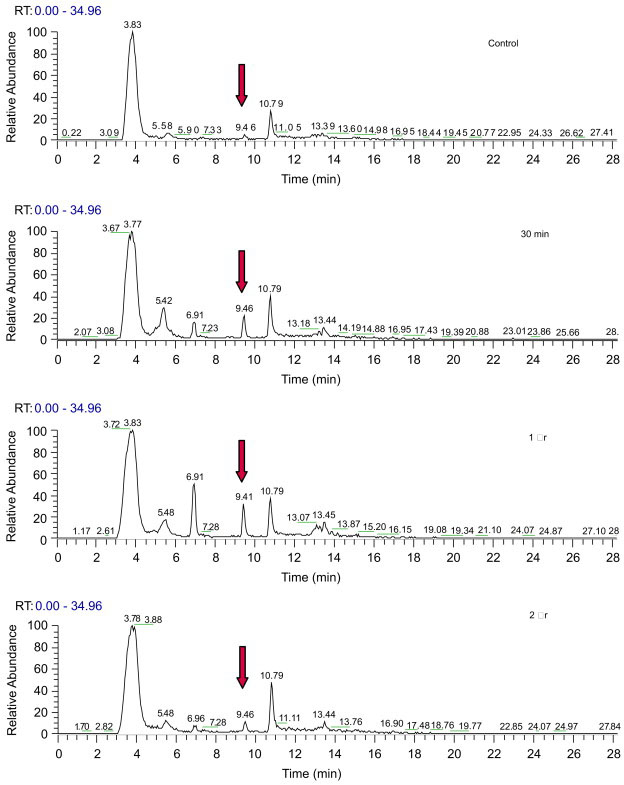
Representative chromatographs showing apocynin in plasma at different times after i.p. injection (5 mg/kg). Arrow points to apocynin peak.
Study was performed to test whether apocynin is converted to glycoconjugate by comparing plasma samples with or without β-glucuronidase treatment. As shown in Figure 6, levels of free apocynin in plasma at 30 and 60 min after injection (5 mg/kg, i.p., n=3) were 20−30 ng/ml as compared to 60−70 ng/ml after β-glucuronidase treatment.
Fig. 6.
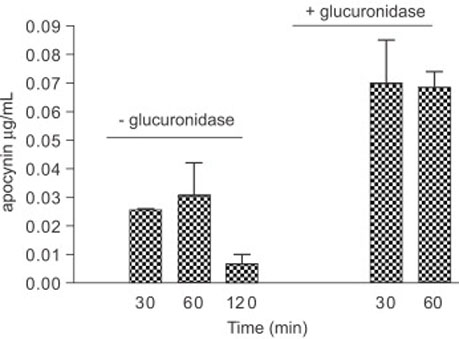
Levels of apocynin in plasma with or without β-glucuronidase treatment.
Results in Figure 7 show the HPLC analysis of apocynin in β-glucuronidase-treated plasma, brain and liver samples in controls and after injection of apocynin (30 min). Data for 2 h samples were not shown because a large part of the apocynin had already diminished at this time (data not shown). Levels of apocynin in the liver were at least 3−5 times higher than those in the brain.
Fig. 7.
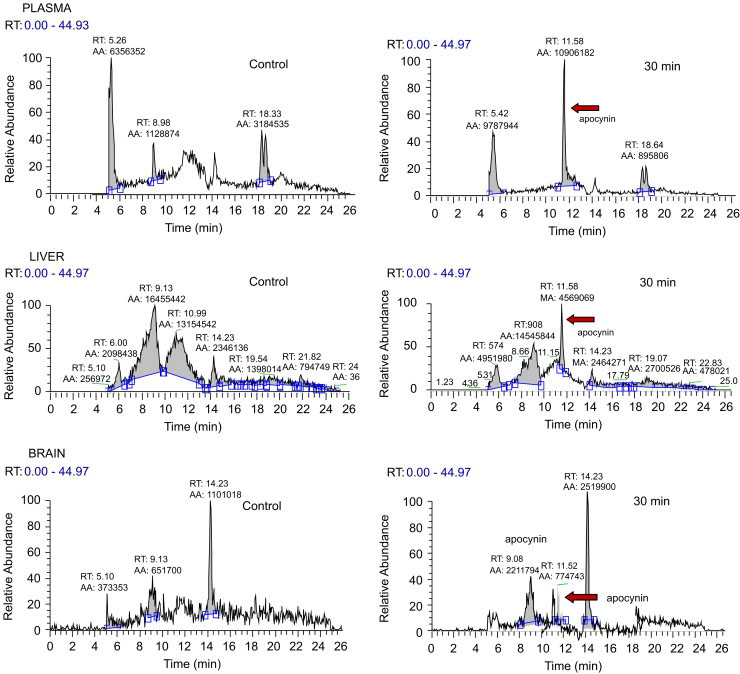
Apocynin in plasma, liver and brain comparing with control and 30 min after i.p. injection (5 mg/kg). Samples were treated with β-glucoronidase prior to analysis by HPLC.
Discussion
Due to their potentially beneficial impact on human health, plant extracts have come into the focus of medicinal interest. However, data on the bioavailability of these plant extracts are largely lacking (Loew and Kaszkin, 2002; Simonyi et al., 2005; Williamson and Manach, 2005). In our previous work, we investigated the bioavailability of two plant polyphenolic compounds, resveratrol and curcumin, extracted from grapes and turmeric, respectively (Wang et al., 2002; Wang et al., 2005). We observed that both polyphenolic compounds form glycoconjugates which were distributed to other organs including the brain. In the present study, we show that apocynin was also rapidly converted to glycoconjugates. Similar to resveratrol and curcumin, apocynin was readily distributed in plasma, liver and brain and reached peak levels around 1 h after injection. The appearance of apocynin in brain is in agreement that this compound can cross the blood–brain barrier and incorporated into brain tissue. The apocynin in brain tissue is unlikely to be due to blood contamination because analysis was carried out after perfusing animal with saline.
In studies in vitro, apocynin was shown to undergo oxidation and converted to diapocynin (Ximenes et al., 2007). In our analysis with plasma samples, we observed a peak corresponding to the retention time for diapocynin in the extract. However, the same peak could be found upon analysis of blank and control samples (without injection of apocynin). Therefore, it is concluded that apocynin was not significantly converted to diapocynin in vivo under these circumstances.
Apocynin is known to inhibit NAPDH oxidase activity by interfering with the assembly of the cytosolic NADPH oxidase components with the membrane components (Stolk et al., 1994). NADPH oxidase has emerged as a major source of oxidative stress in the brain, particularly in neurodegenerative diseases, such as ischemic stroke, Alzheimer's and Parkinson's diseases, HIV dementia, multiple sclerosis, etc (Bedard and Krause, 2007). Recent studies have shown that apocynin placed in the drinking water or in diet are effective at reducing superoxide and oxidative stress in vivo (Cotter and Cameron, 2003; Elmarakby et al., 2005; Hougee et al., 2006; Paliege et al., 2006). No adverse effects were observed with apocynin doses ranging from 2.5 mg/kg/day to 100mg/kg/day and duration between 4 days and 6 weeks (Cotter and Cameron, 2003; Elmarakby et al., 2005; Hougee et al., 2006; Paliege et al., 2006). The therapeutic potential of systemic single administration of apocynin has been demonstrated in a dose range of 2.5−12 mg/kg in different animal models (Sonta et al., 2004; Kimura et al., 2005). In support for the bioavailability of apocynin to the brain, results of our previous study also provided strong evidence for apocynin to offer neuroprotective effects against ischemia-induced neuronal damage (Wang et al., 2006). Taken together, there is increasing support to consider using this phyto-compound as a therapeutic agent for treatment of inflammatory diseases including those in the brain.
Acknowledgement
This work was supported in part by P01 AG18357 from NIH. This work should not be taken as having an impact on FDA policy or regulations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barbieri SS, Cavalca V, Eligini S, Brambilla M, Caiani A, Tremoli E, Colli S. Apocynin prevents cyclooxygenase 2 expression in human monocytes through NADPH oxidase and glutathione redox-dependent mechanisms. Free Radic. Biol. Med. 2004;37:156–165. doi: 10.1016/j.freeradbiomed.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J. Cereb. Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Cotter MA, Cameron NE. Effect of the NAD(P)H oxidase inhibitor, apocynin, on peripheral nerve perfusion and function in diabetic rats. Life Sci. 2003;73:1813–1824. doi: 10.1016/s0024-3205(03)00508-3. [DOI] [PubMed] [Google Scholar]
- Dasari M, Richards KM, Alt MA, Crawford CFP, Schleiden A, Ingram J, Aziz A, Hamidou AAA, Williams A, Chernovitz PA, Luo R, Sun GY, Luchtefeld R, Smith RE. Diapocynin Synthesis. J. Chem. Ed. 2008 in press. [Google Scholar]
- Elbs K, Lerch H. Über dehydrodivanillin. J. Prakt. Chem. 1916;93:1–9. [Google Scholar]
- Elmarakby AA, Loomis ED, Pollock JS, Pollock DM. NADPH oxidase inhibition attenuates oxidative stress but not hypertension produced by chronic ET-1. Hypertension. 2005;45:283–287. doi: 10.1161/01.HYP.0000153051.56460.6a. [DOI] [PubMed] [Google Scholar]
- Hougee S, Hartog A, Sanders A, Graus YM, Hoijer MA, Garssen J, van den Berg WB, van Beuningen HM, Smit HF. Oral administration of the NADPH-oxidase inhibitor apocynin partially restores diminished cartilage proteoglycan synthesis and reduces inflammation in mice. Eur. J. Pharmacol. 2006;531:264–269. doi: 10.1016/j.ejphar.2005.11.061. [DOI] [PubMed] [Google Scholar]
- Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function. Antioxid. Redox Signal. 2006;8:1583–1596. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- Kimura H, Liu S, Yamada S, Uchida K, Matsumoto K, Mukaida M, Yoshida K. Rapid increase in serum lipid peroxide 4-hydroxynonenal (HNE) through monocyte NADPH oxidase in early endo-toxemia. Free Radic. Res. 2005;39:845–851. doi: 10.1080/10715760500161546. [DOI] [PubMed] [Google Scholar]
- Loew D, Kaszkin M. Approaching the problem of bioequivalence of herbal medicinal products. Phytother. Res. 2002;16:705–711. doi: 10.1002/ptr.1248. [DOI] [PubMed] [Google Scholar]
- Paliege A, Pasumarthy A, Mizel D, Yang T, Schnermann J, Bachmann S. Effect of apocynin treatment on renal expression of COX-2, NOS1, and renin in Wistar-Kyoto and spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R694–R700. doi: 10.1152/ajpregu.00219.2005. [DOI] [PubMed] [Google Scholar]
- Picrorhiza kurroa Monograph. Altern. Med. Rev. 2001;6:319–321. [PubMed] [Google Scholar]
- Simonyi A, Wang Q, Miller RL, Yusof M, Shelat PB, Sun AY, Sun GY. Polyphenols in cerebral ischemia: novel targets for neuroprotection. Mol. Neurobiol. 2005;31:135–147. doi: 10.1385/MN:31:1-3:135. [DOI] [PubMed] [Google Scholar]
- Sonta T, Inoguchi T, Tsubouchi H, Sekiguchi N, Kobayashi K, Matsumoto S, Utsumi H, Nawata H. Evidence for contribution of vascular NAD(P)H oxidase to increased oxidative stress in animal models of diabetes and obesity. Free Radic. Biol. Med. 2004;37:115–123. doi: 10.1016/j.freeradbiomed.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am. J. Respir. Cell Mol. Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- Sun AY, Chen YM. Oxidative stress and neurodegenerative disorders. J. Biomed. Sci. 1998;5:401–414. doi: 10.1007/BF02255928. [DOI] [PubMed] [Google Scholar]
- van den Worm E, van den Berg AJJ, Kemeling GM, Beukelman CJ, Halkes SBA, Labadie RP, van Dijk H. Isolation, characterization and activity of diapocynin, an apocynin metabolite. Chapter 5. http://igiturarchive.library.uu.nl/dissertations/1957866/c5.pdf.
- Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sun GY, Sun AY. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002;958:439–447. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- Wang Q, Sun AY, Simonyi A, Jensen MD, Shelat PB, Rottinghaus GE, MacDonald RS, Miller DK, Lubahn DE, Weisman GA, Sun GY. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J. Neurosci. Res. 2005;82:138–148. doi: 10.1002/jnr.20610. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res. 2006;1090:182–189. doi: 10.1016/j.brainres.2006.03.060. [DOI] [PubMed] [Google Scholar]
- Weiss J. Handbook of Ion Chromatography. Weinheim; Wiley-VCH: 2004. p. 196. [Google Scholar]
- Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005;81:243S–255S. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- Ximenes VF, Kanegae MP, Rissato SR, Galhiane MS. The oxidation of apocynin catalyzed by myeloperoxidase: proposal for NADPH oxidase inhibition. Arch. Biochem. Biophys. 2007;457:134–141. doi: 10.1016/j.abb.2006.11.010. [DOI] [PubMed] [Google Scholar]


