Abstract
Intravenous (iv) cocaine mimics salient somato-sensory stimuli in their ability to induce rapid physiological effects, which appear to involve its action on peripherally located neural elements and fast neural transmission via somato-sensory pathways. To further clarify this mechanism, single-unit recording with fine glass electrodes was used in awake rats to examine responses of ventral tegmental area (VTA) neurons, both presumed dopamine (DA) and non-DA, to iv cocaine and tail-press, a typical somato-sensory stimulus. To exclude the contribution of DA mechanisms to the observed neuronal responses to sensory stimuli and cocaine, recordings were conducted during full DA receptor blockade (SCH23390+eticloptide).
Iv cocaine (0.25 mg/kg delivered over 10 s) induces significant excitations of ~63% of long-spike (presumed DA) and ~70% of short-spike (presumed non-DA) VTA neurons. In both subgroups, neuronal excitations occurred with short latencies (4–8 s), peaked at 10–20 s (30–40% increase over baseline) and disappeared at 30–40 s after the injection onset. Most long- (67%) and short-spike (89%) VTA neurons also showed phasic responses to tail-press (5-s). All responsive long-spike cells were excited by tail-press; excitations were very rapid (peak at 1 s) and strong (100% rate increase over baseline) but brief (2–3 s). In contrast, both excitations (60%) and inhibitions (29%) were seen in short-spike cells. These responses were also rapid and transient, but excitations of short-spike units were more prolonged and sustained (10–15 s) than in long-spike cells.
These data suggest that in awake animals iv cocaine, like somato-sensory stimuli, rapidly and transiently excites VTA neurons of different subtypes. Therefore, along with direct action on specific brain substrates, central effects of cocaine may occur via indirect mechanism, involving peripheral neural elements, visceral sensory nerves and rapid neural transmission. Via this mechanism, cocaine, like somato-sensory stimuli, can rapidly activate DA neurons and induce phasic DA release, creating the conditions for DA accumulation by a later occurring and prolonged direct inhibiting action on DA uptake. By providing a rapid neural signal and triggering transient neural activation, such a peripherally driven action might play a crucial role in the sensory effects of COC, thus contributing to learning and development of drug-taking behavior.
Keywords: somato-sensory stimuli, glutamate, visceral sensory nerves, tail-press, dopamine release and uptake, drug-taking behavior, reinforcement
1. Introduction
Monoamine transporters are usually viewed as the most important brain substrates mediating cocaine’s central effects (Iversen, 2006; Reith, 1988; Ritz et al., 1987; Rothman and Baumann, 2003; Wise and Bozarth, 1987). Often overlooked, however, is the drug’s interaction with Na+ and K+ channels (Catterall and Mackie, 1996; Premkumar, 2005; Reith, 1988; Wu et al., 2006) located both centrally, on brain cells, and peripherally, on the terminals of sensory nerves (Lee et al., 2005), which densely innervate blood vessels, peritoneal and nasal cavities (Goder et al., 1993; Michaelis et al., 1994). Given that these peripheral sites are also sites of human cocaine self-administration, the drug’s interaction with peripheral neural substrates, involving neural transmission via somato-sensory pathways, might be important in mediating the rapid psycho-emotional, physiological and behavioral effects of cocaine that occur within seconds after its intravenous (iv) administration, and that are generally resistant to dopamine (DA) receptor blockade.
Our previous thermorecording work has shown that peripheral neural substrates play an important role in the triggering of the central effects of iv cocaine (Brown and Kiyatkin, 2006). Cocaine, at a typical reinforcing dose (1 mg/kg), induces a central temperature response similar to other salient somato-sensory stimuli such as a tail-pinch and social interaction. In each case, temperature rapidly increases in the brain, suggesting metabolic neural activation, but transiently decreases in the skin, suggesting acute vasoconstriction. Importantly, these temperature effects are mimicked by procaine, a structurally similar local anesthetic drug with negligible effects on monoamine transporters (Ritz et al., 1987), and cocaine methiodide, a cocaine derivative that cannot cross the blood-brain barrier (Shriver and Long, 1971). Moreover, this peripherally acting drug fully mimics cocaine in its ability to rapidly increase arterial blood pressure (Dickerson et al., 1999)—a classic CNS-integrated physiological effect (Knuepfer and Branch, 1992). Therefore, it appears that in addition to the direct action in the brain, cocaine can affect central neurons by acting on peripheral, presumably non-monoamine, neural substrates and involving ascending neural pathways that are activated by other somato-sensory stimuli.
In support of this indirect mechanism, most striatal neurons showed rapid, transient excitations (5–25 s) following iv cocaine administration in awake rats (Kiyatkin and Brown, 2007). Neuronal excitations began during an iv infusion— too rapid to reflect a direct interaction with central substrates. While cocaine could rapidly reach brain microvessels (Fowler et al., 1998), more time is necessary to cross the blood-brain barrier and passively diffuse within brain tissue (Spector, 2000). Despite longer latencies, these excitations were similar to those induced by tail-touch and tail-pinch, both typical arousing stimuli, which affect central neurons via ascending somato-sensory pathways. Importantly, cocaine-induced neuronal excitations were mimicked by iv cocaine methiodide, which was even more potent than regular cocaine. These rapid neuronal responses to sensory and drug challenges do not involve DA mechanisms, since single-cell recordings were done during a full DA receptor blockade. However, these effects were strongly attenuated during urethane anesthesia, pointing to their sensory nature and the importance of an awake animal preparation for studying the central effects of sensory stimuli and cocaine.
The primary goal of this study was to further substantiate this mechanism in the ventral tegmental area of the midbrain (VTA), which houses mesocortical and mesolimbic DA neurons and plays a crucial role in behavioral regulation, mediation of psychostimulant effects of cocaine, and regulation of drug-taking behavior (Ritz et al., 1987; Wise and Bozarth, 1987). In contrast to our previous study in which we tested the effects of several somato-sensory stimuli, cocaine, cocaine methiodide and saline both in awake and anesthetized conditions, this study was limited by examination of the effects of iv cocaine and tail-press in awake rat preparation. In contrast to the striatum, which is comprised of primarily GABA-containing spiny cells (Parent and Hazrati, 1995), VTA neuronal population is highly heterogeneous, with cells containing many different neurotransmitters (DA, GABA, glutamate or GLU, peptides) (Bjorklund and Lindvall, 1984; Swanson, 1982). Although VTA cells were extensively studied in vitro and anesthetized preparations (Chiodo, 1988; Grace and Bunney, 1984), data in awake conditions are limited and point at the high variability in their electrophysiological properties and important differences in their activity and responsiveness to sensory stimuli (Dahan et al., 2007; Freeman et al., 1985; Horvitz et al., 1997; Kiyatkin, 1988; Kiyatkin and Rebec, 1998, 2001; Schultz, 1986). By recording impulse activity of single VTA neurons following iv cocaine administration and tail-press stimulation, we tried to answer two primary questions. First, do VTA neurons, both presumed DA and non-DA, show rapid changes in impulse activity following iv cocaine? Second, how does the impulse activity of VTA neurons change following somato-sensory stimulation compared to that induced by cocaine? To further aid in determining possible mechanisms underlying rapid responses of VTA neurons, they were tested with iontophoretic glutamate (GLU) and GABA to examine the pattern of their activity following direct activation of excitatory and inhibitory inputs.
Although awake, freely moving preparation is the best to examine the natural activity and responsiveness of central neurons, single-unit recordings with high-impedance, fine-tip electrodes following exposure to such activating stimuli as tail-press and iv cocaine are virtually impossible under this condition due to robust locomotor activation and muscular activity. The development of multi-wire bundle technology has made long-term neuronal recordings in freely moving rats possible (Nicolelis et al., 1993), but this technique provides a much weaker signal-to-noise ratio, making proper characterization of VTA cell subtypes and accurate assessment of their responses difficult. Therefore, similar to our previous study, recordings were performed in animals administered with a mixture of D1- and D2-selective antagonists (SCH233900 and eticlopride), providing an effective blockade of DA transmission. DA receptor blockade greatly attenuates cocaine-induced motor activation, thus allowing artifact-free neuronal recording, but it keeps neuronal responses to sensory stimuli relatively intact (Kiyatkin and Rebec, 1999; Kiyatkin and Brown, 2007). The use of DA antagonists also excludes any possible contribution of DA mechanisms to the observed neuronal responses to sensory stimuli and cocaine. This could be especially important for a subgroup of DA cells with DA autoreceptors, revealing their responses to cocaine and tail-press when possible influences of changes in DA levels are eliminated. Finally, the use of fine-tip glass electrodes also allows for iontophoretic testing of recorded cells—an important additional tool to study their properties and changes in activity that are mediated via known afferent inputs.
2. Results
2.1. VTA neuronal subgroups and their activity in awake rats during DA receptor blockade
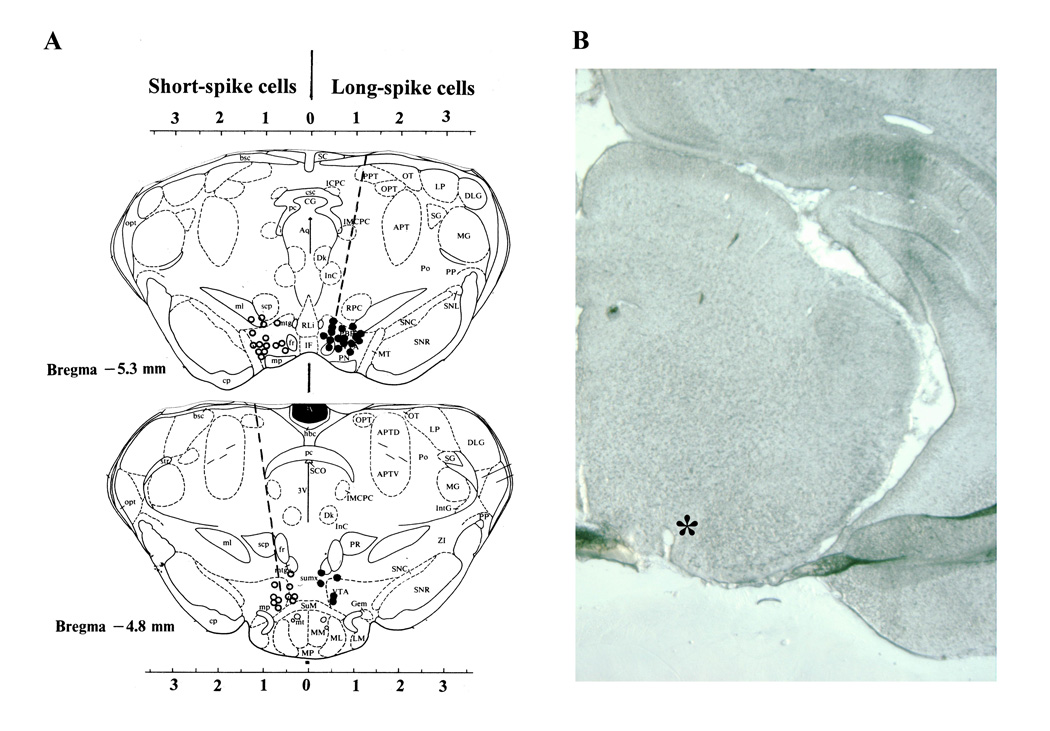
A total of 52 neurons recorded from 8 rats during 12 daily sessions were included in our data sample. Based on histological examination of the electrode tracks, Pontamine Sky Blue depositions and the recording depth, all these cells were located in the VTA (Fig. 1). Of these cells, 38 were tested with cocaine, 43 were tested with tail-press, and 24 with either GLU or GABA. 29 units were tested with both cocaine and tail-press.
Fig. 1.
A. Reconstructed locations of VTA neurons (right side: closed circles - long-spike cells; left side: open circles - short spike cells) recorded in this study. Each point is shown on coronal section of the midbrain tegmentum (modified based on Paxinos and Watson, 1986). Broken lines show the direction of electrode movement. Note that most long-spike neurons are densely located in the medial sector of the VTA, while more a wider distribution is typical of short-spike cells. Several locations are in the neighboring area between the pre- or para-rubral area and the VTA. B shows example of unstained coronal brain slice with an empty spot (asterisk) at the point where Pontamine sky blue was ejected at the final recording site.
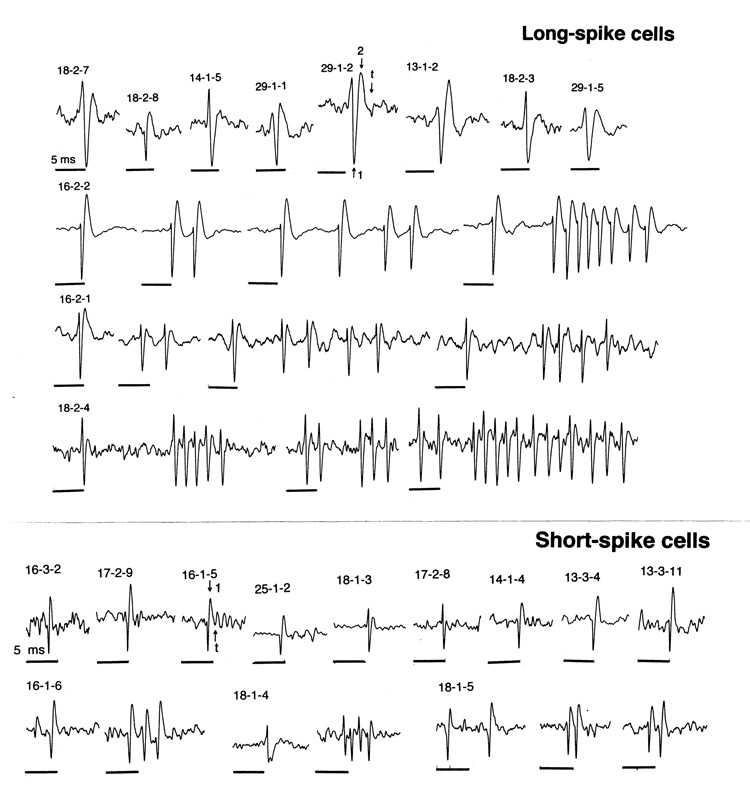
Based on analysis of spike shape and duration, two distinct neuronal subgroups were distinguished in the VTA: neurons with long-duration, triphasic spikes (LS) and neurons with short-duration, primarily biphasic spikes (SS). Total spike duration in LS cells (range: 2.54–5.42 ms; mean: 4.16±0.09 ms) was longer than that in SS cells (range: 0.73–1.79 ms; mean: 1.40±0.05 ms; p<0.001); these subgroups also differed by duration of the first spike phase (0.80±0.08 vs. 0.46±0.08 ms; p<0.01). Although some SS cells had spikes with a less defined third phase (see cell 18-1-3 in Fig. 2), both the first phase and a total duration of their spikes were always shorter than in the LS cells. Dense bursting was another important feature of LS cells. While some cells had relatively long bursts with pronounced spike amplitude decrements (up to 20 imp/burst), other LS cells had very dense bursts (or possibly complex spikes) with very short inter-spike intervals (up to 2–4 ms). These inter-spike intervals within the bursts sometimes could be shorter than the duration of a single spike (# 16-2-2, 16-2-1, 18-2-4). While SS cells usually have a more regular activity pattern, bursting and complex spikes was also seen in several deeply located VTA cells (# 16-1-6, 18-1-5).
Fig. 2.
Individual spikes and spike trains of long- and short-spike VTA neurons. Each record or a group of records shows the cell number. Time scale (black line=5 ms) is identical for all records. Arrows in two spikes shows durations of the first, first+second and a whole spike for LS (29-1-2) and SS (16-1-5) VTA cells.
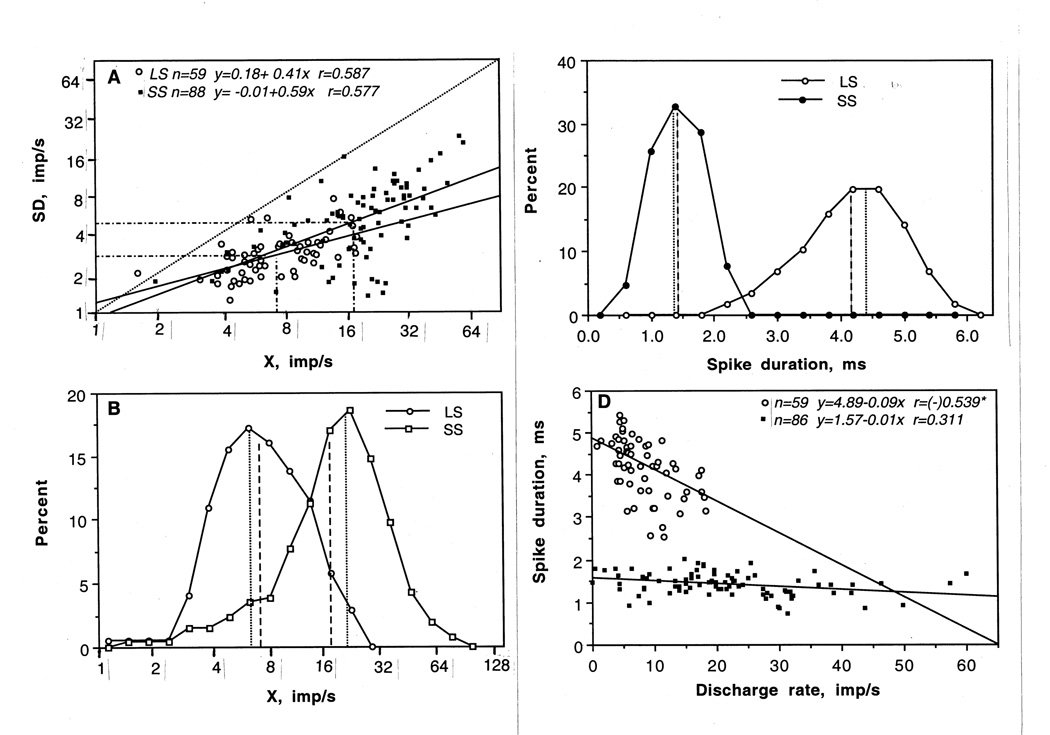
Both LS and SS VTA neurons have highly variable discharge rates (Table 1, Fig. 3). While mean basal rate of LS cells (8.35 imp/s) was significantly lower than in SS cells (21.45 imp/s), this parameter varied in individual cells from 0.90 to 18.30 imp/s. Even higher variability of basal rates (1.95-60 imp/s) was found in the SS cell subgroup. As shown in Fig. 3A, both groups had similarly strong and identical relationships between X and SD, suggesting that all units with higher discharge rate also had higher absolute variability of discharge. In both cases, regression lines descended to zero and they were virtually identical. As shown in Fig. 3B, X values of individual neurons in both VTA subgroups were distributed according to ln-normal law with close values of means and distribution modes. By this parameter, both groups were relatively homogeneous. While in the LS subgroup, distribution was slightly skewed to the right, suggesting a relative prevalence of cells with higher discharge rates, skewing to the left (low rates) was typical of the SS cell subgroup, indicating a relative prevalence of slow-firing cells in this fast-firing neuronal population. As shown in Table 1, mean values of X and SD in SS VTA neurons were significantly higher than in LS cells (p<0.001), while CV was slightly lower (p<0.05), indicating a more regular discharge rate. However, maximal between-group differences were seen in spike duration (Fig. 3C). By this parameter, VTA neurons represented two distinct subgroups with virtually no intersections of distributions. Finally, between-group differences are clear in Fig. 3D, where the relation between discharge rate and spike duration are shown separately for LS and SS VTA cells. In contrast to the wide range of discharge rate in SS cells, LS cells had a dense location of rates, clearly distinguishable from SS cells by large spike duration. While spike duration was independent of discharge rate in SS cells, a weak but significant correlation between these parameters was seen in LS neurons (r=−0.55). Cells with higher rates had shorter spike durations than slower firing cells.
Table 1.
Parameters of single spike and spontaneous impulse activity in long-spike and short-spike VTA neurons in awake rats during DA receptor blockade
| Parameter | Long-spike cells | Short-spike cells |
|---|---|---|
| n cells (values) | 20(59) | 32 (88) |
| Single spike | ||
| -first phase, ms | 0.80±0.08 | 0.46±0.09** |
| -range | 0.46–1.29 | 0.32–0.68 |
| -total, ms | 4.16±0.09 | 1.40±0.05*** |
| -range | 2.54–5.42 | 0.73–1.79 |
| X, imp/s | ||
| -mean, imp/s | 8.35±0.56 | 21.45±1.30 |
| -range, imp/s | 0.90–18.30 | 0.00–60.00 |
| -ln(X) | 2.01±0.07 | 2.87±0.08*** |
| -ln(mean) (mode) | 7.46 (6.50) | 17.64 (20.50) |
| SD, imp/s | ||
| -mean | 2.92±0.15 | 6.07±0.45 |
| -range | 1.23–7.45 | 0.00–23.22 |
| -ln(SD) | 1.00±0.05 | 1.62±0.07*** |
| -ln(mean) mode | 2.72 | 5.05 |
| CV, % | ||
| -mean | 42.39±3.30 | 34.55±3.06 |
| -range | 16.02–165.56 | 5.88–231.42 |
| -ln(CV) | 3.63±0.06 | 3.33±0.07* |
| -ln(mean) mode | 37.71 | 27.94 |
Asterisks show significant differences between groups
p<0.05
p<0.01
p<0.001
Fig. 3.
A. Relationship between mean discharge rate (X) and standard deviation (SD) in long-spike (LS) and short-spike (SS) VTA neurons. While mean values of both parameters significantly differed in each group (hatched lines), cells were intermixed by these parameters and the regression lines were similar with respect to the equality line (hatched). In each group, cells with higher discharge rate had higher absolute variability of discharges. B. Distributions of discharge rates in LS and SS VTA neurons, shown in ln-scale. Both distributions are well approximated by Gaussian (normal) curves, suggesting the ln-normal distribution. While differing in mean (large hatched) and modal values (small hatched), distributions were intersected, suggesting that many cells in both subgroups have similar discharge rates. C. Distributions showing spike durations in LS and SS VTA cells. Distributions virtually do not intersect, suggesting robust between-group differences by this parameter. Differences were also evident by duration of the first phase, but they were much weaker. D. Relationships between mean discharge rate and spike duration for LS and SS VTA neurons. While some SS cells had similarly low and moderate discharge rate as LS cells (2–14 imp/s), their spike duration was consistently low. Graph shows two regression lines with regression equations and coefficients of correlation. LS cells with higher discharge rate have relatively shorter spike duration (r=−0.54, p<0.05). In SS cells, there parameters were not correlated.
2.2. Phasic responses of LS and SS VTA neurons to tail-press
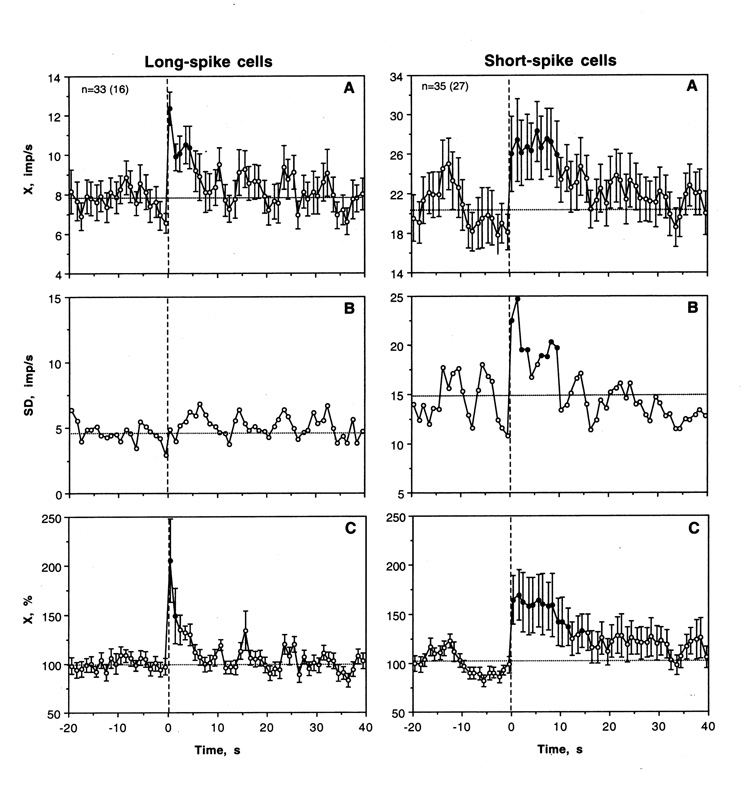
As shown in Table 2 and Fig. 4, more than half of LS VTA neurons (22/33 or 66.7%) showed significant increases in discharge rate following tail-press. In other cases, activity did not change significantly and inhibitions were never seen. Tail-press also had a significant and strong excitatory effect on LS VTA neurons when all cells and tests were superimposed independent of individual changes (F32,362=4.71, p<0.001). Discharge rate rapidly peaked at the first second of stimulation and slowly decreased for the next 5 s (Fig. 5A). This rapid, transient effect was especially evident when analyzed as a relative change in rate (Fig. 5C). In this case, activity rate doubled during the first second and returned to baseline during the next 5 s. In addition to phasic increase, both X and SD showed evident fluctuations after tail-press (Fig. 5A and B).
Table 2.
Phasic responses of VTA neurons to somato-sensory stimuli and cocaine
| Parameters | Long-spike | Short-spike | |
|---|---|---|---|
| Tail-press | |||
| -excitation | 22 (66.7±8.2%) | 21 (60.0±8.3%) | |
| -inhibition | 0 | 10 (28.6±7.6%)* | |
| -no response | 11 (33.3±8.2%) | 4 (11.4.5±4%)* | |
| -total | 33 (100.00%) | 35 (100.00%) | |
| Cocaine | |||
| -excitation | 10 (62.5±12.1%) | 19 (70.4±8.8%) | |
| -inhibition | 0 | 0 | |
| -no response | 6 (37.5±12.1%) | 8 (29.6±8.8%) | |
| -total | 16 (100.00%) | 27 (100.00%) | |
For both tail-press and cocaine, a response’s criterion was is one or more 1-s discharge rate values either higher (excitation) or lower (inhibition) than each of 20 1-s values in pre-stimulus baseline (see Methods for details). Asterisks show significant differences between groups
p<0.05.
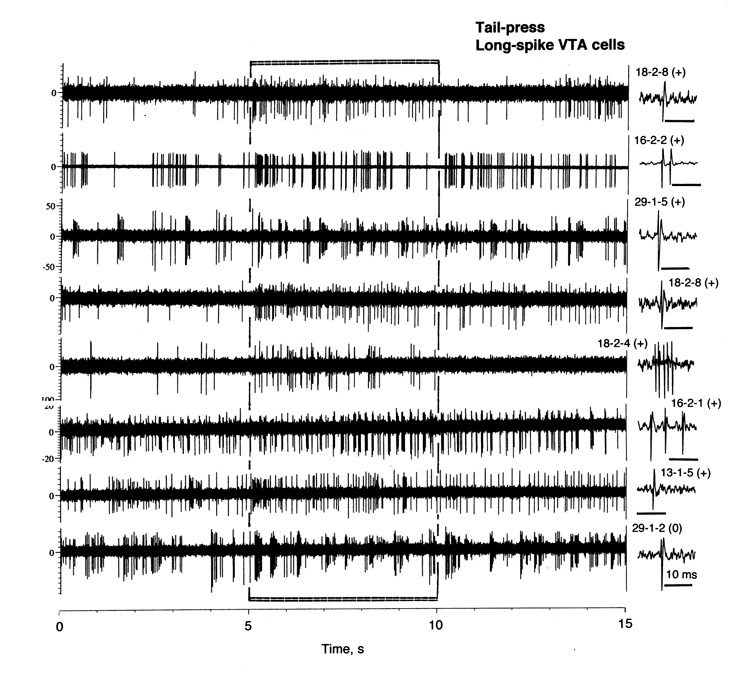
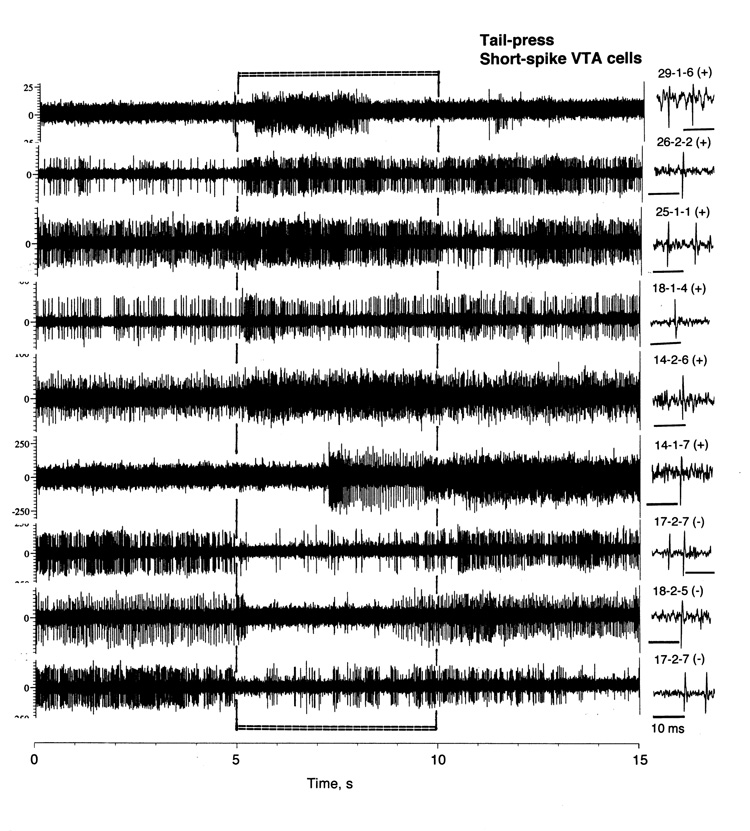
Fig. 4.
Original records of changes in impulse activity of long-spike VTA neurons during tail-press (5–10 s). Each trace was obtained from a different cell, whose number and single spike are shown in a right side. Note that 7 top traces show significant increases (+) and the last trace no change in rate (0) but clear bursting.
Fig. 5.
Mean changes in impulse activity of LS and SS VTA neurons induced by trail-press (onset is shown by a hatched line at 0 s). A, B and C show mean rates (imp/s), standard deviation (imp/s) and percent change (%, baseline=100%) calculated for 20 s before and 40 s after the start of stimulation. Filled symbols show values significantly different from baseline. Statistical significance for SD was assessed based on equation, (t= SD1-SD2/√SD1/2n1+SD2/2n2) with an averaged baseline value calculated for 20 s preceding stimulus presentation.
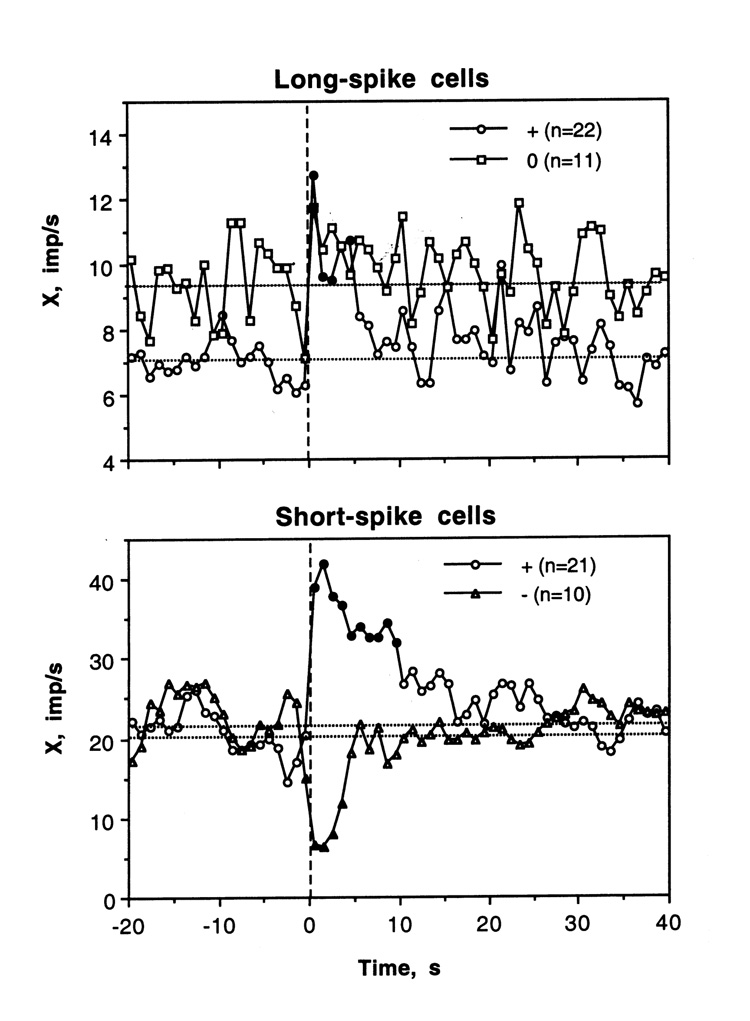
Figure 6 shows changes in discharge rate during tail-press analyzed separately for LS cells that showed phasic excitations (n=22) and no significant change in rate during individual analysis (n=11). Cells that showed excitations had a lower basal rate (7.01±0.68 imp/s) and relatively shorter spike duration (3.78±0.20 ms) than non-responsive cells (respectively, 9.31±1.14 imp/s; p<0.1; 4.75±0.16 ms; p<0.01), and their activity increased rapidly and strongly at the start of stimulation but also quickly returned to baseline. Interestingly, when analyzed as a group, “non-responsive” LS cells also showed an increase in discharge rate, which was significant during the first second of tail-press. Some of these “non-responsive” cells showed dense bursting without changes in mean rate (see #29-1-2 in Fig. 4).
Fig. 6.
Mean changes in discharge rate calculated separately for cells with different responses to tail-press (LS cells: excitations and no changes; SS cells: excitations and inhibitions). Filled symbols show values significantly different from baseline.
Tail-press also significantly affected most SS VTA cells (31/35 or 88.6%), with both phasic excitations (21/35 or 60.0%) and inhibitions (10/35 or 28.6%). Despite opposite effects in individual cells (see examples in Fig. 7), tail-pinch had a significant excitatory effect on SS cells when analyzed as a group (F34,734=1.80, p<0.05). In addition to a significant increase in mean rate (1–8 s), SD also showed robust increase, which was evident for ~10 s after the start of tail-press (Fig. 5A and B). The excitatory effect of tail-press was also evident as a relative change (Fig. 5C). Although discharge rate also peaked immediately after the stimulus onset, this excitatory effect was more prolonged and less phasic than in LS cells.
Fig. 7.
Original records of changes in impulse activity of short-spike VTA neurons during tail-press (5–10 s). Each trace was obtained from a different cell, whose number and single spike are shown in right side.
Response heterogeneity of SS VTA neurons is evident in Fig. 6, which shows mean changes in discharge rate in cells that either excited or inhibited following tail-press. In the first subgroup, discharge rate rapidly peaked after the start of tail-press and slowly decreased toward baseline during the next 10–15 s. Inhibition was also rapid but shorter (4–5 s). Interestingly, in both subgroups of SS neurons, discharge rate also changed immediately before tail-press. In cells that will be either activated or inhibited, discharge rate either slightly increased or decreased immediately before stimulus presentation. These changes obviously reflect the procedural factors associated with sensory stimulation in awake animals. Interestingly, SS cells that showed opposite responses to tail-pinch did not differ in either basal discharge rate (see Fig. 6) or spike duration (1.36±0.06 and 1.41±0.08 ms for excitations and inhibitions, respectively).
2.3. Phasic responses of LS and SS VTA neurons to iv cocaine
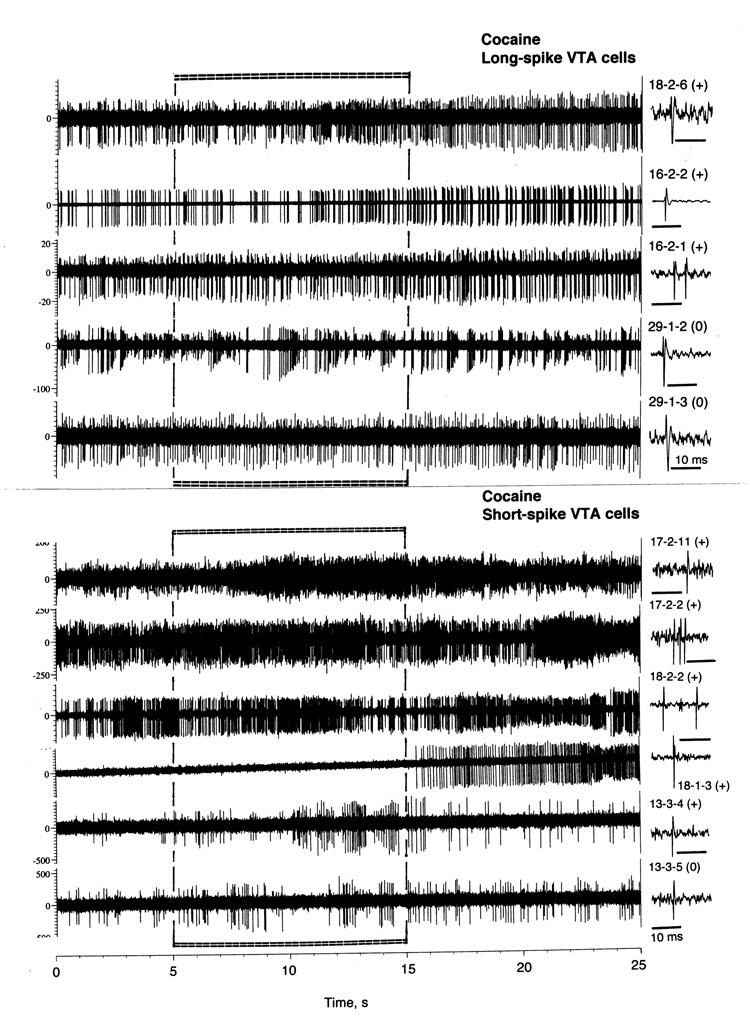
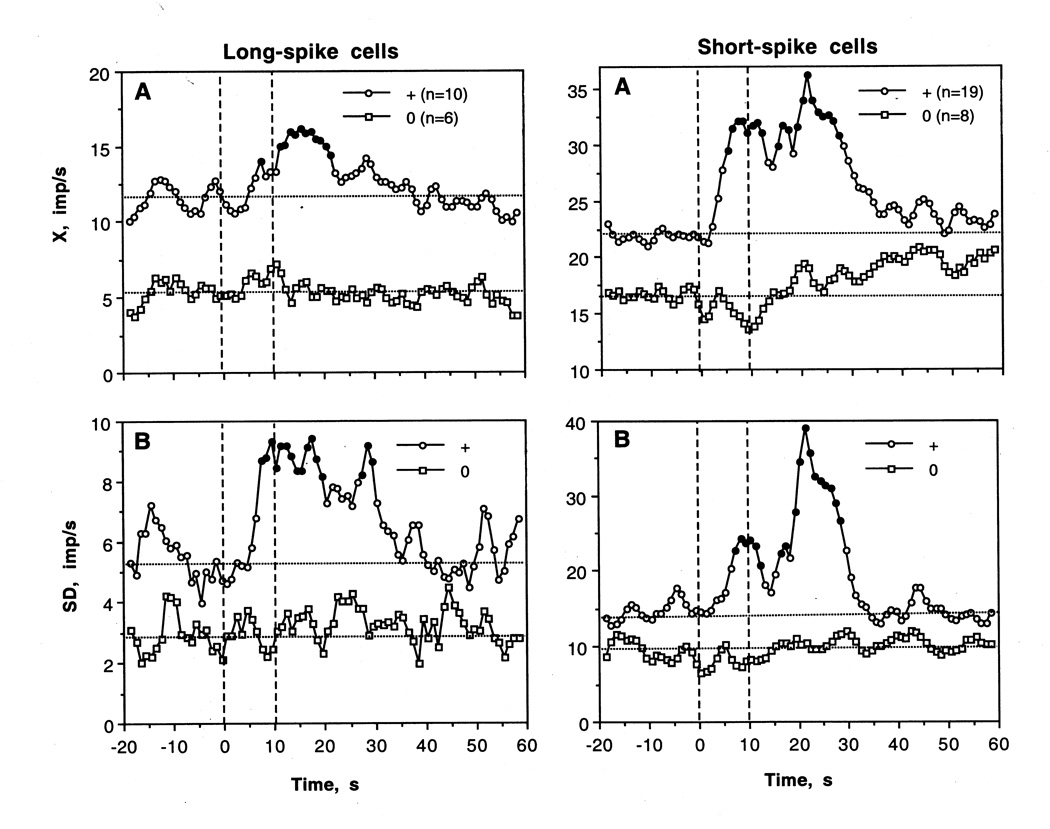
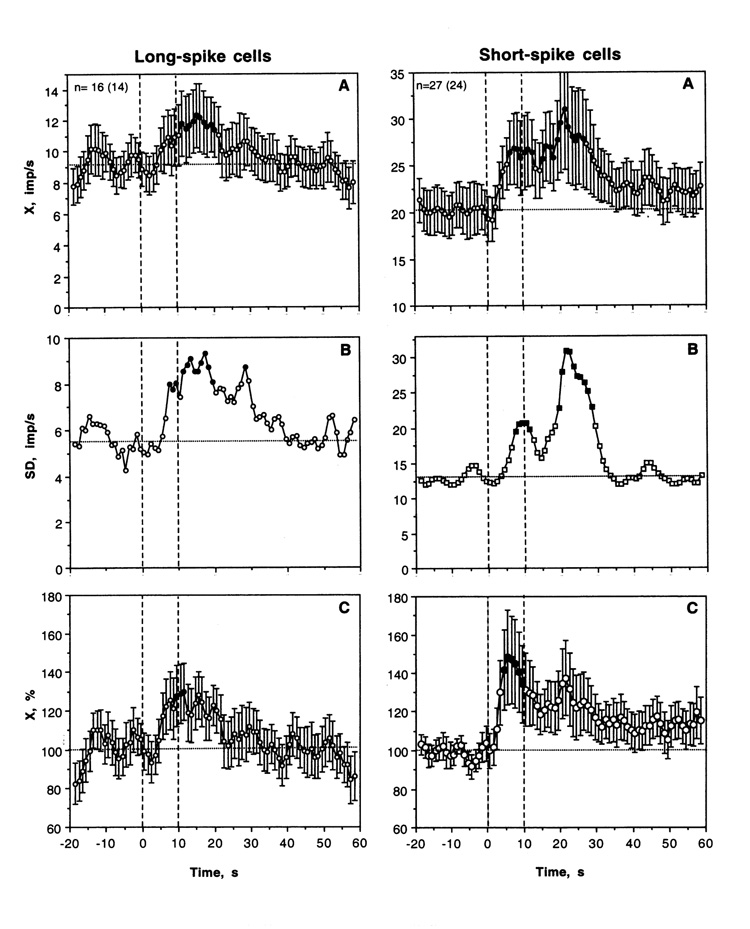
As shown in Table 2 and Fig. 8, most LS VTA neurons showed significant increases in discharge rate after iv cocaine injection. The excitatory effect of cocaine was also significant when all cases were superimposed and analyzed with one-way ANOVA with repeated measures with respect to mean baseline (F15,335=1.69, p<0.05). Discharge rate began to increase from ~6 s, was significantly higher than baseline from 11 to 20 s and then decreased to baseline during the next 10 s. Then the activity remained at relatively stable levels without evident tonic change. Phasic increase was also evident when discharge rate was analyzed in terms of relative change (Fig. 5C; basal activity=100%). In this case, discharge rate transiently increased (~+30%) at the end of a 10-s injection. In addition to increase in X, cocaine induced even stronger increase in SD, with significance between 7–17 s. Then, SD slowly decreased and reached baseline levels at ~40 s after injection start. Although the number of LS cells with no significant changes in rate was smaller than that for cells with excitations, transient episodes of dense bursting were seen after cocaine injections on some “non-responsive” cells (see #29-1-2 and 29-1-3 on Fig. 8).
Fig. 8.
Original records of changes in impulse activity of long-spike and short-spike VTA neurons following iv cocaine injection (0.25 mg/kg, 5–15 s). Each trace was obtained from a different cell, whose number and single spike are shown in right side. Note that in LS cells three top traces show significant increases (+) but the two lower traces show no change in rate (0) but clear bursting.
As shown in Fig. 10, LS VTA cells that were excited by cocaine had significantly higher basal rate and variability than non-responsive cells (X=11.49±1.41 vs. 5.27±0.74 imp/s; SD= 5.38±0.27 vs. 2.92±0.25 imp/s; p<0.01 for both cases). Both X and SD significantly increased ~ 5 s after the start of cocaine injection, but the increase was weaker in amplitude (30–40% for X and 60% for SD) but more prolonged than that seen after tail-press. Interestingly, a weak increase in discharge rate at ~10 s was also seen in “non-responsive” LS cells. While LS cells activated by cocaine had a tendency to have shorter spike duration than non-responsive cells (4.18±0.20 vs. 4.41±0.15 ms), these differences were not significant.
Fig. 10.
Fig. Mean changes in discharge rate (A, X imp/s) and standard deviation of rate (B, SD, imp/s) calculated separately for long-spike and short-spike VTA cells with excitations and no responses to iv cocaine. Filled symbols show values significantly different from baseline. Each graph shows injection interval (vertical hatched line 0 and 10 s) and basal rates in each unit subgroup.
As shown in Table 2 and Fig 8 and 9, most SS VTA neurons also significantly increased their activity rate following cocaine injection, and the effect was significant when all cases were superimposed (F26,566=1.72, p<0.03). In contrast to tail-pinch, inhibitions were never seen. The increase was more rapid than in LS cells (4–5 s latencies), stronger (both in terms of absolute and relative change) and more prolonged (up to 30–40 s after the injection start). In addition, SS units showed the second peak of rate increase (20–30 s), which was related to very high discharge rates (up to 120–130 imp/s) seen in several units. The increase in X was also mirrored by increases in SD (B), which became significantly higher than baseline at ~8 s and also showed the second peak at ~30 s.
Fig. 9.
Mean changes in impulse activity of LS and SS VTA neurons induced by iv cocaine (hatched lines from 0 to 10 s). A, B and C show mean rates (imp/s), standard deviation (imp/s) and percent change (%, baseline=100%) calculated for 20 s before and 60 s after the start of injection. Filled symbols show values significantly different from baseline. Statistical significance for SD was assessed based on the previous equation (see caption to Fig. 5), with an averaged baseline point calculated for 20 s preceding cocaine injection.
Similar to LS cells, cocaine-induced changes were analyzed separately for SS VTA cells showing significant increases and no changes in activity (Fig. 10). Like LS cells, SS cells that were excited by cocaine had significantly higher discharge rate and more variable discharge pattern than non-responsive cells (X=22.03±3.03 vs. 16.66±2.98; SD=14.70±0.70 vs. 9.38±0.41 imp/s; p<0.05 for both cases), but their spikes were virtually identical in length (1.39±0.06 vs. 1.41±0.08 ms). While X and SD rapidly increased in the excitation subgroup, no changes or even a weak tendency to decrease was seen in “non-responsive” cells.
2.4. Responses of LS and SS VTA neurons to GLU and GABA
Similar to other central neural cells, most VTA neurons showed excitatory responses to iontophoretic GLU and inhibitory responses to GABA. However, these responses have some distinct features and differences in LS and SS subgroups.
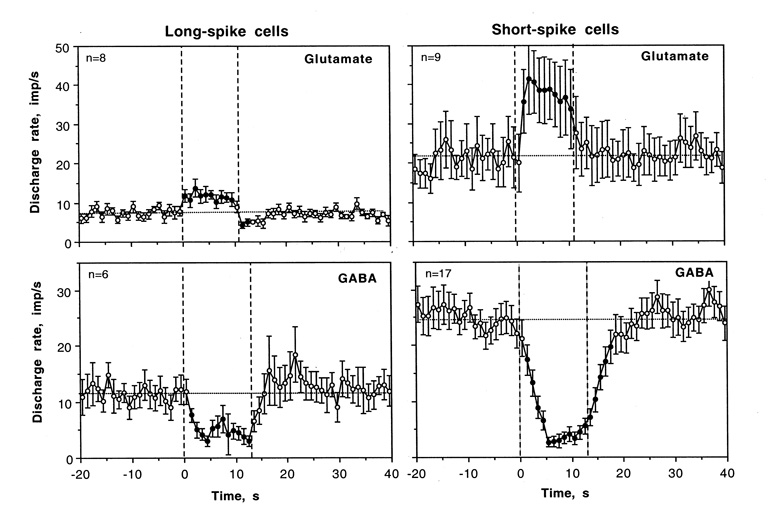
As shown in Table 3 and Fig. 11, GABA dose-dependently inhibited both LS and SS neurons. The GABA-induced inhibition had short latencies, reached a plateau at ~5 s, and maintained for some time after ejection current was turned off. While the number of cells tested at the same low currents was relatively low, it appears that GABA-induced inhibition is more variable in LS cells and followed by a compensatory, rebound-like increase in activity. In SS cells, GABA-induced inhibition was stronger and continued for several seconds after the ejection current was off. In most cases, GABA application to LS VTA cells resulted in increases in spike amplitude and relative regularization of impulse flow. In contrast, most SS units showed a “typical” GABA-induced inhibition, similar to that seen in other central neurons.
Table 3.
Iontophoretic responses of long-spike and short-spike VTA neurons in awake rats during DA receptor blockade
| Transmitter | Short-spike cells | Long-spike cells |
|---|---|---|
| GABA | ||
| mean current, nA | 13.53 | 10 |
| n | 17 (100.0%) | 6 (100.0%) |
| − | 17 (100.0%) | 5 (83.3%) |
| 0 | 0 | 1 (16.7%) |
| GLU | ||
| mean current, nA | 12.78 | 7.86 |
| n | 9 (100.0%) | 11 (100.0%) |
| + | 8 (88.9±10.5%) | 4 (36.4±14.51%)* |
| 0 | 1 (11.1%) | 6 (63.6±14.51%)* |
| − | 0 | 1 |
Asterisks show significance of between-group differences
p<0.05
Fig. 11.
Averaged responses of long-spike (LS) and short-spike (SS) VTA neurons to GLU and GABA ejected at the same small currents (10±5 nA). Each graph shows changes in discharge rate before, during (two vertical hatched line) and after iontophoretic application. Filled symbols show values significantly different from baseline and n shows number of responses analyzed.
In contrast to relatively similar responses induced by GABA in LS and SS units, significantly fewer LS cells were excited by GLU. While in virtually all SS cells GLU induced dose-dependent excitation, which quickly disappeared after the current was switched off, many LS units showed no changes in activity at low GLU currents and strong decreases in spike amplitude at higher currents. Sometimes, the decrease was to the point of almost full disappearance of spikes, greatly complicating data analyses. The averaged GLU response at the same low currents (Fig. 11) was relatively weak in amplitude (+30–40%), much weaker than that in SS cells. Moreover, the activity transiently decreased after GLU current was turned off, possibly reflecting rebound-like post-excitatory inhibition. Such an effect was absent on SS cells.
3. Discussion
This study demonstrates that iv cocaine transiently excites most VTA neurons in awake rats. This excitation occurs with latencies too short (4–8 s) to reflect direct interaction of cocaine with centrally located neural substrates. While cocaine quickly reaches brain vessels after iv delivery (Fowler et al., 1998), much more time is necessary to cross the blood-brain barrier, passively diffuse to brain receptor sites, and interact with them. Therefore, although it is usually believed that all centrally mediated effects of cocaine result from direct interaction with brain substrates, the present data support the view that iv delivered cocaine first interacts with peripherally located neural elements, triggering an ascending neural drive and thereby affecting central neurons prior to drug arrival in the brain. This mechanism is supported by the general similarity in VTA cell responses to tail-press—a typical arousing somato-sensory stimulus. In this case, short-latency responses are triggered via activation of sensory nerve afferents, involving spinothalamic and thalamo-cortical sensory pathways and general activation mechanisms. Hence, in addition to direct interaction with brain substrates, iv cocaine appears to mimic somato-sensory stimuli in their ability to induce rapid central effects via interaction with receptors of visceral sensory nerves, involving fast-conducting neural pathways that are engaged in processing of other sensory signals.
It appears that phasic responses of VTA neurons to sensory stimuli and iv cocaine are specific to awake conditions, representing part of a generalized phenomenon that involves multiple central neurons. Most striatal neurons show rapid, transient excitations of a similar amplitude and time course following tail-press, tail-pinch, iv cocaine and cocaine methiodide in awake conditions, but these effects are dramatically attenuated or fully blocked during urethane anesthesia (Kiyatkin and Brown, 2007). While this study was limited to the awake conditions and we were unable to test the effects of peripherally acting cocaine methiodide (experiments in progress), it is reasonable to assume that general anesthesia, in addition to modulating spontaneous impulse activity, should inhibit the sensory responses of VTA neurons. We are also confident that phasic neuronal responses induced by cocaine injection result from pharmacological action and not from some “non-specific” procedural or vehicle factors. Cocaine was injected via catheter and extending tubing, in low volume and at low speed, and special care has been taken to exclude any possible sensory cues associated with drug injection. Although the complexity of VTA cell recording in the awake conditions made it difficult to evaluate the effects of saline on each cellular subgroup, we previously showed that such injections performed at the same conditions did not affect animal behavior, brain, skin, and muscle temperatures (Brown and Kiyatkin, 2006) as well as impulse activity of striatal neurons (Kiyatkin and Brown, 2007). No effects of low-volume saline injections on VTA cell activity have been also documented in our heroin self-administration studies (Kiyatkin and Rebec, 2001).
However, DA antagonists used in this study should affect the activity and responsiveness of DA and possibly non-DA neurons. Although DA receptor blockade greatly attenuates motor responses to arousing stimuli and cocaine, it keeps sensory systems generally intact and could not make VTA cells sensitive to either tail-press or cocaine. Consistent responses of VTA neurons to iv cocaine seen during DA receptor blockade, moreover, suggest that the peripheral trigger initiating its rapid sensory effects has a non-DA nature.
3.1. VTA cell heterogeneity and the issue of neuronal phenotype
Consistent with literature (Bunney et al., 1973; Chiodo, 1988; Grace and Bunney, 1983; Kiyatkin, 1988; Kiyatkin and Rebec, 1998, 2001; Steinfels et al., 1983), spike duration is the most important parameter for classifying VTA neurons into two distinct subgroups. LS and SS cells also have contrasting properties by most other parameters. In addition to the unusually long, triphasic spikes, LS cells have relatively low basal discharge rates and bursting pattern, with large fluctuations in spike amplitude. Most of these cells show phasic excitatory responses to both tail-press and cocaine. The amplitude of these responses, however, is relatively low. LS neurons also show relatively low-amplitude excitations induced by iontophoretic GLU at any current (to 12 imp/s or 40–50% increase). These excitations, moreover, are followed by rebound-like inhibition, and spike amplitude dramatically decreases with a virtual disappearance of firing at higher GLU currents. The latter effect could reflect depolarization inactivation—the phenomenon described in DA neurons (Grace, 1992) and previously seen with GLU in LS VTA cells in awake, drug-free conditions (Kiyatkin and Rebec, 1998). DA antagonists, by blocking autoreceptors, could make DA cells more predisposed to this effect. Iontophoretic GABA slowed and regularized the activity of all LS cells, but the inhibition was also relatively weak in terms of magnitude and followed by a transient hyperactivity. These response patterns well agree with the autoactivity of DA neurons (Grace and Bunney, 1984)—the feature that makes them resistant to the direct activation of excitatory and inhibitory inputs.
Although without histochemical identification the neurochemical nature (phenotype) of the recorded VTA neurons remains unclear, the properties of LS cells generally match the major electrophysiological characteristics of DA neurons, which comprise about 65% of VTA somata (Swanson, 1982; Yamaguchi et al., 2007). LS VTA neurons in this study, however, have slightly higher and more varying discharge rates (mode 7.5 imp/s; range 0.9–18.3 imp/s) and stronger bursting than usually reported in anesthetized preparations (see Fig. 2). Although LS VTA cells with higher than usual rates were described in awake conditions (Kiyatkin and Rebec, 1998) and the mesoprefrontal DA cells have much higher discharge rates and bursting than nigrostriatal cells even during anesthesia (9.3 vs. 3.1 imp/s; Chiodo 1988; Bannon and Roth, 1983), DA receptor blockade, which should release DA cells from autoinhibition, could be another important factor in higher discharge rates and strong busting. Compared to many studies, in which DA cells were recorded from the substantia nigra pars compacts (SNc) or a lateral segment of the VTA, most of our cells were recorded from its medial portion (see Fig. 1), where the fast-firing mesocortical cells are preferentially located (Chiodo et al. 1984). However, several recent studies questioned the traditional criteria of indirect DA cell identification as less reliable and absolute than was originally thought (Ungless et al., 2004; Margolis et al., 2006). Using Neurobiotin staining with subsequent histochemical identification of the recorded cells in anesthetized conditions, Ungless et al (2004) showed that not all VTA cells with long, triphasic cells (10/18) are DA-containing, although they found that spike duration is relatively longer in identified DA cells compared to non-DA neurons. Therefore, conservatively we can assume that some LS VTA cells recorded in this study could be non-DA cells and such a possibility has been suggested in our previous work (Kiyatkin and Rebec, 1998). However, we can also reasonably assume that the majority or at least a significant part of these cells are DA neurons. Importantly, these cells as a group drastically differed from other VTA cells not only by their spikes, discharge rates and patterns, but also by the specifics of their responses to tail-pinch, cocaine and iontophoretically applied GLU and GABA. The similarity of these features within the group suggests their basic homogeneity, which is unlikely if they represent a mixture of neurochemically different cells.
In contrast, SS VTA cells represent a much more heterogeneous population, possibly combining cells of different phenotypes. In addition to GABA-containing cells, which are usually viewed as the second major VTA subgroup (Swanson, 1982; Olson and Nestler, 2007), a significant number of GLU-containing cells (~25%) have been recently identified in the VTA (Yamaguchi et al., 2007). These cells, moreover, outnumbered GABA cells in the medial VTA, where most of our SS neurons were recorded. The electrophysiological and functional properties of these GLU cells remain fully unclear now. In addition to short, biphasic spikes, higher and much more varying basal rates (mode 17.6 imp/s; range 0–60 imp/s) with both regular and busting patterns, SS neurons showed both excitatory and inhibitory responses to tail-press. Although transient inhibitions induced by arousing stimuli and correlated with EMG activity have been previously described on the fast-firing VTA (Kiyatkin, 1988) and GABA-containing SNr cells (Rebec, 2006), pointing at possible GABA identity of this subgroup, SS neurons with opposite response patterns to tail-press did not differ from each other neither in their rate or spike duration. Identified GLU VTA neurons also did not differ from other non-DA cells in their morphology and size of cell somata (Yamaguchi et al., 2007). Although the nature of SS cells remains unclear and we believe that they combine different neurochemical phenotypes, we can conclude that iv cocaine has phasic, transient excitatory effect on both DA and non-DA VTA neurons.
3.2. Sensory responses of LS and SS VTA neurons
Most of LS VTA neurons (67%) showed significant increases in discharge rate following tail-press, but the real number of responses could be higher because some cells with initially lower rate showed intense bursting with no change in mean rate. Such a response pattern has been previously shown on presumed DA VTA neurons during active wakefulness and paradoxical sleep compared to slow-wave sleep (Dahan et al., 2007). Because of this, the excitatory effect of tail-press was significant not only for all LS cells, but also for a subgroup of “non-responders” (see Fig. 5). The excitation induced by tail-press was very phasic and discharge rate more than doubled during the first second of a 5-s stimulation, rapidly returning to baseline. However, in terms of absolute magnitude, the increase was relatively week (~12 imp/s). This response pattern well agrees with preferential excitations of presumed DA neurons induced by arousing somato-sensory stimuli in awake animals, as shown both in the SNc (Freeman et al., 1985; Freeman and Bunney, 1987; Steinfels et al., 1983; Strecker et al., 1985; Schultz, 1986; Trulson, 1985) and VTA (Guarraci and Kapp, 1999; Freeman and Bunney, 1987; Kiyatkin, 1988; Horvitz et al., 1997; Schultz, 1986). These data are also consistent with large body of neurochemical data (DA metabolism, [DA] evaluated by microdialysis or electrochemistry) suggesting increased DA release, especially in cortical projections, associated with arousing and aversive stimulation (Abercrombie et al., 1989; Deutch et al., 1985; Fadda et al., 1978; Feenstra et al., 2000, 2001; Kiyatkin, 1995; Le Moal and Simon, 1991; Thierry et al., 1976). It appears that this response is typical to the awake conditions when sensory effects and activation mechanisms are intact and functional; somatosensory-evoked discharges typical to most striatal neurons were greatly eliminated during general anesthesia (see Kiyatkin and Brown, 2007; West, 1996). During general anesthesia presumed DA cells generally show no changes in activity following mild sensory stimulation (Coizet et al., 2006; Schultz and Romo, 1987; Tsai et al., 1980) but are both inhibited and activated by very strong, “noxious” stimuli (Chiodo et al, 1980; Coizet et al., 2006; Hommer and Bunney, 1980; Maeda and Mogenson, 1982; Schultz and Romo, 1987; Ungless et al., 2004). Although the ratio of opposite responses widely varied, the excitations were usually much stronger than inhibitions in terms of magnitude (Chiodo et al., 1979; Mantz et al., 1989) and they were predominant in mesocortical DA cells (Mantz et al., 1989).
Most SS VTA neurons (89%) were also sensitive to tail-press, showing both excitations (21/35) and inhibitions (10/35). In contrast to the LS cells, the excitations of SS cells were much stronger in terms of absolute rate increase (40 imp/s) and more prolonged (10–15 s), but as a relative change they were even weaker (~+70%) than in LS cells. More rarely occurring inhibitions were equally rapid but more transient, typically disappearing at the end of 5-s stimulation. While these data suggest that non-DA VTA cells also receive sensory input, the opposite responses could be indicative that they represent a mixture of neurochemically different cells. This presumed heterogeneity, however, was not reflected in either spike durations or spontaneous impulse activity.
3.3. Sensory effects of iv cocaine on different subgroups of VTA neurons
Most VTA neurons showed phasic, transient excitations following iv cocaine, and the excitatory effect was significant for both LS and SS units. Despite certain between-group differences, the activity began to increase 4–6 s after the start of the 10-s injection, peaked at ~15 s and slowly returned to baseline 10–20 s after the injection. Although the onset latencies were definitely longer, these effects were generally similar to those occurring after tail-press in this study and their time-course greatly resembled that seen on striatal neurons (~6 s latency, ~20-s duration) after the same-dose cocaine injections (Kiyatkin and Brown, 2007). Although the number of significant excitations on LS cells was relatively lower than in SS cells (63% vs. 70%), similar to tail-press, some LS cells showed intense bursting without a change in mean rate. This increase in discharge variability was clear in SD, which showed a stronger response than X. On average, the increase was more rapid (3 vs. 6 s), stronger and more prolonged in SS than LS cells. The cocaine-induced excitations of LS cells were relatively weak in terms of absolute (~12 imp/s) and relative response amplitude (~+30%), and they were more often and stronger in cells with initially higher discharge rate and intense bursting (see Fig. 10). These highly bursting cells had relatively shorter spike durations than “non-responsive” cells, but both values are well within the range of LS cells, sharply differing from the SS cells. This difference is not surprising since LS cells with higher rates have relatively shorter spike durations (see Fig. 3D). Similar to the LS subgroup, cocaine-induced excitations of SS cells were also more often in cells with higher basal rate, while more slower firing cells showed no response or a weak tendency to decrease at the end of cocaine injection. However, these subgroups did not differ by their spike duration.
Cocaine-induced excitations of LS VTA neurons seen in this study appear to contrast to mainly inhibitory responses of presumed DA neurons to iv cocaine reported in the SNc and VTA in anesthetized animals (Einhorn et al., 1988; Hinerth et al., 2000; Pitts and Marwah, 1987; Shi et al., 2004). However, this latter effect was much slower (latencies 20–40 s), much more prolonged (10–20 min), relatively weak both on SNc and VTA neurons (ED50 8.8 and 1.2 mg/kg, respectively; Hinerth et al., 2000), and blocked by D2 antagonists (Zhou et al., 2006), suggesting its central pharmacological determination. While it is tempting to find out how iv cocaine affects identified DA cells in awake, drug-free conditions as well as in anesthetized conditions without DA antagonist treatment (experiments in progress), it appears that these effects reflect quite different phenomena with different mechanisms. Since anesthesia is known to block the arousing effects of sensory stimuli (West, 1998), it should eliminate or drastically reduce the rapid effects of iv cocaine seen at a low dose (0.25 mg/kg)—the effect clearly demonstrated on stiatal neurons with respect to both cocaine and peripherally acting cocaine methiodide (Kiyatkin and Brown, 2007). On the other hand, such low doses of cocaine (~20% of ED50) are too small to induce evident pharmacological effects, making it unlikely to observe cellular inhibition. Treatment with the DA antagonists used in our study should also prevent a slowly developing inhibition, which is presumably mediated via DA autoreceptors. In fact, instead of inhibition, cocaine increased the activity of presumed DA VTA neurons in anesthetized animals treated with raclopride, a selective D2 antagonist (Shi et al., 2004). In contrast to rapid, transient excitations produced by cocaine at low dose in our experiments, this increase was tonic and much weaker despite the much higher drug dose.
3.4. Mechanisms underlying rapid, transient effects of iv cocaine on VTA cell activity and their possible consequences
Phasic GLU release appears to be the most probable cause for rapid excitation of DA cells (see Grace and Bunney, 1984; Redgrave and Gurney, 2006), and this input could be responsible for excitation of these cells induced by tail-press and cocaine. While the exact source of GLU release remains unknown, it could come from multiple structures (Geisler et al., 2007) because sensory stimuli activate different cells within the midbrain and reticulo-thalamo-cortical activation system (see Redgrave and Gurney, 2006 for review), most of which are GLU-containing. In contrast to the very phasic excitation of LS cells induced by tail-press, cocaine-induced excitation is much more prolonged, pointing to multiple sources of excitatory input (i.e. parabrachial nucleus, superior colliculus, pedunculo-pontine nucleus, thalamus, cortex, etc.). Since SS VTA cells had shorter onset latencies of cocaine-induced responses than both LS VTA and striatal neurons and at least some of SS cells could be GLU neurons, their activation could provide intra-VTA GLU release, thus contributing to DA cell activation. It is currently unknown, however, whether or not these VTA GLU cells make synaptic connections to DA cells. Neuronal excitation could also occur due to rapid decrease in GABA input (auto-inhibition), the effect clearly shown on presumed DA neurons with local applications of GABA receptor antagonists (Mogenson et al., 1980). However, this mechanism seems unlikely because striatal and accumbal neurons, which should provide GABA input to the VTA, are activated by both sensory stimuli and cocaine, as well as most SS VTA cells, which at least in part should be GABA-containing.
If most or at least a portion of LS VTA neurons represent DA cells, their excitation following iv cocaine injection may suggest that, in addition to the well-known action on the DA transporters and subsequent inhibition of DA uptake, cocaine is able to mimic somato-sensory stimuli in their ability to induce phasic activation of DA neurons and phasic DA release. Although cocaine has no effect on DA release in vitro (Heikkila et al., 1975), some data suggest that it can increase DA release in vivo. By using fast-cyclic voltammetry in awake, drug-naive rats, iv cocaine was found to increase NAcc extracellular DA levels at surprisingly short latencies (Heien et al., 2005). The effect was clearly evident at 10–20 s post-injection and grew afterwards and, while dose-dependent, it was seen after cocaine at low dose (0.3 mg/kg) similar to that used in our study. Although this effect was interpreted solely as the consequence of DA uptake inhibition, it could also result from rapid DA release associated with cocaine injection, with a subsequent DA accumulation due to reuptake inhibition. If iv cocaine in fact induces rapid, transient activation of DA cells via an indirect, sensory mechanism, it provides DA release that is potentiated by a slower developing and much more prolonged direct action on DA uptake (Kiyatkin et al., 2000; Wakazono and Kiyatkin, 2008), increasing DA levels. Therefore, the DA system could be rapidly activated by cocaine, just like by other arousing sensory stimuli, via the peripheral, DA-independent mechanism. While activation of the DA system is important, equally important is rapid sensory input to non-DA cells. In addition to regulating the activity of DA cells, some of these non-DA cells are definitely projection cells, which could affect neuronal activity in various target structures.
3.5. Functional implications: Sensory effects of cocaine and their importance for the reinforcing potential of this drug
This study supports the view that iv cocaine, by providing a rapid, neurally mediated signal and triggering generalized neural activation, may display its acute sensory properties. This signal originates from direct cocaine action on a number of ionic channels (K+, but possibly Na+, Ca++ and TRP) on terminals of sensory nerves and it could be involved in mediating rapid motor, autonomic and psycho-emotional effects of iv cocaine. EEG desynchronization, acute increase in arterial blood pressure, peripheral vasoconstriction and acute euphoria all occur either within or in a few seconds after iv cocaine (Herning et al., 1985; Kiyatkin and Brown, 2005; Lukas et al., 1990; Matsuzaki et al., 1978; Poon and van den Buuse, 1998; Zernig et al., 2003) and, in contrast to prolonged effects on monoamine transporters, all these effects are relatively brief. All these effects, moreover, are generally resistant to DA receptor blockade and in cocaine-experienced subjects they could be mimicked by procaine (Adamec and Start-Adamec, 1987; Adinoff et al., 1998; Fischman and Schuster, 1983; Gawin 1986; Sherer et al., 1989), which is not an inhibitor of monoamine uptake, suggesting a leading role of peripheral non-DA neural mechanisms in its mediation. Some of rapid, centrally mediated effects of cocaine, moreover, are mimicked by cocaine methiodide (Dickerson et al., 1999; Brown and Kiyatkin, 2006), which cannot cross the blood-brain barrier.
The present study indicates that VTA DA cells could be rapidly excited following iv cocaine injection independent of its direct action on DA uptake. The interaction of these two independent and temporally separated actions converging on the same neural substrates may play an important role in cocaine reinforcement. While usually envisioned as an interaction between the drug’s pharmacological actions and associated sensory stimuli or voluntary movement acts, our data suggest that cocaine itself can provide its own pharmacologically mediated sensory signal, which may interact with other drug actions. At neuronal and neurochemical levels, this is an interaction between rapid, generalized but transient neuronal activation and subsequent slow and more prolonged pharmacological actions mediated via direct drug action on brain substrates. While the cellular mechanisms underlying these interactions following repeated drug use remain unclear, they may play important roles in the development of drug-seeking behavior and the enhancement of both the psycho-physiological experience and behavioral effects induced by repeated cocaine administration. This does not exclude the role external stimuli play in development of self-administration behavior. Our data, however, point out the crucial role that sensory effects of cocaine might play in reinforcement and their importance in the development and maintenance of drug-seeking and drug-taking behavior.
4. Experimental procedures
4.1. Animals and surgery
Data were obtained from 9 male Long-Evans rats (420±40 g) supplied by Charles River Laboratories (Greensboro, NC). All animals were housed individually under standard laboratory conditions (12-hr light cycle beginning at 07:00) with free access to food and water. Protocols were performed in compliance with the Guide for the Care and Use of Laboratory Animals (NIH, Publication 865-23) and were approved by the NIDA-IRP Animal Care and Use Committee. Maximal care was taken to minimize the number of animals used and their possible suffering.
Single-unit recording combined with microiontophoresis was performed with a microdriver (Rebec et al., 1993) that allowed fine manual movements of four-barrel glass microelectrodes within the recording track. The surgical procedures used have been described previously (Kiyatkin and Rebec, 2001). Briefly, under general anesthesia (Equithesin 3.3 ml/kg i.p.; dose of sodium pentobarbital 32.5 mg/kg and chloral hydrate 145 mg/kg), rats were implanted with a plastic, cylindrical hub, designed to mate with a microdriver during recording. This hub was centered over a hole drilled above the VTA (5.3±0.3 mm posterior and 2.0 mm lateral to bregma with 10° angle). The electrode insertion was angled to reach the medial sector of the VTA, while avoiding the sinus and possible associated bleeding. During the same surgery session, each rat was implanted with a chronic jugular catheter, which was run subcutaneously to the head mount and secured with dental cement. After a 3–4 day period of recovery and two-three days of habituation to the experimental chamber, recording sessions were held once daily over the next 2–3 days.
4.2. Experimental Protocol
Electrophysiological recordings were performed in awake, non-anesthetized conditions after systemic administration of a mixture of D1-like (SCH23390 1 mg/kg) and D2-like (eticlopride 1 mg/kg) DA antagonists. Both drugs were obtained from Sigma (St. Louis, MO), freshly dissolved, mixed, and injected subcutaneously. Neuronal data obtained between 20 min and 3 h following the injection of DA antagonists were accepted as occurring during full DA receptor blockade, as confirmed by the antagonism of striatal neuronal responses to iontophoretic DA (Kiyatkin and Rebec, 1999). DA blockade was maintained by additional injection of DA antagonists at half the dose, three hours after the initial administration.
4.3. Single-unit recording and iontophoresis
Four-barrel, microfilament-filled, glass pipettes (Omega Dot 50744, Stoelting, Wood Dale, IL), pulled and broken to a diameter of 4±1 µm, were used for single-cell recording and iontophoresis. The recording barrel contained 2% Pontamine Sky Blue (BDH Chemicals Ltd, Poole, England) in 3 M NaCl; the balance barrel contained 0.25 M NaCl. The remaining two barrels were filled with 0.25 M solution l-GLU monosodium salt (dissolved in distilled water, pH 7.5) and 0.25M solution of GABA (dissolved in 0.125M saline, pH 4.2). The resistance, measured at 100 Hz, was 3–5 MΩ for the recording barrel and 10–35 MΩ for the iontophoretic barrels. GLU and GABA were ejected by anionic and cationic currents, respectively (5–40 nA for ~10 s), with a continuous retaining current of opposite polarity (±8–10 nA), using constant current generators (Ion 100 and Ion 100T, Dagan, Minneapolis, MN). Each multibarrel pipette was filled with fresh drug solutions less than one hour before use and fixed in a microdrive assembly that later was inserted into the skull-mounted hub. The electrode was then advanced 7.0 mm below the skull surface to the starting point of unit recording.
Neuronal discharge signals were sent to a head-mounted preamplifier (OPA 404KP, Burr Brown, Tucson, AZ) and then additionally amplified and filtered (band pass: 300–3,000 Hz) with a Neurolog System (Digitimer, Hertfordshire, UK). The filtered signal was then recorded using a Micro 1401 MK2 interface (Cambridge Electronic Design, Cambridge, UK). Spike activity was monitored with a digital oscilloscope and audio amplifier and analyzed using a Spike2 interface (Cambridge Electronic Design).
After isolating a signal-unit discharge and recording basal impulse activity, several stimuli were administered. These stimuli included: a 5-s tail-press by a wooden clothespin, iv cocaine administration (0.25 mg/kg in 0.1 ml saline during 10-s infusion via catheter) and GLU or GABA applications (5–40 nA). While we tried to test each recorded cell with all stimuli, in most cases it was not possible and our stimulus subgroups include different numbers of cells and tests. In some cells, stimuli were used several times; intervals between iontophoretic applications and tail-press were at least 60 s and between cocaine injections at least 8 min. While the cocaine dose used in this study (0.25 mg/kg) is lower than a typical “reinforcing” dose, cocaine at this dose is detected and self-administered by both rats and humans, and induces behavioral activation, acute cardio-vascular effects (Poon and van den Boose, 1998) and striatal neuronal responses (Kiyatkin and Brown, 2007).
4.4. Histology
After the last recording session, animals were anesthetized and Pontamine sky blue was deposited by current ejection (−20 µA for 20 min) at the last recording site. Rats were sacrificed and the brains placed in a formalin solution. Coronal 30µm tissue sections were prepared at −20°C using a microtome cryostat. The Paxinos and Watson atlas (Paxinos and Watson, 1998) served as the basis for histological analyses.
4.5. Data analysis
Each histologically verified VTA neuron was characterized by parameters of single spikes (shape and duration) and three parameters of spontaneous impulse activity (mean rate or X; standard deviation or SD; coefficient of variation or CV) calculated based on twenty 1-s values of discharge rate preceding drug injection, stimulus presentation, and iontophoretic applications. These values were grouped together and further analyzed by using standard statistical procedures (i.e., mean and modal group values, ln-transformations, variability, distributions, Student’s t-test). Since spike shape and duration are the best predictors of subgroup type within the population of VTA neurons (Bunney et al., 1973; Chiodo, 1988; Grace and Bunney, 1983; Kiyatkin, 1988; Kiyatkin and Rebec, 1998; Steinfels et al., 1983), these parameters were used for primary cell separation and grouping. For each cell, we determined the duration of the first phase of a spike and total spike duration, allowing a comparison of cells with biphasic and triphasic spikes. Since major statistical parameters of spontaneous impulse activity in cells of many brain structures (i.e., thalamus, hypothalamus, central gray matter, striatum, VTA) are distributed according to the ln-normal law (Kiyatkin, 1984; Kiyatkin and Rebec, 1998, 1999; Nakahama et al., 1971; Werner and Mountcastle, 1963,1965), their ln-derivatives were used to additionally describe impulse activity of VTA neurons and determine between-group differences.
Responses to tail-press and cocaine were statistically analyzed both for each presentation in individual units and in a cell subgroup. Individual analysis was based on differences in discharge rates between pre-stimulus and post-stimulus conditions. Because individual neurons tested with tail-press and especially cocaine showed changes in activity that widely varied in latency, duration, amplitude and even in direction, a sign criterion (z, one or more consecutive values higher or lower than each of 20 values in pre-stimulus baseline) was used to recognize a unit response. Since this approach provides an approximate evaluation of response following a single presentation, one-way ANOVA with repeated measures (followed by Fisher post-hoc test) was used for quantitative evaluation of the effects of stimuli and drugs in groups as well as between-group differences in effects. All neurons, independent of presence or absence of the response and the response direction (excitation or inhibition) were included for group analyses. To provide an equal contribution of each recorded unit in group analyses, only one test was included for evaluating the effects of sensory stimuli and, if several drug injections were performed on individual neurons, changes in activity were averaged and then included as one data point to evaluate cocaine-induced changes in activity.
While most units were exposed to several GLU or GABA applications at the same or different currents, statistical analysis of iontophoretic effects was performed only for equal moderate currents, and only one response per unit was used for group analyses. Each iontophoretic test was statistically evaluated, and the response was accepted (i.e., excitation or inhibition) if the mean firing rate during iontophoresis (10 s) differed significantly (p<0.05; two-tail Student’s t-test) from baseline activity (20 s) immediately preceding the iontophoretic application. These responses were also assessed in terms of absolute and relative magnitude, onset and offset latencies, and response time-course. One-way ANOVA with repeated measures was used to assess the effects of GLU and GABA on discharge rate in cell groups. If not defined otherwise, data are expressed as mean±SEM.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, NIDA. We wish to thank Dr. Roy A. Wise for valuable comments on the matter of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine frelease in striatum, nucleus accumbens, and medial frontal cortex. J. Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Adamec RE, Stark-Adamec C. The effects of procaine HCl on population cellular and evoked response activity within the limbic system of the cat. Evidence for differential excitatory action of procaine in a variety of limbic circuits. Prog. Neuropsychoparmacol. Biol. Psychiatry. 1987;11:345–364. doi: 10.1016/0278-5846(87)90012-1. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Brady K, Sonne S, Mirabella RF, Kellner CH. Cocaine-like effects of intravenous procaine in cocaine addicts. Addiction Biol. 1998;3:189–196. doi: 10.1080/13556219872245. [DOI] [PubMed] [Google Scholar]
- Bannon MJ, Roth RH. Pharmacology of mesocortical dopamine neurons. Pharmacol. Rev. 1983;35:53–68. [PubMed] [Google Scholar]
- Brown PL, Kiyatkin EA. The role of peripheral Na+ channels in triggering the central excitatory effects of intravenous cocaine. Eur. J. Neurosci. 2006;24:1182–1192. doi: 10.1111/j.1460-9568.2006.05001.x. [DOI] [PubMed] [Google Scholar]
- Bunney BS, Walters JR, Roth RH, Aghajanian GK. Dopaminergic neurons: effect of antipsychotic drugs and amphetamine on single cell activity. J. Pharmacol. Exp. Ther. 1973;185:560–571. [PubMed] [Google Scholar]
- Catterall W, Mackie K. Local anesthetics. In: Hardman JC, Limbird LE, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 9th Ed. NY: McGraw; 1996. pp. 331–347. [Google Scholar]
- Chiodo LA. Dopamine-containing neurons in the mammalian central nervous system: electrophysiology and pharmacology. Neurosci. Biobehav. Rev. 1988;12:49–91. doi: 10.1016/s0149-7634(88)80073-3. [DOI] [PubMed] [Google Scholar]
- Chiodo LA, Antelman SM, Caggiula AR, Lineberry CG. Sensory stimuli alter the discharge rate of dopamine (DA) neurons: Evidence for two functional types of DA cells in the substantia nigra. Brain Res. 1980;189:544–549. doi: 10.1016/0006-8993(80)90366-2. [DOI] [PubMed] [Google Scholar]
- Chiodo LA, Bannon MJ, Grace AA, Roth RH, Bunney BS. Evidence for the absence of impulse-regulating somatodendritic and synthesis-modulating nerve terminal autoreceptors on subpopulations of mesocortical dopamine neurons. Neuroscience. 1984;12:1–16. doi: 10.1016/0306-4522(84)90133-7. [DOI] [PubMed] [Google Scholar]
- Couzet V, Dommett EJ, Redgrave P, Overton PG. Nociceptive responses of midbrain dopamine neurons are modulated by the superior colliculus in the rat. Neuroscience. 2006;139:1479–1493. doi: 10.1016/j.neuroscience.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Dahan L, Astier B, Vautrelle N, Urbain N, Kocsis B, Chouvet G. Prominent burst firing of dopaminergic neurins in the ventral tegmental are during paradoxical sleep. Neuropsychopharmacology. 2007;32:1232–1241. doi: 10.1038/sj.npp.1301251. [DOI] [PubMed] [Google Scholar]
- Dickerson LW, Rodak DJ, Kuhn FE, Wahlstrom SK, Tessel RE, Visner MS, Schaer GL, Gillis RA. Cocaine-induced cardiovascular effects: Lack of evidence for a central nervous system site of action based on hemodynamic studies with cocaine methiodide. J. Cardiovasc. Pharmacol. 1999;33:36–42. doi: 10.1097/00005344-199901000-00006. [DOI] [PubMed] [Google Scholar]
- Einhorn LC, Johansen PA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: studies in the ventral tegmental area. J. Neurosci. 1988;8:100–112. doi: 10.1523/JNEUROSCI.08-01-00100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda F, Argiolas A, Melis MR, Tissari AH, Onali PL, Gessa GL. Stress-induced increase in 3.4-dihydroxyphenylacetic acid (DOPAC) levels in the cerebral cortex and in n. accumbens: reversal by diazepam. Life Sci. 1978;23:2219–2224. doi: 10.1016/0024-3205(78)90207-2. [DOI] [PubMed] [Google Scholar]
- Feenstra MGP. Dopamine and noradrenaline release in the prefrontal cortex in relation to unconditioned and conditioned stress and reward. Prog. Brain Res. 2000;126:133–163. doi: 10.1016/S0079-6123(00)26012-3. [DOI] [PubMed] [Google Scholar]
- Feenstra MGP, Vogel M, Bottenblom MHA, Joosten RNJMA, de Bruin JPC. Dopamine and noradrenaline efflux in the rat prefrontal cortex after classical aversive conditioning to the auditory cue. Eur. J. Neurosci. 2001;13:1051–1054. doi: 10.1046/j.0953-816x.2001.01471.x. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Schuster CR. A comparison of the subjective and cardiovascular effects of cocaine and procaine in humans. Pharmacol. Biochem. Behav. 1983;18:711–716. doi: 10.1016/0091-3057(83)90011-4. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Logan J, Gatley SJ, Pappas N, King P, Ding Y-S, Wang G-J. Measuring dopamine transporter occupancy by cocaine in vitro: radiotracer considerations. Synapse. 1998;28:111–116. doi: 10.1002/(SICI)1098-2396(199802)28:2<111::AID-SYN1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Freeman AS, Meltzer LT, Bunney BS. Firing properties of substantia nigra dopaminergic neurons in freely moving rats. Life Sci. 1985;36:1983–1994. doi: 10.1016/0024-3205(85)90448-5. [DOI] [PubMed] [Google Scholar]
- Freeman AS, Bunney BS. Activity of A9 and A10 dopaminergic neurons in unrestrained rats: further characterization and effects of apomorphine and cholecystokinin. Brain Res. 1987;405:46–55. doi: 10.1016/0006-8993(87)90988-7. [DOI] [PubMed] [Google Scholar]
- Gawin F. Neuroleptic reduction of cocaine-induced paranoia but not euphoria? Psychopharmacology. 1986;90:142–143. doi: 10.1007/BF00172886. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral regmental area in the rat. J. Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goder R, Habler HJ, Janig W, Michaelis M. Receptor properties of afferent nerve fibers associated with the rat saphenous vein. Neurosci Lett. 1993;164:175–178. doi: 10.1016/0304-3940(93)90885-o. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons -- 1. Identification and characterization. Neuroscience. 1983;10:301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J. Neurosci. 1984;4:3877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarraci FA, Kapp BS. An electrophysiological characterization of ventral tegmental area dopaminergic neurons during different Pavlovian fear conditioning in the awake rabbit. Beh. Brain Res. 1999;99:169–179. doi: 10.1016/s0166-4328(98)00102-8. [DOI] [PubMed] [Google Scholar]
- Heien MLAV, Khan AS, Ariansen JL, Cheer JF, Phillips PEM, Wassum KM, Weightman RM. Real-time measurements of dopamine fluctuations after cocaine in the brain of behaving rats. Proc. Natnl. Acad. Sci. USA. 2005;102:10023–10028. doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinerth MA, Collins HA, Baniecki M, Hanson RM, Waszczak BL. Novel in vivo electrophysiological assay for the effects of cocaine and putative “cocaine antagonists” on dopamine transporter activity of substantia nigra and ventral tegmental area dopamine neurons. Synapse. 2000;38:305–312. doi: 10.1002/1098-2396(20001201)38:3<305::AID-SYN9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Hommer DW, Bunney BS. Effect of sensory stimuli on the activity of dopaminergic neurons: Involvement of non-dopaminergic nigral neurons and striatonigral pathways. Life Sci. 1980;27:377–386. doi: 10.1016/0024-3205(80)90185-x. [DOI] [PubMed] [Google Scholar]
- Horvitz JC, Stewart T, Jacobs BL. Burst activity of ventral tegmental neurons is elicited by sensory stimuli in the awake cat. Brain Res. 1997;759:251–258. doi: 10.1016/s0006-8993(97)00265-5. [DOI] [PubMed] [Google Scholar]
- Iversen L. Neurotransmitter transporters and their impact on the development of psychopharmacology. Br. J. Pharmacol. 2006;147 Suppl. 1:S82–S88. doi: 10.1038/sj.bjp.0706428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA. Functional properties of presumed dopamine-containing and other ventral tegmental area neurons in conscious rats. Int. J. Neurosci. 1988;42:21–43. doi: 10.3109/00207458808985756. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA. Functional significance of mesolimbic dopamine. Neurosci. Biobehav. Rev. 1995;19:573–598. doi: 10.1016/0149-7634(95)00029-1. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL. Dopamine-dependent and dopamine-independent actions of cocaine as revealed by brain thermorecording in freely moving rats. Eur. J. Neurosci. 2005;22:930–938. doi: 10.1111/j.1460-9568.2005.04269.x. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL. I.v. cocaine induces rapid, transient excitation of striatal neurons via its action on peripheral neural elements: Single-cell, iontophoretic study in awake and anesthetized rats. Neuroscience. 2007;148:978–995. doi: 10.1016/j.neuroscience.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Kiyatkin DE, Rebec GV. Phasic inhibition of dopamine uptake in nucleus accumbens induced by intravenous cocaine in freely behaving rats. Neuroscience. 2000;98:729–741. doi: 10.1016/s0306-4522(00)00168-8. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Heterogeneity of ventral tegmental area neurons: single-unit recording and iontophoresis in awake, unrestrained rats. Neuroscience. 1998;85:1285–1309. doi: 10.1016/s0306-4522(98)00054-2. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Striatal neuronal activity and responsiveness to dopamine and glutamate after selective blockade of D1 and D2 dopamine receptors in freely moving rats. J. Neurosci. 1999;19:3594–3609. doi: 10.1523/JNEUROSCI.19-09-03594.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Impulse activity of ventral tegmental area neurons during heroin self-administration in rats. Neuroscience. 2001;102:565–580. doi: 10.1016/s0306-4522(00)00492-9. [DOI] [PubMed] [Google Scholar]
- Lee Y, Lee C-H, Oh U. Painful channels in sensory neurons. Mol. Cells. 2005;20:315–324. [PubMed] [Google Scholar]
- Le Moal M, Simon H. Mesocortical dopaminergic network: Functional and regulatory roles. Physiol Rev. 1991;71:155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Mendelson JH, Amass L, Benedikt R. Behavioral and EEG studies of acute cocaine administration: comparisons with morphine, amphetamine, pentobarbital, nicotine, ethanol and marijuana. NIDA Res. Monogr. 1990;95:146–152. [PubMed] [Google Scholar]
- Maeda H, Mogenson GJ. Effects of peripheral stimulation on the activity of neurons in the ventral tegmental area, substantia nigra and midbrain reticular formation of rats. Brain Res. Bul. 1982;8:7–14. doi: 10.1016/0361-9230(82)90021-1. [DOI] [PubMed] [Google Scholar]
- Mantz J, Thierry AM, Glowinski J. Effect of noxious tail pinch on the discharge rate of mesocortical and mesolimbic dopamine neurons: selective activation of the mesocortical system. Brain Res. 1989;476:377–381. doi: 10.1016/0006-8993(89)91263-8. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopamine neurons? J. Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Spingler PJ, Whitlock EG, Misra AL, Mule SJ. Comparative effects of cocaine and pseudococaine on EEG activities, cardiorespiratory functions, and self-administration behavior in the rhesus monkey. Psychopharmacology (Berlin) 1978;57:12–20. doi: 10.1007/BF00426951. [DOI] [PubMed] [Google Scholar]
- Michaelis M, Goder R, Habler HJ, Janig W. Properties of afferent nerve fibers supplying the saphenous vein in the cat. J. Physiol. (London) 1994;474:233–243. doi: 10.1113/jphysiol.1994.sp020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Jones D, Yim CY. From motivation to action: Functional interface between the limbic system and the motor system. Prog. Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Nakahama H, Ishii N, Yamamoto M, Saito H. Stochastic properties of spontaneous impulse activity in central single neurons. Tohoku J. Expt. Med. 1971;104:373–391. doi: 10.1620/tjem.104.373. [DOI] [PubMed] [Google Scholar]
- Olson VG, Nestler EJ. Topographical organization of GABAergic neurons within the ventral tegmental area of the rat. Synapse. 2007;61:87–95. doi: 10.1002/syn.20345. [DOI] [PubMed] [Google Scholar]
- Paxinos J, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney: Academic Press; 1998. [Google Scholar]
- Pitts DK, Marwah J. Cocaine modulation of central monoaminergic transmission. Pharmacol. Biochem. Behav. 1987a;26:453–461. doi: 10.1016/0091-3057(87)90147-x. [DOI] [PubMed] [Google Scholar]
- Pitts DK, Udom CE, Marwah J. Cardiovascular effects of cocaine in anesthetized and conscious rats. Life Sci. 1987b;40:1099–1111. doi: 10.1016/0024-3205(87)90573-x. [DOI] [PubMed] [Google Scholar]
- Poon J, van den Buuse M. Autonomic mechanisms in the acute cardiovascular effects of cocaine in conscious rats. Eur. J. Pharmacol. 1998;363:147–152. doi: 10.1016/s0014-2999(98)00804-8. [DOI] [PubMed] [Google Scholar]
- Premkumar LS. Block of a Ca++-activated potassium channel by cocaine. J. Membr. Biol. 2005;204:129–136. doi: 10.1007/s00232-005-0755-6. [DOI] [PubMed] [Google Scholar]
- Rebec GV. Behavioral electrophysiology of psychostimulants. Neuropsychopharmacology. 2006;31:2341–2348. doi: 10.1038/sj.npp.1301160. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Langley PE, Pierce RC, Wang Z, Heidenreich BA. A simple micromanipulator for multiple uses in freely moving rats: electrophysiology, voltammetry, and simultaneous intracerebral infusions. J. Neurosci. Methods. 1993;47:53–59. doi: 10.1016/0165-0270(93)90021-i. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Gurney K. The short-latency dopamine signal: a role in discovering novel actions? Nature Rev. Neurosci. 2006;7:967–975. doi: 10.1038/nrn2022. [DOI] [PubMed] [Google Scholar]
- Reith ME. Cocaine receptors on monoamine transporters and sodium channels. NIDA Res. Monogr. 1988;88:23–43. [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors of dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Romo R, Schultz W. Somatosensory input to dopamine neurons of the monkey midbrain: responses to pain pinch under anesthesia and to active touch in behavioral context. Prog. Brain Res. 1989;80:473–478. doi: 10.1016/s0079-6123(08)62245-1. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur. J. Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Schultz W. Responses of midbrain dopamine neurons to behavior trigger stimuli in the monkey. J. Neurophysiol. 1986;56:1439–1461. doi: 10.1152/jn.1986.56.5.1439. [DOI] [PubMed] [Google Scholar]
- Schultz W, Romo R. Responses of nigrostriatal dopamine neurons to high-intensity somatosensory stimulation in the anesthetized monkey. J. Neurophysiol. 1987;57:201–207. doi: 10.1152/jn.1987.57.1.201. [DOI] [PubMed] [Google Scholar]
- Shi W-X, Pun C-L, Zhou Y. Psychostimulants induce low-frequency oscillations in the firing activity of dopamine neurons. Neurophychopharmacology. 2004;29:2160–2167. doi: 10.1038/sj.npp.1300534. [DOI] [PubMed] [Google Scholar]
- Sherer MA, Kumor KM, Jaffe JH. Effects of intravenous cocaine are partially attenuated by haloperidol. Psychiatry Res. 1989;27:117–125. doi: 10.1016/0165-1781(89)90127-3. [DOI] [PubMed] [Google Scholar]
- Shriver DA, Long IP. A pharmacological comparison of some quaternary derivatives of cocaine. Arch. Int. Pharmacodyn. 1971;189:198–208. [PubMed] [Google Scholar]
- Spector R. Drug transport in the mammalian central nervous system: multiple complex systems. A critical analysis and commentary. Pharmacology. 2000;60:58–73. doi: 10.1159/000028349. [DOI] [PubMed] [Google Scholar]
- Steinfels GF, Heym J, Stricker RE, Jacobs BL. Behavioral correlates of dopaminergic unit activity in freely moving cats. Brain Res. 1983;258:217–228. doi: 10.1016/0006-8993(83)91145-9. [DOI] [PubMed] [Google Scholar]
- Strecker RE, Jacobs BL. Substantia nigra dopaminergic unit activity in behaving cats: effect of arousal on spontaneous discharge and sensory evoked activity. Brain Res. 1985;361:339–350. doi: 10.1016/0006-8993(85)91304-6. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projection of the ventral tegmental area and adjacent regions: A combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res. Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Tassin JP, Blanc G, Glowinski J. Selective activation of the mesolimbic dopamine system by stress. Nature. 1976;263:242–244. doi: 10.1038/263242a0. [DOI] [PubMed] [Google Scholar]
- Trulson ME. Activity of dopamine-containing substantia nigra neurons in the freely moving cats. Neurosci. Biobehav. Rev. 1984;9:283–297. doi: 10.1016/0149-7634(85)90051-x. [DOI] [PubMed] [Google Scholar]
- Tsai CT, Nakamura S, Iwama K. Inhibition of neuronal activity of the substantia nigra by noxious stimuli and its modification by the caudate nucleus. Brain Res. 1980;195:299–311. doi: 10.1016/0006-8993(80)90066-9. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Wakazono Y, Kiyatkin EA. Electrophysiological evaluation of the time-course of dopamine uptake inhibition induced by intravenous cocaine at a reinforcing dose. Neuroscience. 2008;151:824–835. doi: 10.1016/j.neuroscience.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner G, Mountcastle VB. The variability of central neural activity in a sensory system, and its implications for the central reflection of sensory events. J. Neurophysiol. 1963;26:958–977. doi: 10.1152/jn.1963.26.6.958. [DOI] [PubMed] [Google Scholar]
- Werner G, Mountcastle VB. Neural activity in mechanoreceptive cutaneous afferents: stimulus-response relations, Weber functions, and information transmission. J. Neurophysiol. 1965;28:359–397. doi: 10.1152/jn.1965.28.2.359. [DOI] [PubMed] [Google Scholar]
- West MO. Anesthetics eliminate somatosensory-evoked discharges on neurons in the somatotopically organized sensorimotor striatum of the rat. J. Neurosci. 1998;18:9055–9068. doi: 10.1523/JNEUROSCI.18-21-09055.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol. Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Wu SN, Chang HD, Sung BJ. Cocaine-induced inhibition of ATP-sensitive K+ channels in rat ventricular myocytes and in heart-derived H9c2 cells. Basic Clin. Pharmacol. Toxicol. 2006;98:510–517. doi: 10.1111/j.1742-7843.2006.pto_354.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur. J. Neurosci. 2007;25:106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernig G, Giacomuzzi S, Riemer Y, Wakonigg G, Sturm K, Saria A. Intravenous drug injection habits: Drug users’ self-reports versus researchers’ perception. Pharmacology. 2003;68:49–56. doi: 10.1159/000068731. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Bunney BS, Shi W-X. Differential effects of cocaine on firing rate and pattern of dopamine neurons: Role of α1 receptors and comparison with L-Dopa and apomorphine. J. Pharmacol. Exp. Ther. 2006;317:196–201. doi: 10.1124/jpet.105.094045. [DOI] [PubMed] [Google Scholar]