Abstract
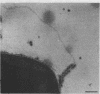
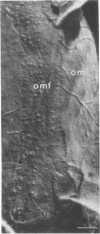
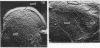
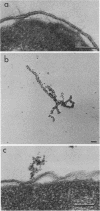
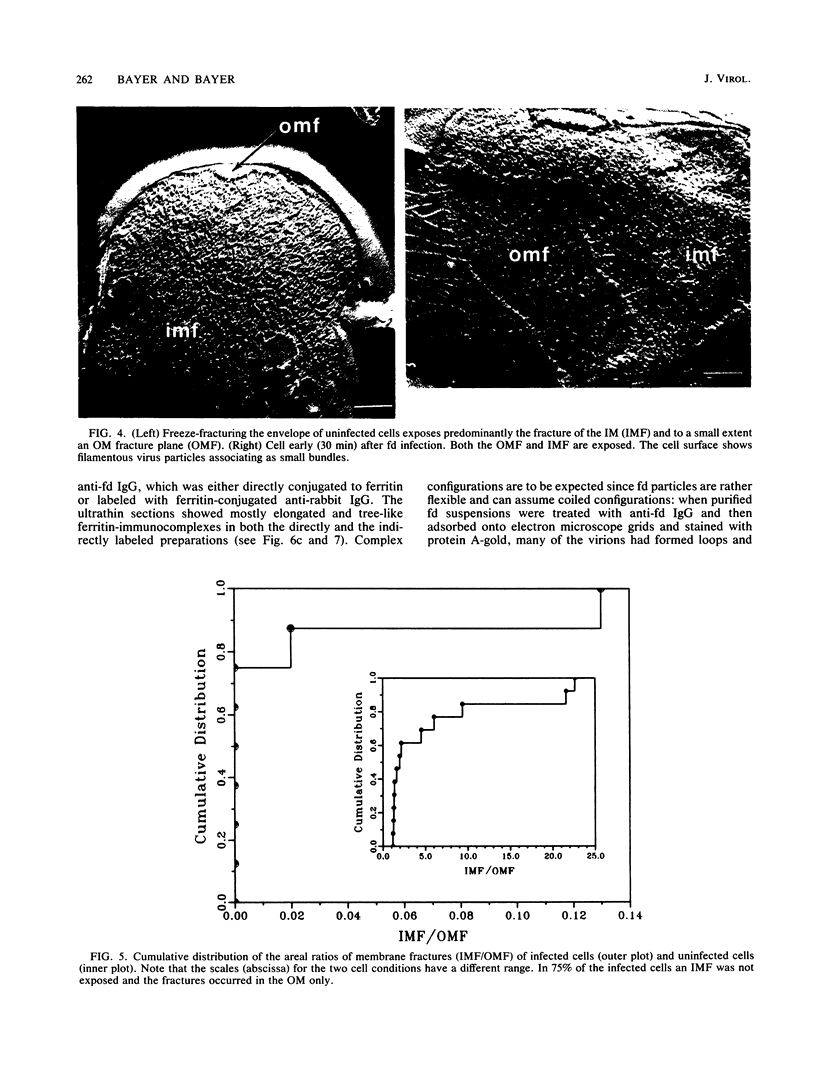
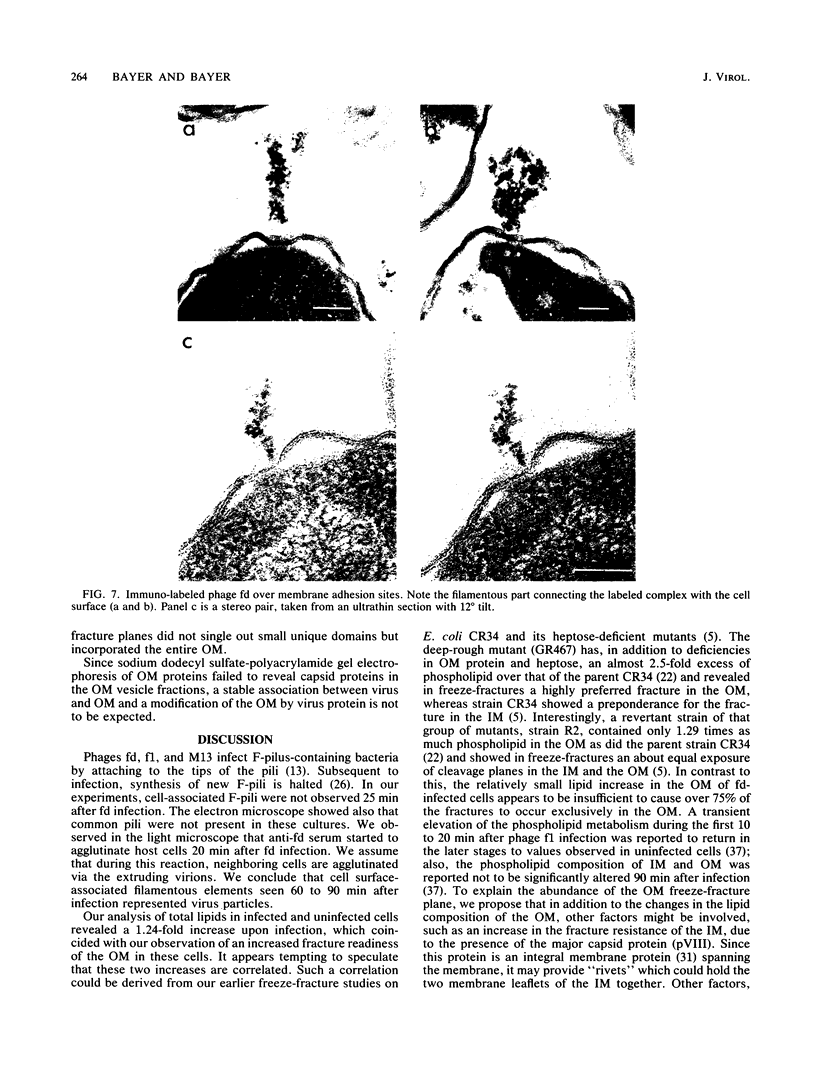
Phage fd-infected host bacteria revealed three characteristic changes in their envelope. (i) The preferred cleavage plane during freeze-fracturing shifted from the inner to the outer membrane (OM). (ii) The total lipids of the OM of the infected cells increased by 25% without major alterations in the relative concentration of phospholipids. We propose that such an increase would to some extent contribute to the change in the freeze-fracture behavior of the OM; however, additional factors will have to play a role in the apparent fracture resistance of the inner membrane. (iii) Ultrathin sectioning and immunolabeling methods revealed that extrusion of fd phages takes place at membrane adhesion sites of the infected cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Banner D. W., Nave C., Marvin D. A. Structure of the protein and DNA in fd filamentous bacterial virus. Nature. 1981 Feb 26;289(5800):814–816. doi: 10.1038/289814a0. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Cummings D. J. Structural aberrations in T-even bacteriophage. VIII. Surface morphology of T4 lollipops. Virology. 1977 Feb;76(2):767–780. doi: 10.1016/0042-6822(77)90257-4. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Koplow J., Goldfine H. Alterations in envelope structure of heptose-deficient mutants of Escherichia coli as revealed by freeze-etching. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5145–5149. doi: 10.1073/pnas.72.12.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Starkey T. W. The adsorption of bacteriophage phi X174 and its interaction with Escherichia coli; a kinetic and morphological study. Virology. 1972 Jul;49(1):236–256. doi: 10.1016/s0042-6822(72)80026-6. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Starkey T. W. The adsorption of bacteriophage phi X174 and its interaction with Escherichia coli; a kinetic and morphological study. Virology. 1972 Jul;49(1):236–256. doi: 10.1016/s0042-6822(72)80026-6. [DOI] [PubMed] [Google Scholar]
- Bayer M. E. Structural and functional evidence of cooperativity between membranes and cell wall in bacteria. Int Rev Cytol Suppl. 1981;12:39–70. doi: 10.1016/b978-0-12-364373-5.50012-3. [DOI] [PubMed] [Google Scholar]
- Bayer M. H., Bayer M. E. Phosphoglycerides and phospholipase C in membrane fractions of Escherichia coli B. J Bacteriol. 1985 Apr;162(1):50–54. doi: 10.1128/jb.162.1.50-54.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. H., Costello G. P., Bayer M. E. Isolation and partial characterization of membrane vesicles carrying markers of the membrane adhesion sites. J Bacteriol. 1982 Feb;149(2):758–767. doi: 10.1128/jb.149.2.758-767.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., Model P., Zinder N. D. Effects of bacteriophage f1 gene III protein on the host cell membrane. Mol Gen Genet. 1982;186(2):185–192. doi: 10.1007/BF00331849. [DOI] [PubMed] [Google Scholar]
- Bradley D. E., Dewar C. A. Intracellular changes in cells of Escherichia coli infected with a filamentous bacteriophage. J Gen Virol. 1967 Apr;1(2):179–188. doi: 10.1099/0022-1317-1-2-179. [DOI] [PubMed] [Google Scholar]
- Fodor S. P., Dunker A. K., Ng Y. C., Carsten D., Williams R. W. Lipid-tail group dependent structure of the fd gene 8 protein. Prog Clin Biol Res. 1981;64:441–455. [PubMed] [Google Scholar]
- Grant R. A., Webster R. E. Minor protein content of the gene V protein/phage single-stranded DNA complex of the filamentous bacteriophage f1. Virology. 1984 Mar;133(2):315–328. doi: 10.1016/0042-6822(84)90398-2. [DOI] [PubMed] [Google Scholar]
- HOFFMANN-BERLING H., DUERWALD H., BEULKE I. EIN FAEDIGER DNS-PHAGE (FD) UND EIN SPHAERISCHER RNS-PHAGE (FR) WIRTSSPEZIFISCH FUER MAENNLICHE STAEMME VON E. COLI. III. BIOLOGISCHES VERHALTEN VON FD UND FR. Z Naturforsch B. 1963 Nov;18:893–898. [PubMed] [Google Scholar]
- HOFSCHNEIDER P. H., PREUSS A. M 13 BACTERIOPHAGE LIBERATION FROM INTACT BACTERIA AS REVEALED BY ELECTRON MICROSCOPY. J Mol Biol. 1963 Oct;7:450–451. doi: 10.1016/s0022-2836(63)80038-8. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J., Webster R. E. Assembly site of bacteriophage f1 corresponds to adhesion zones between the inner and outer membranes of the host cell. J Bacteriol. 1985 Sep;163(3):1270–1274. doi: 10.1128/jb.163.3.1270-1274.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J., Webster R. E. Morphogenesis of filamentous bacteriophage f1: orientation of extrusion and production of polyphage. Virology. 1983 May;127(1):177–193. doi: 10.1016/0042-6822(83)90382-3. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanninga N. Ultrastructure of the cell envelope of Escherichia coli B after freeze-etching. J Bacteriol. 1970 Jan;101(1):297–303. doi: 10.1128/jb.101.1.297-303.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Russel M., Model P. A bacterial gene, fip, required for filamentous bacteriophage fl assembly. J Bacteriol. 1983 Jun;154(3):1064–1076. doi: 10.1128/jb.154.3.1064-1076.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilowitz H., Carson J., Robbins P. W. Association of newly synthesized major f1 coat protein with infected host cell inner membrane. J Supramol Struct. 1972;1(1):8–18. doi: 10.1002/jss.400010103. [DOI] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits M. A., Simons G., Konings R. N., Schoenmakers J. G. Expression of bacteriophage M13 dna in vivo. I. Synthesis of phage-specific RNA and protein in minicells. Biochim Biophys Acta. 1978 Nov 21;521(1):27–44. doi: 10.1016/0005-2787(78)90246-0. [DOI] [PubMed] [Google Scholar]
- Webster R. E., Cashman J. S. Abortive infection of Escherichia coli with the bacteriophage f1: cytoplasmic membrane proteins and the f1 DNA-gene 5 protein complex. Virology. 1973 Sep;55(1):20–38. doi: 10.1016/s0042-6822(73)81005-0. [DOI] [PubMed] [Google Scholar]
- Webster R. E., Grant R. A., Hamilton L. A. Orientation of the DNA in the filamentous bacteriophage f1. J Mol Biol. 1981 Oct 25;152(2):357–374. doi: 10.1016/0022-2836(81)90247-3. [DOI] [PubMed] [Google Scholar]
- Woolford J. L., Jr, Cashman J. S., Webster R. E. F1 Coat protein synthesis and altered phospholipid metabolism in f1 infected Escherichia coli. Virology. 1974 Apr;58(2):544–560. doi: 10.1016/0042-6822(74)90088-9. [DOI] [PubMed] [Google Scholar]