Abstract
A screen of indole-based structures revealed the natural product brassinin to be a moderate inhibitor of indoleamine 2,3-dioxygenase (IDO), a new cancer immunosuppression target. A structure-activity study was undertaken to determine which elements of the brassinin structure could be modified to enhance potency. Three important discoveries have been made which will impact future IDO inhibitor development: (1) the dithiocarbamate portion of the brassinin lead is a crucial moiety, which may be binding to the heme iron of IDO; (2) an indole ring is not necessary for IDO inhibition; (3) substitution of the S-methyl group of brassinin with large aromatic groups provides inhibitors that are three times more potent in vitro than the most commonly used IDO inhibitor, 1-methyl-tryptophan.
Introduction
Understanding tumor evasion of the host immune system has been a central focus of cancer research. Recently, the enzyme indoleamine 2,3-dioxygenase (IDO; EC 1.13.11.42) has been implicated in tumor immunosuppression.1 Several reports identify IDO as playing a role in undermining a more vigorous immune response to tumor growth. Consequently, we have been focused on identifying novel, potent IDO inhibitors with the goal of developing a new therapeutic approach to cancer treatment. IDO inhibitors alone or in combination with other chemotherapeutics might provide another tool in the oncology armamentarium.
IDO is an extrahepatic enzyme that catalyzes the initial and rate-limiting step in the degradation of tryptophan along the kynurenine pathway that leads to the biosynthesis of nicotinamide adenine dinucleotide (NAD+).2 IDO does not, however, handle dietary catabolism of tryptophan, which is instead the role of the structurally unrelated liver enzyme tryptophan dioxygenase (TDO2; 1.13.11.11). IDO is a monomeric 45 kDa heme-containing oxidase that is active with the heme iron in the ferrous (Fe+2) form. The ferric (Fe+3) form of IDO is inactive and substrate inhibition is believed to result from tryptophan (Trp) binding to ferric IDO.3 The primary catalytic cycle of IDO does not involve redox changes, nevertheless IDO is prone to autooxidation and therefore a reductant is necessary to reactivate the enzyme. In vivo, IDO purportedly relies on a flavin or tetrahydrobiopterin co-factor. In vitro, methylene blue and ascorbic acid are believed to substitute for the natural flavin or tetrahydrobiopterin co-factor.
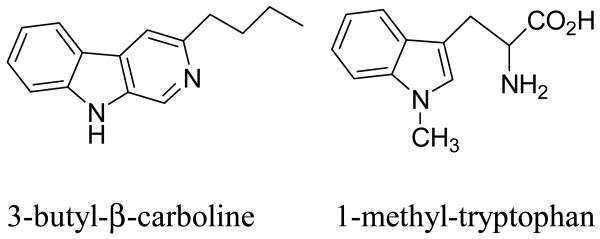
Inhibition of IDO has previously been targeted for other therapies, most notably neurological disorders.2b Several metabolites of the kynurenine pathway are neurotoxic or are implicated in neurodegeneration, e.g. quinolinic acid, and therefore attention has focused on IDO. A recent review4 summarizes the range of compounds that have been tested as IDO inhibitors. Strikingly, almost all IDO inhibitors, whether competitive or non-competitive, retain the indole ring of the natural substrate. Currently, the most potent IDO inhibitor reported is 3-butyl-β-carboline (Figure 1), a non-competitive inhibitor with a Ki=3.3 μM.5 However, the most commonly used IDO inhibitor is 1-methyl-tryptophan,6 (Figure 1) a commercially available compound that is a competitive inhibitor with a Ki=34 μM.
Figure 1.
IDO Inhibitors
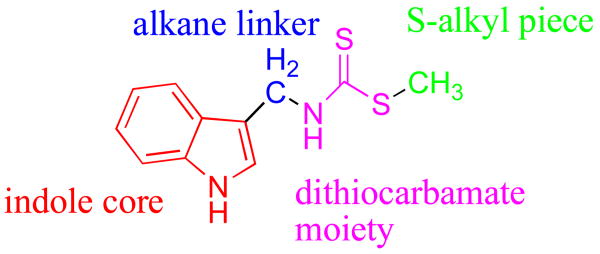
We undertook a screen of commercially available indole-based molecules to find novel IDO inhibitors. Interestingly, the natural product brassinin (1, Figure 2) was found to be a moderately active competitive inhibitor, Ki=97.7 μM. Brassinin is a phytoalexin in the cruciferous plants7 and has demonstrated some anti-fungal8 and anti-cancer activity.9 We undertook a structure-activity relationship study of brassinin with the goal of obtaining a more potent IDO inhibitor. We divided the brassinin structure into four components: the indole core, the alkane linker, the dithiocarbamate moiety and the S-alkyl piece. Analogs of brassinin that varied each of the four components were synthesized. The study determined the significance and flexibility of each of the four portions of the brassinin structure. The experimentation led to a more potent inhibitor and several important developments in the field of IDO inhibition. Details of this study and our findings are reported herein.
Figure 2.
Brassinin (1) Structure
Results and Discussion
Chemistry
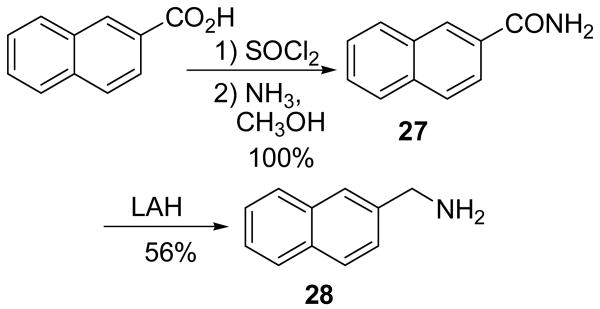
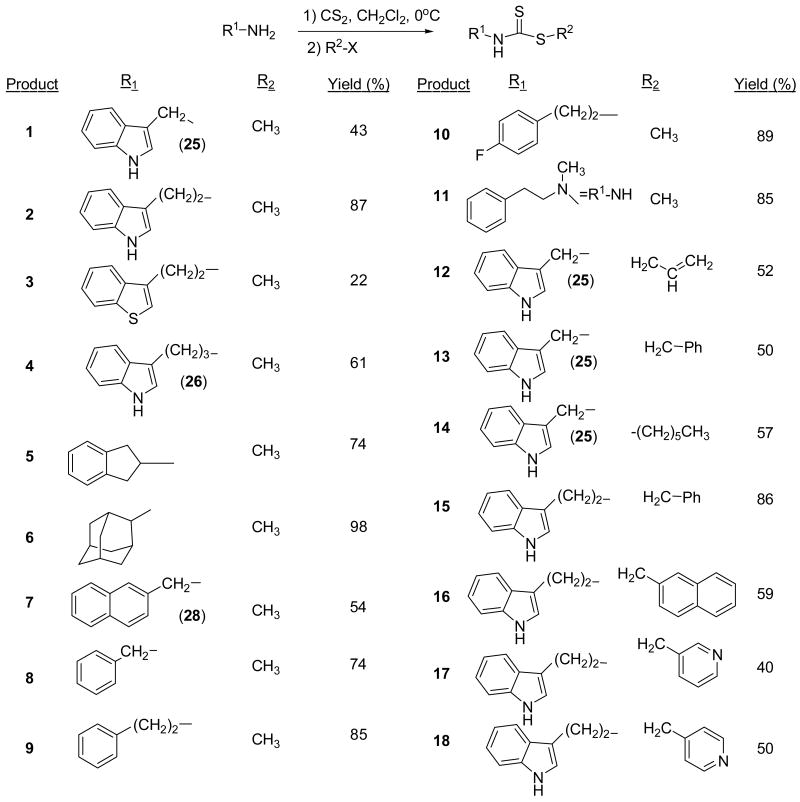
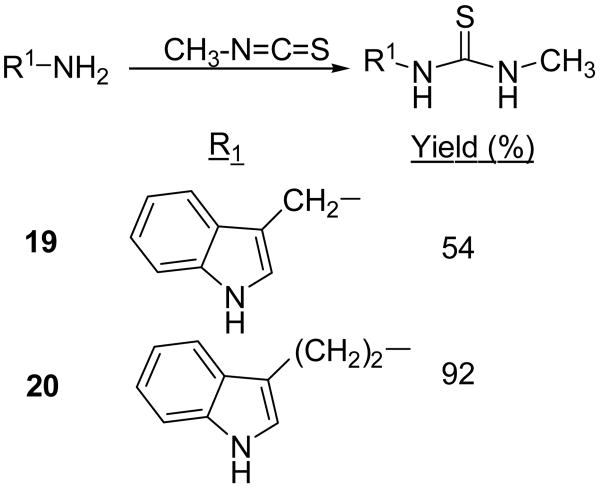
Brassinin dithiocarbamate analogs were synthesized by adding an amine to carbon disulfide at 0°C, stirring for one hour, and then adding an alkyl halide. Modification of the indole core or alkane linker occurred by adding different amines. Many amines were commercially available, although several required synthesis. The indole-3-methanamine 25 of brassinin 1 was prepared through the reductive amination of indole-3-carboxaldehyde. Although there are several different reductive amination procedures reported in the literature,9,10 we found Mehta's procedure9 to be the most effective. Homotryptamine 26, the amine reagent for 4, was synthesized in three steps from indole-3-propanoic acid following literature precedent.11 2-Aminomethyl-naphthalene 28 was also synthesized in three steps from 2-naphthoic acid (Scheme 2). Modifications of the S-alkyl piece occurred by substituting various alkyl halides for iodomethane, e.g. 12-18 (Scheme 1).
Scheme 2.
Scheme 1.
Dithiocarbamate Synthesis
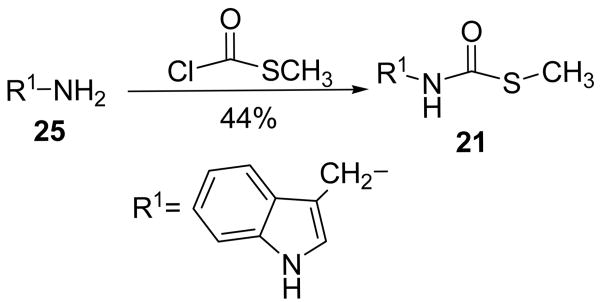
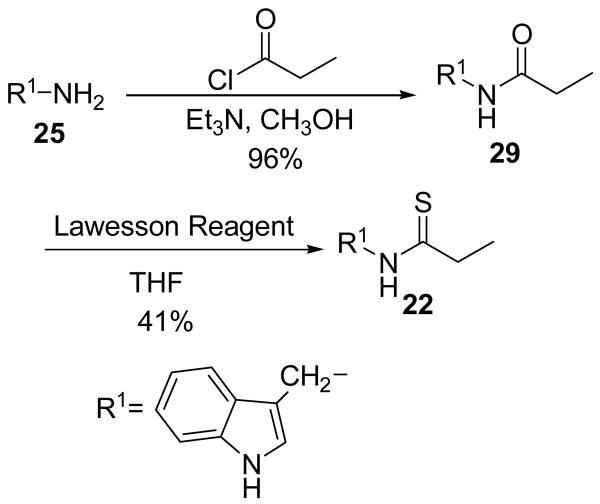
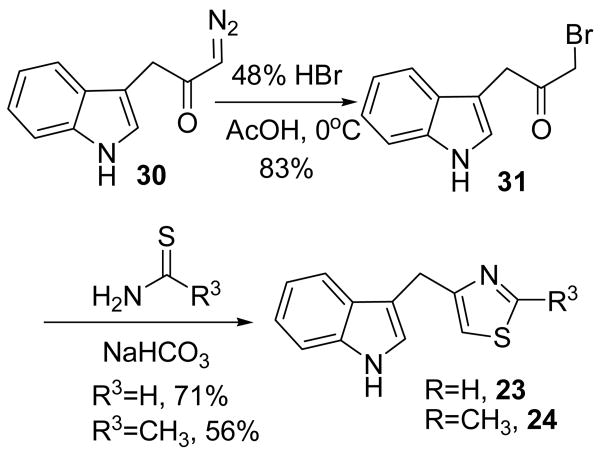
Modifications to the dithiocarbamate moiety included thioureas (19 and 20, Scheme 3), S-alkyl thiocarbamates (21, Scheme 4), thioamides (22, Scheme 5) and thiazoles (23 and 24, Scheme 6). The thioureas were synthesized by reacting amines with methyl isothiocyanate (Scheme 3).35 The S-alkyl thiocarbamate 21, a phytoalexin called brassitin, came from the reaction of S-alkyl thiochloroformate with 25 (Scheme 4). The thioamide 22 was synthesized by reaction of 25 with an acid chloride and then treatment with Lawesson's reagent (Scheme 5). Thiazoles were synthesized by reaction of thioformamide or thioacetamide with α-bromoketones 31 (Scheme 6). The α-bromoketones 31 were generated from the corresponding α-diazoketone derivative 30,12 which was synthesized in three steps from indole-3-acetic acid following a literature procedure.13
Scheme 3.
Thiourea Synthesis
Scheme 4.
Brassitin Synthesis
Scheme 5.
Thioamide Synthesis
Scheme 6.
Thiazole Synthesis
Enzyme studies
Brassinin analogs were analyzed for inhibition of extracted and purified γ-interferon-induced human IDO. The assay was conducted according to a literature protocol,14 with ascorbic acid and methylene blue serving the role of reductant.15 Catalase was added to prevent IDO decomposition from peroxide side products.16 The enzyme assay monitored for formation of N-formylkynurenine by hydrolyzing the formyl group and spectrophotometrically analyzing for the conjugated imine generated from kynurenine and 4-(dimethylamino)benzaldehyde. In all cases where inhibition was seen, the brassinin analogs demonstrated competitive inhibition. The inhibitory constants shown are an average of two or three trials. The array of analogs tested allowed for an evaluation of the four components of the brassinin structure and resulted in some important discoveries (Table 1).
Table 1.
IDO Inhibition Data
| Compound | Ki (μM) | Compound | Ki (μM) |
|---|---|---|---|
| 1 | 97.7 | 13 | 13.22 |
| 2 | 82.54 | 14 | 363.6 |
| 3 | 40.95 | 15 | 17.15 |
| 4 | 33.97 | 16 | 11.55 |
| 5 | 42.06 | 17 | 28.38 |
| 6 | 179.6 | 18 | 20.48 |
| 7 | 47.57 | 19 | N. I.a |
| 8 | 72.41 | 20 | 342.3 |
| 9 | 62.36 | 21 | N. I. |
| 10 | 149.35 | 22 | 202 |
| 11 | 1267 | 23 | 1292 |
| 12 | 36.95 | 24 | 328.7 |
N. I.=no inhibition detected
Variation of the indole core
One of the most suprising discoveries was the range of groups that could be substituted for indole and still retain some IDO inhibitory activity. Indeed, not only were flat aromatic structures (e.g. 5, 7-10) effective substitutes, but the adamantyl structure 6 could also bind in the substrate pocket, based on the competitive inhibition witnessed for all these analogs. Although IDO is relatively promiscuous, there are still very few reports of substrates or inhibitors that lack the indole core.4 The current demonstration of inhibition with benzene and cycloalkyl based structures expands the range of structures that behave as IDO inhibitors. Furthermore, benzene aromatic structures are more easily derivatized with available synthetic methods than indole compounds. Indole derivatives can also be a liability given the neuroactivity of some indole containing compounds, e.g. serotonin and related indolealkylamines.
Variation of the alkane linker
Linker variation was possible and, in the brassinin series, it was found that the longer linker lead to more potent compounds, c.f. 1 vs. 2 vs. 4. However, analogs that modified two brassinin components did not replicate this trend (c.f. 13 vs. 15). Taken together the results with the alkane linker modifications and the indole core changes indicate the IDO active site is rather accommodating.
Variation of the dithiocarbamate
The most interesting results came from isosteric modifications of the dithiocarbamate. The transformation of brassinin's dithiocarbamate moiety to a thiourea (19, 20), thiocarbamate (21), thioamide (22) or thiazole (23, 24) led to weaker or no inhibition. Notably the S-methyl-thiocarbamate analog 21 (brassitin29), suffered a complete loss in inhibitory activity with the substitution of a carbonyl for the thiocarbonyl group. Given the recognized metal coordinating properties of dithiocarbamates,17,18 it is likely that the dithiocarbamate moiety is chelating to the heme iron at the active site of IDO.19 In fact, pyrrolidine dithiocarbamate, reportedly inhibits IDO,17a besides being a well-known anti-oxidant and NF-kB inhibitor.20
Computational experiments to explore electronic nature of dithiocarbamate
If the dithiocarbamate moiety is binding to the heme iron, then the electronic nature or charge of the group should be important to achieve optimum binding. We performed several computational experiments to understand the electronic nature of the dithiocarbamate sulfur versus the sulfur/oxygen in the isosteric analogs. To reduce the computational time, most of the experiments involved simplified analogs 32-36 (Figure 3), which lacked the indole ring. Figure 4 shows the molecular electrostatic potential mapped onto the electron distribution of 32, the dithiocarbamate analog. Clearly the sulfur of 32 projects the greatest electron density and therefore is the richest and most available Lewis basic site for iron binding.
Figure 3.
Dithiocarbamate Analogs
Figure 4.
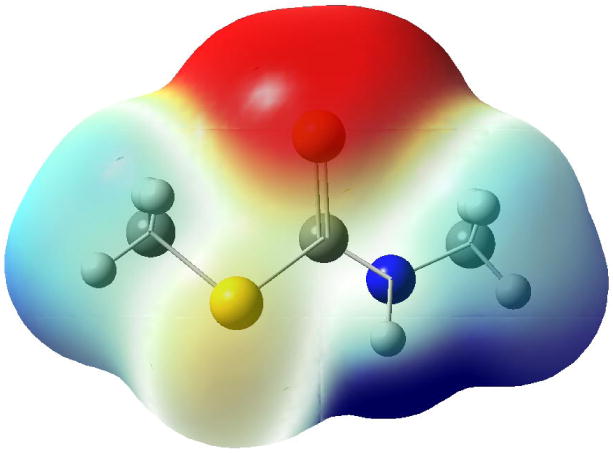
Molecular electrostatic potential mapped onto electron distribution for 32
In Table 2, the electrostatic potential (ESP) and natural bond order (NBO) charge values were derived for 32-36. Elimination of the indole group allowed for more rapid computational experiments and did not affect the trend witnessed.21 For compounds 32-35 a clear trend can be seen for both ESP and NBO charge calculations: the smaller the charge on sulfur/oxygen, the better the inhibition. Compound 36 breaks the trend; however the conformationally rigid thiazole ring may prevent an effective interaction between the sulfur of the thiazole and the heme iron. The trend witnessed with compounds 32-35 may seem counterintuitive, i.e. a more electron rich sulfur/oxygen should be a better electron donor to the Lewis acid heme iron. Nevertheless, optimum inhibition may arise from a softer Lewis base coordinating with the softer active ferrous form of the enzyme.22 Future inhibitor design will be guided by the insights from these computational experiments.
Table 2.
Charge Data for Dithiocarbamate Analogs
| Compound | ESP Charge | NBO Charge | Ki of related analog, μM (compound no.) |
|---|---|---|---|
| 32 | -0.466 | -0.277 | 97.7 (1) |
| 33 | -0.518 | -0.318 | 202 (22) |
| 34 | -0.550 | -0.390 | N. I. (19) |
| 35 | -0.661 | -0.711 | N. I. (21) |
| 36 | -0.133 | 0.290 | 1292 (23) |
Variation of the S-alkyl piece
The greatest increases in potency were realized in modifications of the S-alkyl group. Although alkyl groups that were longer than the methyl in brassinin were less active, S-allyl brassinin 12 was two times more potent than brassinin and the benzyl analog 13 was almost one order of magnitude more potent. Moreover, the tryptamine/naphthyl analog 16 was as potent as 13; pyridyl analogs 17 and 18 also demonstrated modest inhibition. Since all these compounds behaved as competitive inhibitors, these analogs reveal a large additional pocket in the IDO active site capable of accommodating flat, aromatic groups.
Sono has reported that β-carboline, a non-competitive inhibitor, binds to the heme iron at the active site, but not in the same space as the substrate.23 Moreover, the β-carboline reportedly acts as a nitrogen donor ligand and competes with O2 for binding to the heme iron. It is possible that the large aromatic S-alkyl pieces are binding in the same pocket that accommodates the tricyclic aromatic β-carboline structure. Nevertheless, the pyridyl analogs 17 and 18 failed to demonstrate stronger inhibition despite their similarity to the pyridyl ring of β-carboline.
Conclusion
A systematic study of the IDO inhibitory activity of brassinin has been undertaken and three important discoveries have been made. Contrary to most previously reported IDO inhibitors, an indole ring was not necessary for inhibitory activity with the dithiocarbamate analogs of brassinin. Although indole-containing derivatives were still the most active inhibitors (i.e. 13 and 16), the inhibitory activity retained by analogs, such as 5, 7, 8 and 9, create new opportunities to further inhibitor development. Importantly, new analogs might be possible that avoid the pharmacological liabilities of the indole ring and leverage the wealth of chemical methods for benzene substitution. The dithiocarbamate moiety is an optimum group for IDO inhibition and probably chelates to the active site iron. Large unsaturated groups on the dithiocarbamate sulfur can be accommodated in the active site and lead to more potent inhibitors of IDO. Although only small increases in potency were achieved through the structure-activity study, the new inhibitors (i.e. 13 and 16) are three times more potent than the most commonly used IDO inhibitor. In addition, the SAR discoveries should greatly advance the search for more potent IDO inhibitors, an exciting new cancer target.
Experimental Section
Chemistry
All reactants and reagents were commercially available and were used without further purification unless otherwise indicated. Anhydrous THF was obtained by distillation from benzophenone-sodium under argon immediately before use. Anhydrous CH2Cl2 and Et3N were obtained by distillation from calcium hydride under argon. Methanol was dried over Mg and distilled under argon. A saturated solution of HCl in CH3OH was made by bubbling HCl through a drying tube, filled with CaCl2, into a cooled flask of anhydrous CH3OH under a stream of argon. A saturated solution of NH3 in CH3OH was made by bubbling anhydrous NH3 into an Erlenmyer flask with a predetermined volume of CH3OH. Concentrated refers to the removal of solvent with a rotary evaporator at normal water aspirator pressure followed by further evacuation with a two-stage mechanical pump unless otherwise indicated. Yields refer to chromatographically and spectroscopically pure (>95%) compounds, except as otherwise indicated. All new compounds were determined to be >95% pure by NMR, HPLC and/or GC as indicted (see supplementary materials). Melting points were determined using an open capillary and are uncorrected. 1H and 13C NMR spectra were recorded at 300 and 75 MHz, respectively. Chemical shifts are reported in δ values (ppm) relative to an internal reference (0.05% v/v) of tetramethylsilane (TMS) for 1H NMR and the solvent peak in 13C NMR, except where noted. Peak splitting patterns in the NMR are reported as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad. Rotamer peaks (about 1/4 intensity) were seen for all dithiocarbamate structures.24 Normal phase HPLC (NP-HPLC) analysis was performed with UV detection at 254 nm and a 5μ silica gel column (250 × 4.6 mm); eluted with 90:10 n-hexane:IPA (or gradient) at 1 mL/min. Reverse phase HPLC (RP-HPLC) analysis was performed with UV detection at 254 nm and a C18 column (300 × 3.9 mm); eluted with a gradient of H2O + 0.1% TFA and CH3CN + 0.1% TFA at 1 mL/min., unless otherwise indicated. GC analyses were performed with an EI-MS detector fitted with a 30 m × 0.25 mm column filled with crosslinked 5% PH ME siloxane (0.25 μm film thickness); gas pressure 7.63 psi He. IR data was obtained with an FT-IR spectrometer. Thin layer chromatography was performed using silica gel 60 A precoated glass backed plates (0.25 mm thickness) with fluorescent indicator, which were scored and cut. Developed TLC plates were visualized with UV light (254 nm), iodine or KMnO4. Flash column chromatography was conducted with the indicated solvent system using normal phase silica gel 60 A, 230-400 mesh. All reactions were carried out under an inert atmosphere of argon or nitrogen unless otherwise indicated.
Indole-3-methanamine (25)
Indole-3-carboxaldehyde (189 mg, 1.3 mmol) and NH4OH • HCl (113 mg, 1.63 mmol) were dissolved in a Parr flask with 15 mL of MeOH, which was previously saturated with anhydrous ammonia. The flask was stoppered and placed on a Parr shaker for 5 hours. To the resulting solution was added 200 mg of Raney Nickel (50% slurry in H2O) and the flask was pressurized to 60 psi with H2 and allowed to shake overnight. The next day, the resulting mixture was filtered through celite and volatiles were removed to yield a yellow solid, 190 mg (100% yield). The product was unstable, so it was used immediately in subsequent reactions without further purification. 1H NMR (CDCl3/CD3OD) δ 8.04 (br s, 1H, NH), 7.67 (d, 1H, ArH, J = 7.8), 7.39 (d, 1H, ArH, J = 7.1), 7.16 (3H, ArH), 4.07 (d, 2H, ArCH2 J = 7.4 Hz).
General Method for the Synthesis of Dithiocarbamates
The amine (1.0 eq.) was dissolved in pyridine (2-3 mL) and the solution was cooled to 0° C. Triethylamine (1.0-1.1 eq) and carbon disulfide (1.1 eq) were added and the solution was stirred at 0° C. After 30 minutes, iodomethane (1.0-1.2 eq) was added and the reaction was allowed to slowly warm to rt overnight. The reaction was poured into 1M H2SO4 and extracted with EtOAc (3x). The organic layer was washed with brine, dried with Na2SO4, and filtered. Concentration afforded a crude product that was chromatographed as described.
Brassinin (1)
Brassinin was formed from 25 according to the general method. The crude yellow solid was chromatographed on silica with EtOAc/hexanes (1:3) and the resulting yellow solid was further purified by recrystallization from CH2Cl2/hexanes to yield rose colored crystals (43% yd). 1H and 13C NMR spectra, IR spectrum and m.p. matched a previous report for 1.25
N-[2-(Indol-3-yl)ethyl]-S-methyl-dithiocarbamate (2).26
The general method was used with tryptamine and CH2Cl2 as the solvent. The crude product was chromatographed with CH2Cl2/hexanes (2:1) to afford a waxy yellow-white solid (87% yield). m.p.=59-64°C. 1H NMR (CDCl3) δ 8.05 (br s, 1H, NH), 7.61 (d, 1H, ArH, J=7.8 Hz), 7.36 (d, 1H, ArH, J=8.0 Hz), 7.22 (dt, 1H, ArH, J=8, 1 Hz), 7.14 (dt, 1H, ArH, J=8, 1 Hz), 7.01 (br s, 1H, NH), 4.05 (q, 2H, CH2NH, J=12.4, 6.6 Hz), 3.11 (t, 2H, ArCH2, J=6.7 Hz), 2.56 (s, 3H, CH3) and signals due to a minor rotamer (ca. 29%) at 3.73 (m), 2.68 (s). 13C NMR (CDCl3) δ 198.8, 136.4, 127.1, 122.4, 122.1, 119.7, 118.7, 112.3, 111.3, 47.2, 23.9, 18.0, and signals due to a minor rotamer at 201.6, 126.8, 118.4, 46.1, 24.6, 18.9. GC: r.t.=15.00 min. EI-MS m/z (%) 202 (27, M+-SCH3), 143 (4), 130 (100). NP-HPLC r.t.=5.9 min.
N-[2-(benzo[b]thiophen-3-yl)ethyl]-S-methyl-dithiocarbamate (3)
The general method was used with 2-(benzo[b]thiophen-3-yl)ethanamine and CH2Cl2 as the solvent. The crude product was chromatographed with EtOAc/hexanes (1:9) to yield a light amber oil which slowly crystallized (22% yield). m.p.=81-84°C. 1H NMR (CDCl3) δ 7.90 (m, 1H, ArH), 7.79 (m, 1H, ArH), 7.43 (m, 3H, ArH), 7.02 (br s, 1H, NH), 5.18 (d, 2H, ArCH2CH2, J = 4.17 Hz), 2.63 (m, 5H, SCH3 overlapping with ArCH2) and a signal due to a minor rotamer (ca. 17%) at 4.91 (m). 13C NMR (CDCl3) δ 199.2, 140.6, 137.7, 130.7, 125.9, 124.9, 124.7, 123.1, 121.7, 45.1, 18.3, 14.2 and signals due to minor rotamer peaks at 60.4, 21.0. IR (KBr) vmax cm-1: 3336, 3229, 3079, 2995, 2916, 1499, 1379, 1302, 1075, 926. NP-HPLC r.t.= 4.8 min. RP-HPLC r.t.= 12.1 min.
N-[3-(Indol-3-yl)propyl]-S-methyl-dithiocarbamate (4)
The general method was used with 3-(indol-3-yl)-propan-1-amine, 2 eq. of Et3N and MeOH as the solvent. After the reaction was complete, the volatiles were removed and the residue was dissolved in EtOAc (60 mL). The solution was washed with 0.5M HCl (2 × 30 mL), H2O (20 mL), and brine (20 mL). The organic solution was dried with Na2SO4, filtered and concentrated. The crude product was chromatographed with EtOAc/hexanes (1:3) to yield an off-white oil which crystallized overnight (61% yield). m.p.=54-56°C. 1H NMR (CDCl3) δ 8.04 (br s, 1H, NH), 7.60 (d, 1H, ArH, J = 7.6), 7.35 (d, 1H, ArH, J = 8.0), 7.20 (m, 1H, ArH), 7.12 (m, 1H, ArH), 7.04 (m, 1H, ArH), 6.91 (br s, 1H, NH), 3.82 (q, 2H, ArCH2CH2CH2, J = 7.0 Hz), 2.86 (t. 2H, ArCH2CH2J = 7.2 Hz), 2.52 (s, 3H, SCH3), 2.1 (m, 2H, ArCH2CH2) and signals due to a minor rotamer (ca. 30%) 3.50 (q, J=6.3 Hz), 2.68 (s). 13C NMR (CDCl3) δ 198.7, 136.4, 127.1, 122.2, 121.6, 119.4, 118.7, 115.1, 111.3, 47.2, 28.3, 22.7, 18.0 and signals due to a minor rotamer at 46.0, 28.9, 18.7. IR (KBr) vmax cm-1: 3410, 3321, 2919, 1888, 1504, 1337, 1094. EI-MS: m/z (%) 216 (57, M+−SCH3), 183 (5), 156 (10), 131 (23). NP-HPLC r.t.= 12.3 min. RP-HPLC r.t.= 11.9 min.
N-(Indan-2-yl)-S-methyl-dithiocarbamate (5)
The general method was used with 2-aminoindan HCl. The crude product in EtOAc was decolorized with charcoal, filtered through celite and the volatiles were removed to yield a clear oil. The oil was chromatographed with EtOAc/hexanes (1:9) to yield an off-white solid (74% yield). m.p.=106-108°C. 1H NMR (CDCl3) δ 7.23 (4H, ArH), 7.10 (br s, 1H, NH), 5.31 (m, 1H, CH2CHCH2), 3.44 (m, 2H, CHCHCH), 2.98 (dd, 2H, CHCHCH, J = 16.5 Hz, 3.7 Hz), 2.62 (s, 3H, SCH3) and signals due to a minor rotamer (ca. 38%) 4.78 (m), 2.70 (s). 13C NMR (CDCl3) δ 198.6, 140.5, 127.0, 124.9, 57.9, 39.4, 18.2 and signals due to a minor rotamer at 57.1, 39.8, 18.5. IR (KBr) vmax cm-1: 3226, 2948, 2916, 2088, 1483, 1371, 1337, 1070. NP-HPLC r.t.= 4.5 min; RP-HPLC r.t.= 12.2 min.
N-(Adamant-2-yl)-S-methyl-dithiocarbamate (6)
The general method was used with 2-adamantylamine HCl and 2 eq. of Et3N to afford a white solid (98% yield). m.p. 128-129°C. 1H NMR (CDCl3) δ 7.25 (br s, 1H, NH), 4.65 (t, 1H, CHNH, J = 3.6 Hz), 2.63 (s, 3H, SCH3), 2.13 (m, 2H, CH2), 1.73 (m, 12H, CH2) and signals due to a minor rotamer (ca. 36%) 4.08 (m), 2.68 (s). 13C NMR (CDCl3) δ 197.8, 97.5, 61.1, 37.3, 32.7, 31.4, 27.4, 18.5 and signals due to a minor rotamer at 37.8, 32.0, 27.3, 19.3. IR (KBr) vmax cm-1: 3351, 2918, 2852, 1497, 1384, 1117, 942. NP-HPLC r.t.= 4.1 min. RP-HPLC r.t.= 13.2 min.
N-[(Naphth-2-yl)methyl]-S-methyl-dithiocarbamate (7)
The general method was used with 28, 2 eq. of Et3N and MeOH as the solvent. The crude product was chromatographed on silica with EtOAc/hexanes (15:85) to yield a yellow solid (54% yield). m.p. 70-72°C. 1H NMR, 13C NMR, and IR spectra matched a previous report for 7.27
N-Benzyl-S-methyl-dithiocarbamate (8)
The general method was used with benzylamine and the crude product was chromatographed with EtOAc/hexanes (1:10) to yield an off-white oil (74% yield). 1H NMR, 13C NMR, and IR spectra matched a previous report for 8.28
N-Phenethyl-S-methyl-dithiocarbamate (9)
The general method was used with phenethylamine and the crude product was chromatographed with EtOAc/hexanes (1:10) to yield an off-white solid (85% yield). m.p. 50-51°C. 1H NMR (CDCl3) δ 7.28 (5 overlapping H, ArH), 6.91 (br s, 1H, NH2), 4.02 (t, 2H, ArCH2CH2, J = 6.9), 2.98 (t, 2H, ArCH2CH2, J = 7.0), 2.61 (s, 3H, SCH3) and signals due to a minor rotamer (ca. 24%) at 3.71 (m), 2.69 (s). 13C NMR (CDCl3) δ 199.1, 138.2, 128.8, 128.7, 126.8, 48.0, 34.2, 18.1 and signals due to a minor rotamer at 47.3, 34.9, 18.5. IR (KBr) vmax cm-1: 3340, 3240, 3026, 2918, 1946, 1496, 1337, 1095. NP-HPLC r.t.= 4.6 min. RP-HPLC r.t.= 11.5 min.
N-4-Fluorophenethyl-S-methyl-dithiocarbamate (10)
The general method was used with 4-fluorophenethylamine and the solvent was CH2Cl2. The volatiles were removed and the residue was dissolved in EtOAc. The organic layer was washed with 1M H2SO4 (40 mL), H2O (40 mL) and brine (30 mL). The resulting organic solution was dried with Na2SO4 and filtered. The volatiles were removed to yield a beige solid which was chromatographed with EtOAc/hexanes (8/92) to yield a white solid (89% yield). m.p. 59-60°C. 1H NMR (CDCl3) δ 7.18 (m, 2H, ArH), 7.01 (m, 2H, ArH), 3.96 (q, 2H, ArCH2CH2, J = 7.0 Hz), 2.96 (t, 2H, ArCH2, J = 7.1 Hz), 2.62 (s, 3H, SCH3) and signals due to a minor rotamer (ca. 24%) at 3.69 (q, J=6.6 Hz), 2.68 (s). 13C NMR (CDCl3) δ 199.4, 161.8 (d, J=243 Hz), 133.9, 130.2, 115.8, 48.0, 33.5, 18.1 and signals due to a minor rotamer at 130.1, 47.2, 30.9, 18.5. IR (KBr) vmax cm-1: 3250, 3002, 2921, 1886, 1506, 1385, 1222, 940.8. NP-HPLC r.t.= 5.1 min. RP-HPLC r.t.= 11.6 min.
N,S-Dimethyl-N-phenethyldithiocarbamate (11)
The general method was used with N-methylphenthylamine and the solvent was CH2Cl2. The crude product was chromatographed with EtOAc/hexanes (1/19) to yield a white oil (85% yield). 1H NMR (CDCl3) δ 7.29 (m, 5H, ArH), 4.25 (t, 2H, ArCH2CH2, J = 6.9 Hz), 3.20 (s, 3H, NCH3), 3.01 (q, 2H, ArCH2, J = 6.8 Hz), 2.66 (s, 3H SCH3) and signals due to a minor rotamer (ca. 42%) at 3.89 (m), 3.47 (s). 13C NMR (CDCl3) δ 198.6, 138.9, 138.1, 129.3, 129.2, 129.1, 127.0, 59.5, 40.9, 32.9, 20.7 and signals due to a minor rotamer at 56.6, 44.6, 34.0. IR (KBr) vmax cm-1: 3025, 2917, 1949, 1808, 1485, 1386, 1292, 1185, 1100, 992.5. NP-HPLC r.t.= 4.2 min. RP-HPLC r.t.= 12.7 min.
S-Allyl-brassinin(12)
The general method was used with 25, but allyl bromide was substituted for iodomethane. The crude product was purified by chromatography on silica with EtOAc/hexanes (3/7) to afford an orange oil (52% yield). 1H NMR (CDCl3) δ 8.17 (br s, 1H, NH), 7.64 (d, 1H, ArH, J = 7.8 Hz), 7.43 (d, 1H, ArH, J = 8.1 Hz), 7.21 (3H, ArH), 7.03 (br s, 1H, NH), 5.93 (m, 1H, SCH2CH=CH2), 5.22 (m, 2H, SCH2CH=CH2), 5.05 (d, 2H, ArCH2, J = 4.4 Hz), 3.92 (d, 2H, SCH2CH=CH2, J = 7.7 Hz) and signals due to a minor rotamer (ca. 16%) at 4.69 (m), 4.09 (d, J=7 Hz). 13C NMR (CDCl3) δ 196.3, 136.2, 132.7, 126.4, 122.7, 120.2, 118.6, 118.5, 111.5, 110.3, 43.1, 38.3 and signals due to a minor rotamer at 41.0, 39.5. IR (KBr) vmax cm-1: 3402, 2915, 1852, 1635, 1377, 1063. NP-HPLC r.t.= 6.7 min. RP-HPLC r.t.= 11.6 min.
S-Benzyl-brassinin (13)
The general method was used with 25, but benzyl bromide was substituted for iodomethane and CH2Cl2 was used as the solvent. The crude product was chromatographed on silica EtOAc/hexanes (3/7) to yield a translucent, yellow oil which slowly solidified. Recrystallization from CH2Cl2/hexanes yielded a bright yellow solid (50% yield). m.p. 101-102°C. 1H NMR (CDCl3) δ 8.22 (br s, 1H, NH), 7.62 (d, 1H, ArH, J = 7.9 Hz), 7.28 (9H, ArH + PhH), 6.98 (br s, 1H, NH), 5.11 (d, 2H, ArCH2, J = 3.9 Hz), 4.55 (s, 2H, CH2Ph) and signals due to a minor rotamer (ca.19%) at 4.77 (d, J=4.5 Hz), 4.67 (s). 13C NMR (CDCl3) δ 196.4, 136.6, 136.3, 129.0, 128.6, 127.5, 126.5, 124.0, 122.8, 120.3, 118.7, 111.4, 110.7, 43.2, 39.9. IR (KBr) vmax cm-1: 3417, 3334, 3058, 1890, 1494, 1455, 1067. NP-HPLC, r.t.= 6.8 min. RP-HPLC, r.t.= 12.5 min.
S-Hexyl-brassinin (14)
The general method was used with 25, but 1-iodohexane was substituted for iodomethane. The crude product was chromatographed on silica with EtOAc/hexanes (3/7) to yield a golden oil (57% yield). 1H NMR (CDCl3) δ 8.18 (br s, 1H, NH), 7.65 (d, 1H, ArH, J = 7.8 Hz), 7.43 (d, 1H, ArH, 8.1 Hz), 7.22 (3H, ArH), 6.99 (br s, 1H, NH), 5.06 (d, 2H, ArCH2, J = 4.4 Hz), 3.26 (t, 2H, SCH2, J = 7.5 Hz), 1.70 (m, 2H, SCH2CH2CH2CH2CH2CH3), 1.39 (6H, SCH2CH2CH2CH2CH2CH3), 0.88 (t, 3H, CH3, J = 7.5 Hz) and signals due to a minor rotamer (ca. 19%) at 4.79 (d, J=4.8 Hz), 3.39 (t, J=7.5 Hz). 13C NMR (CDCl3) δ 197.6, 136.2, 126.4, 124.0, 122.6, 120.1, 118.6, 111.5, 110.5, 43.0, 35.4, 29.0, 28.5, 22.5, 14.0 and signals due to a minor rotamer at 42.0, 36.5. IR (KBr) vmax cm-1: 3409, 3328, 2955, 2927, 2855, 1620, 1494, 1456, 1379, 1094. NP-HPLC r.t.= 6.0 min. RP-HPLC r.t.= 13.8 min.
N-[2-(indol-3-yl)ethyl]-S-benzyl-dithiocarbamate (15)
The general method was used with tryptamine as the amine and CH2Cl2 as the solvent. Benzyl bromide was used as the alkylating agent in place of iodomethane. The crude product was chromatographed with EtOAc/hexanes (1:4) to yield white crystals (86% yield). Further purification was accomplished by recrystallization in EtOAc/hexanes to afford a 73% yield. m.p.=79-81°C. 1H NMR (CDCl3) δ 8.02 (br s, 1 H, NH), 7.58 (m, 1 H, ArH), 7.37 (m, 6 H, ArH), 7.32 – 7.18 (m, 1 H, ArH), 7.13 (t, 1 H, ArH, J = 9.0 Hz), 6.99 (m, 2 H, ArH), 4.48 (s, 2 H, SCH2), 4.05 (q, J = 6.0 Hz, 1 H, ArCH2CH2), 3.09 (m, 2 H, ArCH2), and signals due to a minor rotamer (ca. 24%) at 4.59 (s), 3.74 (q, J = 6.0 Hz). 13C NMR (CDCl3) δ 197.2, 136.5, 136.3, 129.3, 128.9, 128.6, 127.6, 127.4, 127.1, 122.4, 122.1, 119.7, 118.7, 112.2, 111.3, 47.2, 39.8, 24.6, 23.9, and signals due to a minor rotamer at 135.7, 127.6, 118.4, 41.0, 24.6. IR (KBr) vmax cm-1: 3394, 3179, 1618, 1503, 1455, 1332, 1095, 936. EI-MS: m/z (%) 130 (100), 202 (37). GC: r.t. 14.8 minutes. NP-HPLC: r.t. 7.6 min. RP-HPLC: r.t. 12.9 min. Anal. Calcd for C18H18N2S2: C, 66.22; H, 5.56; N, 8.58; S, 19.64. Found: C, 66.19; H, 5.43; N, 8.42; S, 19.87.
N-[2-(indol-3-yl)ethyl]-S-[(naphth-2-yl)methyl]-dithiocarbamate (16)
The general method was used with tryptamine as the amine and CH2Cl2 as the solvent. 2-(Bromomethyl)naphthalene was used as the alkylating agent in place of iodomethane. The crude product was chromatographed with EtOAc/hexanes (1:4) to afford the pure product (59% yield). Further purification was accomplished by recrystallization in EtOAc/hexanes to afford white crystals (29% yield). m.p.=158-160°C. 1H (CDCl3) δ 8.05 (br s, 1 H, NH), 7.90 (m, 1 H, ArH), 7.79 (t, J= 9.4 Hz, 4 H, ArH), 7.46 (m, 5 H, ArH), 7.11-7.35 (m, 4 H, ArH), 6.97 (s, 1 H, ArH), 4.64 (s, 2 H, SCH2), 4.08 (q, ArCH2CH2, J = 6.0 Hz), 3.12 (t, 2 H, ArCH2, J = 6.0 Hz), and signals due to a minor rotamer (ca. 25%) at 4.77 (s), 3.78 (q, J = 6.0 Hz).13C (CDCl3) δ 197.3, 136.6, 134.2, 133.5, 132.9, 128.7, 128.0, 127.9, 127.3, 127.2, 126.5, 126.2, 122.6, 122.4, 119.9, 118.9, 112.5, 111.5, 47.4, 40.3, 24.1, 1.2 and signals due to a minor rotamer (ca. 20%) at δ 46.0, 42.0. IR (KBr) vmax cm-1: 3436, 3191, 2914, 2837, 1592, 1515, 1451, 1387, 1358, 1326, 1300, 1204, 1089, 999, 935, 816, 736. EI-MS: m/z (%)130 (100), 202 (24). GC: r.t. 14.7 minutes. NP-HPLC: r.t. 5.7 min. RP-HPLC: r.t. 13.2 min.
N-[2-(indol-3-yl)ethyl]-S-[(pyrid-3-yl)methyl]-dithiocarbamate (17)
The general method was used with tryptamine as the amine and CH2Cl2 as the solvent. 3-(Bromomethyl)pyridine, HBr salt, was used as the alkylating agent in place of iodomethane and 2.0 eq. of Et3N were used. The crude product was chromatographed with EtOAc/hexanes (3:1) to afford a powdery tan solid (21% yield). m.p.=°C. 1H NMR (CDCl3) 8.5 (m, 2 H, ArH), δ 8.16 (br s, 1 H), 7.69 (m, 1 H, ArH), 7.58 (t, 1 H, ArH, J = 6.0 Hz), 7.37 (d, 1 H, ArH, J = 6.0 Hz), 7.24 – 7.12 (m, 4 H, ArH), 7.03 (m, 1 H, ArH), 4.52 (s, 2 H, SCH2), 4.08 (m, 2 H, ArCH2CH2), 3.12 (m, 2H, ArCH2), and signals due to a minor rotamer (ca. 25%) at 4.58 (s), 3.75 (m).13C NMR (CDCl3) δ 197.0, 150.3, 148.8, 136.8, 133, 127, 123.6, 122.7, 122.4, 120, 118.9, 112.5, 111.6, 53.5, 47.7, 36.9, 24.2 and a signal due to a minor rotamer at 54.0. IR (KBr) vmax cm-1: 3403, 3306, 3164, 2917, 1724, 1619, 1500, 1455, 1421, 1392, 1332, 1257, 1089, 926, 851, 739. EI-MS: m/z (%)130 (100), 202 (35). GC: r.t. 14.8 min. NP-HPLC: r.t. 27.9 min. RP-HPLC: r.t. 9.4 min.
N-[2-(indol-3-yl)ethyl]-S-[(pyrid-4-yl)methyl]-dithiocarbamate (18)
The general method was used with tryptamine as the amine and CH2Cl2 as the solvent. 4-(Bromomethyl)pyridine, HBr salt, was used as the alkylating agent in place of iodomethane and 2.0 eq. of Et3N were used. The crude product was recrystallized with EtOAc/hexanes (3:1) to afford tan crystals (50% yield). m.p.=125-7°C. 1H NMR (CDCl3) 8.51 (m, 2 H, ArH), 8.08 (br s, 1 H), 7.59 (m, 1 H, ArH), 7.39 (d, 1 H, ArH, J= 6.9 Hz), 7.28-7.00 (m, 6 H, ArH), 4.51 (s, 2 H, SCH2), 4.07 (m, ArCH2CH2, J = 6.0 Hz), 3.14 (m, 2 H, ArCH2), and signals due to a minor rotamer (ca. 25%) at 4.60 (s), 3.75 (m).13C NMR (CDCl3) δ 196.3, 150.1, 146.7, 136.7, 127.4, 124.3, 124.1, 122.7, 122.4, 120.0, 118.9, 112.5, 111.6, 47.9, 38.5, 24.2, 19.8. IR (KBr) vmax cm-1: 3404, 3299, 2917, 2851, 2178, 2099, 1600, 1508, 1455, 1416, 1337, 1225, 1091, 1002, 927, 743. EI-MS: m/z (%)130 (100), 202 (29). GC: r.t. 14.7 min. NP-HPLC: r.t. 28.9 min. RP-HPLC: r.t. 9.4 min.
General Method for the Synthesis of Thioureas
The amine was dissolved/suspended in CH2Cl2, cooled to 0°C and treated with Et3N (2.1-2.2 eq). Methyl isothiocyanate (1.1-1.5 eq) was added about 5 min. later and the reaction was allowed to slowly warm to r.t. while stirring overnight.
N-[1-(Indol-3-yl)methyl]-N′-methyl-thiourea (19)
The general method was used with 25. The volatiles were removed from the reaction and the crude residue was recrystallized from EtOAc/hexanes to yield a gold, crystalline solid (54% yield). m.p. 148-150°C. 1H NMR (DMSO-d6) δ 10.9 (br s, 1H, NH), 7.65 (m, 1H, ArH), 7.36 (m, 2H, ArH), 7.10 (t, 1H, ArH, J = 7.2 Hz), 4.75 (br s, 2H, ArCH2), 2.85 (br s, 3H, NHCH3). 13C NMR (DMSO-d6) δ 183.4, 137.1, 124.9, 124.4, 122.1, 119.4, 112.7, 111.9, 40.1 (overlapped with CDCl3), 31.5. IR (KBr) vmax cm-1: 3210, 1565, 1456, 1300, 1089. NP-HPLC (isocratic) r.t.= 23.9 min. NP-HPLC (gradient) r.t.= 22.5 min.
N-[1-(Indol-3-yl)ethyl]-N′-methyl-thiourea (20)
The general method was used with tryptamine HCl. The crude product was isolated by washing the reaction mixture with 1 M H2SO4 (2x), saturated NaHCO3, and brine and drying with Na2SO4. After concentration, the crude product was further purified by chromatography with EtOAc/hexanes (gradient, 1/1 to 3/1) to afford an oil which crystallizes on sitting to a light brown solid (92% yield). m.p.=102-6°C. 1H NMR (CDCl3) δ 8.13 (s, 1H, NH), 7.60 (d, 1H, ArH, J=7.8 Hz), 7.37 (d, 1H, ArH, J=8.1 Hz), 7.21 (t, 1H, ArH, J=7.0 Hz), 7.12 (t, 1H, ArH, J=7.0 Hz), 7.04 (s, 1H, ArH), 5.75 (br s, 2H, NH-C=S), 3.79 (br d, 2H, ArCH2CH2, J=5.4 Hz), 3.06 (t, 2H, ArCH2, J=6.6 Hz), 2.79 (br d, 3H, CH3, J=4.5 Hz). 13C NMR (CDCl3) δ 182.3, 136.3, 127.1, 122.4, 122.3, 119.6, 118.5, 112.4, 111.4, 44.8, 30.5, 24.8. IR (KBr) vmax cm-1: 3394, 3320, 3323, 3051, 1561, 1342. NP-HPLC r.t.= 24.2 min. RP-HPLC (1/1 MeOH/H2O) r.t.= 6.1 min.
Brassitin (21).29
Freshly made 25 (190 mg, 1.3 mmol) and Et3N (271 μL, 1.95 mmol) was dissolved in anhydrous MeOH (10 mL). The flask was cooled to 0° C and methyl chlorothiolformate (116 μL, 1.36 mmol) was added dropwise followed by stirring at rt for 6 h. A few drops of H2O were added to quench excess reagent and the volatiles were evaporated. The residue was dissolved in EtOAc (35 mL) and washed with 0.5 M HCl (2 × 20 mL), sat. NaHCO3 (20 mL), and brine (15 mL). The organic solution was dried with Na2SO4, filtered and concentrated to afford a crude brownish-orange solid (270 mg). After recrystallization from CH2Cl2/hexanes, beige crystals: 125 mg, 44% yd. mp 110-111°C. 1H NMR (CDCl3) δ 8.15 (br s, 1H, NH), 7.64 (d, 1H, ArH J = 7.9), 7.39 (d, 1H, ArH J = 7.1), 7.23 (m, 1H, ArH), 7.18 (m, 1H, ArH), 7.13 (m, 1H, ArH), 5.52 (br s, 1H, CH2NHC), 4.67 (d, 2H, ArCH2, J = 5.1), 2.38 (s, 3H, SCH3). 13C NMR (CDCl3) δ 167.6, 136.3, 126.3, 123.3, 122.5, 119.9, 118.7, 112.1, 111.3, 36.9, 12.4. EI-MS m/z (%) 220 (37, M+), 205 (9), 172 (12, M+-SCH3), 130 (100). NP-HPLC r.t.= 9.8 min. RP-HPLC (1/1 CH3CN/H2O + 0.1% TFA) r.t.=9.5 min.
N-[(Indol-3-yl)methyl]propanamide (29)
Compound 25 (1.00 g, 6.84 mmol) and Et3N (1.4 mL, 10.26 mmol) were dissolved in MeOH and cooled to 0° C. Propionyl chloride (633 mg, 6.84 mmol) was added dropwise and the reaction was stirred at rt for 4 h. The volatiles were removed and the residue was taken up in CH2Cl2 (40 mL), washed with 10% citric acid (20 mL), satd. NaHCO3 (20 mL) and brine (20 mL). The organic layer was dried with Na2SO4, filtered and the volatiles were removed to yield 1.33 g of a white, crystalline solid (1.33 g, 96% yield). An analytical sample was recrystallized form EtOAc/hexanes. m.p. 91-92°C. 1H NMR (CDCl3) δ 8.80 (br s, 1H, NH), 7.62 (d, 1H, ArH, J = 7.85 Hz), 7.38 (d, 1H, ArH, J = 7.2 Hz), 7.20 (3H, ArH), 5.80 (br s, 1H, NH), 4.60 (d, 2H, ArCH2, J = 5.1 Hz), 2.20 (q, 2H, COCH2CH3, J = 7.6 Hz),1.14 (t, 3H, COCH2CH3, J = 7.6 Hz). 13C NMR (CDCl3) δ 173.6, 136.5, 126.6, 123.3, 122.5, 119.9, 118.8, 112.8, 111.4, 35.2, 29.7, 9.9. IR (KBr) vmax cm-1: 3405, 1891, 1634, 1532, 1097.
N-[(Indol-3-yl)methyl]propanethioamide (22)
Amide 29 (190 mg, 0.94 mmol) was dissolved in THF (20 mL). Lawesson reagent (304 mg, 0.75 mmol) was added to the resulting solution and the reaction was stirred for 2 h at room temperature. The volatiles were removed and the residue was dissolved in CH2Cl2 (20 mL) and washed with H2O (12 mL). The organic layer was dried with Na2SO4 and filtered. After standing, a white precipitate formed which was filtered and the filtrate concentrated. The resulting residue (380 mg) was chromatographed with EtOAc/hexanes (1:1) to yield a clear oil which slowly crystallizes (85 mg, 41% yield). m.p. 132-134°C. 1H NMR (CDCl3) δ 8.23 (br s, 1H, NH), 7.63 (1H, ArH), 7.41 (1H, ArH), 7.23 (3H, ArH), 4.98 (d, 2H, ArCH2, J = 4.5 Hz), 2.68 (q, 2H, CSCH2CH3, J = 7.5 Hz), 1.30 (t, 3H, CSCH2CH3, J = 7.5 Hz). 13C NMR (CDCl3) δ 205.9, 136.3, 126.5, 124.0, 122.8, 120.3, 118.7, 111.5, 110.8, 42.2, 40.0, 13.5. IR (KBr) vmax cm-1: 3331, 2975, 2931, 1523, 1413, 1090. EI-MS m/z (%) 218 (49, M+), 163 (8), 131 (12), 130 (100). NP-HPLC r.t.= 10.9 min. RP-HPLC r.t.= 10.4 min.
2-Naphthoyl chloride
A 100 mL round bottom flask was charged with 2-naphthoic acid (2 g, 11.6 mmol) and SOCl2 (15 mL). The solution was refluxed for 4 hours and then concentrated to yield a yellow solid which was used without further purification (2.21 g, 100% yield); 1H NMR (CDCl3) δ 8.76 (s, 1H, ArH), 8.04 (2H, ArH), 7.93 (d, 2H, ArH, J = 8.9 Hz), 7.66 (m, 2H, ArH).
2-Naphthamide (27)
2-Naphthoyl chloride (2.21 g, 11.6 mmol) was dissolved in a MeOH/NH3 solution (2 M, 20 mL) and was allowed to stir overnight. Volatiles were removed and the resulting white solid was triturated with EtOAc. The solid was filtered and washed with cold EtOAc to yield a white solid which was used without further purification (1.98 g, 100% yield). m.p. 191-192°C. 1H NMR (CDCl3) δ 8.39 (s, 1H, ArH), 7.90 (4H, ArH), 7.57 (m, 2H, ArH). 13C NMR (CDCl3) δ 169.3, 135.0, 132.6, 130.5, 129.0, 128.6, 128.1, 127.9, 127.8, 126.9, 123.7. IR (nujol) vmax cm-1: 3400, 3210, 1650, 1628, 1512, 1510.
2-Aminomethylnaphthalene (28)
Compound 27 (1.00 g, 5.8 mmol) in THF (20 mL) was added slowly to a solution of LAH (1.76 g, 46.4 mmol) in THF (45 mL) at 0° C. The solution was allowed to warm to room temperature and the reaction was stirred overnight. The reaction was cooled to 0° C and quenched with H2O. The solids were filtered from the solution through celite and washed with hot THF. The filtrate was concentrated and the residue was dissolved in EtOAc (80 mL) and washed with 1M HCl (3 × 30 mL). The aqueous layer was basified with 6M NaOH to a pH of 12 and the precipitate was extracted with EtOAc (3 × 30 mL). The resulting organic solution was washed with brine (40 mL), dried with Na2SO4 and filtered. Concentration afforded a slightly yellow solid (510 mg, 56% yield). m.p. 55-56°C. 1H NMR (CDCl3) δ 7.80 (3H, ArH), 7.72 (s, 1H, ArH), 7.43 (m, 3H, ArH), 4.00 (s, 2H, ArCH2). 13C NMR (CDCl3) δ 140.6, 133.5, 132.5, 128.2, 127.7, 126.1, 125.8, 125.5, 125.1, 46.6. IR (KBr) vmax cm-1: 3362, 3291, 3050, 2915, 1950, 1596, 1507, 1358, 1273. GC: r.t.=9.0 min. EI-MS m/z (%) 157 (83, M+), 156 (100), 141 (15), 129 (49), 128 (40), 127 (24), 115 (10).
1-Bromo-3-(indol-3-yl)propanone (31)
The diazoketone 30 (379 mg, 1.90 mmol) was dissolved in acetic acid (4 mL) and cooled to 0°C. HBr (48%, 0.51 mL) was added dropwise. Forty minutes later the reaction was diluted with H2O and then quenched at 5°C with saturated. NaHCO3. The reaction mixture was extracted with CH2Cl2 (2X), washed with saturated. NaHCO3, H2O, brine, dried with Na2SO4, filtered and concentrated to a brown oil (398 mg, 83% yield). The crude product was used immediately in the next step. 1H NMR (CDCl3) δ 8.26 (br s, 1H, NH), 7.56 (d, 1H, ArH, J=7.8 Hz), 7.39 (d, 1H, ArH, J=7.8 Hz), 7.27-7.13 (m, 3H, ArH), 4.07 (s, 2H, CH2Br), 3.95 (s, 2H, ArCH2).
General Method for the Synthesis of Thiazoles
alpha-Bromoketone 31 was dissolved in EtOH and treated with thioamide (1.5 eq) and NaHCO3 (1.5 eq). The resulting mixture was heated at reflux overnight. Upon cooling the reaction material was partitioned between EtOAc and half saturated NaHCO3. The aqueous layer was extracted with EtOAc and the combined organic layers were washed with H2O and brine, dried with MgSO4, filtered and concentrated to a brown oily solid. The crude thiazole product was purified by chromatography with EtOAc/hexanes (1:2).
4-[(Indol-3-yl)methyl]thiazole (23)
The general method was used with thioformamide30 to afford a 71% yield. 1H NMR (CDCl3) δ 8.76 (d, 1H, SCHN, J=2.0 Hz), 8.11 (br s, 1H, NH), 7.52 (d, 1H, ArH, J=7.6 Hz), 7.36 (d, 1H, ArH, J=8.0 Hz), 7.18 (t, 1H, ArH, J=7.1 Hz), 7.08 (t, 2H, ArH, J=7.4 Hz), 6.90 (s, 1H, ArH), 4.34 (s, 2H, ArCH2). 13C NMR (CDCl3) δ 157.5, 152.5, 122.5, 122.1, 119.4, 119.1, 113.8, 113.5, 111.2, 27.6. IR (CH2Cl2) vmax cm-1: 3626, 3470, 3051, 2987, 1420, 1264. GC: r.t.=15.1 min. EI-MS m/z (%) 214 (100, M+), 213 (86), 186 (15), 154 (14), 130 (51). RP-HPLC (1/1 CH3CN/H2O + 0.1% TFA) r.t.=4.4 min.
4-[(Indol-3-yl)methyl]-2-methyl-thiazole (24)
The general method was used with thioacetamide to afford a 56% yield. 1H NMR (CDCl3) δ 8.17 (br s, 1H, NH), 7.53 (d, 1H, ArH, J=7.8 Hz), 7.34 (d, 1H, ArH, J=8.1 Hz), 7.17 (t, 1H, ArH, J=7.0 Hz), 7.10-7.04 (m, 2H, ArH), 6.63 (s, 1H, ArH), 4.23 (s, 2H, CH2), 2.69 (s, 3H, CH3). 13C NMR (CDCl3) δ 165.6, 162.3, 156.1, 136.4, 127.3, 122.6, 122.0, 119.3, 119.1, 113.4, 111.1, 27.7, 19.1. IR (KBr) vmax cm-1: 3247, 3090, 2919, 1527, 1454, 1429, 1188. GC: r.t.=15.4 min. EI-MS m/z (%) 228 (100, M+), 227 (71), 186 (22), 154 (23), 130 (39). RP-HPLC (1/1 MeOH/H2O) r.t.= 18.6 min.
Computational Procedure
All electronic structure calculations were carried out using the Gaussian 03 suite of programs.31 Natural bond orbital (NBO) population analysis was done with NBO 3.1 as implemented in Gaussian 03.32 All compounds with terminal methyl groups were optimized at the HF/6-31G*//HF/6-31G33 level. ESP34 and NBO atomic charges were computed. The HF/6-31G* molecular electrostatic potential surface was mapped onto the total density surface.
Inhibition Assays with Indoleamine 2,3-Dioxygenase
The inhibition assays were performed in a 96-well microtiter plate as described by Littlejohn et.al.14 with a small modification. Briefly, the reaction mixture contained 50 mM potassium phosphate buffer (pH 6.5), 40 mM ascorbic acid, 400 μg/ml catalase, 20 μM methylene blue and purified recombinant IDO(1) optimized based on its activity. The reaction mixture was added to the substrate, l-tryptophan (l-Trp), and the inhibitor. The l-Trp was serially diluted from 200 to 25 μM and the inhibitors were tested at two concentrations, 200 and 400 μM. The reaction was carried out at 37°C for 60 min and stopped by adding 30% (w/v) trichloroacetic acid. The plate was heated at 65 °C for 15 min to convert formylkynurenine to kynurenine and then was spun at 6000 g for 5 min. Finally 100 μl supernatant from each well was transferred to a new 96 well plate and mixed with 2% (w/v) p-dimethylamino-benzaldehyde in acetic acid. The yellow color generated from the reaction with kynurenine was measured at 490 nm using a Synergy HT microtiter plate reader (Bio-Tek, Winooski, VT). The data was analyzed using Graph Pad Prism 4 software (Graph Pad Software Inc., San Diego, CA)
Supplementary Material
Copies of 1H NMR spectra for compounds 1-24, 27-29 and 31. Copies of 13C NMR spectra for compounds 1-24 and 27-29. Copies of HPLC data for compounds 1-24. Copies of GC data for compounds 15, 17, 18, 23, 24 and 28. Copies of MS data for compounds 15, 17, 18, 23, 24, and 28. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We thank the National Institutes of Health (NCI R01-CA 109542) and Bryn Mawr College (Faculty Research Fund) for financial support of this work. The authors are grateful to Prof. Michelle Francl of Bryn Mawr College for assistance with the computational experimentation.
References
- 1.a Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of Indoleamine 2,3-Dioxygenase, an Immunoregulatory Target of the Cancer Suppression Gene Bin1, Potentiates Cancer Chemotherapy. Nat Med. 2005;11:312–9. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]; b Munn DH, Mellor AL. IDO and Tolerance to Tumors. Trends in Molecular Medicine. 2004;10:15–18. doi: 10.1016/j.molmed.2003.11.003. [DOI] [PubMed] [Google Scholar]; c Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a Tumoral Immune Resistance Mechanism Based on Tryptophan Degradation by Indoleamine 2,3-Dioxygenase. Nat Med. 2003;9:1269–74. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]; d Friberg M, Jennings R, Alsarraj M, Dessureault S, Cantor A, Extermann M, Mellor AL, Munn DH, Antonia SJ. Indoleamine 2,3-Dioxygenase Contributes to Tumor Cell Evasion of T Cell-mediated Rejection. International Journal of Cancer. 2002;101:151–155. doi: 10.1002/ijc.10645. [DOI] [PubMed] [Google Scholar]
- 2.a Sono M, Roach MP, Coulter ED, Dawson JH. Heme-containing Oxygenases. Chem Rev. 1996;96:2841–87. doi: 10.1021/cr9500500. [DOI] [PubMed] [Google Scholar]; b Botting NP. Chemistry and Neurochemistry of the Kynurenine Pathway of Tryptophan Metabolism. Chem Soc Rev. 1995:401–12. [Google Scholar]; c Sono M, Hayaishi O. The Reaction Mechanism of Indoleamine 2,3-Dioxygenase. Biochemical Reviews. 1980;50:173–81. [Google Scholar]
- 3.a Sono M, Taniguchi T, Watanabe Y, Hayaishi O. Indoleamine 2,3-Dioxygenase. Equilibrium Studies of the Tryptophan Binding to the Ferric, Ferrous, and CO-bound Enzymes. J Biol Chem. 1980;255:1339–45. [PubMed] [Google Scholar]; b Kobayashi K, Hayashi K, Sono M. Effects of Tryptophan and pH on the Kinetics of Superoxide Radical Binding to Indoleamine 2,3-Dioxygenase Studied by Pulse Radiolysis. J Biol Chem. 1989;264:15280–3. [PubMed] [Google Scholar]
- 4.a Muller AJ, Malachowski WP, Prendergast GC. Indoleamine 2,3-Dioxygenase in Cancer: Targeting Pathological Immune Tolerance with Small-molecule Inhibitors. Expert Opin Ther Targets. 2005;9:831–849. doi: 10.1517/14728222.9.4.831. [DOI] [PubMed] [Google Scholar]; b Malachowski WP, Metz R, Prendergast GC, Muller AJ. Drugs of the Future. 2005 in press. [Google Scholar]
- 5.Peterson AC, La Loggia AJ, Hamaker LK, Arend RA, Fisette PL, Ozaki Y, Will JA, Brown RR, Cook JM. Evaluation of Substituted β-Carbolines as Noncompetitive Indoleamine 2,3-Dioxygenase Inhibitors. Med Chem Res. 1993;3:473–82. [Google Scholar]
- 6.a Cady SG, Sono M. 1-Methyl-DL-tryptophan, beta-(3-Benzofuranyl)-DL-alanine (the Oxygen Analog of Tryptophan), and beta-[3-Benzo(b)thienyl]-DL-alanine (the Sulfur Analog of Tryptophan) are Competitive Inhibitors of Indoleamine 2,3-Dioxygenase. Arch Biochem Biophys. 1991;291:326–33. doi: 10.1016/0003-9861(91)90142-6. [DOI] [PubMed] [Google Scholar]; b Peterson AC, Migawa MT, Martin MJ, Hamaker LK, Czerwinski KM, Zhang W, Arend RA, Fisette PL, Ozaki Y, Will JA, Brown RR, Cook JM. Evaluation of functionalized tryptophan derivatives- and related compounds as competitive inhibitors of indoleamine 2,3-dioxygenase. Med Chem Res. 1994;3:531–544. [Google Scholar]
- 7.a Pedras MSC, Jha M, Ahiahonu PWK. The synthesis and biosynthesis of phytoalexins produced by cruciferous plants. Current Organic Chemistry. 2003;7:635–1647. [Google Scholar]; b Ruszkowska J, Wrobel JT. Tryptophan-derived sulfur-containing phytoalexins: A general overview. Advances in Experimental Medicine and Biology. 2003;527:629–636. doi: 10.1007/978-1-4615-0135-0_72. [DOI] [PubMed] [Google Scholar]; c Pedras MSC, Okanga FI, Zaharia IL, Khan AQ. Phytoalexins from Crucifers: Synthesis, Biosynthesis, and Biotransformation. Phytochemistry. 2000;53:161–76. doi: 10.1016/s0031-9422(99)00494-x. [DOI] [PubMed] [Google Scholar]
- 8.a Pedras MSC, Jha M. Concise Syntheses of the Cruciferous Phytoalexins Brassilexin, Sinalexin, Wasalexins and Analogues: Expanding the Scope of the Vilsmeier Formylation. J Org Chem. 2005;70:1828–34. doi: 10.1021/jo0479866. [DOI] [PubMed] [Google Scholar]; b Pedras MSC, Sorenson JL. Phytoalexin Accumulation and Antifungal Compunds from the Crucifer Wasabi. Phytochemistry. 1998;49:1959–65. [Google Scholar]
- 9.a Mehta RG, Liu J, Constantinou A, Thomas CF, Hawthorne M, You M, Gerhauser C, Pezzuto JM, Moon RC, Moriarty RM. Cancer Chemopreventive Activity of Brassinin, a Phytoalexin from Cabbage. Carcinogenesis. 1995;16:399–404. doi: 10.1093/carcin/16.2.399. [DOI] [PubMed] [Google Scholar]; b Mezencev R, Mojzis J, Pilatova M, Kutschy P. Antiproliferative and cancer chemopreventive activity of phytoalexins: focus on indole phytoalexins from crucifers. Neoplasma. 2003;50:239–245. [PubMed] [Google Scholar]
- 10.a Schallenberg J, Meyer E. Simple Synthesis of 3-Substituted Indoles and Their Application for High Yield Carbon-14 Labeling. Z Naturforsch. 1983;38b:108–12. [Google Scholar]; b Yamada F, Kobayashi K, Shimizu A, Aoki N, Somei M. A Synthesis Method of Indole-3-methanamine and/or Gramine from Indole-3-carboxaldehyde, and its Application for the Syntheses of Brassinin, its 4-Substituted Analogs, and 1,3,4,5-Tetrahdyropyrrolo[4,3,2-de]quinoline. Heterocycles. 1993;36:2783–2804. [Google Scholar]; c Kutschy P, Dzurilla M, Takasugi M, Torok M, Achbergerova I, Homzova R, Racova M. New Syntheses of Indole Phytoalexins and Related Compounds. Tetrahedron. 1998;54:3549–66. [Google Scholar]
- 11.Dornyei G, Incze M, Kajtar-Peredy M, Szantgay Csaba. Intramolecular Mannich Reaction of 2-Oxotryptamine and Homologues with Oxo Reagents Yielding Spiro Compounds. Part II. Coll Czech Chem Comm. 2002;67:1669–80. [Google Scholar]
- 12.Lutz RE, Wilson JW. Antimalarials. Aliphatic Amino Ketones and Alcohols. J Org Chem. 1947;12:767–70. doi: 10.1021/jo01170a004. [DOI] [PubMed] [Google Scholar]
- 13.Cuevas-Yanez E, Muchowski JM, Cruz-Almanza R. Rhodium(II) Catalyzed Intramolecular Insertion of Carbenoids Derived from 2-Pyrrolyl and 3-Indolyl α-Diazo-β-ketoesters and α-Diazoketones. Tetrahedron. 2004;60:1505–1511. [Google Scholar]
- 14.Littlejohn TK, Takikawa O, Skylas D, Jamie JF, Walker MJ, Truscott RJW. Expression and Purification of Recombinant Human Indoleamine 2,3–Dioxygenase. Protein Expression and Purification. 2000;19:22–29. doi: 10.1006/prep.2000.1214. [DOI] [PubMed] [Google Scholar]
- 15.Sono M. The Roles of Superoxide Anion and Methylene Blue in the Reductive Activation of Indoleamine 2,3-Dioxygenase by Ascorbic Acid or by Xanthine Oxidase-Hypoxanthine. J Biol Chem. 1989;264:1616–1622. [PubMed] [Google Scholar]
- 16.Ohnishi T, Hirata F, Hayaishi O. Indoleamine 2,3-Dioxygenase: Potassium Superoxide as Substrate. J Biol Chem. 1977;252:4643–4647. [PubMed] [Google Scholar]
- 17.For leading examples of metalloenzyme inhibition by a dithiocarbamate see: Thomas SR, Salahifar H, Mashima R, Hunt NH, Richardson DR, Stocker R. Antioxidants Inhibit Indoleamine 2,3-Dioxygenase in IFN-g-Activated Human Macrophages: Posttranslational Regulation by Pyrrolidine Dithiocarbamate. J Immunol. 2001;166:6332–40. doi: 10.4049/jimmunol.166.10.6332.Warshawsky A, Rogachev I, Patil Y, Baszkin A, Weiner L, Gressel J. Copper-specific chelators as synergists to herbicides: 1. Amphiphilic dithiocarbamates, synthesis, transport through lipid bilayers, and inhibition of Cu/Zn superoxide dismutase activity. Langmuir. 2001;17:5621–35.Diaz GJ, Squires EJ. Metabolism of 3-Methylindole by Porcine Liver Microsomes: Responsible Cytochrome P450 Enzymes. Toxicological Sciences. 2000;55:284–92. doi: 10.1093/toxsci/55.2.284.
- 18.For general leading references on metal chelation by dithiocarbamates see: Paleologos EK, Giokas DL, Tzouwara-Karayanni SM, Karayannis MI. Micelle Mediated Methodology for the Determination of Free and Bound Iron in Wines by Flame Atomic Absorption Spectrometry. Anal Chim Acta. 2002;458:241–8.Furuta S, Ortiz F, Sun XZ, Wu HH, Mason A, Momand J. Copper Uptake is Required for Pyrrolidine Dithiocarbamate-mediated Oxidation and Protein Level Increase of p53 in Cells. Biochemical Journal. 2002;365:639–48. doi: 10.1042/BJ20011251.Iseki A, Kambe F, Okumura K, Miwata S, Yamamoto R, Hayakawa T, Seo H. Pyrrolidine Dithiocarbamate Inhibits TNF-α-dependent Activation of NF-kB by Increasing Intracellular Copper Level in Human Aortic Smooth Muscle Cells. Biochem Biophys Res Comm. 2000;276:88–92. doi: 10.1006/bbrc.2000.3452.Kim CH, Kim JH, Xu J, Hsu CY, Ahn YS. Pyrrolidine Dithiocarbamate Induces Bovine Cerebral Endothelial Cell Death by Increasing the Intracellular Zinc Level. J Neurochem. 1999;72:1586–92. doi: 10.1046/j.1471-4159.1999.721586.x.
- 19.Interestingly, there is a report of IDO acceleration in the presence of diethyldithiocarbamate. The acceleration results from inhibition of superoxide dismutase which can remove superoxide, an IDO activator. Tanigucchi T, Hirata F, Hayaishi O. Intracellular Utilization of Superoxide Anion by Indoleamine 2,3-Dioxygenase of Rabbit Enterocytes. J Biol Chem. 1977;252:2774–6.
- 20.a Nurmi A, Vartiainen N, Pihlaja R, Goldsteins G, Yrjaenheikki J, Koistinaho J. Pyrrolidine Dithiocarbamate Inhibits Translocation of Nuclear Factor Kappa-B in Neurons and Protects Against Brain Ischemia with a Wide Therapeutic Time Window. J Neurochem. 2004;91:755–65. doi: 10.1111/j.1471-4159.2004.02756.x. [DOI] [PubMed] [Google Scholar]; b Hayakawa M, Miyashita H, Sakamoto I, Kitagawa M, Tanaka H, Yasuda H, Karin M, Kikugawa K. Evidence that Reactive Oxygen Species Do Not Mediate NF-B Activation. EMBO J. 2003;22:3356–66. doi: 10.1093/emboj/cdg332. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Liu SF, Ye X, Malik AB. In vivo Inhibition of Nuclear Factor-B Activation Prevents Inducible Nitric Oxide Synthase Expression and Systemic Hypotension in a Rat Model of Septic Shock. J Immunol. 1997;159:3976–83. [PubMed] [Google Scholar]; d Ziegler-Heitbrock HW, Sternsdorf T, Liese J, Belohradsky B, Weber C, Wedel A, Schreck R, Bauerle P, Strobel M. Pyrrolidine Dithiocarbamate Inhibits NF-B Mobilization and TNF Production in Human Monocytes. J Immunol. 1993;51:6986–93. [PubMed] [Google Scholar]; e Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA. Dithiocarbamates as Potent Inhibitors of Nuclear Factor B Activation in Intact Cells. J Exp Med. 1992;175:1181–94. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calculations with 1 and 22 showed an almost identical change (slightly more negative) in ESP and NBO charges versus 32 and 33.
- 22.Pearson RG. Hard and Soft Acids and Bases. J Am Chem Soc. 1963;85:3533–39. [Google Scholar]
- 23.Sono M, Cady SG. Enzyme Kinetic and Spectroscopic Studies of Inhibitor and Effector Interactions with Indoleamine 2,3-Dioxygenase. 1. Norharman and 4-Phenylimidazole Binding to the Enzyme as Inhibitors and Heme Ligands. Biochemistry. 1989;28:5392–5399. doi: 10.1021/bi00439a012. [DOI] [PubMed] [Google Scholar]
- 24.Holloway CE, Gitlitz MH. Rotational barrier in dithiocarbamate esters. Can J Chem. 1967;45:2659–63. [Google Scholar]
- 25.Takasugi M, Monde K, Katsui N, Shirata A. Novel Sulfur-Containing Phytoalexins from the Chinese Cabbage Brassica campestris L. ssp. pekinensis (Cruciferae) Bull Chem Soc Jpn. 1988;61:285–89. [Google Scholar]
- 26.a Pedras MSC, Okanga FI. Probing the Phytopathogenic Blackleg Fungus with a Phytoalexin Homolog. J Org Chem. 1998;63:416–7. doi: 10.1021/jo971702i. [DOI] [PubMed] [Google Scholar]; b Pedras MSC, Okanga FI. Metabolism of Analogs of the Phytoalexin Brassinin by Plant Pathogenic Fungi. Can J Chem. 2000;78:338–46. [Google Scholar]
- 27.Pedras MSC, Ahiahonu PWK, Hossain M. Detoxification of the Cruciferous Phytoalexin Brassinin in Sclerotinia Sclerotiorum Requires an Inducible Glucosyltransferase. Phytochemistry. 2004;65:2685–2694. doi: 10.1016/j.phytochem.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 28.a Mohanta PK, Dhar S, Samal SK, Ila H, Junjappa H. 1-(Methyldithiocarbonyl)imidazole: a Useful Thiocarbonyl Transfer Reagent for Synthesis of Substituted Thioureas. Tetrahedron. 2000;56:629–637. [Google Scholar]; b Burrows AA, Hunter L. The Associating Effect of the Hydrogen Atom. XV. The S.sbd.H.sbd.N bond. Esters of Thion- and Dithiocarbamic acids. Journal of the Chemical Society, Abstracts. 1952:4118–22. [Google Scholar]; c Thorn GD, Ludwig RA. The Dithiocarbamates and Related Compounds. Elsevier; New York: 1962. p. 78. [Google Scholar]
- 29.Monde K, Takasugi M, Shirata A. Three Sulphur-Containing Stress Metabolites from Japanese Radish. Phytochemistry. 1995;39:581–6. [Google Scholar]
- 30.Londergan TE, Hause NL, Schmitz WR. A New Synthesis of the Thiazole Fragment of Vitamin B1. J Am Chem Soc. 1953;75:4456–8. [Google Scholar]
- 31.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA. Gaussian 03, Revision B.05. Gaussian, Inc.; Wallingford CT: 2004. [Google Scholar]
- 32.Glendening ED, Reed AE, Carpenter JE, Weinhold F. NBO Version 3.1. [Google Scholar]
- 33.First-row elements: Hahrihan PC, Pople JA. Influence of Polarization Functions on MO Hydrogenation Energies. Theor Chim Acta. 1973;28:213. Second-row elements: Franc1, Pietro WJ, Hehre WJ, Binkley JS, Defrees DJ, Pople JA, Gordon MS. Self-consistent Molecular Orbital Methods. XXIII. A Polarization-type Basis Set for Second-row Elements. J Chem Phys. 1982;77:3654.
- 34.a Chirlian LE, Francl MM. Atomic Charges Derived from Electrostatic Potentials: a Detailed Study. J Comp Chem. 1987;8:894. [Google Scholar]; b Breneman CM, Wiberg KB. Determining Atom-centered Monopoles from Molecular Electrostatic Potentials. The Need for High Sampling Density in Formamide Conformational Analysis. J Comp Chem. 1990;11:361. [Google Scholar]
- 35.Schroeder DC. Thioureas. Chem Rev. 1955;55:181. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Copies of 1H NMR spectra for compounds 1-24, 27-29 and 31. Copies of 13C NMR spectra for compounds 1-24 and 27-29. Copies of HPLC data for compounds 1-24. Copies of GC data for compounds 15, 17, 18, 23, 24 and 28. Copies of MS data for compounds 15, 17, 18, 23, 24, and 28. This material is available free of charge via the Internet at http://pubs.acs.org.