Abstract
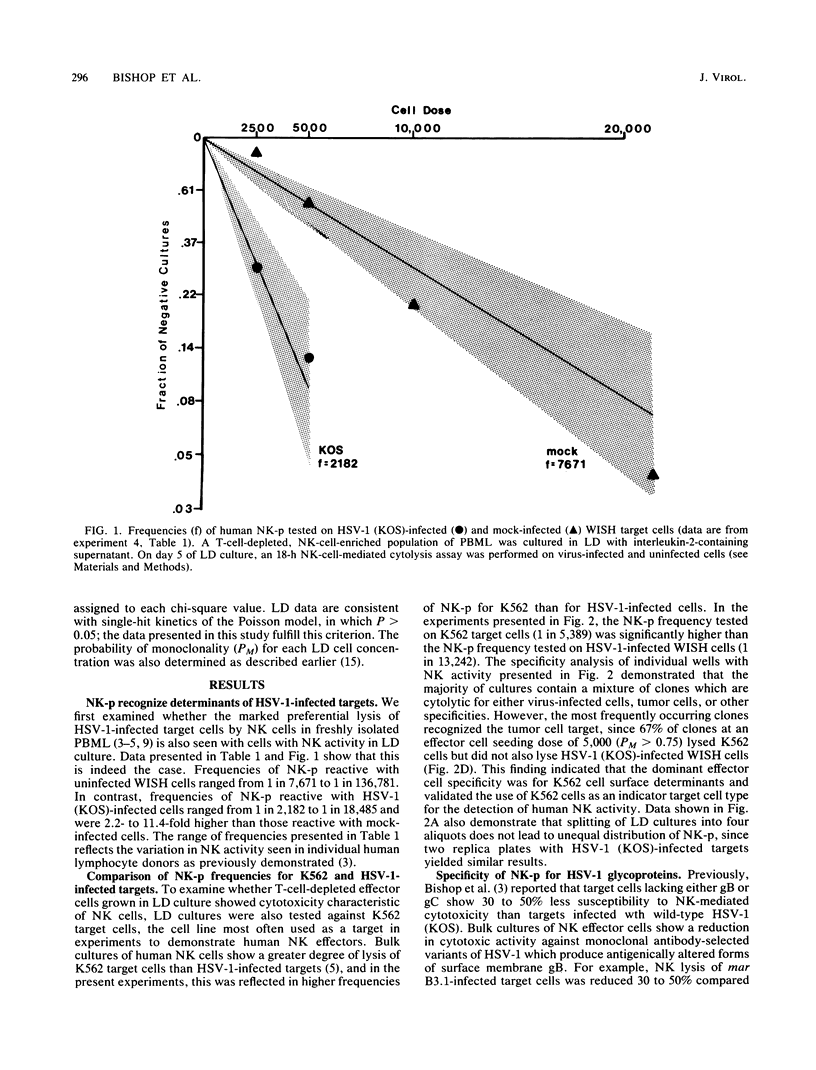
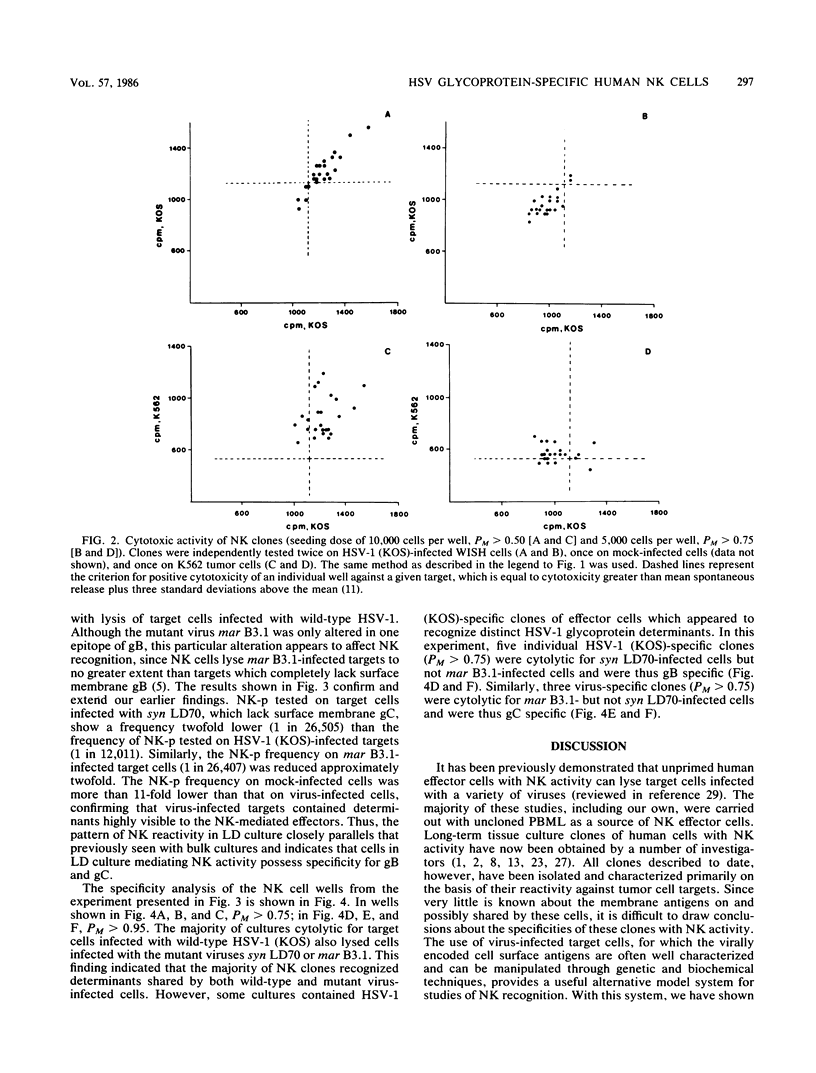
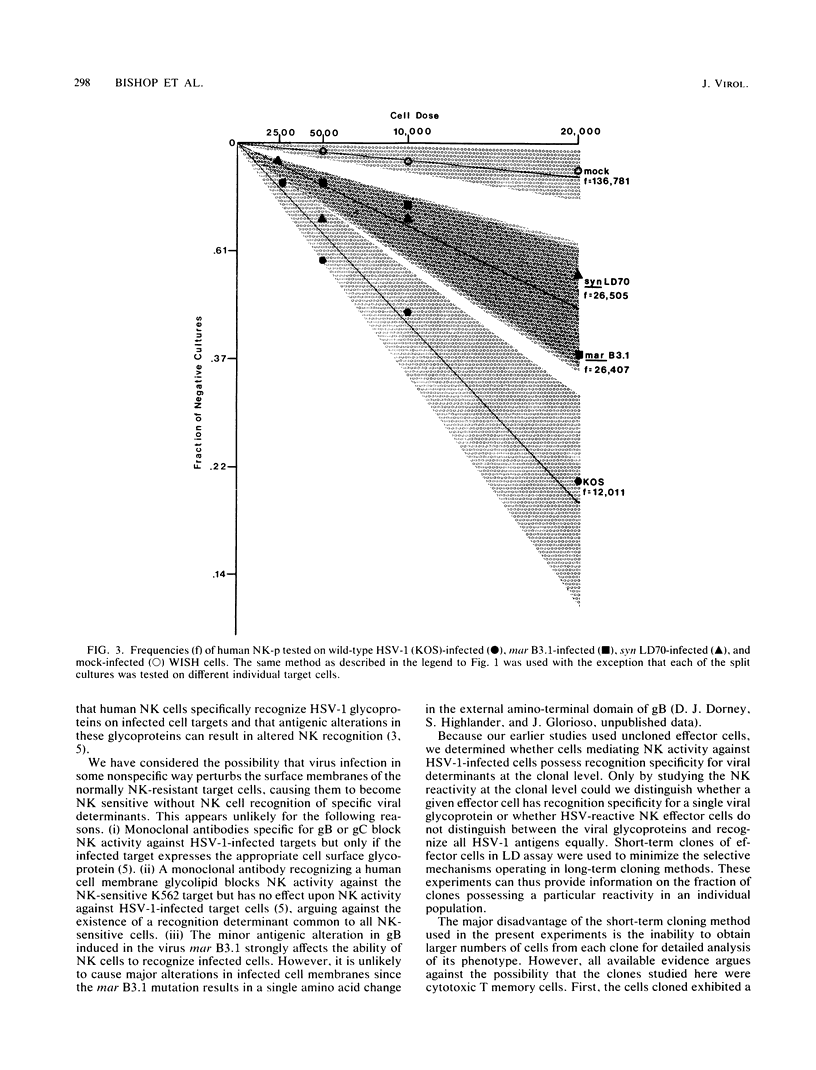
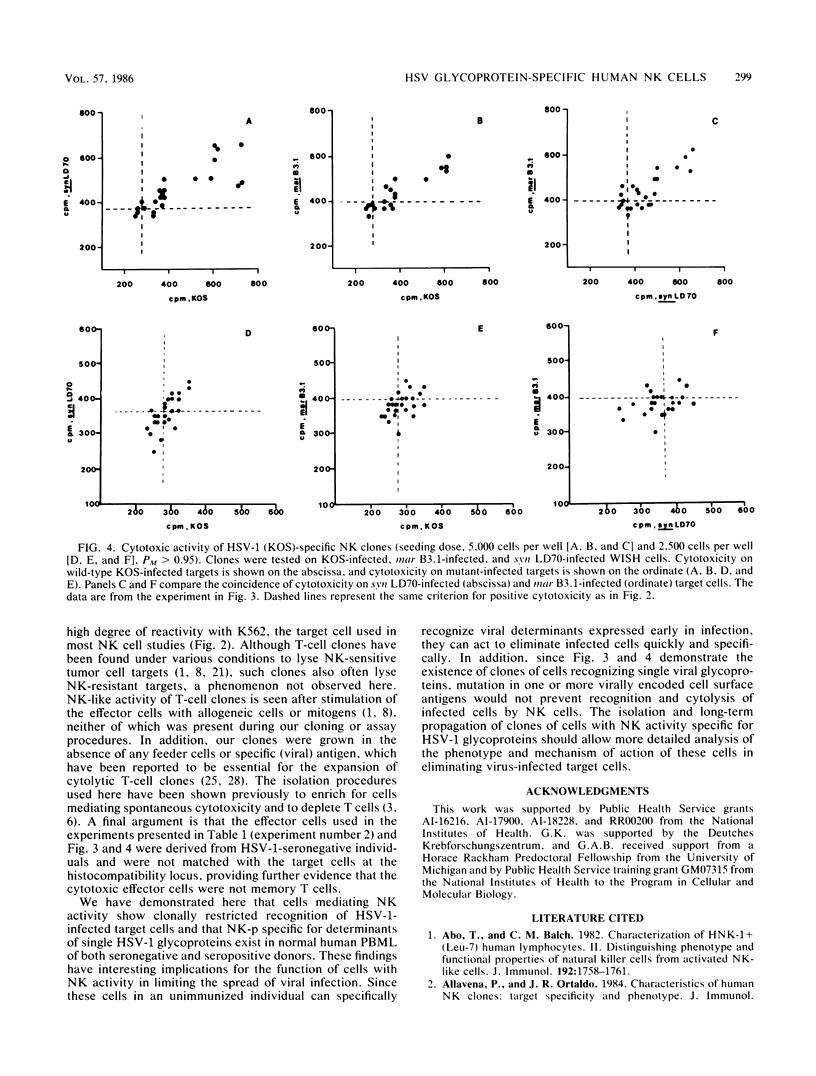
The frequency and specificity of human cells with natural killer (NK) cytotoxic activity for herpes simplex virus type 1 (HSV-1)-infected targets was measured by limiting dilution culture. The frequency of NK cell precursors (NK-p) reactive with HSV-1-infected cells was 2- to 11-fold higher than that of NK-p reactive with mock-infected cells. The frequency of NK-p reactive with infected target cells lacking viral glycoprotein C or presenting an antigenically altered glycoprotein B was approximately twofold lower than that with wild-type virus-infected cells. Specificity analysis demonstrated that NK cells with a high statistical probability of being monoclonal were reactive with either glycoprotein B or C. These results provide the first evidence that cells with human NK activity possess clonal specificity for HSV-1-infected target cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. Characterization of HNK-1+ (Leu-7) human lymphocytes. II. Distinguishing phenotypic and functional properties of natural killer cells from activated NK-like cells. J Immunol. 1982 Oct;129(4):1758–1761. [PubMed] [Google Scholar]
- Bishop G. A., Glorioso J. C., Schwartz S. A. Relationship between expression of herpes simplex virus glycoproteins and susceptibility of target cells to human natural killer activity. J Exp Med. 1983 May 1;157(5):1544–1561. doi: 10.1084/jem.157.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop G. A., Glorioso J. C., Schwartz S. A. Role of interferon in human natural killer activity against target cells infected with HSV-1. J Immunol. 1983 Oct;131(4):1849–1853. [PubMed] [Google Scholar]
- Bishop G. A., Marlin S. D., Schwartz S. A., Glorioso J. C. Human natural killer cell recognition of herpes simplex virus type 1 glycoproteins: specificity analysis with the use of monoclonal antibodies and antigenic variants. J Immunol. 1984 Oct;133(4):2206–2214. [PubMed] [Google Scholar]
- Bishop G. A., Schwartz S. A. Enhancement of human natural killer cells by interferon requires RNA and protein synthesis. Clin Immunol Immunopathol. 1982 Dec;25(3):374–385. doi: 10.1016/0090-1229(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Burns G. F., Triglia T., Werkmeister J. A. In vitro generation of human activated lymphocyte killer cells: separate precursors and modes of generation of NK-like cells and "anomalous" killer cells. J Immunol. 1984 Sep;133(3):1656–1663. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Ching C., Lopez C. Natural killing of herpes simplex virus type 1-infected target cells: normal human responses and influence of antiviral antibody. Infect Immun. 1979 Oct;26(1):49–56. doi: 10.1128/iai.26.1.49-56.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorioso J. C., Levine M., Holland T. C., Szczesiul M. S. Mutant analysis of herpes simplex virus-induced cell surface antigens: resistance to complement-mediated immune cytolysis. J Virol. 1980 Sep;35(3):672–681. doi: 10.1128/jvi.35.3.672-681.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorioso J., Kees U., Kümel G., Kirchner H., Krammer P. H. Identification of herpes simplex virus type 1 (HSV-1) glycoprotein gC as the immunodominant antigen for HSV-1-specific memory cytotoxic T lymphocytes. J Immunol. 1985 Jul;135(1):575–582. [PubMed] [Google Scholar]
- Hercend T., Reinherz E. L., Meuer S., Schlossman S. F., Ritz J. Phenotypic and functional heterogeneity of human cloned natural killer cell lines. Nature. 1983 Jan 13;301(5896):158–160. doi: 10.1038/301158a0. [DOI] [PubMed] [Google Scholar]
- Holland T. C., Homa F. L., Marlin S. D., Levine M., Glorioso J. Herpes simplex virus type 1 glycoprotein C-negative mutants exhibit multiple phenotypes, including secretion of truncated glycoproteins. J Virol. 1984 Nov;52(2):566–574. doi: 10.1128/jvi.52.2.566-574.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kees U., Kynast G., Weber E., Krammer P. H. A method for testing the specificity of influenza A virus-reactive memory cytotoxic T lymphocyte (CTL) clones in limiting dilution cultures. J Immunol Methods. 1984 Apr 27;69(2):215–227. doi: 10.1016/0022-1759(84)90320-x. [DOI] [PubMed] [Google Scholar]
- Ly I. A., Mishell R. I. Separation of mouse spleen cells by passage through columns of sephadex G-10. J Immunol Methods. 1974 Aug;5(3):239–247. doi: 10.1016/0022-1759(74)90108-2. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Herberman R. B. Heterogeneity of natural killer cells. Annu Rev Immunol. 1984;2:359–394. doi: 10.1146/annurev.iy.02.040184.002043. [DOI] [PubMed] [Google Scholar]
- Phillips W. H., Ortaldo J. R., Herberman R. B. Selective depletion of human natural killer cells on monolayers of target cells. J Immunol. 1980 Nov;125(5):2322–2327. [PubMed] [Google Scholar]
- Roder J. C., Rosén A., Fenyö E. M., Troy F. A. Target-effector interaction in the natural killer cell system: isolation of target structures. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1405–1409. doi: 10.1073/pnas.76.3.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoli D., Trinchieri G., Lief F. S. Cell-mediated cytotoxicity against virus-infected target cells in humans. I. Characterization of the effector lymphocyte. J Immunol. 1978 Aug;121(2):526–531. [PubMed] [Google Scholar]
- Schneider E. M., Pawelec G. P., Liangru S., Wernet P. A novel type of human T cell clone with highly potent natural killer-like cytotoxicity divorced from large granular lymphocyte morphology. J Immunol. 1984 Jul;133(1):173–179. [PubMed] [Google Scholar]
- Sheehy M. J., Quintieri F. B., Leung D. Y., Geha R. S., Dubey D. P., Limmer C. E., Yunis E. J. A human large granular lymphocyte clone with natural killer-like activity and T cell-like surface markers. J Immunol. 1983 Feb;130(2):524–526. [PubMed] [Google Scholar]
- Suzuki R., Handa K., Itoh K., Kumagai K. Natural killer (NK) cells as a responder to interleukin 2 (IL 2). I. Proliferative response and establishment of cloned cells. J Immunol. 1983 Feb;130(2):981–987. [PubMed] [Google Scholar]
- Takasugi M., Koide D., Ramseyer A. Specificities in natural cell-mediated cytotoxicity by the cross-competition assay. Int J Cancer. 1977 Mar 15;19(3):291–297. doi: 10.1002/ijc.2910190303. [DOI] [PubMed] [Google Scholar]
- Vose B. M., Bonnard G. D. Limiting dilution analysis of the frequency of human T cells and large granular lymphocytes proliferating in response to interleukin 2. I. The effect of lectin on the proliferative frequency and cytotoxic activity of cultured lymphoid cells. J Immunol. 1983 Feb;130(2):687–693. [PubMed] [Google Scholar]
- Welsh R. M. Natural cell-mediated immunity during viral infections. Curr Top Microbiol Immunol. 1981;92:83–106. doi: 10.1007/978-3-642-68069-4_6. [DOI] [PubMed] [Google Scholar]
- Zaunders J., Werkmeister J., McCarthy W. H., Hersey P. Characterization of antigens recognized by natural killer cells in cell-culture supernatants. Br J Cancer. 1981 Jan;43(1):5–12. doi: 10.1038/bjc.1981.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Griend R. J., van Krimpen B. A., Ronteltap C. P., Bolhuis R. L. Rapidly expanded activated human killer cell clones have strong antitumor cell activity and have the surface phenotype of either T gamma, T-non-gamma, or null cells. J Immunol. 1984 Jun;132(6):3185–3191. [PubMed] [Google Scholar]


