Abstract
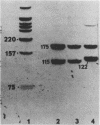
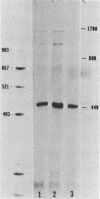
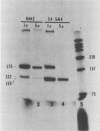
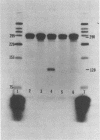
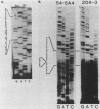
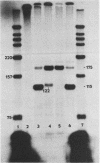
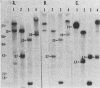
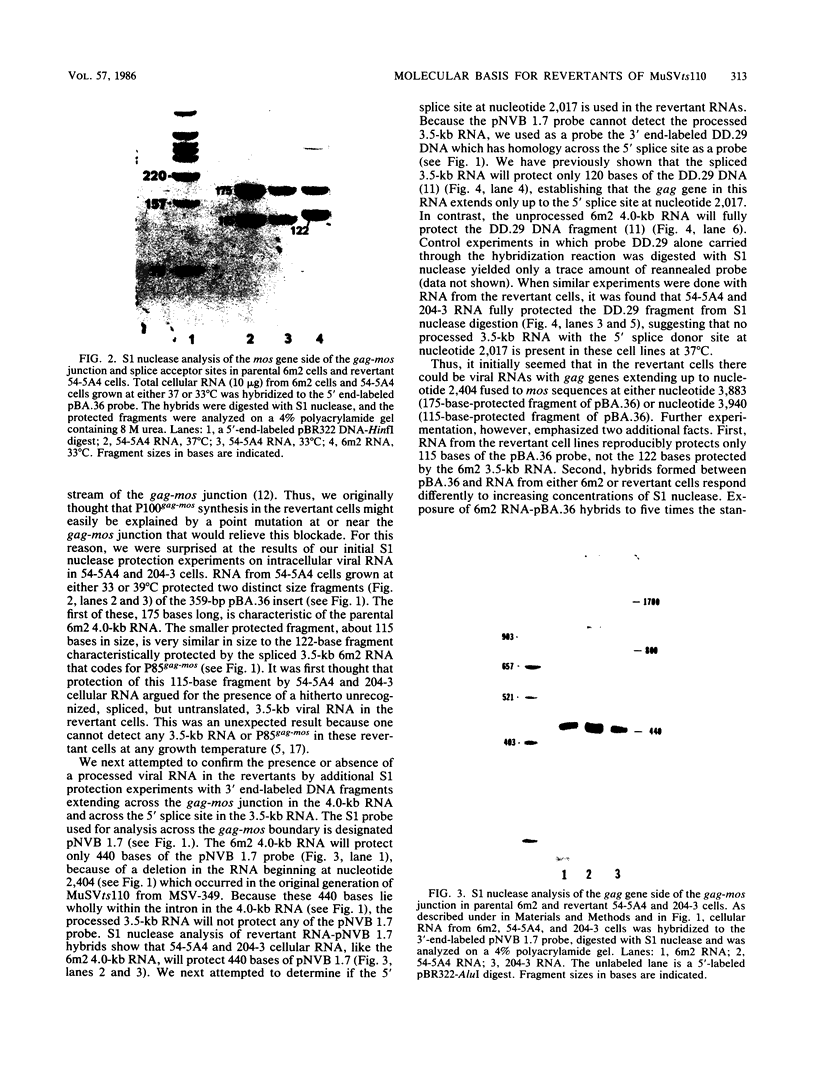
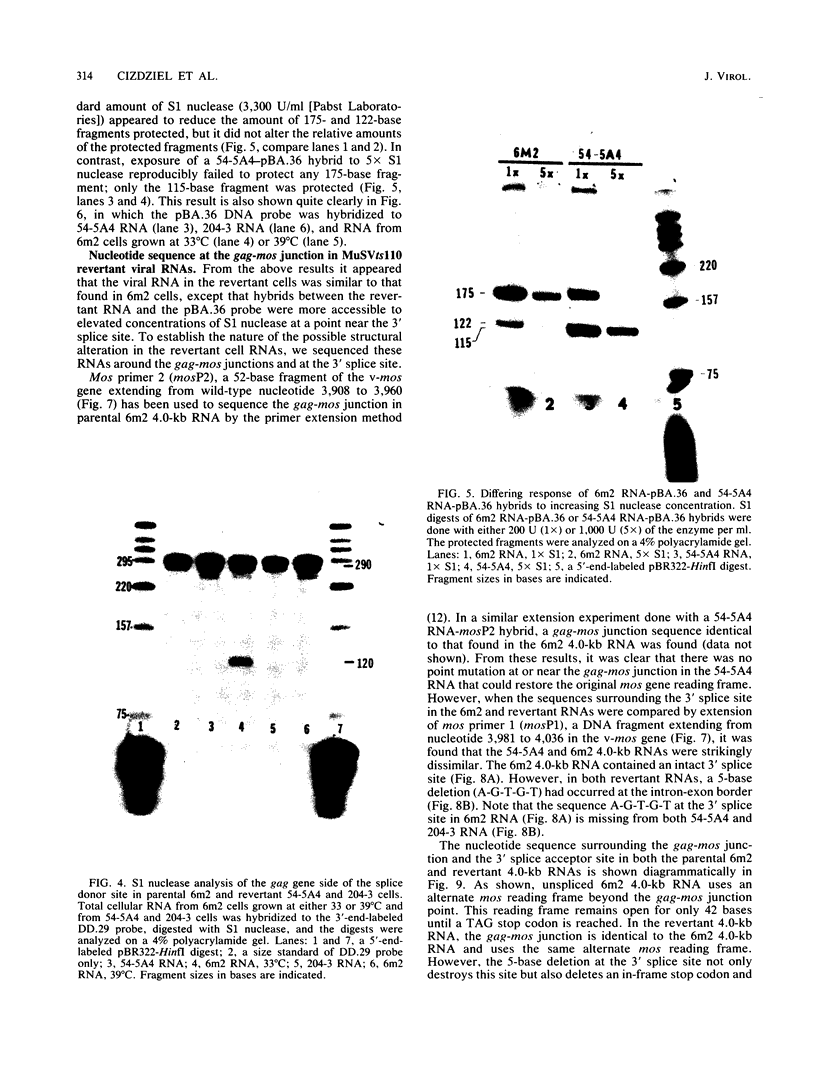
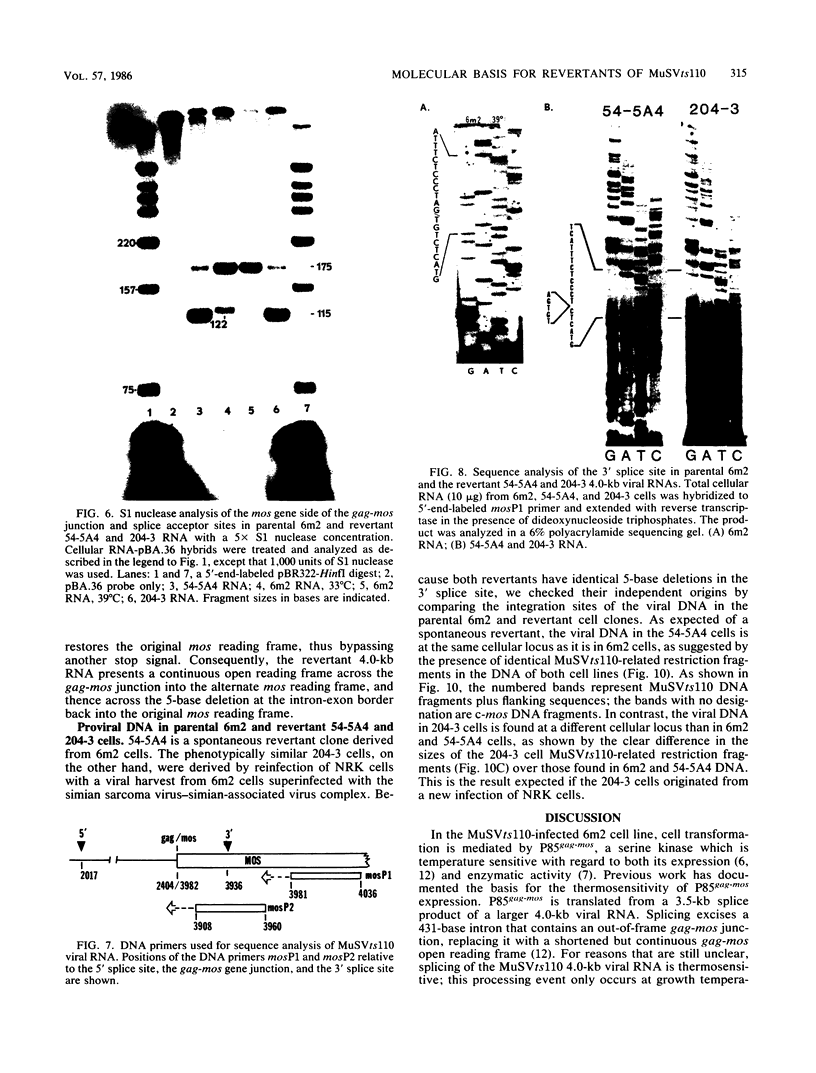
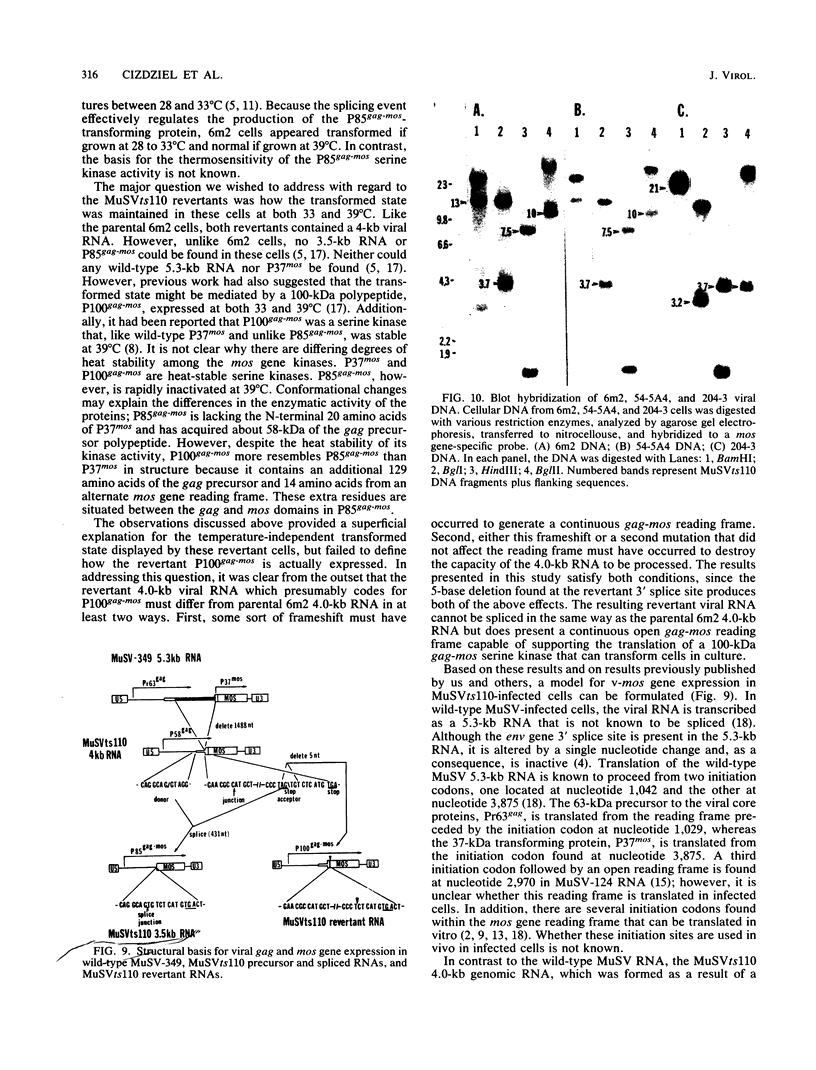
We examined the molecular basis for phenotypic reversion in cells infected with a transformation mutant of murine sarcoma virus, MuSVts110. In MuSVts110-infected NRK cells (6m2 cells), the manifestation of the transformed phenotype at 33 degrees C and the normal phenotype at 39 degrees C is governed by thermosensitive splicing of the MuSVts110 primary transcript, a 4.0-kilobase (kb) RNA which contains the gag and mos genes joined out of frame. At 33 degrees C, selectively, the 4.0-kb RNA is processed to a spliced 3.5-kb RNA in which the gag and mos genes are rejoined in a continuous open reading frame, thus allowing synthesis of the P85gag-mos-transforming protein. In contrast, the MuSVts110 revertant cell lines (designated 54-5A4 and 204-3) appear transformed at all growth temperatures from 33 to 39 degrees C and express a P100gag-mos-transforming protein from an apparently unprocessed 4.0-kb viral RNA. In the current study we established both by S1 nuclease analysis and primer extension sequencing that the revertant 54-5A4 and 204-3 4.0-kb viral RNAs suffered a 5-base deletion at the intron-exon border of the 3' splice site. The effect of this deletion is twofold. First, because of the damage to the 3' splice site, the revertant viral 4.0-kb RNAs cannot be processed to the spliced 3.5-kb RNA and, consequently, cannot be translated to P85gag-mos. Second, the 5-base deletion excises an in-frame stop codon positioned at the intron-exon border in the parental RNA and restores the original mos gene reading frame. The net effect is to produce a continuous open reading frame from the gag, alternate mos, and authentic mos gene reading frames which are fused together in the revertant 4.0-kb RNA. This continuous open reading frame can be translated into the P100gag-mos-transforming protein at any growth temperature.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blair D. G., Hull M. A., Finch E. A. The isolation and preliminary characterization of temperature-sensitive transformation mutants of Moloney sarcoma virus. Virology. 1979 Jun;95(2):303–316. doi: 10.1016/0042-6822(79)90486-0. [DOI] [PubMed] [Google Scholar]
- Cremer K., Reddy E. P., Aaronson S. A. Translational products of Moloney murine sarcoma virus RNA: identification of proteins encoded by the murine sarcoma virus src gene. J Virol. 1981 May;38(2):704–711. doi: 10.1128/jvi.38.2.704-711.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue D. J. Demonstration of biological activity and nucleotide sequence of an in vitro synthesized clone of the Moloney murine sarcoma virus mos gene. J Virol. 1982 May;42(2):538–546. doi: 10.1128/jvi.42.2.538-546.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue D. J., Hunter T. A generalized method of subcloning DNA fragments by restriction site reconstruction: application to sequencing the amino-terminal coding region of the transforming gene of Gazdar murine sarcoma virus. Nucleic Acids Res. 1982 Apr 24;10(8):2549–2564. doi: 10.1093/nar/10.8.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin R., Brizzard B. L., Nash M. A., Murphy E. C., Jr, Arlinghaus R. B. Temperature-sensitive viral RNA expression in Moloney murine sarcoma virus ts110-infected cells. J Virol. 1985 Feb;53(2):616–623. doi: 10.1128/jvi.53.2.616-623.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn J. P., Wood T. G., Murphy E. C., Jr, Blair D. G., Arlinghaus R. B. A selective temperature-sensitive defect in viral RNA expression in cells infected with a ts transformation mutant of murine sarcoma virus. Cell. 1981 Jul;25(1):37–46. doi: 10.1016/0092-8674(81)90229-4. [DOI] [PubMed] [Google Scholar]
- Kloetzer W. S., Maxwell S. A., Arlinghaus R. B. Further characterization of the P85gag-mos -associated protein kinase activity. Virology. 1984 Oct 15;138(1):143–155. doi: 10.1016/0042-6822(84)90154-5. [DOI] [PubMed] [Google Scholar]
- Kloetzer W. S., Maxwell S. A., Arlinghaus R. B. P85gag-mos encoded by ts110 Moloney murine sarcoma virus has an associated protein kinase activity. Proc Natl Acad Sci U S A. 1983 Jan;80(2):412–416. doi: 10.1073/pnas.80.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons D. D., Murphy E. C., Jr, Mong S. M., Arlinghaus R. B. The translation products of Moloney murine sarcoma virus-124 RNA. Virology. 1980 Aug;105(1):60–70. doi: 10.1016/0042-6822(80)90156-7. [DOI] [PubMed] [Google Scholar]
- Murphy E. C., Jr, Arlinghaus R. B. Comparative tryptic peptide analysis of candidate P85gag-mos of ts110 Moloney murine sarcoma virus and P38-P23 mos gene-related proteins of wild-type virus. Virology. 1982 Sep;121(2):372–383. doi: 10.1016/0042-6822(82)90175-1. [DOI] [PubMed] [Google Scholar]
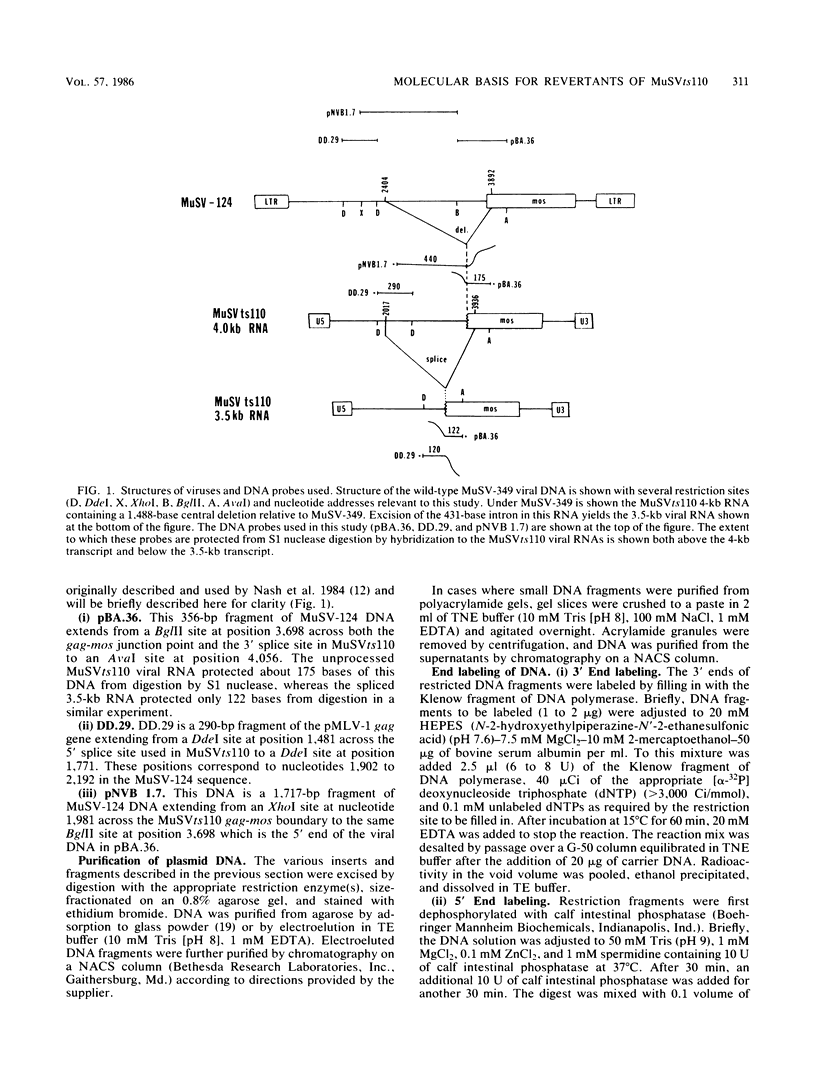
- Nash M. A., Brizzard B. L., Wong J. L., Murphy E. C., Jr Murine sarcoma virus ts110 RNA transcripts: origin from a single proviral DNA and sequence of the gag-mos junctions in both the precursor and spliced viral RNAs. J Virol. 1985 Feb;53(2):624–633. doi: 10.1128/jvi.53.2.624-633.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash M., Brown N. V., Wong J. L., Arlinghaus R. B., Murphy E. C., Jr S1 nuclease mapping of viral RNAs from a temperature-sensitive transformation mutant of murine sarcoma virus. J Virol. 1984 May;50(2):478–488. doi: 10.1128/jvi.50.2.478-488.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J., Lai M. H., Hunter T., Verma I. M. Analysis of transforming gene products from Moloney murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):109–119. doi: 10.1016/0092-8674(81)90365-2. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Verma I. M., Hunter T. Detection of a transforming gene product in cells transformed by Moloney murine sarcoma virus. Cell. 1982 Jun;29(2):417–426. doi: 10.1016/0092-8674(82)90158-1. [DOI] [PubMed] [Google Scholar]
- Singh L., Jones K. W. The use of heparin as a simple cost-effective means of controlling background in nucleic acid hybridization procedures. Nucleic Acids Res. 1984 Jul 25;12(14):5627–5638. doi: 10.1093/nar/12.14.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanker L. H., Horn J. P., Gallick G. E., Kloetzer W. S., Murphy E. C., Jr, Blair D. G., Arlinghaus R. B. Gag-mos Polyproteins encoded by variants of the Moloney strain of mouse sarcoma virus. Virology. 1983 Apr 15;126(1):336–347. doi: 10.1016/0042-6822(83)90483-x. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Galleshaw J. A., Verma I. M. Nucleotide sequence of the genome of a murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):97–108. doi: 10.1016/0092-8674(81)90364-0. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin E. H., Wong-Staal F., Gelmann E. P., Dalla-Favera R., Papas T. S., Lautenberger J. A., Eva A., Reddy E. P., Tronick S. R., Aaronson S. A. Expression of cellular homologues of retroviral onc genes in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2490–2494. doi: 10.1073/pnas.79.8.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]