Abstract
Surfactant protein B (SP-B) is an essential component of pulmonary surfactant. Synthetic surfactant peptide KL4, a peptide based on a C-terminal amphipathic helical region of human SP-B, efficiently mimics some functional properties of SP-B and is included in therapeutic surfactant preparations used in trials to treat respiratory distress syndrome. The membrane orientation of this peptide is controversial. We used an in vitro transcription-translation system to study the insertion of hydrophobic sequences into microsomal membranes, and showed that the KL4 sequence integrates efficiently with a transmembrane orientation despite the presence of intermittent lysines throughout the sequence. In contrast, the precise sequence of the C-terminal SP-B amphipathic region failed to integrate, indicating a nontransmembrane orientation. Differences in the membrane insertion between KL4 and the SP-B-inspiring sequence match predictions from calculated free energies of insertion of the two sequences into membranes.
Pulmonary surfactant is a mixture of lipids and proteins that lowers surface tension at the air/liquid interface, thereby reducing the work involved in breathing and reducing the risk of collapse at expiration. The main lipid constituent and surface tension-reducing component is dipalmitoylphosphatidylcholine (1). Dipalmitoylphosphatidylcholine, however, is extremely inefficient at adsorbing into the interface. Two small hydrophobic proteins, surfactant protein B (SP-B) and surfactant protein C (SP-C), facilitate the rapid movement of lipids between membranes and the interface and help respread the compressed state of the films during subsequent cycling (2). Surfactant membranes also contain anionic phosphatidylglycerol, which is thought to establish functionally important interactions with cationic SP-B and SP-C (3).
Pulmonary surfactant is essential for survival, and its deficiency or dysfunction contributes to severe respiratory pathologies such as infant respiratory distress syndrome (IRDS) in neonates or acute respiratory distress syndrome. Surfactant therapy using exogenous surfactants is a routine clinical procedure for the prevention and treatment of IRDS. The hydrophobic SP-B and SP-C proteins are essential components for the physiological activity of most clinical surfactants (4), which are obtained from animal sources. Because the use of animal-derived surfactants bears serious concerns in terms of potential infections, immunological responses, and compositional consistency, the development of synthetic surfactants is a topic of intense investigation (5).
The structural complexity of SP-B has thus far precluded the expression of functionally active recombinant SP-B. However, synthetic surfactants that contain peptide mimics of SP-B segments have been developed (Surfaxin), exhibiting some efficacy in cases of IRDS (6). The peptide included in this synthetic surfactant (KL4) was based on the C-terminal region of mature SP-B, a hydrophobic sequence with intermittent positively charged residues, which improved the interfacial activity of synthetic phospholipids (7). KL4 was designed as a similar sequence pattern of hydrophobic/cationic residues, consisting of a stretch of four hydrophobic leucines interspersed with cationic lysine to create a 21-residue sequence ([lysine-(leucine)4]4-lysine). KL4 has been proposed to stabilize interfacial lipid layers via electrostatic interactions between the lysines and anionic headgroups, and hydrophobic interactions between the leucines and the phospholipid acyl chains (8). Nevertheless, the real orientation of this synthetic peptide in surfactant membranes and films has been a topic of discussion. KL4 exhibited a high α-helical content as studied by infrared and circular dichroism spectroscopies in the presence of micelles or bilayers, and exhibited an orientation parallel to the lipid acyl chains in bilayers (9). In contrast, infrared reflection-absorption spectroscopy studies showed an antiparallel β-sheet structure of KL4 in interfacial films with a transition toward an α-helical conformation in the interfacial region of multilayers formed under compression (10). Conformation and lipid-protein interactions of KL4 depend on membrane lipid composition, bilayer packing density, and peptide concentration in the membrane (11,12). Thus, further studies are needed to determine the exact secondary structure and orientation of KL4 in biological membranes. This study examined the ability of the KL4 peptide sequence to integrate into endoplasmic reticulum (ER) membranes and compared its behavior with that of the SP-B sequence that inspired its design.
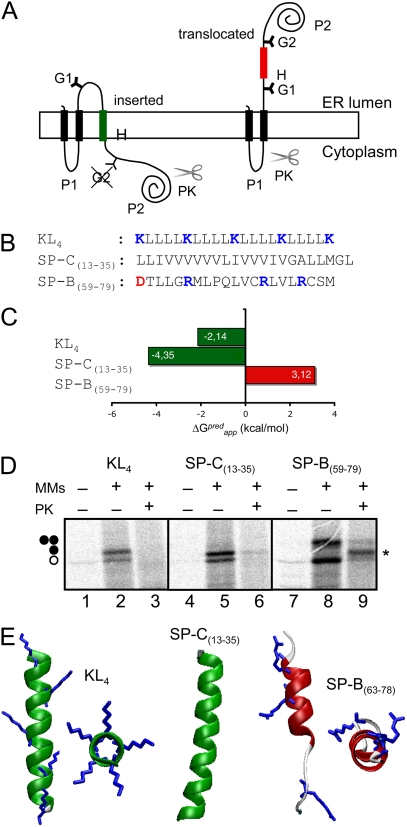
The membrane insertion capacity of the surfactant KL4 peptide was investigated using an experimental system based on the Escherichia coli inner membrane protein leader peptidase (Lep), which accurately reports the integration of transmembrane (TM) helices into ER membranes (13). Lep consists of two TM segments connected by a cytoplasmic loop (P1) and a large C-terminal domain (P2), and inserts into ER-derived microsomal membranes (MMs) with both termini located in the lumen (Fig. 1 A). The segment analyzed (H-segment) is engineered into the luminal P2 domain and is flanked by two acceptor sites (G1 and G2) for N-linked glycosylation. Single glycosylation (i.e., membrane integration) results in a molecular mass increase of ∼2.5 kDa relative to the observed molecular mass of Lep expressed in the absence of microsomes; the molecular mass shifted ∼5 kDa upon double glycosylation (i.e., membrane translocation of the H-segment). Proteinase K (PK) added to MM vesicles will digest the cytoplasmic exposed, nonglycosylated form of the P2 domain (Fig. 1 A, left) to produce a protected, double-glycosylated P2 fragment when the P2 domain is located in the lumen of the MMs (Fig. 1 A, right).
FIGURE 1.
(A) Schematic of the engineered leader peptidase (Lep) model protein. (B) The H-segment sequences examined in this study. (C) Calculated ΔGapp values for the assessed sequences using the ΔG Prediction Server (see text). (D) In vitro translation in the presence (+) or absence (−) of MMs and PK. Nonglycosylated protein bands are indicated by a white dot; single and double glycosylated proteins are indicated by one and two black dots, respectively. The protected double-glycosylated P2 fragment is indicated by an asterisk (*). (E) Left, KL4 canonical helix structure. Center, SP-C helical region (residues 13–35) from the structure determined by NMR (Protein Data Bank: 1SPF) (15). Right, SP-B region (residues 63–78) based on the structure of Mini-B in sodium dodecylsulfate micelles (Protein Data Bank code: 2DWF). Side chains of Arg and Lys residues are shown.
Given the amino acid sequence of an H-segment, TM orientation can be predicted using the apparent free energy difference (ΔGapp) for insertion into ER membranes (14). The predicted ΔGapp for the KL4 sequence was computed as −2.14 kcal/mol (Fig. 1 C, calculated using the ΔG Prediction Server v1.0, http://www.cbr.su.se/DGpred/) (13,14). The negative ΔGapp predicts the KL4 sequence as a TM segment, resembling the TM orientation of surfactant protein SP-C (15) rather than SP-B in terms of ER membrane integration. In fact, sequence 13–35 of SP-C (Fig. 1 B) is predicted to be TM (Fig. 1 C), whereas the SP-B segment that inspired KL4 (segment 59–79; Fig. 1 B) is predicted to not be integrated into membranes (Fig. 1 C).
The translation of constructs harboring the KL4 sequence (constructed using double-stranded oligonucleotides as previously described (16)) clearly resulted in single glycosylated forms (Fig. 1 D, lane 2). PK treatment of this sample rendered complete loss of detectable fragments (Fig. 1 D, lane 3), confirming membrane integration of the KL4 sequence. The dispersion of charged lysine residues in the KL4 sequence around the circumference of the helix (Fig. 1 E, left) has been argued to inhibit partition of the peptide into the core of the bilayer (10). Nevertheless, the strong hydrophobic contribution of the abundant leucine residues in KL4 may reduce the free energy of membrane integration. Interestingly, recent computer simulation studies suggest that the transfer of ∼4 leucines from water to the bilayer interior could be sufficient to balance the transfer of a cationic residue (17). Translation of a construct bearing the TM segment of SP-C in the presence of MMs yielded single-glycosylated forms sensitive to PK digestion (Fig. 1 D, lanes 5 and 6), indicative of TM integration. We also examined the SP-B 59–79 sequence. As shown in Fig. 1 D (lanes 7–9), translation of this construct in the presence of MMs rendered double-glycosylated forms (lane 8), and PK treatment of this sample generated a protected double-glycosylated P2 fragment (lane 9), indicating membrane translocation of this H-segment.
One possibility is that the particular chemical character of lysine residues may be important for the membrane integration process of TM segments. To test this idea, we replaced the central lysine residue in KL4 with either aspartic acid or arginine. As shown in Fig. S1 of the Supplementary Material, both sequences inserted in the membrane in a manner similar to KL4 (lanes 2–4), in good agreement with computed values, arguing against specific effects of the lysine itself. Furthermore, insertion of a second lysine in the central region of the KL4 sequence, which reduced the predicted ΔGapp by 1.62 kcal/mol (Fig. S1), retained insertion of ∼85% of the molecules (Fig. S1, lane 5).
As stated, some controversy surrounds the actual orientation of KL4 peptide in surfactant membranes and films (18,19). Despite the fact that this peptide was originally designed to mimic amphipathic peripheral helices in SP-B, significant structural differences exist between KL4 and the C-terminal amphipathic helices of SP-B. In fact, a model of the SP-B 63–78 residue region based on the structure of Mini-B (a minimized version of the SP-B protein, Protein Data Bank code 2DWF) (20) shows a noncanonical helix conformation with an amphipathic distribution of the cationic residues (Fig. 1 E, right), which can account for the nonintegration effect observed in our experimental system.
This work shows that KL4 adopts a TM orientation in MMs, and therefore can hardly be considered as an analog of surfactant protein SP-B. However, as a TM peptide, KL4 could still efficiently promote the transfer of phospholipids from bilayers into interfacial films, similar to SP-C (21). Whether KL4 could be actually transferred into the interface upon adsorption of peptide/lipid complexes remains to be demonstrated. Most studies have characterized the interfacial behavior of SP-C or KL4 in films formed from lipid/peptide organic mixtures, which may not entirely reflect their behavior when films are formed from lipid/peptide aqueous suspensions. SP-B has been suggested to have an active role in stabilizing compressed interfacial structures, contributing to reach the lowest tensions required to stabilize respiratory mechanics (22). The structure of KL4 was proposed to mimic the electrostatic and hydrophobic interactions associated with high mechanical stability in SP-B-containing films (8). Our results suggest that the analogy between the behavior of KL4 and SP-B at the interface has yet to be demonstrated, particularly in films formed from lipid/protein structures in which the two polypeptides could have very different orientations. Further studies will explore the minimal sequence variations required for KL4-like peptides to mimic the orientation of SP-B sequences with respect to membranes and the dependence of parameters defining optimal surfactant activity on specific peptide-membrane orientations.
SUPPLEMENTARY MATERIAL
To view all of the supplemental files associated with this article, visit www.biophysj.org.
Acknowledgments
We are grateful for Prof. Gunnar von Heijne for providing us with the original Lep constructs.
This work was supported by grants BMC2006-08542 (to I.M.) and BIO20006-03130 (to J.P.-G.) from the Spanish Ministry of Education and Science, and ACOMP07/119 (to I.M.) from the Generalitat Valenciana.
Editor: Anthony Watts.
References
- 1.Johansson, J., and T. Curstedt. 1997. Molecular structures and interactions of pulmonary surfactant components. Eur. J. Biochem. 244:675–693. [DOI] [PubMed] [Google Scholar]
- 2.Serrano, A. G., and J. Perez-Gil. 2006. Protein-lipid interactions and surface activity in the pulmonary surfactant system. Chem. Phys. Lipids. 141:105–118. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Gil, J., C. Casals, and D. Marsh. 1995. Interactions of hydrophobic lung surfactant proteins SP-B and SP-C with dipalmitoylphosphatidylcholine and dipalmitoylphosphatidylglycerol bilayers studied by electron spin resonance spectroscopy. Biochemistry. 34:3964–3971. [DOI] [PubMed] [Google Scholar]
- 4.Robertson, B., and H. L. Halliday. 1998. Principles of surfactant replacement. Biochim. Biophys. Acta. 1408:346–361. [DOI] [PubMed] [Google Scholar]
- 5.Mingarro, I., D. Lukovic, M. Vilar, and J. Perez-Gil. 2008. Synthetic pulmonary surfactant preparations: new developments and future trends. Curr. Med. Chem. 15:393–403. [DOI] [PubMed] [Google Scholar]
- 6.Cochrane, C. G., S. D. Revak, T. A. Merritt, G. P. Heldt, M. Hallman, M. D. Cunningham, D. Easa, A. Pramanik, D. K. Edwards, and M. S. Alberts. 1996. The efficacy and safety of KL4-surfactant in preterm infants with respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 153:404–410. [DOI] [PubMed] [Google Scholar]
- 7.Revak, S. D., T. A. Merritt, M. Hallman, G. Heldt, R. J. La Polla, K. Hoey, R. A. Houghten, and C. G. Cochrane. 1991. The use of synthetic peptides in the formation of biophysically and biologically active pulmonary surfactants. Pediatr. Res. 29:460–465. [DOI] [PubMed] [Google Scholar]
- 8.Cochrane, C. G., and S. D. Revak. 1991. Pulmonary surfactant protein B (SP-B): structure-function relationships. Science. 254:566–568. [DOI] [PubMed] [Google Scholar]
- 9.Gustafsson, M., G. Vandenbussche, T. Curstedt, J. M. Ruysschaert, and J. Johansson. 1996. The 21-residue surfactant peptide (LysLeu4)4Lys(KL4) is a transmembrane α-helix with a mixed nonpolar/polar surface. FEBS Lett. 384:185–188. [DOI] [PubMed] [Google Scholar]
- 10.Cai, P., C. R. Flach, and R. Mendelsohn. 2003. An infrared reflection-absorption spectroscopy study of the secondary structure in (KL4)4K, a therapeutic agent for respiratory distress syndrome, in aqueous monolayers with phospholipids. Biochemistry. 42:9446–9452. [DOI] [PubMed] [Google Scholar]
- 11.Saenz, A., O. Canadas, L. A. Bagatolli, M. E. Johnson, and C. Casals. 2006. Physical properties and surface activity of surfactant-like membranes containing the cationic and hydrophobic peptide KL4. FEBS J. 273:2515–2527. [DOI] [PubMed] [Google Scholar]
- 12.Saleem, M., M. C. Meyer, D. Breitenstein, and H. J. Galla. 2008. The surfactant peptide KL4 in lipid monolayers: phase behavior, topography, and chemical distribution. J. Biol. Chem. 283:5195–5207. [DOI] [PubMed] [Google Scholar]
- 13.Hessa, T., H. Kim, K. Bihlmaier, C. Lundin, J. Boekel, H. Andersson, I. Nilsson, S. H. White, and G. von Heijne. 2005. Recognition of transmembrane helices by the endoplasmic reticulum translocon. Nature. 433:377–381. [DOI] [PubMed] [Google Scholar]
- 14.Hessa, T., N. M. Meindl-Beinker, A. Bernsel, H. Kim, Y. Sato, M. Lerch-Bader, I. Nilsson, S. H. White, and G. von Heijne. 2007. Molecular code for transmembrane-helix recognition by the Sec61 translocon. Nature. 450:1026–1030. [DOI] [PubMed] [Google Scholar]
- 15.Johansson, J., T. Szyperski, T. Curstedt, and K. Wuthrich. 1994. The NMR structure of the pulmonary surfactant-associated polypeptide SP-C in an apolar solvent contains a valyl-rich α-helix. Biochemistry. 33:6015–6023. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Gil, L., A. Sauri, M. Vilar, V. Pallas, and I. Mingarro. 2007. Membrane insertion and topology of the p7B movement protein of Melon Necrotic Spot Virus (MNSV). Virology. 367:348–357. [DOI] [PubMed] [Google Scholar]
- 17.MacCallum, J. L., W. F. Bennett, and D. P. Tieleman. 2008. Distribution of amino acids in a lipid bilayer from computer simulations. Biophys. J. 94:3393–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merritt, T. A. 2006. Concerning the article by T. Curstedt and J. Johansson: new synthetic surfactants—basic science. Biol. Neonate. 89:257–258. [DOI] [PubMed] [Google Scholar]
- 19.Curstedt, T., and J. Johansson. 2006. Letter to the editor: Reply. Biol. Neonate. 89:258–259. [Google Scholar]
- 20.Sarker, M., A. J. Waring, F. J. Walther, K. M. Keough, and V. Booth. 2007. Structure of mini-B, a functional fragment of surfactant protein B, in detergent micelles. Biochemistry. 46:11047–11056. [DOI] [PubMed] [Google Scholar]
- 21.Lukovic, D., I. Plasencia, F. J. Taberner, J. Salgado, J. J. Calvete, J. Perez-Gil, and I. Mingarro. 2006. Production and characterisation of recombinant forms of human pulmonary surfactant protein C (SP-C): structure and surface activity. Biochim. Biophys. Acta. 1758:509–518. [DOI] [PubMed] [Google Scholar]
- 22.Longo, M. L., A. M. Bisagno, J. A. Zasadzinski, R. Bruni, and A. J. Waring. 1993. A function of lung surfactant protein SP-B. Science. 261:453–456. [DOI] [PubMed] [Google Scholar]