Abstract
Background
Airway eosinophilia is considered a central event in the pathogenesis of asthma. The toxic components of eosinophils are thought to be important in inducing bronchial mucosal injury and dysfunction. Previous studies have suggested an interaction between nitric oxide (NO) and chemokines in modulating eosinophil functions, but this is still conflicting. In the present study, we have carried out functional assays (adhesion and degranulation) and flow cytometry analysis of adhesion molecules (VLA-4 and Mac-1 expression) to evaluate the interactions between NO and CC-chemokines (eotaxin and RANTES) in human eosinophils.
Methods
Eosinophils were purified using a percoll gradient followed by immunomagnetic cell separator. Cell adhesion and degranulation were evaluated by measuring eosinophil peroxidase (EPO) activity, whereas expression of Mac-1 and VLA-4 was detected using flow cytometry.
Results
At 4 h incubation, both eotaxin (100 ng/ml) and RANTES (1000 ng/ml) increased by 133% and 131% eosinophil adhesion, respectively. L-NAME alone (but not D-NAME) also increased the eosinophil adhesion, but the co-incubation of L-NAME with eotaxin or RANTES did not further affect the increased adhesion seen with chemokines alone. In addition, L-NAME alone (but not D-NAME) caused a significant cell degranulation, but it did not affect the CC-chemokine-induced cell degranulation. Incubation of eosinophils with eotaxin or RANTES, in absence or presence of L-NAME, did not affect the expression of VLA-4 and Mac-1 on eosinophil surface. Eotaxin and RANTES (100 ng/ml each) also failed to elevate the cyclic GMP levels above baseline in human eosinophils.
Conclusion
Eotaxin and RANTES increase the eosinophil adhesion to fibronectin-coated plates and promote cell degranulation by NO-independent mechanisms. The failure of CC-chemokines to affect VLA-4 and Mac-1 expression suggests that changes in integrin function (avidity or affinity) are rather involved in the enhanced adhesion.
Background
Airway eosinophilia is considered a central event in the pathogenesis of asthma. The toxic components of eosinophils are thought to be important in inducing bronchial mucosal injury and dysfunction [1]. Evidences suggest that recruitment of eosinophils into sites of inflammation is a multifactorial and multistep process, involving eosinophil-endothelial interactions through adhesion molecules and local generation of chemotactic agents that direct cell migration into the inflamed airways [2]. Thus, adhesion molecules and chemokines play key roles in selective eosinophil accumulation [3]. The integrins macrophage adhesion molecule-1 (Mac-1, CD11b/CD18, αMβ2) and very late antigen-4 (VLA-4, CD49d/CD29, α4β1) are the most important integrins responsible for the firm adhesion of eosinophils to the endothelium through their ligands the intercellular adhesion molecule (ICAM)-1 and the vascular cell adhesion molecule (VCAM)-1 [4]. The integrin VLA-4 is constitutively expressed in eosinophils [5], whereas Mac-1 is expressed in eosinophils stimulated with different class of mediators such as platelet-activating factor (PAF), granulocyte macrophage colony stimulating factor (GM-CSF), and N-formyl-methionyl-leucyl-phenylalanine (fMLP) [6-8]. Both Mac-1 and VLA-4 integrins have a role in the degranulation and superoxide anion production in stimulated eosinophils [6,9], and thus can also be referred as mediators of eosinophil functions. These integrins can also bind to extracellular matrix components such as serum, fibrinogen and fibronectin. Adhesion of eosinophils to fibronectin via VLA-4 [8] prolongs cell survival by Fas antigen signalling, indicating a role for integrin adhesion and signaling in regulating eosinophil function and death [10]. The firm adhesion of eosinophils to fibronectin is mediated through the binding of eosinophil-expressed VLA-4 to the connecting segment 1 (CS-1) region of fibronectin. Eosinophils bind to CS-1 with high avidity, an effect inhibited with neutralizing antibodies to α4 integrins expressed by eosinophils or with neutralizing antibodies to CS-1 [11].
Eotaxin and RANTES (Regulated upon activation normal T cell expressed and secreted) are CC-chemokines responsible for selective eosinophil chemotaxis and transendothelial migration in airways of allergic subjects [12-15]. Selective activation of VLA-4 by eotaxin has provided one mechanism of more efficient recruitment of eosinophils to the lung [16]. In addition, eosinophil priming with interleukin-5 (IL-5) results in a synergistic increase in transendothelial migration in response to RANTES [17] and eotaxin [18]. In fact, interactions of IL-5 and chemokines induce a change in the affinity of VLA-4 in responding leukocytes [5].
Several studies have demonstrated the involvement of nitric oxide (NO) on eosinophil recruitment, but this is still a matter of controversy. Rats treated with the nitric oxide synthase (NOS) inhibitor Nω-nitro-L-arginine methyl ester (L-NAME) shows a marked reduction in rat eosinophil migration in vivo in an allergic inflammation model [19]. In vitro studies have showed that incubation of rat or human eosinophils with NOS inhibitors attenuate the in vitro chemotaxis, suggesting that NO has a role in cell locomotion [20,21]. Previous studies have investigated the functional interactions between NO and CC-chemokines. Young et al. [22] showed that increased number of eosinophils in bronchoalveolar lavage (BAL) fluid of challenged monkeys is markedly increased at 24 h post-challenge, and that is accompanied by higher levels of both exhaled NO and eotaxin in BAL fluid. In rhinitis patients, eotaxin increased the number of eosinophils in the nasal lavage fluid, and that was also accompanied by elevated nasal NO levels [23]. In contrast, in murine models of asthma, selective inhibition of inducible NOS resulted in reduction of the pulmonary eosinophil migration [24,25], which was associated with increased expression of the CC-chemokine monocyte chemoattractant protein-1 (MCP-1) in the lung tissue [26]. Moreover, NO (or NO donors) have also been shown to inhibit the production of RANTES [27]. Nitric oxide via peroxynitrite (ONOO-) formation was also shown to reduce the eotaxin-induced eosinophil migration [28]. However, the modulatory role of NO in the CC-chemokines-mediated eosinophil functions is still conflicting. Therefore, the present study was designed to investigate the modulatory effect of NO in the enhanced eosinophil adhesion and degranulation induced by the CC-chemokines eotaxin and RANTES in vitro, and the expression of VLA-4 and Mac-1 in the eosinophil surface.
Methods
Human eosinophil isolation
Blood was collected from healthy volunteers who were not under medication. Informed consent and approval from the local ethics committee were obtained before the study. Eosinophils were isolated from peripheral blood as originally described by Hansel et al. [29] with minor modifications. Briefly, 60–120 ml of blood collected in 3.13% (w/v) sodium citrate was diluted 1:1 with phosphate buffered saline (PBS) and 35 ml of diluted blood was overlaid onto a 15 ml Percoll gradient (1,088 g/ml, pH 7.4, 340 mosmol/Kg H2O). Gradients were centrifuged at 1000 × g for 20 min, 4°C (Hermle model Z360k centrifuge, Germany) and the pellet containing red cells and granulocytes was collected. Red cells were lysed with lysing buffer (155 mM NH4Cl, 10 mM KHCO3 0.1 mM EDTA), and the remaining granulocytes were washed and then incubated with anti-CD16 immunomagnetic microbeads for 30 min before passing on a steel-matrix column in a magnetic field and CD16-negative eosinophils were collected. Eosinophils were resuspended in RPMI-1640 medium (without phenol red), pH 7.2 (> 92% eosinophils, contaminating cells were mononuclear cells).
Experimental Protocols
After the purification of human isolated eosinophils, the following in vitro assays were carried out: cell viability (MTT assay), adhesion to fibronectin-coated plates, EPO release (cell degranulation), cGMP level measurement and flow cytometry analysis for the adhesion molecules VLA-4 and Mac-1. These assays have been performed in eosinophils stimulated with either eotaxin or RANTES, in the absence and/or in the presence of L-NAME, as detailed below.
MTT assay
Cell viability was estimated using the tetrazolium salt reduction test (MTT assay) by eosinophils after exposure to chemokines. Isolated eosinophils were resuspended to a concentration of 2 × 106 cells/ml in RPMI-1640 medium (without phenol red) and then were exposed or not to eotaxin or RANTES (10, 100 and 1000 ng/ml each) for 2, 3 or 4 h at 37°C in a humified atmosphere. Eosinophils (100 μl/well), treated or not, and 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT, 10 μl/well, 5 mg/ml in PBS) were added in triplicate to a 96-well plates. Cells were allowed to incubate for 3 h at 37°C and 5% CO2. After incubation, 100 μl of 10% SDS in 0.01 M HCl was added to each well. Cell samples were then incubated for 18 h at 37°C, 5% CO2 and absorbance measured at 540 nm in a microplate reader (Multiscan MS, Labsystems, USA).
Eosinophil adhesion assay
96-well plates were prepared by coating individual wells with 60 μl of fibronectin solution (20 μg/ml in PBS) overnight at 4°C. Wells were then washed twice with PBS before blocking non-coated sites with 0.1% (w/v) BSA for 60 min at 37°C. Wells were washed twice again with PBS before allowing plates to dry. Eosinophils (50 μL of 7 × 104 cells/ml) were incubated with either eotaxin (10–1000 ng/ml) or RANTES (10–1000 ng/ml) for 2, 3 and 4 h in the absence or in the presence of 0.1 mM of L-NAME (or its inactive enantiomer D-NAME) prior to addition to fibronectin-coated wells. Platelet-activating factor (PAF; 10-6 M) was used as a positive control [30]. After 2, 3 or 4 h of incubation, the eosinophils were added in a volume of 50 μl of RPMI-1640 medium without phenol red (7 × 104 cells/ml) to the coated wells. Cells were allowed to adhere to wells for 30 min at 37°C, 5% CO2. After incubation, non-adhered cells were removed and the remaining cells were washed twice with PBS. Fifty μl of RPMI-1640 medium were added to each well, and varying concentrations of the original cell suspension were added to empty wells to form a standard curve. Eosinophil adhesion was calculated by measuring residual eosinophil peroxidase (EPO) activity of adherent cells [9]. Fifty μl of EPO substrate (1 mM H2O2, 1 mM o-phenylenediamine and 0.1% Triton X-100 in Tris buffer, pH 8.0) were added to each well. After 30 min at room temperature, 25 μl of 4 M H2SO4 were added to each well to stop the reaction and absorbance measured at 490 nm in a microplate reader. Adherence was calculated by comparing absorbance of unknowns to that of the standard curve. In order to exclude the possibility that enhanced eosinophil adhesion to chemokines or L-NAME reflects augmented EPO activity, varying number of eosinophils (20 to 200 μL of 7 × 104cells/ml) were incubated with RPMI alone, eotaxin (100 ng/ml), RANTES (100 ng/ml) or L-NAME (0.1 mM) 4 h in non-coated wells. The EPO measurement in all samples did not differ significantly among each other in any condition used (data not shown), indicating that EPO activity can be used as a marker of eosinophil adhesion. Each experiment was carried out in triplicate (n = 6 different individuals).
Eosinophil degranulation assay
Eosinophil peroxidase release was measured in the supernatants by the o-phenylenediamine (OPD) method [31]. All tubes used were precoated with 2.5% human serum albumin for 2 h at 37°C to block nonspecific activation of eosinophils, and were washed with 1 ml of PBS once before aliquots of eosinophils were added. Ninety μl of eosinophils (5 × 106 cells/ml in RPMI-1640 medium without phenol red) stimulated or not with eotaxin (100 ng/ml) or RANTES (100 ng/ml), in the absence or in the presence of 0.1 mM of L-NAME (or of 0.1 mM D-NAME), were incubated for 4 h at 37°C, 5% CO2. The calcium ionophore A23187 (5 μM, 30 min) was used as a positive control [32]. At the end of the incubation period the reaction was stopped by cooling on ice for 3 min. The samples were centrifuged at 250 × g for 15 min at 4°C and the cells discarded. Aliquots of the supernatants (50 μl; triplicate) were dispensed into each well of a microplate, and 50 μl of eosinophil peroxidase substrate (1 mM H2O2, 1 mM OPD and 0.1% Triton X-100 in Tris buffer, pH 8.0) was added to each well. Measurement of EPO activity was measured at 490 nm using a microplate reader (Multiscan MS, Labsystems, USA), as described above. Each experiment was carried out in triplicate (n = 4 different individuals).
Flow cytometry
Eosinophils were incubated with either eotaxin (100 ng/ml) or RANTES (100 ng/ml) for 4 h in the absence and/or in the presence of L-NAME. Eosinophils were also incubated with PAF (10-6 M) using the same experimental conditions. Expression of adhesion molecules on the surface of eosinophils was detected using flow cytometry. After 2, 3 or 4 h of incubation, eosinophils were washed and incubated (100 μL of 3 × 106 cells/ml) for 20 min at 4°C with 5 μl of human serum. Cells were washed and incubated for 20 min at 4°C with 5 μl of RPE-conjugated mouse IgG1, k monoclonal immunoglobulin isotype control, RPE-conjugated mouse anti-human CD11b/Mac-1 monoclonal antibody or RPE-conjugated anti-human CD49d monoclonal antibody, after which they were washed and fixed in 0.3 ml 0.5% paraformaldehyde. Cells were analysed on a Becton-Dickinson FACScalibur (San Jose, USA). The mean fluorescence intensity was compared to that of isotype control reacted cells.
Extraction and measurement of cyclic guanosine-3', 5'-monophosphate (cGMP) from eosinophils
Eosinophils were isolated and resuspended to a concentration of 1 × 107 cells/ml in PBS. Cells were incubated with the phosphodiesterase inhibitor 3-isobutyl-l-methyl-xanthine (IBMX; 2 mM) for 30 min at room temperature. Then, eosinophils were incubated (37°C, humidified atmosphere) for 30 min or 4 h with or without eotaxin (or RANTES) and/or L-NAME. The NO donor sodium nitroprusside (SNP; 0.1 mM) were used as positive control in the cGMP assays. Next, the reaction was interrupted by the addition of cold acidified absolute ethanol to a final concentration of 67% (v/v), and samples were vigorously agitated by hand for 30 sec. Cell samples were then incubated on ice for 30 min before centrifuging at 4000 g for 30 min at 4°C. The supernatants were collected, retained and the precipitates washed with 0.5 ml 67% (v/v) acidified ethanol before centrifuging again at 14,000 g for 5 min at room temperature. The supernatants from these washed samples were collected and added to the first supernatants collected and dried at 55–60°C under a stream of nitrogen in a water bath and stored at -20°C until measurement of cGMP. Cyclic GMP in 1.5 × 106 cells/well was measured using Cayman kit (Ann Arbor, USA). The samples for measurement of cGMP were previously acetylated, following the manufacturer recommendations. Each experiment was carried out in triplicate using 03 healthy volunteers.
Materials
The system used for eosinophil purification, complete with columns and microbeads, was acquired from Miltenyi Biotec Inc. (Auburn, CA, USA). The antibodies used to flow cytometry assays were purchased from BD Biosciences Pharmingen (RPE-conjugated mouse IgG1, k monoclonal immunoglobulin isotype control, RPE-conjugated mouse antihuman CD11b/Mac-1 monoclonal antibody and RPE-conjugated anti-human CD49d monoclonal antibody; San Jose, USA). Recombinant human eotaxin and RANTES were obtained from R&D Systems (Saint Louis, USA). All other products were purchased from Sigma Chem. Co (St. Louis, MO, USA), unless otherwise stated.
Statistical analysis
Results are expressed as means ± S.E.M and analysis of variance (ANOVA) for multiple comparisons followed by Tukey's test, or unpaired Student's t-test were performed when appropriate. A value of P < 0.05 was taken as significant.
Results
Cell viability
The MTT reduction assay was performed to ascertain the eosinophil viability during incubation periods of 2, 3 and 4 h in the presence of either eotaxin or RANTES (10–1000 ng/ml). Our data showed that eosinophils remained viable in all concentrations and incubation periods studied (Table 1). A concentration-dependent increase (P < 0.05) in cell activity was rather detected in most of our experimental conditions.
Table 1.
MTT reduction assay in stimulated eosinophils in vitro.
| Treatment | MTT Reduction (% control) | ||
| 2 h | 3 h | 4 h | |
| Eotaxin | |||
| (10 ng/ml) | 84.6 ± 6.6 | 113.0 ± 6.1 | 91.0 ± 2.7 |
| (100 ng/ml) | 113.8 ± 5.0* | 130.7 ± 2.3* | 127.3 ± 3.6* |
| (1000 ng/ml) | 124.7 ± 5.0* | 138.4 ± 2.0* | 146.0 ± 11.8* |
| RANTES | |||
| (10 ng/ml) | 104.8 ± 1.6 | 128.8 ± 8.0* | 105.8 ± 1.0 |
| (100 ng/ml) | 118.9 ± 2.8* | 133.4 ± 3.6* | 138.4 ± 6.0* |
| (1000 ng/ml) | 117.4 ± 3.8* | 118.0 ± 1.0* | 147.0 ± 6.9* |
Eosinophil suspension (2 × 106cells/ml) was incubated for 2, 3 or 4 h with eotaxin (10–1000 ng/ml) or RANTES (10–1000 ng/ml). Each experiment was carried out in triplicate (n = 3). Results are expressed as MTT reduction (% control). *P < 0.05 compared to respective untreated cells.
Eotaxin and RANTES-induced eosinophil adhesion to fibronectin
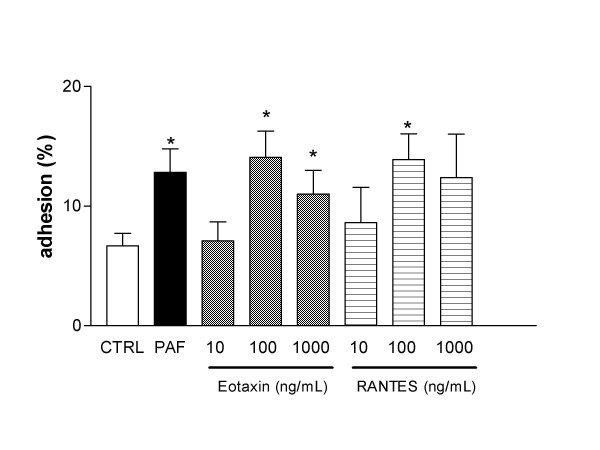
Eosinophils (50 μL of 7 × 104 cells/ml) were incubated with either eotaxin (10–1000 ng/ml) or RANTES (10–1000 ng/ml) for 2, 3 and 4 h prior to addition to fibronectin-coated wells. At 2 and 3 h incubation, neither eotaxin nor RANTES significantly increased the eosinophil adhesion to fibronectin in comparison with non-stimulated cells (not shown; n = 6 each). On the other hand, at 4 h incubation, eotaxin and RANTES significantly (P < 0.05) increased the eosinophil adhesion compared with non-stimulated cells (Figure 1). Maximal eosinophil adhesion to eotaxin (14.1 ± 2.2%) and RANTES (13.9 ± 2.1%) was observed with the concentration of 100 ng/ml of each. In the same experimental conditions, incubation of eosinophils with PAF (10-6 M), used as a positive control, significantly increased the eosinophil adhesion (12.8 ± 2.0%; Figure 1). For further experiments, the time of 4 h, and chemokine concentrations of 100 ng/ml were chosen as best incubation period and optimum concentrations.
Figure 1.
Adhesion of human eosinophils to fibronectin-coated plates. Eosinophils (3.5 × 103cells/well) were incubated with eotaxin (10, 100 and 1000 ng/ml), RANTES (10, 100 and 1000 ng/ml) or platelet-activating factor (PAF; 10-6 M) for 4 h (37°C, 5%CO2) and then allowed to adhere to fibronectin-coated wells for 30 min. Results are expressed as mean adhered cell percentages of total cell number ± SEM (n = 6). * P < 0.05 compared to control (CTRL).
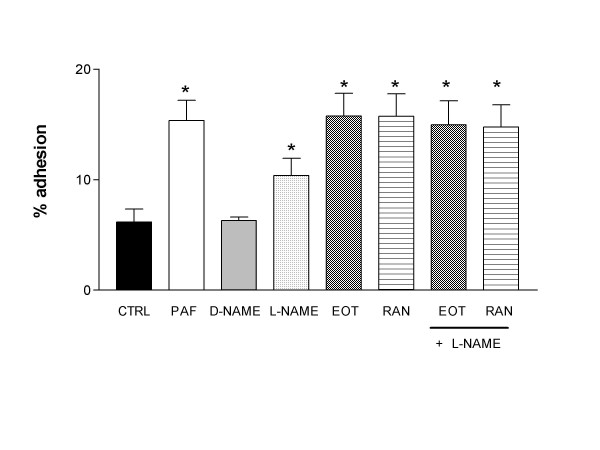
Figure 2 shows that incubation of eosinophils with L-NAME alone (0.1 mM) increased significantly the adhesion of eosinophils to fibronectin (10.4 ± 1.6%; P < 0.05) compared with non-stimulated cells (6.2 ± 1.2%), whereas its inactive enantiomer D-NAME (0.1 mM) had no effect (6.3 ± 0.3%). However, co-incubation of L-NAME with eotaxin or RANTES did not affect the increased adhesion seen with the chemokines alone (Figure 2).
Figure 2.
Lack of effect of Nω-nitro-L-arginine methyl ester (L-NAME) upon eotaxin- and RANTES-induced eosinophil adhesion to fibronectin-coated plates. Eosinophils (3.5 × 103 cells/well) were incubated with platelet-activating factor (PAF; 10-6M), D-NAME; 0.1 mM), L-NAME (0.1 mM), eotaxin (EOT; 100 ng/ml) or RANTES (RAN; 100 ng/ml) for 4 h (37°C, 5%CO2), and then allowed to adhere to fibronectin-coated wells for 30 min. Results are expressed as mean adhered cell percentages of total cell number ± SEM (n = 6). * P < 0.05 compared to control (CTRL).
Eotaxin and RANTES-induced eosinophil degranulation
Table 2 shows that incubation of human eosinophils (90 μL of 5 × 106 cells/ml) with either eotaxin (100 ng/ml) or RANTES (100 ng/ml) caused a significant cell degranulation (P < 0.001) in comparison with basal response. Incubation of eosinophils with 0.1 mM of L-NAME alone (but not with the inactive enantiomer D-NAME) also increased significantly (P < 0.001) cell degranulation when compared with non-stimulated cells. Co-incubation of L-NAME with either eotaxin or RANTES did not further affect the increased degranulation seen with the chemokines alone (Table 2). Using the same experimental conditions, the calcium ionophore A23187 (5 μM), used as a positive control, increased by 135% the EPO release above basal values.
Table 2.
Effect of L-NAME and CC-chemokines upon eosinophil peroxidase (EPO) release.
| Treatment | Optical density |
| CTRL | 0.39 ± 0.02 |
| A23187 (5 μM) | 0.87 ± 0.04* |
| D-NAME (0.1 mM) | 0.27 ± 0.01 |
| L-NAME (0.1 mM) | 0.70 ± 0.04* |
| Eotaxin (100 ng/ml) | 0.70 ± 0.03* |
| RANTES (100 ng/ml) | 0.71 ± 0.05* |
| Eotaxin+L-NAME | 0.77 ± 0.02* |
| RANTES+L-NAME | 0.69 ± 0.04* |
Eosinophils (4.5 × 105 cells) were incubated or not (CTRL) with Nω-nitro-L-arginine methyl ester (L-NAME; 0.1 mM), eotaxin (100 ng/ml) or RANTES (100 ng/ml) for 4 h (37°C, 5%CO2). After incubation the released peroxidase in 50 μl of supernatant was measured and expressed as mean ± SEM of optical density (n = 4). *P < 0.05 compared to control (CTRL).
Expression of integrins in human eosinophils
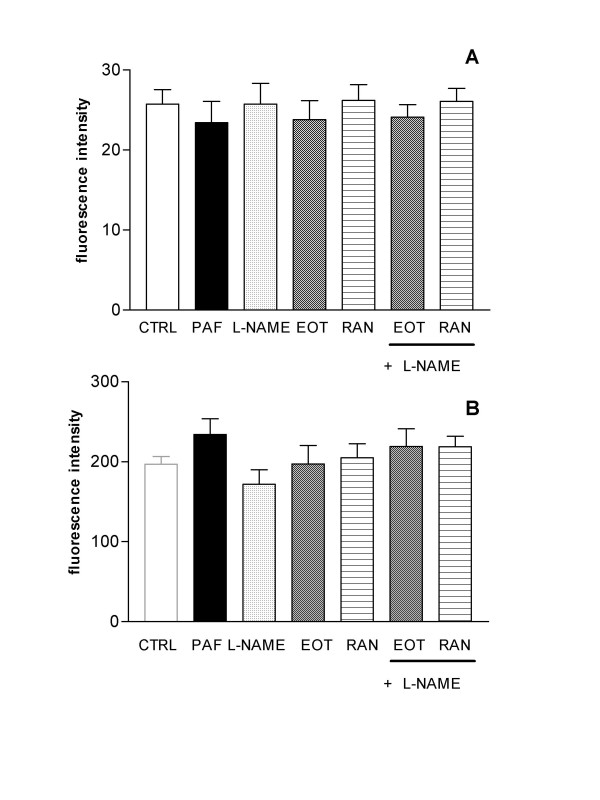
Expression of α4 (CD49d) and αM (CD11b/Mac-1) subunits on the eosinophil surface was determined by fluorescent-immunolabelling cells and detection by flow cytometry. Eosinophils, treated or not with either eotaxin or RANTES during 4 h, were incubated with the respective monoclonal antibodies, and the average mean fluorescence were detected by flow cytometry. Neither eotaxin (100 ng/ml) nor RANTES (100 ng/ml) affected the expression of α4 (Figure 3A) or αM subunits (Figure 3B) on eosinophils in any of the incubation periods.
Figure 3.
Effect of L-NAME and CC-chemokines upon eosinophil expression of α4 and αM subunits. Eosinophils (3.0 × 105 cells) were incubated or not (CTRL) with platelet-activating factor (PAF; 10-6M), Nω-nitro-L-arginine methyl ester (L-NAME; 0.1 mM), eotaxin (EOT; 100 ng/ml) or RANTES (RAN; 100 ng/ml) for 4 h (37°C, 5%CO2), and then were labelled with anti-α 4 (VLA-4; Panel A) or anti-αM (Mac-1; Panel B) antibodies. The results are expressed as mean fluorescence ± SEM (n = 4).
To verify the effect of L-NAME upon integrin expression of chemokine-stimulated and non-stimulated, eosinophils were incubated for 4 h with L-NAME alone or in the presence of eotaxin (or RANTES). No changes were detected in the expression of α4 or αM subunits (Figure 3A,B).
In eosinophils treated with PAF (10-6 M; used as a positive control) an increase in the αM expression about of 20% was found (fluorescence intensity of 234.1 ± 19.8) compared with untreated cells (fluorescence intensity of 196.9 ± 9.6; Figure 3B).
Effect of chemokines and L-NAME upon intracellular cGMP levels in eosinophils
Incubation of eosinophils for either 30 min or 4 h with eotaxin (100 ng/ml) did not significantly increase the concentration of cyclic cGMP (2.9 ± 0.5 and 4.2 ± 0.5 pmol/ml, respectively) above baseline (2.3 ± 0.3 and 4.4 ± 0.5 pmol/ml, respectively; n = 3). Incubation of eosinophils with eotaxin (100 ng/ml each) in the presence of L-NAME (0.1 mM) also failed to affect the cGMP production (2.7 ± 0.2 and 4.3 ± 0.4 pmol/ml, for 30 min and 4 h-incubation, respectively; n = 3).
RANTES (100 ng/ml) also failed to significantly increase the cGMP levels above baseline (2.6 ± 0.4 and 4.5 ± 0.6 pmol/ml, respectively, for 30 min and 4 h incubation, respectively). Incubation of eosinophils with RANTES (100 ng/ml each) in the presence of L-NAME (0.1 mM) also failed to affect the cGMP production (2.1 ± 0.2 and 4.2 ± 0.4 pmol/ml, for 30 min and 4 h-incubation, respectively; n = 3).
Incubation of eosinophils with the NO-donor SNP (0.1 mM; positive control) markedly increased (P < 0.001) the cGMP levels above baseline at 30 min and 4 h (7.7 ± 0.7 and 6.2 ± 0.5 pmol/ml, respectively).
Discussion
The roles of chemokines and NO in eosinophil functions in allergic processes have been largely studied, but studies focusing on the interaction between CC-chemokines (particularly eotaxin and RANTES) and the NO-cGMP pathway have been poorly explored. In the present study, using human eosinophils in vitro, our findings are clear to suggest that NO modulates neither the enhanced adhesion and degranulation in response to eotaxin or RANTES, nor the Mac-1 and VLA-4 expression in the eosinophil surface.
Among the eosinophil-active chemoattractants described to date, the CC-chemokines, particularly RANTES, eotaxin, eotaxin-2, MCP-3 and MCP-4 have been suggested to contribute to bronchial eosinophilia in patients with asthma [33,34]. In general, chemokines can induce integrin expression on the surface of leucocytes, allowing firm adhesion to the endothelium near to the inflamed focus [35], and then stimulating the transendothelial migration [36]. In murine eosinophils of IL-5 transgenic mice, eotaxin increases the CD11b and VLA-4 expressions, with maximal responses observed 1 h (CD11b) and 2 h (VLA-4) after incubation with eotaxin, suggesting that Mac-1/ICAM-1 interactions are dominant at early time, whereas VLA-4/VCAM-1 becomes predominant at later time-points during eotaxin-induced eosinophil recruitment [37]. In human eosinophils, a flow cytometry study showed that 15 min-incubation with eotaxin (300 ng/ml) caused increased surface expression of CD11b [38]. In an apparent contrast with this work, our present study showed that the CD49d and CD11b expression in human eosinophils incubated with eotaxin remained unchanged in all incubation periods studied. This discrepancy could reflect differences in the animal species (IL-5-transgenic mice vs human) and source of eosinophils used (peritoneal vs peripheral blood), as well as incubation-time used (30 min vs 4 h). On the other hand, a previous study also failed to demonstrate an increased expression of α4 integrin in human eosinophils treated for 30 min with eotaxin [11]. Similarly, our data from RANTES-stimulated eosinophils demonstrated no change on expression of CD49d or CD11b, which are in accordance with Jia et al. [37] in murine eosinophils. Additionally, in human eosinophils in vitro RANTES was shown to induce transendothelial migration without affecting the CD11b expression [17].
The action of NO is generally carried out through the activation of the soluble guanylyl cyclase, thereby increasing intracellular levels of cGMP, which acts as its second messenger. This signal transduction pathway underlies the majority of physiological actions attributed to NO, including regulation of leukocyte rolling, adhesion, and extravasation [39,40]. Immunohistochemical studies revealed that rat and human eosinophils express inducible and endothelial NO synthases, and activated rat peritoneal eosinophils are able to release NO [20,41]. Furthermore, the NO synthesis inhibitor L-NAME reduces the experimental eosinophil migration in vivo, suggesting a fundamental role for NO in eosinophil chemotactic responses [19,24] that has been associated with increased expression of MCP-1 and MIP-2 in the lung tissue [26]. On the other hand, L-NAME has been shown to enhance the in vivo cat leukocyte adhesion and the human eosinophil adhesion to fibronectin-coated plates [8,40]. However, no study exists investigating a potential role of NO-cGMP signaling pathways modulating the enhanced eosinophil adhesion in response to CC-chemokines. In the present study, at 4 h-incubation time, L-NAME itself increased the eosinophil adhesion, confirming a previous study [8]; however, L-NAME failed to affect the enhanced adhesion in response to both eotaxin and RANTES. Both of these CC-chemokines also failed to elevate the cGMP concentrations above the baseline. Additionally, treatment of eosinophils with L-NAME alone or in combination with eotaxin (or RANTES) had no effect in the cell integrin expressions (αM or α4 subunits), all suggesting that CC-chemokines eotaxin and RANTES increases the in vitro eosinophil adhesion to fibronectin-coated plates by NO-independent mechanisms. It is known that Granulocyte-macrophage colony-stimulating factor (GM-CSF) up-regulates the binding of eosinophils to VCAM-1 or fibronectin (CS-1 region) by up-regulating the binding affinity of VLA-4 to VCAM-1 (or to CS-1), which is not associated with changes in the integrin expression [11]. The binding of eosinophils to CS-1 is inhibited with neutralizing antibodies to α4 integrins expressed by eosinophils or with neutralizing antibodies to CS-1 [11]. Moreover, in the presence of eotaxin, a transient increase in the force of eosinophil adhesion to CS-1 is followed by a sustained reduction in the force of eosinophil adhesion to CS-1. This latter phase was not due to alterations in VLA-4 receptor number or alterations in VLA-4 receptor distribution [11]. This suggested to authors that conformational changes in VLA-4 receptor may be responsible for the change in binding affinity. In our study, whether such mechanism operates in L-NAME- and CC-chemokines-treated human eosinophils requires additional studies. We also would like to emphasize that a small fraction of stimulated cells were able to adhere, which seems to be a common feature of in vitro models exploring cell adhesion. The basal (non-stimulated) adhesion in models of serum-, fibrinogen-, fibronectin- or recombinant VCAM1-coated wells ranges always from 5 to 30%, depending of the cell type used (eosinophils, neutrophils, platelets) and experimental conditions [8,42-46]. Most importantly, cell stimulation with different agents usually enhances the adhesion about of 20–100% above baseline.
Eotaxin and RANTES are also important chemotactic factors for eosinophils, and are reported to largely contribute to the movement of eosinophils from the blood to the sites of inflammation. Once recruited to tissues, eosinophils receive signals to promote degranulation, causing the release of eosinophil granule proteins such as major basic protein (MBP), eosinophil peroxidase (EPO) and eosinophil cationic protein (ECP) that has been associated with the induction of epithelial damage, edema, and airways hyperreactivity, playing therefore a pivotal role in the pathogenesis of allergic disorders [47]. Accordingly, the increased presence of cationic granule in the asthmatic lung correlates with severity of bronchial asthma [48,49]. Amongst the inflammatory mediators postulated to contribute to eosinophil degranulation, eotaxin and/or RANTES have been shown to release CCR3-dependent granule proteins [50,51] in equivalent amounts to PAF, which is considered a potent eosinophil activator [52]. In a human eosinophil cell line (15-HL-60), eotaxin, eotaxin-2 and eotaxin-3 also produced concentration-dependent degranulation of EPO [53]. Since the chemokines may reach high amounts in the allergic inflammatory site, it is accepted that they greatly contribute to local eosinophil degranulation [52]. Our findings showed that both eotaxin and RANTES caused a significant eosinophil degranulation, thus confirming the literature data. However, the failure of L-NAME to modify the eotaxin- and RANTES-induced degranulation indicates that NO does not modulate degranulation in CC-chemokine-activated eosinophils. It is interestingly, however, that L-NAME itself significantly increased the EPO release in equivalent amounts to the CC-chemokines. While in other cell types such as mast cells, NO synthesis inhibitors are reported to cause cell degranulation [54,55], to our understanding the present study is the first to show that in vitro NO inhibition in human eosinophils leads to cell degranulation. It is known that exocytosis of stored granule proteins of eosinophils takes place by three distinct mechanisms, namely classical exocytosis, piecemeal degranulation and cytolysis [56]. Further studies are thus required to elucidate the mechanism(s) by which NO influences the eosinophil degranulation.
Conclusion
In summary, our study shows that the CC-chemokines eotaxin and RANTES increases the in vitro eosinophil adhesion and degranulation by NO-independent mechanisms. The failure of CC-chemokine to affect VLA-4 and Mac-1 expression on eosinophil surface indicates that changes in the integrin function (avidity or affinity) are rather involved in the enhanced adhesion.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
LL carried out the functional studies and helped to draft the manuscript. GF and AN participated in the flow cytometry. CA–N, AZ and GdN participated in the design of the study. EA participated in its design and supervision, and helped to draft the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
Letícia Lintomen and Edson Antunes thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial support.
Contributor Information
Letícia Lintomen, Email: lintomen2001@yahoo.com.br.
Gilberto Franchi, Email: gfran@unicamp.br.
Alexandre Nowill, Email: aenowill@unicamp.br.
Antonio Condino-Neto, Email: condino@unicamp.br.
Gilberto de Nucci, Email: denucci@dglnet.com.br.
Angelina Zanesco, Email: azanesco@rc.unesp.br.
Edson Antunes, Email: antunes@fcm.unicamp.br.
References
- Spina D, Page CP. Asthma – a need for a rethink? Trends Pharmacol Sci. 2002;23:311–315. doi: 10.1016/S0165-6147(02)02022-9. [DOI] [PubMed] [Google Scholar]
- Gonglur U, Efeoglu T. Vascular adhesion and transendothelial migration of eosinophil leukocytes. Cell Tissue Res. 2004;318:473–482. doi: 10.1007/s00441-004-0925-3. [DOI] [PubMed] [Google Scholar]
- Tachimoto H, Ebisawa M, Bochner BS. Cross-talk between integrins and chemokines that influences eosinophil adhesion and migration. Int Arch Allergy Immunol. 2002;128:18–20. doi: 10.1159/000059414. [DOI] [PubMed] [Google Scholar]
- Lampinen M, Carlson M, Hakansson LD, Venge P. Cytokine-regulated accumulation of eosinophils in inflammatory disease. Allergy. 2004;59:793–805. doi: 10.1111/j.1398-9995.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- Chigaev A, Blenc AM, Braaten JV, Kumaraswamy N, Kepley CL, Andrews RP, Oliver JM, Edwards BS, Prossnitz ER, Larson RS, Sklar AS. Real time analysis of the affinity regulation of alpha 4-integrin. The physiologically activated receptor is intermediate in affinity between resting and Mn2+ or antibody activation. J Biol Chem. 2001;276:48670–48678. doi: 10.1074/jbc.M103194200. [DOI] [PubMed] [Google Scholar]
- Horie S, Kita H. CD11b/CD18 (Mac-1) is required for degranulation of human eosinophils induced by human recombinant granulocyte-macrophage colony-stimulating factor and platelet-activating factor. J Immunol. 1994;152:5457–5467. [PubMed] [Google Scholar]
- Berends C, Dijkhuizen B, Monchy JGR, Dubois AEJ, Gerritsen J, Kauffman HF. Inhibition of PAF-induced expression of CD11b and shedding of L-selectin on human neutrophils and eosinophils by the type IV selective PDE inhibitor, rolipran. Eur Respir J. 1997;10:1000–1007. doi: 10.1183/09031936.97.10051000. [DOI] [PubMed] [Google Scholar]
- Conran N, Ferreira HH, Lorand-Metze I, Thomazzi SM, Antunes E, De Nucci G. Nitric oxide regulates human eosinophil adhesion mechanisms in vitro by changing integrin expression and activity on the eosinophil cell surface. Br J Pharmacol. 2001;134:632–638. doi: 10.1038/sj.bjp.0704295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata M, Sedgwick JB, Bates ME, Kita H, Busse WW. Eosinophil adhesion to vascular cell adhesion molecule-1 activates superoxide anion generation. J Immunol. 1995;155:2194–2202. [PubMed] [Google Scholar]
- Higashimoto I, Chihara J, Kakazu T, Kawabata M, Nakajima S, Osame M. Regulation of eosinophil cell death by adhesion to fibronectin. Int Arch Allergy Immunol. 1996;111:66–69. doi: 10.1159/000237420. [DOI] [PubMed] [Google Scholar]
- Sung K-LP, Yang L, Kim J, Ko D, Stachnick G, Castaneda D, Nayar J, Broide DH. Eotaxin induces a sustained reduction in the functional adhesive state of very late antigen 4 for the connecting segment 1 region of fibronectin. J Allergy Clin Immunol. 2000;106:933–940. doi: 10.1067/mai.2000.110797. [DOI] [PubMed] [Google Scholar]
- Lamkhioued B, Renzi PM, Abi-Younes S, Garcia-Zepada EA, Allakhverdi Z, Ghaffar O, Rothenberg MD, Luster AD, Hamid Q. Increased expression of eotaxin in bronchoalveolar lavage and airways of asthmatics contributes to the chemotaxis of eosinophils to the site of inflammation. J Immunol. 1997;159:4593–4601. [PubMed] [Google Scholar]
- Minshall EM, Cameron L, Lavigne F, Leung DY, Hamilos D, Garcia-Zepada EA, Rothenberg M, Luster AD, Hamid Q. Eotaxin mRNA and protein expression in chronic sinusitis and allergen-induced nasal responses in seasonal allergic rhinitis. Am J Respir Cell Mol Biol. 1997;17:683–690. doi: 10.1165/ajrcmb.17.6.2865. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Takanashi S, Hasegawa Y, Kanehira Y, Kaizuka M, Okumura K. Eotaxin level in induced sputum is increased in patients with bronchial asthma and in smokers. Respiration. 2003;70:600–605. doi: 10.1159/000075205. [DOI] [PubMed] [Google Scholar]
- Tateno H, Nakamura H, Minematsu N, Nakajima T, Takahashi S, Nakamura M, Fukunaga K, Asano K, Lilly CM, Yamaguchi K. Plasma eotaxin level and severity of asthma treated with corticosteroid. Respir Med. 2004;98:782–790. doi: 10.1016/j.rmed.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Lilly CM, Nakamura H, Belostotsky OI, Haley KJ, Garcia-Zepeda EA, Luster AD, Israel E. Eotaxin expression after segmental allergen challenge in subjects with atopic asthma. Am J Respir Crit Care Med. 2001;163:1669–1675. doi: 10.1164/ajrccm.163.7.9812044. [DOI] [PubMed] [Google Scholar]
- Ebisawa M, Yamada T, Bickel C, Klunk D, Schleimer RP. Eosinophil transendothelial migration induced by cytokines. Effect of the chemokine RANTES. J Immunol. 1994;153:2153–2160. [PubMed] [Google Scholar]
- Costa GG, Silva RM, Franco-Penteado CF, Antunes E, Ferreira HHA. Interactions between eotaxin and interleukin-5 in the chemotaxis of primed and non-primed human eosinophils. Eur J Pharmacol. 2007;566:200–205. doi: 10.1016/j.ejphar.2006.09.075. [DOI] [PubMed] [Google Scholar]
- Ferreira HHA, Bevilacqua E, Gagioti SM, De Luca IM, Zanardo RC, Teixeira CE, Sannomiya P, Antunes E, De Nucci G. Nitric oxide modulates eosinophil infiltration in antigen-induced airway inflammation in rats. Eur J Pharmacol. 1998;358:253–259. doi: 10.1016/S0014-2999(98)00575-5. [DOI] [PubMed] [Google Scholar]
- Zanardo RC, Costa E, Ferreira HHA, Antunes E, Martins AR, Murad F, De Nucci G. Pharmacological and immunohistochemical evidence for a functional nitric oxide synthase system in rat peritoneal eosinophils. Proc Natl Acad Sci USA. 1997;94:14111–14114. doi: 10.1073/pnas.94.25.14111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomazzi S, Ferreira HHA, Conran N, De Nucci G, Antunes E. Role of nitric oxide on in vitro human eosinophil migration. Biochem Pharmacol. 2001;62:1417–1421. doi: 10.1016/S0006-2952(01)00782-1. [DOI] [PubMed] [Google Scholar]
- Young SS, Ritacco G, Skeans S, Chapman RW. Eotaxin and nitric oxide production as markers of inflammation in allergic cynomolgus monkeys. Int Arch Allergy Immunol. 1999;120:209–217. doi: 10.1159/000024269. [DOI] [PubMed] [Google Scholar]
- Hanasawa T, Antuni JD, Kharitonov SA, Barnes PJ. Intranasal administration of eotaxin increases nasal eosinophils and nitric oxide in patients with allergic rhinitis. J Allergy Clin Immunol. 1999;105:58–64. doi: 10.1016/S0091-6749(00)90178-7. [DOI] [PubMed] [Google Scholar]
- Feder LS, Stelts D, Chapman RW, Manfra D, Crawley Y, Jones H, Minnicozzi M, Fernandez X, Paster P, Egan RW, Kreutner W, Kung TT. Role of nitric oxide on eosinophilic lung inflammation in allergic mice. Am J Respir Cell Mol Biol. 1997;17:436–442. doi: 10.1165/ajrcmb.17.4.2845. [DOI] [PubMed] [Google Scholar]
- Iijima H, Duguet A, Eum S-Y, Hamid Q, Eidelman DH. Nitric oxide and protein nitration are eosinophil dependent in allergen-challenged mice. Am J Respir Crit Care Med. 2001;163:1233–1240. doi: 10.1164/ajrccm.163.5.2003145. [DOI] [PubMed] [Google Scholar]
- Trifilieff A, Fujitani Y, Mentz F, Dugas B, Fuentes M, Bertrand C. Inducible nitric oxide synthase inhibitors suppress airway inflammation in mice through down-regulation of chemokine expression. J Immunol. 2000;165:1526–1533. doi: 10.4049/jimmunol.165.3.1526. [DOI] [PubMed] [Google Scholar]
- Frank S, Kampfer H, Wetzler C, Satllmeyer B, Pfeilschifter Large induction of the chemotactic cytokine RANTES during cutaneous wound repair: a regulatory role for nitric oxide in keratinocyte-derived RANTES expression. Biochem J. 2000;347:265–273. doi: 10.1042/0264-6021:3470265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato E, Simpson KL, Grisham MB, Koyama S, Robbins RA. Effects of reactive oxygen and nitrogen metabolites on eotaxin-induced eosinophil chemotactic activity in vitro. Am J Respir Cell Mol Biol. 2000;22:61–67. doi: 10.1165/ajrcmb.22.1.3644. [DOI] [PubMed] [Google Scholar]
- Hansel TT, De Vries IJ, Iff T, Rihs S, Wandzilak M, Betz S, Blaser K, Walker C. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J Immunol Meth. 1991;145:105–110. doi: 10.1016/0022-1759(91)90315-7. [DOI] [PubMed] [Google Scholar]
- Anwar AR, Walsh GM, Cromwell O, Kay AB, Wardlaw AJ. Adhesion to fibronectin primes eosinophils via alpha 4 beta 1 (VLA-4) Immunology. 1994;82:222–228. [PMC free article] [PubMed] [Google Scholar]
- Kroegel C, Yukawa T, Dent G, Venge P, Chung KF, Barnes PJ. Stimulation of degranulation from human eosinophils by platelet-activating factor. J Immunol. 1989;142:3518–3526. [PubMed] [Google Scholar]
- Adamko DJ, We Y, Gleich GJ, Lacy P, Moqbel R. The induction of eosinophil peroxidase release: improved methods of measurement and stimulation. J Immunol Methods. 2004;291:101–108. doi: 10.1016/j.jim.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Ying S, Meng Q, Zeibecoglou K, Robinson DS, Macfarlane A, Humbert M, Kay AB. Eosinophil chemotactic chemokines (eotaxin, eotaxin-2, RANTES, monocyte chemoattractant protein-3 (MCP-3), and MCP-4), and C-C chemokine receptor 3 expression in bronchial biopsies from atopic and nonatopic (Intrinsic) asthmatics. J Immunol. 1999;163:6321–6329. [PubMed] [Google Scholar]
- Wardlaw AJ. Molecular basis for selective eosinophil trafficking in asthma: A multistep paradigm. Allergy Clin Immunol. 1999;104:J917–926. doi: 10.1016/S0091-6749(99)70069-2. [DOI] [PubMed] [Google Scholar]
- Ono SJ, Nakamura T, Ohbayashi M, Dawson M, Toda M. Chemokines: Role in leukocyte development, trafficking, and effector function. J Allergy Clin Immunol. 2003;111:1185–1199. doi: 10.1067/mai.2003.1594. [DOI] [PubMed] [Google Scholar]
- Gale LM, McColl SR. Chemokines: extracellular messengers for all occasions? Bioessays. 1999;21:17–28. doi: 10.1002/(SICI)1521-1878(199901)21:1<17::AID-BIES3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Jia G-Q, Gonzalo J-A, Hidalgo A, Wagner D, Cybulsky M, Gutierrez-Ramos JC. Selective eosinophil transendothelial migration triggered by eotaxin via modulation of Mac-1/ICAM-1 and VLA-4/VCAM-1 interactions. Int Immunol. 1999;11:1–10. doi: 10.1093/intimm/11.1.1. [DOI] [PubMed] [Google Scholar]
- Liu J, Munõz NM, Meliton AY, Zhu X, Lambertino AT, Xu C, Myo S, Myou S, Boetticher E, Johnson M, Leff AR. β2-Integrin adhesion caused by eotaxin but not IL-5 is blocked by PDE-4 inhibition and β2-adrenoceptor activation in human eosinophils. Pulm Pharmacol Ther. 2004;17:73–79. doi: 10.1016/j.pupt.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giembycz MA, Lindsay MA. Pharmacology of the eosinophil. Pharmacol Rev. 1999;51:213–340. [PubMed] [Google Scholar]
- Del Pozo MA, Sanchez-Mateos P, Sanchez-Madrid F. Cellular polarization induced by chemokines: a mechanism for leukocyte recruitment? Immunol Today. 1996;17:127–131. doi: 10.1016/0167-5699(96)80604-9. [DOI] [PubMed] [Google Scholar]
- Sano H, Zhu X, Sano A, Boetticher EE, Shioya T, Jacobs B, Munoz NM, Leff AR. Extracellular signal-regulated kinase 1/2-mediated phosphorylation of cytosolic phospholipase A2 is essential for human eosinophil adhesion to fibronectin. J Immunol. 2001;166:3515–3521. doi: 10.4049/jimmunol.166.5.3515. [DOI] [PubMed] [Google Scholar]
- Conran N, Gambero A, Ferreira HH, Antunes E, de Nucci G. Nitric oxide has a role in regulating VLA-4-integrin expression on the human neutrophil cell surface. Biochem Pharmacol. 2003;66:43–50. doi: 10.1016/S0006-2952(03)00243-0. [DOI] [PubMed] [Google Scholar]
- Canalli AA, Conran N, Fattori A, Saad ST, Costa FF. Increased adhesive properties of eosinophils in sickle cell disease. Exp Hematol. 2004;32:728–734. doi: 10.1016/j.exphem.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Sedgwick JB, Jansen KJ, Kennedy JD, Kita H, Busse WW. Effects of the very late adhesion molecule 4 antagonist WAY103 on human peripheral blood eosinophil vascular cell adhesion molecule 1-dependent functions. J Allergy Clin Immunol. 2005;116:812–819. doi: 10.1016/j.jaci.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Marcondes S, Cardoso MH, Morganti RP, Thomazzi SM, Lilla S, Murad F, De Nucci G, Antunes E. Cyclic GMP-independent mechanisms contribute to the inhibition of platelet adhesion by nitric oxide donor: a role for alpha-actinin nitration. Proc Natl Acad Sci USA. 2006;103:3434–3439. doi: 10.1073/pnas.0509397103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- Filley WV, Holley KE, Kephart GM, Gleich GJ. Identification by immunofluorescence of eosinophil granule major basic protein in lung tissues of patients with bronchial asthma. Lancet. 1982;2:11–16. doi: 10.1016/S0140-6736(82)91152-7. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Kubo K, Matsuzawa Y, Sekiguchi M. Eosinophil cationic protein levels in induced sputum correlate with the severity of bronchial asthma. Chest. 1997;112:1241–1247. doi: 10.1378/chest.112.5.1241. [DOI] [PubMed] [Google Scholar]
- Horie S, Gleich GJ, Kita H. Cytokines directly induce degranulation and superoxide production from human eosinophils. J Allergy Clin Immunol. 1996;98:371–381. doi: 10.1016/S0091-6749(96)70161-6. [DOI] [PubMed] [Google Scholar]
- Fujisawa T, Kato Y, Nagase H, Atsuta J, Terada A, Iguchi K, Kamiya H, Morita Y, Kitaura M, Kawasaki H, Yoshie O, Hirai K. Chemokines induce eosinophil degranulation through CCR3. J Allergy Clin Immunol. 2000;106:507–513. doi: 10.1067/mai.2000.108311. [DOI] [PubMed] [Google Scholar]
- El-Shazly A, Masuyama K, Nakano K, Eura M, Samejima Y, Ishikawa T. Human eotaxin induces eosinophil-derived neurotoxin release from normal human eosinophils. Int Arch Allergy Immunol. 1998;117:55–58. doi: 10.1159/000053573. [DOI] [PubMed] [Google Scholar]
- Badewa AP, Hudson CE, Heiman AS. Regulatory effects of eotaxin, eotaxin-2, and eotaxin-3 on eosinophil degranulation and superoxide anion generation. Exp Biol Med (Maywood) 2002;227:645–651. doi: 10.1177/153537020222700814. [DOI] [PubMed] [Google Scholar]
- Salvemini D, Masini E, Pistelli A, Mannaioni PF, Vane JR. Nitric oxide: a regulatory mediator of mast cell reactivity. J Cardiovasc Pharmacol. 1991;17:S258. doi: 10.1097/00005344-199100001-00046. [DOI] [Google Scholar]
- Kimura M, Mitani H, Bandoh T, Totsuka T, Hayashi S. Mast cell degranulation in rat mesenteric venule: effects of L-NAME, methylene blue and ketotifen. Pharmacol Res. 1999;39:397–402. doi: 10.1006/phrs.1999.0451. [DOI] [PubMed] [Google Scholar]
- Clark K, Simson L, Newcombe N, Koskinen AML, Mattes J, Lee NA, Lee JJ, Dent LA, Matthaei KI, Foster PS. Eosinophil degranulation in the allergic lung of mice primarily occurs in the airway lumen. J Leukoc Biol. 2004;75:1001–1009. doi: 10.1189/jlb.0803391. [DOI] [PubMed] [Google Scholar]