Abstract
Background
Helicobacter pylori is a common pathogen, and its prevalence varies with socioeconomic conditions (10–80%). It has recently been recognized as a class I carcinogen in relation to gastric cancer. The aim of this study was to investigate the presence of Helicobacter pylori in neoplasms of the colon by immunohistochemical methods.
Methods
The polypectomy materials of 51 patients (19 male and 32 female) who had undergone colonoscopic polypectomy were retrieved for retrospective examination. The endoscopic size and colonic localization of the polyps were recorded. Hematoxylin and eosin stains were evaluated according to histological type and grade of dysplasia. Biopsy stains were immunohistochemically treated with Helicobacter pylori antibodies by the streptavidine-biotin immunoperoxidase technique. Helicobacter pylori staining in the gastric mucosa was used as the control for the immunohistochemical method. Specimens were classified according to the presence of Helicobacter pylori under an optical microscope, and Helicobacter pylori positive specimens were stratified according to the respective staining pattern.
Results
Mean age was 61.88 ± 10.62 (40–82) years. Polyp sizes were 1.45 ± 0.92 (1–4) cm; and 25.5% of polyps were localized in the right colon, 68.6% in the left colon and 5.9% in the transverse colon. Presence of Helicobacter pylori was not correlated with localization (p > 0.05) or size of the polyps (p > 0.05).
Eleven (21.6%) of all specimens included in the study were Helicobacter pylori positive by immunohistochemical methods. Of the Helicobacter pylori positive specimens, the staining pattern was diffuse: Equivocal in 90.9%, nonspecific with a finely granular type concentrated on the luminal surface in 90.9%, dot-like granular in 54.5%, and spiral in 9.1%. Of the tubular polyps, 17.9% were H. pylori positive, and the staining pattern was equivocal in 100%, luminal in 85.7%, and dot-like granular in 57.1%. Of the villous polyps, 60% were H. pylori positive, and the staining pattern was inconclusive in 66.7%, luminal in 100%, dot-like granular in 33.3%, and spiral in 33.3%. Of the cancerous cases, 25% were H. pylori positive and showed an equivocal, luminal, and dot-like granular staining pattern. No significant correlation was determined between histologic types and prevalence of H. pylori (p > 0.05).
Conclusion
The presence of H. pylori in colon polyps did not yield any correlation with polyp size, colonic localization or histopathologic type. The higher rate of H. pylori positivity in villous polyps does not present a causal relationship. We were able to determine H. pylori existence in colon polyps by immunohistochemical methods, albeit with no statistical significance.
Background
Helicobacter pylori (H. pylori) is a class I carcinogen giving rise to gastric adenocarcinoma [1,2]. In humans, apart from the gastric mucosa, it has been isolated from cholestatic liver parenchyma [3]. Experimental studies have demonstrated a relationship between certain Helicobacter species with inflammatory bowel disease and colonic adenocarcinoma development [4-6]. H. pylori infections have been considered as a risk factor for development of colorectal neoplasms (CRNs) such as colon polyps and colon cancer (CC) due to the high prevalence of serologically positive H. pylori infection among CRN patients in some uncontrolled studies [4,7,8]. Nonetheless, data pertaining to the association between CRN and H. pylori is limited and insufficient. Even though there exist human studies that support [7,9] or reject [10,11] a relationship between CRN and H. pylori, a direct colonization of the colon by H. pylori that would suggest a causal role for CRN has not been shown [4,7,8]. In this study, the presence of H. pylori in CRNs, and a potential relationship with histopathologic types of the neoplasms by immunohistochemical (IHC) methods specific to H. pylori was investigated.
Methods
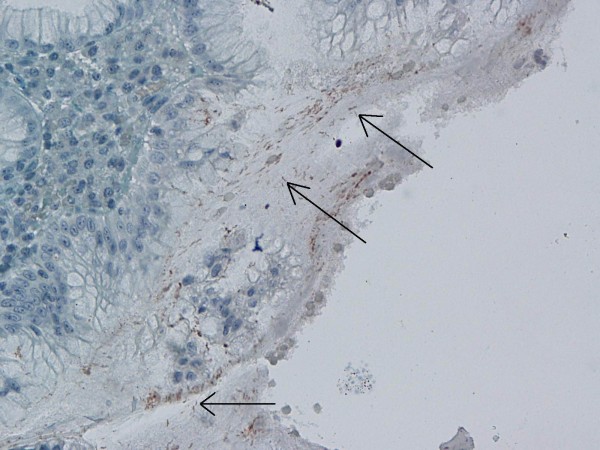
Biopsy specimens of 51 patients (19 female and 32 male) who had been administered polypectomy following detection of polyps by colonoscopy were included in the analysis. Pseudopolyps in inflammatory bowel diseases, inflammatory polyps, juvenile polyposis syndrome, familial adenomatous polyposis and colon neoplasms that did not appear polypoid by endoscopy or colon carcinomas were excluded. Polyps in all colonic localizations were included. Polyps localized in the descending colon, sigmoid colon and rectum were classified as 'left colon', those in the transverse colon as 'transverse colon' and those in the cecum and ascending colon as 'right colon' localization. The mean age of the patients was 61.88 ± 10.62 years (range: 40–82 years). Endoscopic polyp size and localization were categorized. Haematoxylin and eosin (H&E) stains obtained from paraffin blocks were retrospectively evaluated by two pathologists and classified according to histopathologic type and degree of dysplasia. In the IHC analysis following this assessment of the H&E stains, specimens were treated with H. pylori antibodies (ready to use, polyclonal, Biogen, Union City, CA, USA) by the streptavidine-biotin immunoperoxidase technique. Specimens were incubated with primary antibody at a dilution of 200 μg/ml at room temperature for twenty minutes [12]. The control testing for the IHC method consisted of H. pylori staining in the gastric mucosa (Figure 1, 2). H. pylori positive specimens were separated according to the IHC staining pattern of H. pylori under optical microscopy. An institutional ethics committee approval was obtained prior to initiation of the study.
Figure 1.
Immunohistochemical H. pylori positivity in gastric control biopsies (×40).
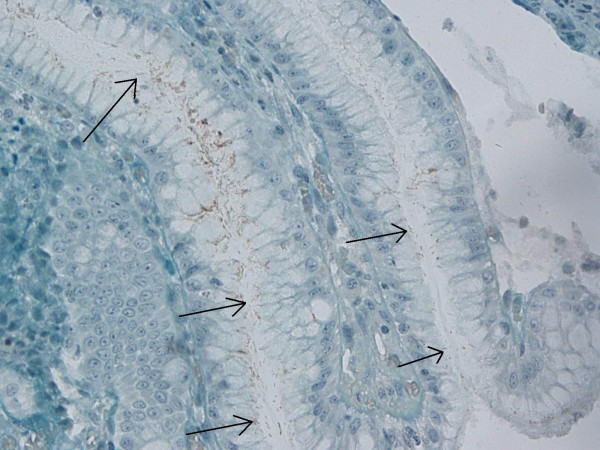
Figure 2.
Immunohistochemical H. pylori positivity in gastric control biopsies (×40).
Statistical Analysis
To perform the statistical analyses, descriptive statistical methods, the Kruskal-Wallis test, the Mann-Whitney U test, and the chi-square test were used. Results were evaluated at a 95% confidence interval; and a p < 0.05 value was recognized as the statistical significance level.
Results
During endoscopic investigation, polyp sizes were 1.45 ± 0.92 cm (range: 1–4 cm), 25.5% of the polyps were localized in the right colon, 68.6% in the left colon and 5.9% in the transverse colon. H. pylori positivity/negativity in the polyps was not correlated to localization in the colon (p > 0.05) or polyp size (p > 0.05). The distribution of histopathologic types was 76.5% tubular, 9.8% villous, 5.9% tubulovillous, and 7.8% adenocarcinoma. Of the 39 tubular cases, 38 (97.4%) had low grade dysplasia (LGD) and 1 (2.6%) high grade dysplasia (HGD); of the 5 villous polyps, 1 (20%) had LGD and 4 (80%) HGD; and finally, all 3 (100%) of the tubulovillous polyps had LGD. Adenocarcinoma was established in four polyps.
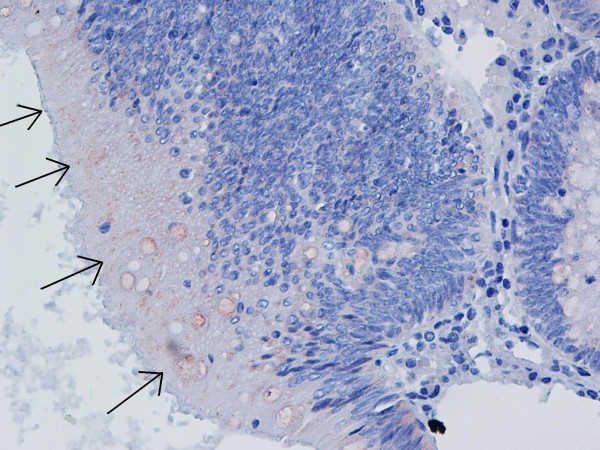
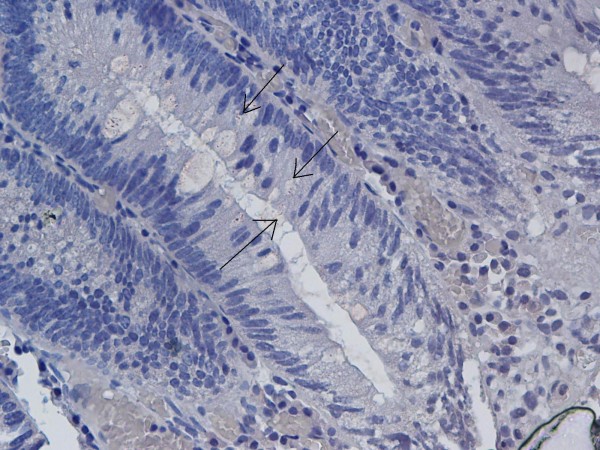
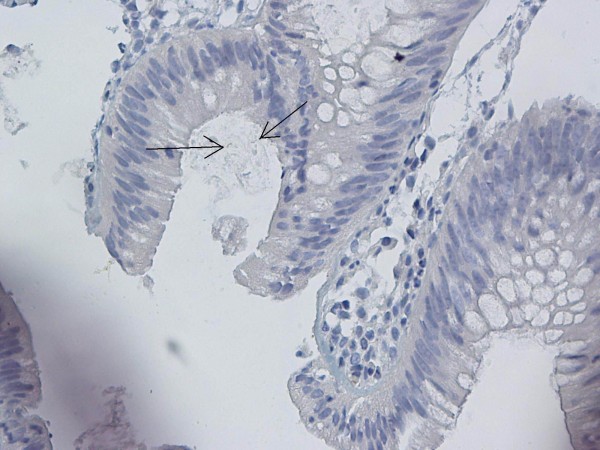
11 (21.6%) of the polyps were determined to be H. pylori positive by IHC evaluation. Of these 11 specimens, 10 (90.9%) had an equivocal diffuse mucosal staining pattern, 10 (90.9%) had nonspecific finely granular staining concentrated on the luminal surface (Figure 3), and 6 (54.5%) also had a dot-like granular staining pattern. The weak dark yellow/light brown staining in a spiral form in 1 (9.1%) specimen was evaluated as positive (Figure 4, 5). Staining patterns of H. pylori positive specimens according to histopathological polyp types are summarized in Table 1.
Figure 3.
Nonspecific finely granular staining concentrated on the luminal surface by IHC staining (×40).
Figure 4.
Spiral-form dark yellow/light brown staining in lumen of adenomatous polyp (×40).
Figure 5.
Spiral-form dark yellow/light brown staining in lumen of adenomatous polyp (×40).
Table 1.
Positivity for H. pylori and staining patterns according to histopathologic type
| Tubular (n = 7) | Villous (n = 3) | Cancer (n = 1) | |
| n (%) | n (%) | n (%) | |
| H. pylori presence (p = 0.137) | |||
| Positive | 17.9% | 60.0% | 25.0% |
| Negative | 82.1% | 40.0% | 75.0% |
| Staining patterns of H. pylori (+) specimens | |||
| Equivocal | 7 (100%) | 2 (66.7%) | 1 (100%) |
| Luminal | 6 (85.7%) | 3 (100%) | 1 (100%) |
| Dot-like Granular | 4 (57.1%) | 1 (33.3%) | 1 (100%) |
| Spiral | - | 1 (33.3%) | - |
The prevalence of H. pylori was higher in villous type polyps (60%) compared to other histologic types, but this difference was not statistically significant. We were not able to establish a significant correlation between the polyps' histologic types and the prevalence of H. pylori (p > 0.05).
Discussion
Epidemiological studies have confirmed a causal relationship between H. pylori and gastric cancer [13,14] and colonic phenotype of H. pylori related intestinal metaplasia (IM) has been associated with gastric cancer [1,15]. Thus, association of H. pylori in various gastrointestinal system organ cancers has been investigated and Helicobacter DNAs were positive in 52.6% of the hepatobiliary cancer cases. This positivity suggests that Helicobacter species may play a role in the pathogenesis of hepatobiliary cancer through an acceleration of biliary cell kinetics [16]. Helicobacter species, which may colonize the biliary tract, have been implicated as a possible cause of hepatobiliary diseases ranging from chronic cholecystitis and primary sclerosing cholangitis to gall-bladder carcinoma and primary hepatic carcinomas [17]. Therefore the hypothesis that H. pylori would also be associated with intestinal polypoid structures needs to be investigated. Furthermore, there exist several studies demonstrating the co-existence of CRNs and H. pylori seropositivity supports this suggestion. The potential mechanism of the significant association between colon polyps and serologic H. pylori positivity has been attributed to the remote trophic effect of the elevated gastrin level on the colonic mucosa [9]. This study has been designed as to investigate an association between H. pylori and extragastric intestinal neoplasms and colonization in cases of direct colonic dysplasia has been investigated by specific IHC methods.
Shmuely et al. reported that H. pylori CagA+ seropositivity is enhanced in gastric and colon cancers [18], while Fireman et al. demonstrated a correlation between H. pylori seropositivity and CA19-9 elevation in patients with CC [19]. In the evaluation by Mizuno et al. of the colon pathologies of 332 patients with high-resolution colonoscopy, the increase in the incidence of adenomatous polyps in H. pylori IgG seropositive patients and diminution of normal colonoscopy findings was found more significant than in seronegative patients [20]. While the underlying mechanism is not clear, the prevalence of gastric H. pylori infection was found to be increased, especially in colonic adenomas (71.4% in polyps, 55% in cancers) [21]. In case reports of Cap polyposis that support the correlation between CRN and H. pylori, despite the inability to demonstrate H. pylori in polyps by IHC methods, eradication of the infection led to improvements in symptoms and polyps [22,23]. Although direct and indirect relationship between H. pylori and CRNs have been widely recognized, only the relationship between the presence of serologically positive H. pylori and CRNs have been demonstrated [4,8]. It has also been reported that the remote and local consequences of H. pylori infection might also have a synergistic effect on the emergence or development of these neoplasms under certain conditions [4,8]. A study of 374 patients with GI cancers evaluated serologic H. pylori positivity according to localization, serologic H. pylori positivity was found to be unrelated to CC localization [24]. In an investigation for H. pylori specific 16S rDNA with PCR in CC biopsies, Grahn et al. revealed H. pylori DNA in 27% of cancer tissue specimens. They could not ascertain any correlation between H. pylori positivity and the stage or colonic localization of the cancer [25]. We also found no association with the size or colonic localization of polyps determined to be H. pylori positive by IHC methods.
In a study by Bulajic et al. on 83 subjects with CC that investigated H. pylori DNA by PCR in biopsy specimens from CC and normal mucosal tissues, H. pylori IgG antibody was positive in 36 patients while H. pylori was determined by PCR in the tissues of 1 patient with CC and 5 specimens of normal mucosa. However, no correlation between H. pylori positivity and CC could be demonstrated [10]. There exist serologic and colon tissue PCR studies that support [11,20,26] or reject [5,10,27] a correlation between H. pylori and CRNs.
The Giemsa staining method is specific for the determination of gastric spiral forms of the organism. However, IHC methods have been found to better identify non-spiral forms of the bacterium [7,28-32]. It has been suggested that a dot-like staining pattern with the IHC method represents the coccoid form of H. pylori, and this method of staining makes it impossible to differentiate mucus or debris on the lumen from antigenic structures with H. pylori-like reactions or stain precipitates. Although IHC staining is more specific than the Giemsa method routinely used for the evaluation of gastric H. pylori, false positives may be difficult to conclusively differentiate [7,28,29]. In our study, H. pylori showed various staining patterns with IHC method. Therefore we regarded a dot-like granular staining pattern as a positive finding together with the coexistence of other luminal or equivocal staining patterns.
Similar to our study, in a trial investigating presence of H. pylori in colon polyps by IHC methods, H. pylori was determined in tubular and tubulovillous adenomas, but not in villous polyps. This result was deemed insignificant and interpreted to be potentially related to the micro-environment [7]. Similarly, as with the low prevalence of H. pylori in gastric IM sites or some gastric fundic polyps, the micro-lining in villous adenomas may also be inopportune for H. pylori[7,33]. Contrary to this finding, we found a higher prevalence of H. pylori positivity in villous polyps compared with other histologic types. A similar situation is present within studies investigating presence of H. pylori in gastric IM [34,35]. Whilst some studies support the assumption that H. pylori is absent in gastric IM; in studies using IHC method presence of H. pylori in gastric IM have been demonstrated [28,33,36]. Furthermore, the absence of H. pylori in villous adenomas as reported by several authors does not necessarily indicate the lack of an association, as this might be supportive of the hypothesis about H. pylori migration after development of the lesion [7,28,37]. Despite reports of positivity for H. pylori in tubular and tubulovillous adenomas [7] and the lack of a significant association with cellular types of polyps in our study, reports that have directly demonstrated the presence of H. pylori in villous adenomas (60%) by IHC methods exist in medical literature. The small number of materials in our study may account for the lack of statistical significance. However, the lack of a correlation between cellular types of polyps and H. pylori presence indicates that the micro-environment may indeed have a role, and H. pylori strains may vary.
Conclusion
This study has demonstrated the presence of H. pylori in colon polyps by IHC methods, albeit with no statistical significance. Our findings do not suffice for the assertion of a definitive association between H. pylori positivity and CRNs. However, even in the absence of a causal relationship, our results are suggestive of a correlation between colon polyps and H. pylori. Further cellular studies that are supported by molecular biological techniques are needed to clarify the presence or absence of such an association.
Abbreviations
CC: colon cancer; CRN: colorectal neoplasm; GI: gastrointestinal; H&E: haematoxylin and eosin; HGD: high grade dysplasia; H. pylori: Helicobacter pylori; IHC: immunohistochemical; IM: intestinal metaplasia; LGD: low grade dysplasia.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AS planned and coordinated the study, and prepared manuscript; SO prepared and stained specimens, and evaluated specimens; HA, KD and MK were involved in the compilation of colonoscopic polypectomy cases; NY was involved in data entry; ABK was involved in literature search. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Aliye Soylu, Email: aliyesoylu@superonline.com.
Selvinaz Ozkara, Email: selvinazo@gmail.com.
Halil Alıs, Email: halilalis@gmail.com.
Kemal Dolay, Email: dolayk@yahoo.com.
Mustafa Kalaycı, Email: mukalayci@hotmail.com.
Nurgul Yasar, Email: yasarnurgul@windowslive.com.
A Baki Kumbasar, Email: abakikumbasar@yahoo.com.
References
- Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Gologan A, Graham DY, Sepulveda AR. Molecular markers in Helicobacter pylori-associated gastric carcinogenesis. Clin Lab Med. 2005;25:197–222. doi: 10.1016/j.cll.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Nilsson HO, Taneera J, Castedal M, Glatz E, Olsson R, Wadström T. Identification of Helicobacter pylori and other Helicobacter species by PCR, hybridization, and partial DNA sequencing in human liver samples from patients with primary sclerosing cholangitis or primary biliary cirrhosis. J Clin Microbiol. 2000;38:1072–1076. doi: 10.1128/jcm.38.3.1072-1076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio-Price L, Treuting P, Zeng W, Tsang M, Bielefeldt-Ohmann H, Iritani BM. Helicobacter infection is required for inflammation and colon cancer in SMAD3-deficient mice. Cancer Res. 2006;66:828–838. doi: 10.1158/0008-5472.CAN-05-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SJ, Chisholm SA, Owen RJ, Borriello SP, Kamm MA. Evaluation of Helicobacter species in inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18:481–486. doi: 10.1046/j.1365-2036.2003.01703.x. [DOI] [PubMed] [Google Scholar]
- Erdman SE, Poutahidis T, Tomczak M, Rogers AB, Cormier K, Plank B, Horwitz BH, Fox JG. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol. 2003;162:691–702. doi: 10.1016/S0002-9440(10)63863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M, Helliwell P, Pritchard C, Tharakan J, Mathew J. Helicobacter pylori in colorectal neoplasm: is there an aetiological relationship? World J Surg Oncol. 2007;5:51. doi: 10.1186/1477-7819-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VP, Poutahidis T, Ge Z, Nambiar PR, Boussahmain C, Wang YY, Horwitz BH, Fox JG, Erdman SE. Innate immune inflammatory response against enteric bacteria Helicobacter hepaticus induces mammary adenocarcinoma in mice. Cancer Res. 2006;66:7395–7400. doi: 10.1158/0008-5472.CAN-06-0558. [DOI] [PubMed] [Google Scholar]
- Breuer-Katschinski B, Nemes K, Marr A, Rump B, Leiendecker B, Breuer N, Goebell H. Helicobacter pylori and the risk of colonic adenomas. Colorectal Adenoma Study Group. Digestion. 1999;60:210–215. doi: 10.1159/000007661. [DOI] [PubMed] [Google Scholar]
- Bulajic M, Stimec B, Jesenofsky R, Kecmanovic D, Ceranic M, Kostic N, Schneider-Brachert W, Lowenfels A, Maisonneuve P, Löhr JM. Helicobacter pylori in colorectal carcinoma tissue. Cancer Epidemiol Biomarkers Prev. 2007;16:631–633. doi: 10.1158/1055-9965.EPI-06-1031. [DOI] [PubMed] [Google Scholar]
- Siddheshwar RK, Muhammad KB, Gray JC, Kelly SB. Seroprevalence of Helicobacter pylori in patients with colorectal polyps and colorectal carcinoma. Am J Gastroenterol. 2001;96:84–88. doi: 10.1111/j.1572-0241.2001.03355.x. [DOI] [PubMed] [Google Scholar]
- Immunohistology Protocol, LabVision http://www.labvision.com/pdf/Immunochemistry.pdf
- Parsonnet J. Helicobacter pylori. Infect Dis Clin North Am. 1998;12:185–97. doi: 10.1016/S0891-5520(05)70417-7. [DOI] [PubMed] [Google Scholar]
- Testino G, Cornaggia M, Valentini M. Helicobacter pylori, pre-neoplastic changes, gastric cancer: a point of view. Eur J Gastroenterol Hepatol. 1999;11:357–359. doi: 10.1097/00042737-199903000-00024. [DOI] [PubMed] [Google Scholar]
- Craanen ME, Dekker W, Blok P, Ferwerda J, Tytgat GN. Intestinal metaplasia and Helicobacter pylori: an endoscopic bioptic study of the gastric antrum. Gut. 1992;33:16–20. doi: 10.1136/gut.33.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Kuroki T, Tajima Y, Tsuneoka N, Kitajima T, Matsuzaki S, Furui J, Kanematsu T. Comparative analysis of Helicobacter DNAs and biliary pathology in patients with and without hepatobiliary cancer. Carcinogenesis. 2002;23:1927–1931. doi: 10.1093/carcin/23.11.1927. [DOI] [PubMed] [Google Scholar]
- Leong RW, Sung JJ. Review article: Helicobacter species and hepatobiliary diseases. Aliment Pharmacol Ther. 2002;16:1037–1045. doi: 10.1046/j.1365-2036.2002.01282.x. [DOI] [PubMed] [Google Scholar]
- Shmuely H, Passaro D, Figer A, Niv Y, Pitlik S, Samra Z, Koren R, Yahav J. Relationship between Helicobacter pylori CagA status and colorectal cancer. Am J Gastroenterol. 2001;96:3406–3410. doi: 10.1111/j.1572-0241.2001.05342.x. [DOI] [PubMed] [Google Scholar]
- Fireman Z, Trost L, Kopelman Y, Segal A, Sternberg A. Helicobacter pylori: seroprevalence and colorectal cancer. Isr Med Assoc J. 2000;2:6–9. [PubMed] [Google Scholar]
- Mizuno S, Morita Y, Inui T, Asakawa A, Ueno N, Ando T, Kato H, Uchida M, Yoshikawa T, Inui A. Helicobacter pylori infection is associated with colon adenomatous polyps detected by high-resolution colonoscopy. Int J Cancer. 2005;117:1058–1059. doi: 10.1002/ijc.21280. [DOI] [PubMed] [Google Scholar]
- Meucci G, Tatarella M, Vecchi M, Ranzi ML, Biguzzi E, Beccari G, Clerici E, de Franchis R. High prevalence of Helicobacter pylori infection in patients with colonic adenomas and carcinomas. J Clin Gastroenterol. 1997;25:605–607. doi: 10.1097/00004836-199712000-00011. [DOI] [PubMed] [Google Scholar]
- Oiya H, Okawa K, Aoki T, Nebiki H, Inoue T. Cap polyposis cured by Helicobacter pylori eradication therapy. J Gastroenterol. 2002;37:463–466. doi: 10.1007/s005350200067. [DOI] [PubMed] [Google Scholar]
- Akamatsu T, Nakamura N, Kawamura Y, Shinji A, Tateiwa N, Ochi Y, Katsuyama T, Kiyosawa K. Possible relationship between Helicobacter pylori infection and cap polyposis of the colon. Helicobacter. 2004;9:651–656. doi: 10.1111/j.1083-4389.2004.00273.x. [DOI] [PubMed] [Google Scholar]
- Wang KX, Wang XF, Penq JL, Cui YB, Wang J, Li CP. Detection of serum anti-Helicobacter pylori immunoglobulin G in patients with different digestive malignant tumors. World J Gastroenterol. 2003;9:2501–2504. doi: 10.3748/wjg.v9.i11.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn N, Hmani-Aifa M, Fransén K, Söderkvist P, Monstein HJ. Molecular identification of Helicobacter DNA present in human colorectal adenocarcinomas by 16S rDNA PCR amplification and pyrosequencing analysis. J Med Microbiol. 2005;54:1031–1035. doi: 10.1099/jmm.0.46122-0. [DOI] [PubMed] [Google Scholar]
- Moss SF, Neugut AI, Garbowski GC, Wang S, Treat MR, Forde KA. Helicobacter pylori seroprevalence and colorectal neoplasia: evidence against an association. J Natl Cancer Inst. 1995;87:762–763. doi: 10.1093/jnci/87.10.762. [DOI] [PubMed] [Google Scholar]
- Luzza F, Maletta M, Imeneo M, Monteleone G, Marasco R, Biancone L, Pallone F. Evidence against colonic mucosa colonisation by Helicobacter pylori. Lack of a specific antibody response in homogenates of rectal endoscopic biopsies. Ital J Gastroenterol. 1996;28:447–451. [PubMed] [Google Scholar]
- Jang TJ, Kim JR, Kim DH. Adherence of Helicobacter pylori to areas of type II intestinal metaplasia in Korean gastric mucosa. Yonsei Med J. 1999;40:392–395. doi: 10.3349/ymj.1999.40.4.392. [DOI] [PubMed] [Google Scholar]
- Rotimi O, Cairns A, Gray S, Moayyedi P, Dixon MF. Histological identification of Helicobacter pylori: comparison of staining methods. J Clin Pathol. 2000;53:756–759. doi: 10.1136/jcp.53.10.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzio L, Angelucci D, Grossi L, Diodoro MG, Di Campli E, Cellini L. Anti-Helicobacter pylori specific antibody immunohistochemistry improves the diagnostic accuracy of Helicobacter pylori in biopsy specimen from patients treated with triple therapy. Am J Gastroenterol. 1998;93:223–226. doi: 10.1111/j.1572-0241.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- Saito N, Sato F, Kato M, Takeda H, Sugiyama T, Asaka M. What does the positivity of a monoclonal antibody against H. pylori mean? Helicobacter. 1998;3:143. [PubMed] [Google Scholar]
- Chaput C, Ecobichon C, Cayet N, Girardin SE, Werts C, Guadagnini S, Prévost MC, Mengin-Lecreulx D, Labigne A, Boneca IG. Role of AmiA in the morphological transition of Helicobacter pylori and in immune escape. PLoS Pathog. 2006;2:e97. doi: 10.1371/journal.ppat.0020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shand AG, Taylor AC, Banerjee M, Lessels A, Coia J, Clark C, Haites N, Ghosh S. Gastric fundic gland polyps in south-east Scotland: absence of adenomatous polyposis coli gene mutations and a strikingly low prevalence of Helicobacter pylori infection. J Gastroenterol Hepatol. 2002;17:1161–1164. doi: 10.1046/j.1440-1746.2002.02863.x. [DOI] [PubMed] [Google Scholar]
- Craanen ME, Blok P, Dekker W, Ferwerda J, Tytgat GN. Subtypes of intestinal metaplasia and Helicobacter pylori. Gut. 1992;33:597–600. doi: 10.1136/gut.33.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genta RM. Helicobacter pylori as a protomer of intestinal metaplasia and gastric cancer: an alluring hypothesis in search of evidence. Eur J Gastroenterol Hepatol. 1995;7:25–30. [PubMed] [Google Scholar]
- Genta RM, Gürer IE, Graham DY, Krishnan B, Segura AM, Gutierrez O, Kim JG, Burchette JL., Jr Adherence of Helicobacter pylori to areas of incomplete metaplasia in the gastric mucosa. Gastroenterology. 1996;111:1206–1211. doi: 10.1053/gast.1996.v111.pm8898634. [DOI] [PubMed] [Google Scholar]
- Jung A, Vieth M, Maier O, Stolte M. Fundic gland polyps (Elster's cysts) of the gastric mucosa. A marker for colorectal epithelial neoplasia? Pathol Res Pract. 2002;198:731–734. doi: 10.1078/0344-0338-00328. [DOI] [PubMed] [Google Scholar]