Abstract
The amyloid β-peptide (Aβ) has been suggested to exert its toxicity intracellularly. Mitochondrial functions can be negatively affected by Aβ and accumulation of Aβ has been detected in mitochondria. Because Aβ is not likely to be produced locally in mitochondria, we decided to investigate the mechanisms for mitochondrial Aβ uptake. Our results from rat mitochondria show that Aβ is transported into mitochondria via the translocase of the outer membrane (TOM) machinery. The import was insensitive to valinomycin, indicating that it is independent of the mitochondrial membrane potential. Subfractionation studies following the import experiments revealed Aβ association with the inner membrane fraction, and immunoelectron microscopy after import showed localization of Aβ to mitochondrial cristae. A similar distribution pattern of Aβ in mitochondria was shown by immunoelectron microscopy in human cortical brain biopsies obtained from living subjects with normal pressure hydrocephalus. Thus, we present a unique import mechanism for Aβ in mitochondria and demonstrate both in vitro and in vivo that Aβ is located to the mitochondrial cristae. Importantly, we also show that extracellulary applied Aβ can be internalized by human neuroblastoma cells and can colocalize with mitochondrial markers. Together, these results provide further insight into the mitochondrial uptake of Aβ, a peptide considered to be of major significance in Alzheimer's disease.
Keywords: Alzheimer disease, protein import, human brain biopsies
The amyloid-β peptide (Aβ) is produced by regulated intramembrane proteolysis of the Aβ precursor protein (APP) by the sequential cleavage by β- and γ-secretases (1–2). Plaques consisting mainly of aggregated Aβ are detected in the neuropil in aged subjects and in particular in subjects with Alzheimer's disease (AD) (3–5). Recently, it has been argued that it is Aβ oligomers and fibrils that cause toxicity, loss of synapses, and ultimately neuronal death (6–9). The exact mechanisms of how Aβ damages the neurons are still unknown; however, several lines of evidence implicate that Aβ exerts its toxicity intracellularly (10, 11) and point toward a role of mitochondria in this process (12). It has been reported that mitochondrial Aβ accumulation impairs neuronal function and, thus, contributes to cellular dysfunction in a transgenic APP mouse model (13). It is noteworthy that in AD at an early stage there is already a reduction in the number of mitochondria (14), the brain glucose metabolism is decreased (15), and the activities of both tricarboxylic acid cycle enzymes (16) and cytochrome c oxidase (COX) are reduced (17–20). In vitro studies with isolated mitochondria suggest that Aβ1-42 inhibits COX activity in a copper-dependent manner (21). Furthermore, mitochondrial Aβ-binding alcohol dehydrogenase (ABAD) has been found to be up-regulated in neurons from AD patients (22), and Aβ has been shown to interact with ABAD, resulting in free radical production and neuronal apoptosis. Recently, we have shown that presequence protease (PreP) is responsible for the degradation of the accumulated Aβ in mitochondria (23).
The reported incomplete mitochondrial translocation of APP leaving the Aβ region outside the mitochondrial membrane (24, 25) suggests that Aβ cannot be generated locally in mitochondria. Thus, Aβ has to be taken up by mitochondria. The major pathway for mitochondrial import of precursor proteins with mitochondrial targeting signals involves the translocase of the outer membrane (TOM) and the translocase of the inner membrane (TIM). Targeting signals are first recognized by receptors of TOM, Tom20, Tom22 and Tom70 (26, 27). The receptors are associated to Tom40, the general import pore of TOM, whereupon the precursors are directed to the matrix via the Tim23 complex (27). Another pathway through which metabolites and small molecules can pass into mitochondria is the voltage-dependent anion channel (VDAC). Induction of mitochondrial permeability transition also allows uptake of small molecules (28).
Our experiments using isolated rat mitochondria show that Aβ is imported into mitochondria via the TOM complex. We also demonstrate that extracellulary applied Aβ is internalized in cells and colocalizes with mitochondrial markers. Immunoelectron microscopy studies of human brain biopsies and of mitochondrial fractions after in vitro Aβ import show a consistent localization pattern of Aβ to the mitochondrial cristae. Together, our data suggest that Aβ can be internalized by cells, imported into mitochondria via the TOM complex, and accumulated in the cristae.
Results
Aβ Accumulates in Human Brain Mitochondria.
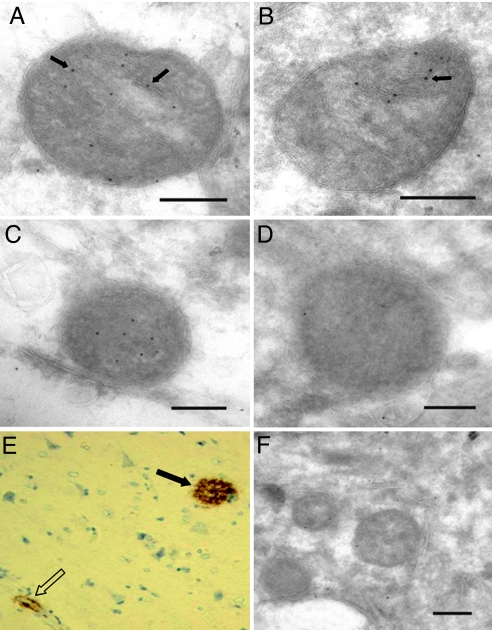
First, we studied the mitochondrial localization of Aβ1-42 in human cortical brain tissue specimens from patients going through neurosurgery because of suspected normal pressure hydrocephalus. The clinical data from these patients are summarized in supporting information (SI) Table S1. In a biopsy from a patient (#1) with amyloid deposits visualized by means of immunohistochemistry (Fig. 1E), Aβ1-42 labeling was apparent in the mitochondrial cristae (Fig. 1 A and B). Preabsorption of the antibody with the Aβ1-42 peptide almost abolished labeling; of the six mitochondria from each sample were counted, there were 27.8 gold particles/μm2 without preabsorption, compared to 3.9 gold particles/μm2 after preabsorption (Fig. 1. C and D). These data show a unique accumulation of Aβ1-42 in mitochondria in surgical specimens obtained from living subjects. Biopsies from a total of seven subjects were examined. Five of these showed amyloidosis similar to Fig. 1E and accumulation of Aβ1-42 in mitochondria similar to Figs. 1 A to C. One patient (#3) showed tauopathy and no amyloidosis. Accordingly, this patient had very low Aβ1-42 labeling in mitochondria (Fig. 1F). Similarly, a patient (#6) without pathology also had very low Aβ1-42 labeling in mitochondria (data not shown).
Fig. 1.
Immunoelectron microscopy of Aβ1-42 localization to mitochondria in a human brain biopsy from patient #1 with amyloidosis using JNAβ1-42 antibody (A and B). Arrows indicate ImmunoGold labeling. Bars: 0.2 μm. Preabsorption of antibody with Aβ1-42 peptide abolished labeling of mitochondria: control (C), preabsorption (D). Bars: 0.2 μm. Protein aggregates in the same frontal cortex biopsy (patient #1) as in A to D visualized by immonohistochemistry applying antibody directed to β-amyloid (clone 6F/3D). Magnification 200×. Both cerebral amyloid angiopathy (open arrow) and a dense aggregate (black arrow) are seen (E). Immunoelectron microscopy of Aβ1-42 localization to mitochondria in a human brain biopsy from patient #3 with tauopathy, using JNAβ1-42 antibody (F). Bar: 0.2 μm.
Aβ Extracellularly Applied to Human Neuroblastoma SH-SY5Y Cells Is Internalized and Taken up by Mitochondria.
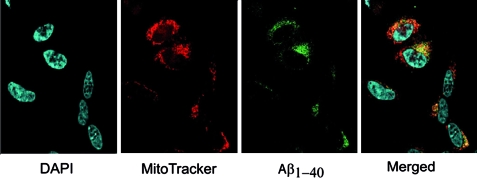
To investigate whether Aβ applied extracellularly can be taken up by cells and reach mitochondria, we incubated human SH-SY5Y neuroblastoma cells with fluorescent Aβ1-40-HiLyte Fluor (Alexa Fluor 488) peptide. The cellular uptake of Aβ1-40- HiLyte Fluor peptide was visualized by laser confocal microscopy. SH-SY5Y neuroblastoma cells incubated with fluorescent 1-μM Aβ1-40-HiLyte Fluor for 18 h showed cellular uptake and colocalization between Aβ1-40 and mitochondria (Fig. 2). Similar results were obtained in mouse embryonic fibroblasts treated with fluorescent Aβ1-40 (data not shown).
Fig. 2.
Confocal immunofluorescence microscopy analysis of human neuroblastoma SH-SY5Y cells treated with 1 μM Aβ1-40-HiLyte Fluor (Alexa Fluor 488) for 18 h. DAPI stains the nuclei and MitoTracker stains mitochondria. The yellow color in the merged image indicates overlap between green (Aβ1-40-HiLyte Fluor) and red (MitoTracker Orange) fluorescence.
Aβ Import Is Dependent on the TOM Machinery.
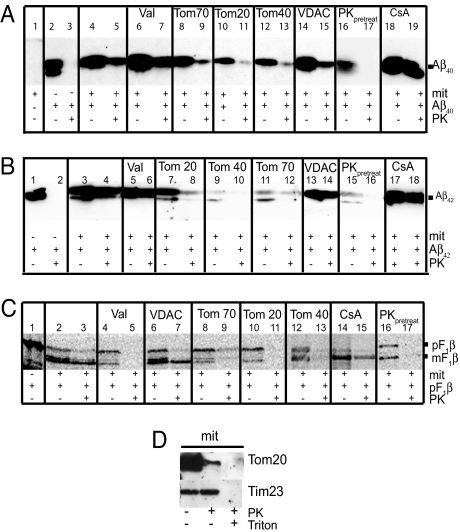
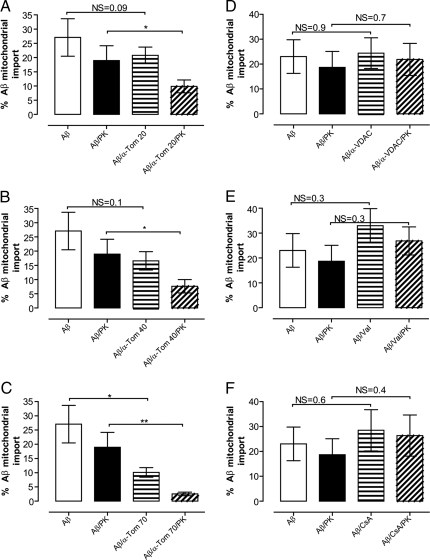
The mitochondrial Aβ content after import was investigated by immunoblotting, immunoelectron microscopy, and flow cytometry analysis. Immunoblot analysis showed that both Aβ1-40 and Aβ1-42, as well as a control protein, the F1β precursor of the ATP synthase from N. plumbaginifolia, were successfully imported into rat liver mitochondria (Fig. 3A, lane 5; Fig. 3B, lane 4; Fig. 3C, lane 3). PK treatment was performed to degrade the Aβ or the F1β precursor that remained on the outside of mitochondria after import. To determine whether Aβ import was dependent on the mitochondrial membrane potential, we pretreated mitochondria with valinomycin, a potassium ionophore that depolarizes the membrane potential. Valinomycin treatment of mitochondria completely inhibited import of the F1β precursor (see Fig. 3C, lane 5), whereas import of Aβ1-40 and Aβ1-42 was not affected (see Fig. 3A: compare lanes 7 and 5; Fig. 3B: compare lanes 6 and 4).
Fig. 3.
In vitro import of Aβ1-40 (A), Aβ1-42 (B), and pF1β (C) detected by immunoblot analysis. Aβ peptides and F1β (pF1β = precursor F1β; mF1β = mature F1β) were imported into isolated mitochondria from rat liver followed by treatment with PK. Valinomycin (Val), CyclosporinA (CsA), antibodies raised toward Tom70, Tom20, Tom40, and VDAC were used as described in Materials and Methods to investigate the import mechanism of Aβ1-40 and Aβ1-42. In some experiments, mitochondria were pretreated with Proteinase K (PKpretreatment) before import. The degradation of mitochondrial receptors was analyzed using antibodies toward Tom20 and Tim23 (D).
Next, we investigated whether the TOM complex is involved in the mitochondrial uptake of Aβ1-40 and Aβ1-42. To this end we preincubated mitochondria with antibodies directed toward Tom20, Tom40, or Tom70 and then performed import assays. Results clearly show a decreased import of both Aβ1-40 and Aβ1-42 in the presence of all of the three types of antibodies (see Fig. 3A: compare lanes 9, 11, and 13 with lane 5; Fig. 3B: compare lanes 8, 10, and 12 with lane 4). In addition, the import of the control protein, the F1β precursor, was decreased after pretreatment with antibodies directed toward the TOM components, as expected for a protein containing a classical N-terminal import signal (see Fig. 3C: compare lanes 9, 11, and 13 with lane 3). In contrast, preincubation of mitochondria with VDAC antibodies did not abolish the import of Aβ1-40, Aβ1-42, and F1β precursor, indicating specificity of the TOM component antibodies to impair import through the TOM complex (see Fig. 3 A: compare lane 15 with lane 5; Fig. 3B: compare lane 14 with lane 4; Fig. 3C: compare lane 7 with lane 3).
To further explore the importance of the TOM complex for mitochondrial Aβ1-40 and Aβ1-42 uptake, mitochondria were pretreated with PK (100 μg/ml) to remove receptors of the outer mitochondrial membrane, Tom20, Tom22, and Tom70. Western blot analysis shows that the Tom20 receptor was degraded after PK treatment, while the inner membrane protein Tim23 remained unaffected by this treatment (Fig. 3D). Results show that both the Aβ1-40 and Aβ1-42 import was abolished in mitochondria pretreated with PK before the import assay (see Fig. 3A: compare lane 17 and 5; Fig. 3B: compare lane 16 and 4), indicating specificity of the Aβ uptake and ruling out association of Aβ with membrane lipids.
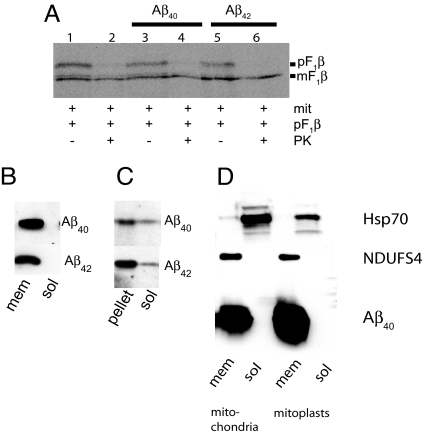
As Aβ contains no classical import signal, we speculated that this peptide might be immobilized in the import pore. Therefore, we performed experiments in which mitochondria were first incubated with Aβ1-40 or Aβ1-42 followed by washing, reisolation of mitochondria, and additional incubation of the mitochondria with the F1β precursor. Interestingly, the import of F1β precursor was not inhibited, suggesting that Aβ1-40 and Aβ1-42 were not immobilized in the import pore (Fig. 4A: compare lanes 4 and 6 with lane 2).
Fig. 4.
Analysis of Aβ localization after in vitro import. pF1β import analysis by phosphoimaging following Aβ1-40 and Aβ1-42 import (A). Mitochondria were fractionated after Aβ import and Proteinase K treatment into a soluble (sol) and a membrane (mem) fraction followed by immunoblot analysis (B). The membrane fraction in (B) was further treated with 0.1-mM Na2CO3 followed by immunoblot analysis (C). Mitochondria and mitoplasts were fractionated into a soluble (sol) and a membrane (mem) fraction after Aβ1-40 import. Grp75 is a mitochondrial matrix marker and Ndufs is an mitochondrial inner membrane marker (D).
To study the intramitochondrial localization of the imported Aβ, the mitochondria were fractionated into a soluble and a membrane fraction. Aβ1-40 and Aβ1-42 were localized to the membrane fraction as analyzed by immunoblotting (Fig. 4B). Upon treatment of the membrane fraction with Na2CO3, a small portion of Aβ1-40 and Aβ1-42 dissociated from the membrane (Fig. 4C), indicating that Aβ was partially peripherally associated with the mitochondrial membrane. To further verify the submitochondrial localization of Aβ, after import mitoplasts were prepared and fractionated into a membrane fraction and a soluble fraction, and probed with markers for the mitochondrial matrix (Grp75; a member of the Hsp70 family of chaperones) and mitochondrial inner membrane [NDUFS4; Complex I (NADH dehydrogenase) subunit] (Fig. 4D). Aβ1-40 was clearly associated with the inner membrane fraction and not present in the matrix fraction.
Flow Cytometry.
Mitochondrial import of Aβ was also studied by flow cytometry analysis. For this purpose we incubated isolated mitochondria with fluorescent Aβ1-40 (Aβ1-40-FITC; 0.5 μM, 30 min). Before flow cytometry, analysis samples were labeled with MitoFlour Red, ensuring that the analysis was restricted to mitochondria. A significant inhibition of Aβ1-40-FITC import was shown by flow cytometry after treatment of mitochondria with the TOM complex component antibodies (Tom20 *, P < 0.05; Tom40 *, P < 0.05; Tom70 **, P < 0.01) (Fig. 5 A–C), supporting the data obtained by immunoblot analysis. As shown above, the import of Aβ1-40-FITC was also insensitive to valinomycin and thus independent of an intact mitochondrial membrane potential (Fig. 5E). Mitochondria were also pretreated with an antibody toward VDAC, but no consistent decrease of Aβ1-40-FITC import could be detected (Fig. 5D), in agreement with immunoblot analysis. Furthermore, inhibition of the membrane permeability transition pore with cyclosporine A did not show any significant decrease of Aβ1 -40-FITC import (Fig. 5F), also in agreement with immunoblot analysis. As expected, the import of the control peptide, the F1β precursor was not affected by the presence of cyclosporine A (Fig. 3C, lane 15).
Fig. 5.
Isolated mitochondria were treated in the presence or absence of proteinase K (PK). MitoFluor Red positive mitochondria were selected and gated for by flow cytometry. A significant inhibition of Aβ/α-Tom20/PK, Aβ/α-Tom40/PK, and Aβ/α-Tom70/PK (*, P < 0.05, *, P < 0.05, **, P < 0.01) as compared to Aβ/PK treated mitochondria is shown (A–C). Pretreatment of mitochondria with VDAC antibody, valinomycin (Val) or cyclosporine A (CsA) had no statistically significant effects on Aβ1-40 import (D–F).
Immunoelectron Microscopy.
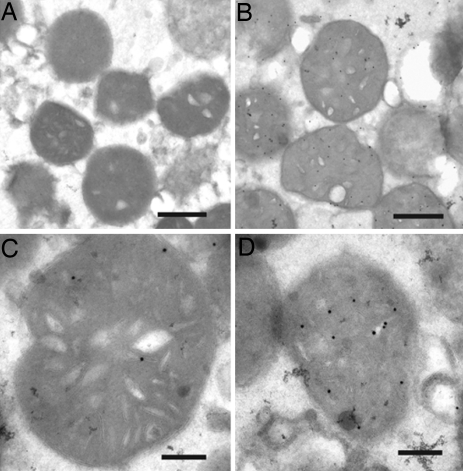
Our results from the in vitro import assay show that Aβ is located to the mitochondrial membrane fraction (see Fig. 4 B and C). To more precisely determine the localization of Aβ after import, we performed immunoelectron microscopy. Gold particle labeling of Aβ1-42 clearly shows that the peptide has been imported into mitochondria (Fig. 6 B–D). Aβ1-42 labeling was mainly detected in association to the inner membranes. Quantification of the distribution of Aβ1-42 inside mitochondria after import showed that ≈75% of the gold particles were detected in cristae, 18% in matrix, and 7% associated with the outer membrane (108 gold particles from 20 mitochondria were counted in total). In control samples treated in the same way as in Fig. 6 B–D, except that no Aβ1-42 was added during import, no labeling with the antibody JN1-42 was detected (Fig. 6A). Interestingly, the labeling pattern of Aβ1-42 in mitochondria was consistent between the mitochondrial fractions and the biopsy material (Fig. 1).
Fig. 6.
Immunoelectron microscopy of in vitro imported Aβ1-42 using JNAβ1-42 antibody. Mitochondria without Aβ1-42 in the import assay (A). Mitochondria after Aβ1-42 import (B–D). Bars: 2 μm (A and B); 0.2 μm (C and D).
Discussion
Aβ has been found in mitochondria in postmortem AD brain and in transgenic mice over-expressing mutant APP (13, 22, 29), but the uptake mechanisms for Aβ in mitochondria have not been clarified. Here we have addressed how Aβ is able to pass the mitochondrial membrane and investigated Aβ uptake into mitochondria using an in vitro import assay. We report that Aβ is taken up by mitochondria both when applied extracellularly (see Fig. 2) or directly to isolated mitochondria (see Figs. 3–6). Aβ1-40 and Aβ1-42 imports were decreased in the presence of antibodies directed toward either the mitochondrial receptors Tom20 or Tom70, or the general mitochondrial import pore of the outer membrane Tom40, suggesting that Aβ is imported through the TOM complex. We also tested whether Aβ is blocking the import pore by the sequential import of first Aβ1-40 or Aβ1-42 followed by import of the F1β precursor. We found that the F1β precursor could be imported after import of Aβ1-40 or Aβ1-42, showing that the outer membrane import pore was not blocked by Aβ. Aβ interaction with the TOM complex was recently suggested by Sirk and colleagues (30), showing that chronic exposure of cells to Aβ inhibits mitochondrial import of nuclear-encoded proteins. Even though the import pore was not blocked by Aβ during our 30 min import assay, it is still possible that chronic exposure could have this effect, as suggested by Sirk and colleagues.
The uptake of matrix proteins containing a classical import signal, such as the F1β precursor, is dependent on the mitochondrial membrane potential. In our experiments, lowering of the mitochondrial membrane potential by valinomycin treatment increased import of Aβ. This may be explained by the fact that Aβ is negatively charged at pH 7 (pI = 5.3) and a decrease of membrane potential, and thus negative ions in matrix, would cause a lower barrier for the peptide to cross. It is also possible that valinomycin could cause a small increase in Aβ import by physically disrupting mitochondrial membranes. Interestingly, Aβ by itself has been reported to cause mitochondrial depolarization (19). Import of matrix proteins (26) and integration of hydrophobic carrier proteins into the inner membrane requires a membrane potential (31). Our studies show that this is not the case for Aβ, which probably does not follow the regular inner membrane protein insertion routes. The C-terminus of Aβ (residues 29–42) is hydrophobic, and we hypothesize that Aβ binds to import receptors through hydrophobic interactions and that its import into mitochondria is facilitated by the positively charged intermembrane space and by the lack of membrane potential. Its insertion into the mitochondrial inner membrane might be dependent on the length, hydrophobicity, and helix potential of the Aβ peptide (32).
Using immunoelectron microscopy we found that most of the imported Aβ1-42 was associated with the inner membrane and that only a small fraction was localized to matrix. Moreover, subfractionation of sonicated mitochondria into a pellet and supernatant showed that Aβ was localized to the membrane fraction. Apparently, some of this Aβ was loosely attached to the membranes because it could be washed out using Na2CO3. In addition, subfractionation of mitoplasts into membrane and soluble fractions showed that Aβ is located to the inner membrane and not present in the matrix. Thus, we suggest that Aβ is inserted into the inner membrane after import and that only a small portion of the Aβ is loosely attached. Interestingly, the integration of Aβ into the inner membrane, where the respiratory chain complexes reside, is in line with results showing that Aβ1-42 may cause inhibition of complex IV (21). On the other hand, it was reported that Aβ colocalize with the mitochondrial matrix protein Hsp60 in mouse and human brain samples (13). One explanation to this discrepancy might be that in the in vitro assay we studied Aβ localization after 30 min of import, whereas Caspersen and colleagues (13) report data from postmortem AD brains and 8-month-old transgenic APP mice. However, our data from brain biopsies obtained from living subjects, which display Aβ aggregates in the neuropil, show Aβ ImmunoGold labeling in association with mitochondrial inner membranes.
One important question is how Aβ can reach the mitochondrial surface. Aβ is generated in the lumen of the endoplasmatic reticulum/intermediate compartment, trans-Golgi network and endosomal/lysosomal pathway, as well as secreted from the plasma membrane (33). Intracellular Aβ1-42 has been shown to accumulate in intracellular multivesicular bodies (10) and it is possible that Aβ leaking from these vesicles could reach the mitochondria. Moreover, our confocal microscopy analysis shows that fluorescent Aβ1-40 applied extracellulary is taken up by the cells and later partly localized to mitochondria. Accordingly, Saavedra and colleagues (34) have recently shown that Aβ1-42 is internalized by primary neurons in the absence of Apolipoprotein E. These data suggest that secreted Aβ can be reinternalized into cells either itself or through some kind of vesicular transport and come in contact with mitochondria. These mechanisms require further investigation.
In summary, we report that mitochondria are able to import Aβ in vitro and that the import occurs through the TOM complex. We also show that Aβ is associated to the mitochondrial inner membrane after import. Importantly, a similar labeling pattern was revealed by immunoelectron microscopic analysis of human brain biopsies. The presented mechanism for mitochondrial Aβ uptake can help the understanding of how Aβ can accumulate and cause mitochondrial dysfunction.
Materials and Methods
Cellular and Mitochondrial Uptake of Aβ1–40-HiLyte Fluor Analyzed by Confocal Microscopy.
Human neuroblastoma SH-SY5Y cells were grown on glass chamber slides (Lab-Tek, Nalge Nunc International Corp.). Aβ1-40-HiLyte Fluor (Alexa Fluor 488) peptide was freshly dissolved in PBS and added to complete culture medium (0.5 ml) at a final concentration of 1 μM for 18 h at 37°C. Subsequently, medium was changed and 585-nM MitoTracker Orange (Molecular Probes Inc.) was added for 30 min at 37°C. Cells were incubated for another 15 min in new medium before washing with PBS and fixation in 2% paraformaldehyde for 5 min. Cells were washed with PBS and mounted using ProLong Gold antifade reagent with DAPI (Molecular Probes Inc). The samples were visualized by an inverted Laser Scanning Microscope (LSM 510 META, Zeiss).
Isolation of Rat Liver Mitochondria.
Male Sprague–Dawley rats (≈200 g) were killed and the liver was dissected and homogenized in buffer B (0.23-M mannitol, 0.07-M sucrose, 20-mM Hepes, 0.5-mM EDTA, 0.1% BSA, pH 7.2). Approval for these experiments was received from the Animal Ethics Committee of South Stockholm, Sweden. All centrifugations were carried out at 4°C. Unbroken cells and cell nuclei were spun down at 500 × g for 5 min. A crude mitochondrial pellet was obtained from the supernatant by centrifugation at 8,000 × g for 10 min. The pellet was resuspended and cell debris was removed by a centrifugation at 500 × g for 5 min. Finally a mitochondrial pellet was collected by a centrifugation at 8,000 × g for 10 min. The pellet was resuspended in 2-ml buffer B and the protein concentration determined.
In Vitro Import Assay.
Samples containing 200-μg protein of isolated rat liver mitochondria resuspended in 200 μl import buffer (0.23-M mannitol, 0.07-M sucrose, 20-mM Hepes-KOH, 1-mM DTT, 1-mM ATP, 5-mM MgCl2,1-mM Succinate, 1-mM methionine, pH 7.2) were prepared. Samples were either pretreated with antibodies raised against VDAC, Tom20, Tom70, or Tom40 (3.75 μg per 200-μg sample) or with valinomycin (1 μM), an ionophore disrupting membrane potential, or with cyclosporine A, CsA (10 μM), an inhibitor of the mitochondrial membrane permeability transition pore, on ice for 20 min. Freshly dissolved Aβ1-40 or Aβ1-42 peptides (0.1 μM) were added and incubated at 25°C for 30 min. For flow cytometry analysis, 300-μg protein of isolated rat liver mitochondria was resuspended in 300-μl import buffer and incubated with 0.5-μM FITC-conjugated Aβ1-40 (rPeptide) at 25°C for 30 min. After import, the samples were centrifuged at 3,000 × g for 5 min to discard excess Aβ peptides and the mitochondrial pellet was resuspended in import buffer. Half of each sample was incubated with proteinase K, PK (60 μg/ml for immunoblot analysis, 60 μg/ml or 10 μg/ml for flow cytometry analysis) on ice for 20 min. The PK-activity was stopped by addition of PMSF (100 μM) and the mitochondrial pellet was reisolated by 5-min centrifugation at 3,000 × g. To investigate the localization of imported Aβ peptides, samples were sonicated on ice for 15 sec before ultracentrifugation at 100,000 × g for 20 min, resulting in a membrane fraction and a soluble fraction. The membrane fraction was incubated with Na2CO3 (0.1 mM, pH 11.5) for 20 min on ice and then spun at 100,000 × g for 20 min. Proteins from the soluble fraction were collected by filter-isolation (size cut off 3,000 MW; Millipore). A second approach to investigate the localization of the imported Aβ was also taken. In this case the mitochondrial pellet was resuspended in import buffer supplemented with 0.5-M NaCl (import buffer N) and then either directly fractionated as described above or treated with osmotic shock to obtain mitoplasts. To prepare mitoplasts, mitochondria were diluted 1:10 times with water and left 20 min on ice. The mitoplast fraction was repelleted at 4000 × g before adding import buffer N, followed by sonication and centrifugation at 100,000 × g. To study whether Aβ accumulates in the import pore, the same mitochondria were treated first with Aβ1-40 or Aβ1-42, followed by import of the F1β precursor protein. Samples were analyzed by immunoblotting (see SI Text). All antibodies used in this study are listed in Table S2.
Flow Cytometry Analysis of Aβ1–40-FITC Import.
After the import assay, mitochondria were suspended in 500-μl analysis buffer [250-nM sucrose, 20-mM 3-(N-morpholino)propanesulfonicacid (Mops), 10-mM Tris-Base, 100-μM Pi(K), 0.5-mM Mg2+, pH 7.0] containing 5-mM succinate (Sigma) and 0.1-μg/ml rotenone (Sigma), as previously described (35). The samples were incubated with MitoFluor Red (Molecular Probes Inc.) as mitochondrial marker for 15 to 10 min followed by immediate analysis without washing. These markers were used to exclude the debris from isolated mitochondria. MitoFlour Red (Molecular Probes Inc.) was prepared and stored according to the manufacturer's instructions. Flow cytometry was performed using a FACS Calibur Cytometry (Becton Dickinson) equipped with a 488-nm argon laser and a 635-nm red diode laser. The import of Aβ1-40-FITC was analyzed in fluorescence channel 1 (FL-1) and MitoFluor Red in FL-4 channel. The FL-1-FL-4 compensation was 2.0 to 4.0%. The samples were gated based on light scattering properties in the side scattering (SSC) and forward scattering (FCS) modes. For each experiment, 100,000 events were counted. In all, preparations more than 80% were MitoFluor Red-positive, indicating some contamination of other organelles. Presented data are based on events gated for mitochondria that were MitoFluor Red positive.
Immunoelectron Microscopy.
Mitochondrial fractions obtained after the import assay or human brain biopsy specimens were fixed in 2% paraformaldehyde + 0.1% glutaraldehyde in 0.1-M phosphate buffer (PB) pH 7.4 over night, rinsed in 0.1-M PB, and soaked in 10% Gelatin at 37°C for 20 min. The pellet was then placed in the refrigerator and fixed into the same fixation as above. The pellet was cut into smaller specimens and infiltrated into 2.3 M of sucrose and frozen in liquid nitrogen. Sectioning was performed according to Tokuyasu (36) at −95°C. Immunolabeling procedure was performed as follows: grids were placed directly on drops of 0.15-M NaCl containing 20-mM glycine followed by incubation in 2% BSA (Sigma fraction V) and 2% Gelatin (IGSS quality, Amersham Biosciences U.K. Ltd.) in 0.1-M PB to block nonspecific binding. Sections were then incubated with the primary antibody diluted 1:50 in 0.1 M of PB containing 0.1% BSA + 0.1% Gelatin overnight in a humidified chamber at room temperature. As control, preabsorption with a competition mix of Aβ1-42 antibody (1:50) and Aβ 1-42 protein (10 μM) was performed as described by Van Noorden (37). The sections were thoroughly washed in the same buffer and bound antibodies were detected with protein A coated with 10-nm gold (Amersham Biosciences U.K. Ltd.) at a final dilution of 1:100. Sections were rinsed in buffer and fixed in 2% glutaraldehyde, contrasted with 0.1% uranyl acetate, embedded in 2% methylcellulose, and examined in a Tecnai 10 (FEI Company, The Netherlands) at 80 kV. Images were taken using a Megaview III digital camera (38).
Statistical Analysis.
Wilcoxon signed-rank test or the nonparametric, two-tailed Mann–Whitney test was used to compare statistical differences in the import assay. Values of *, P < 0.05, **, P < 0.01 and ***, P < 0.001 were considered to be significant. Values are shown as mean ± standard error of the mean.
Supplementary Material
Acknowledgments.
The authors thank Dr. Kjell Hultenby (Karolinska Institutet, Stockholm, Sweden) for excellent help with immunoelectron microscopy, and Dr. Jan Näslund (Astra Zeneca, Södertälje, Sweden) for the kind gift of Aβ antibodies. This work was supported by Dainippon Sumitomo Pharma Company, Ltd., Gamla Tjänarinnor Foundation, Gun och Bertil Stohnes Foundation, The Foundation for Geriatric Diseases at Karolinska Institutet, Wallenbergs Foundation, and the Swedish Research Council (to E.G. and N.A.). The Flow cytometry analysis was performed at Center for Infectious Medicine, which is supported by the Swedish Foundation for Strategic Research.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806192105/DCSupplemental.
References
- 1.Vassar R, et al. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 2.Kimberly WT, et al. Gamma-secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proc Natl Acad Sci USA. 2003;100:6382–6387. doi: 10.1073/pnas.1037392100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glenner GG, Wong CW. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122:1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- 4.Masters CL, et al. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soderberg L, et al. Analysis of single Alzheimer solid plaque cores by laser capture microscopy and nanoelectrospray/tandem mass spectrometry. Biochemistry. 2006;45:9849–9856. doi: 10.1021/bi060331+. [DOI] [PubMed] [Google Scholar]
- 6.Walsh DM, Tseng BP, Rydel RE, Podlisny MB, Selkoe DJ. The oligomerization of amyloid beta-protein begins intracellularly in cells derived from human brain. Biochemistry. 2000;39:10831–10839. doi: 10.1021/bi001048s. [DOI] [PubMed] [Google Scholar]
- 7.Lambert MP, et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh DM, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi RH, et al. Oligomerization of Alzheimer's beta-amyloid within processes and synapses of cultured neurons and brain. J Neurosci. 2004;24:3592–3599. doi: 10.1523/JNEUROSCI.5167-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gouras GK, v Intraneuronal A{beta}42 accumulation in human brain. Am J Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wirths O, Multhaup G, Bayer TA. A modified β-amyloid hypothesis: intraneuronal accumulation of the β-amyloid peptide—the first step of a fatal cascade. J Neurochem. 2004;91:513–520. doi: 10.1111/j.1471-4159.2004.02737.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Su B, Perry G, Smith MA, Zhu X. Insights into amyloid-beta-induced mitochondrial dysfunction in Alzheimer disease. Free Radic Biol Med. 2007;43:1569–1573. doi: 10.1016/j.freeradbiomed.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Caspersen C, et al. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB J. 2005;19:2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 14.Hirai K, et al. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer's disease. FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging. 2005;32:486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 16.Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann Neurol. 2005;57:695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- 17.Parker WD, Jr, Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer's disease. Neurology. 1990;40:1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- 18.Parker WD, Jr, Parks JK. Cytochrome c oxidase in Alzheimer's disease brain: purification and characterization. Neurology. 1995;45:482–486. doi: 10.1212/wnl.45.3.482. [DOI] [PubMed] [Google Scholar]
- 19.Cardoso SM, Santana I, Swerdlow RH, Oliveira CR. Mitochondria dysfunction of Alzheimer's disease cybrids enhances Abeta toxicity. J Neurochem. 2004;89:1417–1426. doi: 10.1111/j.1471-4159.2004.02438.x. [DOI] [PubMed] [Google Scholar]
- 20.Kish SJ, et al. Brain cytochrome oxidase in Alzheimer's disease. J Neurochem. 1992;59:776–779. doi: 10.1111/j.1471-4159.1992.tb09439.x. [DOI] [PubMed] [Google Scholar]
- 21.Crouch PJ, et al. Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta1-42. J Neurosci. 2005;25:672–679. doi: 10.1523/JNEUROSCI.4276-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lustbader JW, et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 23.Falkevall A, et al. Degradation of the amyloid beta-protein by the novel mitochondrial peptidasome, PreP. The J Biol Chem. 2006;281:29096–29104. doi: 10.1074/jbc.M602532200. [DOI] [PubMed] [Google Scholar]
- 24.Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer's amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol. 2003;161:41–54. doi: 10.1083/jcb.200207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer's disease brain is associated with mitochondrial dysfunction. J Neurosci. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neupert W, Herrmann J. M. Translocation of proteins into mitochondria. Annu Rev Biochem. 2007;76:723–749. doi: 10.1146/annurev.biochem.76.052705.163409. [DOI] [PubMed] [Google Scholar]
- 27.Rehling P, Pfanner N, Meisinger C. Insertion of hydrophobic membrane proteins into the inner mitochondrial membrane—a guided tour. J Mol Biol. 2003;326:639–657. doi: 10.1016/s0022-2836(02)01440-7. [DOI] [PubMed] [Google Scholar]
- 28.Rasola A, Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 2007;12:815–833. doi: 10.1007/s10495-007-0723-y. [DOI] [PubMed] [Google Scholar]
- 29.Manczak M, et al. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 30.Sirk D, et al. Chronic exposure to sub-lethal beta-amyloid (Abeta) inhibits the import of nuclear-encoded proteins to mitochondria in differentiated PC12 cells. J Neurochem. 2007;103:1989–2003. doi: 10.1111/j.1471-4159.2007.04907.x. [DOI] [PubMed] [Google Scholar]
- 31.Truscott KN, Brandner K, Pfanner N. Mechanisms of protein import into mitochondria. Curr Biol. 2003;13:R326–R337. doi: 10.1016/s0960-9822(03)00239-2. [DOI] [PubMed] [Google Scholar]
- 32.Lundin C, et al. Stable insertion of Alzheimer Abeta peptide into the ER membrane strongly correlates with its length. FEBS Lett. 2007;581:3809–3813. doi: 10.1016/j.febslet.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gouras GK, Almeida CG, Takahashi RH. Intraneuronal Abeta accumulation and origin of plaques in Alzheimer's disease. Neurobiol Aging. 2005;26:1235–1244. doi: 10.1016/j.neurobiolaging.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 34.Saavedra L, Mohamed A, Ma V, Kar S, de Chaves EP. Internalization of beta-amyloid peptide by primary neurons in the absence of apolipoprotein E. J Biol Chem. 2007;282:35722–35732. doi: 10.1074/jbc.M701823200. [DOI] [PubMed] [Google Scholar]
- 35.Mattiasson G, Friberg H, Hansson M, Elmer E, Wieloch T. Flow cytometric analysis of mitochondria from CA1 and CA3 regions of rat hippocampus reveals differences in permeability transition pore activation. J Neurochem. 2003;87:532–544. doi: 10.1046/j.1471-4159.2003.02026.x. [DOI] [PubMed] [Google Scholar]
- 36.Tokuyasu KT. A technique for ultracryotomy of cell suspensions and tissues. J Cell Biol. 1973;57:551–565. doi: 10.1083/jcb.57.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Noorden S. Tissue preparation and immunostaining techniques for light microscopy. In: Polak JM, Van Noorden S, editors. Immunocytochemistry: Modern Methods and Applications. 2nd Ed. Bristol, England: John Wright & Sons; 1986. [Google Scholar]
- 38.Sodersten F, et al. Ultrastructural immunolocalization of cartilage oligomeric matrix protein (COMP) in relation to collagen fibrils in the equine tendon. Matrix Biol. 2005;24:376–385. doi: 10.1016/j.matbio.2005.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.