Abstract
Cognitive flexibility is a crucial human ability allowing efficient adaptation to changing task challenges. Although a person's degree of flexibility can vary from moment to moment, the conditions regulating such fluctuations are not well understood. Using a task-switching procedure with fMRI, we found several brain regions in which neural activity preceding each trial predicted subsequent cognitive flexibility. Specifically, as pretrial activity increased, performance improved on trials when the task switched but did not improve when the task repeated. Regions from which flexibility could be predicted reliably included the basal ganglia, anterior cingulate cortex, prefrontal cortex, and posterior parietal cortex. Although further analysis revealed similarities across the regions in how flexibility was predicted, results supported the existence of multiple independent sources of prediction. These results reveal distinct neural mechanisms underlying fluctuations in cognitive flexibility.
Keywords: basal ganglia, fMRI, task switching, cognitive control
Juggling several tasks at once, or multitasking, is a fact of everyday life and becomes increasingly salient with the growing use of products such as mobile telephones, wireless e-mail devices, and portable music players. Given the wide array of tasks people successfully pack into their daily routines, the capacity to multitask is among the most remarkable endowments of the human mind. Nevertheless, this capacity, known as “cognitive flexibility,” does come at a cost: empirical investigations of task switching consistently show that performance upon switching to a new task is slower and more error prone than performance when repeating a task (1–3). These behavioral costs are difficult to avoid even when subjects are given ample time to prepare for the upcoming task (1, 3), but variations in the size of this switch cost have been observed within experimental sessions (4, 5), suggesting that an individual's flexibility can fluctuate from moment to moment.
Unfortunately, it is unclear how fluctuations in flexibility can be predicted, although such knowledge would carry considerable value. From a practical standpoint, for instance, productivity could be maximized by reserving multitasking activities for known periods of high flexibility and scheduling single-task activities for periods of low flexibility. From a theoretical standpoint, identifying the predictors of cognitive flexibility could facilitate a deeper understanding of the mechanisms governing cognitive control.
In this study, we used fMRI to predict cognitive flexibility. Because fMRI is noninvasive and imposes minimal additional task demands on subjects, it potentially can reveal variations in flexibility with negligible interference to the task-switching procedure.
Our particular aim was to learn if brain activity preceding task cues could predict task-switching performance. Probing before the cue, when the task to be performed has not yet been revealed to subjects, distinguishes our work from previous studies that have analyzed neural activity after the task is known. Such studies are geared to measure task-specific processing, for which rule representations tailored to the known task are implemented (6, 7). Focusing analysis on the post-cue preparation period has provided critical insights about the role of task-specific processing during task-switching performance (8–10), in some cases linking the magnitude of post-cue activity to the degree of task-switching success (11–14). However, cognitive flexibility relates to the ability to react to any future task challenge rather than to a specifically cued task; cognitive flexibility thus requires that preparatory bias toward performing a specific task, at the expense of other tasks, be minimal. Thus, we focused on neural activity preceding the cue.
Results
Behavior.
Twenty-one subjects performed task switching during a single fMRI session. On each trial they were cued to perform 1 of 2 tasks on a subsequent target digit (a magnitude or a parity judgment; see Methods). The order of trial presentation was randomized so that half of the trials consisted of task switches (e.g., magnitude following parity), and the remaining trials consisted of task repetitions (e.g., magnitude following magnitude). The randomization ensured that the task on each trial could not be predicted before the cue (15).
One subject was excluded because of low accuracy (67.8%). Data from the remaining subjects revealed robust behavioral switch costs, with response times (RT) significantly slower on switch (684 ms) than on repeat trials (642 ms), t (19) = 4.39, P < 0.0004. Accuracy was 96.2% on switch trials and 97.1% on repeat trials, t (19) = 1.74, P < 0.1.
Cognitive Flexibility Predicted by Pretrial fMRI Signal.
In estimating neural activity preceding the task cue, we defined pretrial signal as the blood oxygenation level-dependent (BOLD) fMRI signal collected during the single volume acquisition preceding the task cue (i.e., from −1.5 to 0 s). We then analyzed how this measure separately predicted performance on switch and repeat trials. Given that increases in flexibility should translate to smaller switch costs, RT on switch and repeat trials should converge as flexibility increases. Thus, if pretrial signal carries information about flexibility, its relationship to RT on switch and repeat trials must manifest differently.
For each subject, and at every voxel, linear regression was carried out using pretrial signal as a predictor for RT (16); as noted previously, this regression was done separately for switch and repeat trials. The resulting slope coefficients reflect the degree to which RT changed as a function of pretrial signal (e.g., negative values indicate that increasing pretrial signal predicts decreasing RT). To determine whether the slopes differed between switch and repeat trials, paired t tests were performed for each voxel.
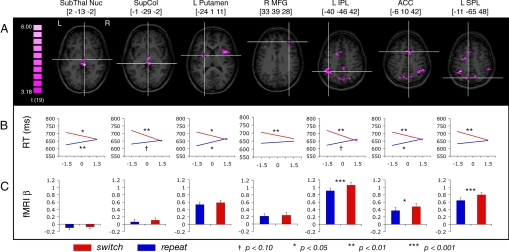
Results confirmed that neural activity preceding task cues indeed could predict cognitive flexibility; a number of brain regions emerged in which slopes on switch and repeat trials differed significantly, including medial and lateral prefrontal cortex (PFC), posterior parietal cortex (PPC), anterior cingulate cortex (ACC), anterior insula, and subcortical structures including the basal ganglia (BG) and superior colliculus (SC) (Fig. 1 A and B; Table 1). Most of these regions were characterized by significantly negative slopes on switch trials and non-negative slopes on repeat trials, a pattern reflective of decreasing switch costs with increasing pretrial signal. This pattern is consistent with the notion that increased flexibility improves performance on trials when task set reconfiguration is needed (i.e., on switch trials).
Fig. 1.
Seven of the regions identified in which pretrial signal differentially predicts RT on switch and repeat trials, with Talairach coordinates (40) indicating center of mass. (A) Axial slices of corresponding regions, with cross-hairs indicating center of mass. (B) Mean regression lines for switch and repeat conditions, drawn from −1.5 to + 1.5 SD of pretrial signal value, for each of the corresponding ROIs. The x-axis indicates pretrial signal value (z-score), and the y-axis indicates RT. Symbols denote significance (see legend) of difference between the slope value versus a hypothesized mean of 0. (C) Trial-evoked BOLD activity for repeat and switch trials. Error bars reflect standard error of the difference.
Table 1.
Regions predicting cognitive flexibility (comparison of switch slope versus repeat slope)
| Region | Coordinates |
Vox, no. | Peak t value | Relationship between pretrial signal and RT |
Trial-evoked BOLD response switch > repeat (P) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Switch |
Repeat |
|||||||||
| X | Y | Z | Δ ms/sd | P | Δ ms/sd | P | ||||
| L IPL | −40 | −46 | 42 | 94 | 5.315 | −23.337 | 0.0016 | 13.252 | 0.0514 | 0.0004 |
| SupCol | −1 | −29 | −2 | 19 | 5.253 | −23.418 | 0.0005 | 8.246 | 0.0838 | 0.1649 |
| L MFG | −39 | 29 | 20 | 38 | 5.166 | −17.476 | 0.0195 | 15.196 | 0.0080 | 0.0111 |
| R SPL | 22 | −67 | 45 | 6 | 5.104 | −13.741 | 0.0382 | 9.342 | 0.0512 | 0.1847 |
| R AI | 37 | 11 | 11 | 50 | 4.825 | −22.047 | 0.0005 | 11.136 | 0.0504 | 0.3528 |
| L SPL | −11 | −65 | 48 | 25 | 4.742 | −18.958 | 0.0011 | 7.035 | 0.1802 | 0.0002 |
| L IPS | −31 | −68 | 37 | 9 | 4.690 | −16.155 | 0.0317 | 8.566 | 0.0148 | 0.0102 |
| ACC | −6 | 10 | 42 | 18 | 4.600 | −16.114 | 0.0098 | 14.376 | 0.0188 | 0.0140 |
| L Putamen | −24 | 1 | 11 | 16 | 4.582 | −16.885 | 0.0187 | 16.235 | 0.0148 | 0.1040 |
| R SPL-2 | 4 | −70 | 41 | 19 | 4.516 | −5.272 | 0.1670 | 15.365 | 0.0191 | 0.0001 |
| L SFS | −23 | −10 | 51 | 15 | 4.396 | −15.646 | 0.0080 | 19.163 | 0.0070 | 0.0149 |
| R MFG | 33 | 39 | 28 | 7 | 4.359 | −13.042 | 0.0057 | 4.307 | 0.3169 | 0.4787 |
| SubThalNuc | 2 | −13 | −2 | 7 | 4.352 | −14.533 | 0.0131 | 11.552 | 0.0050 | 0.4020 |
| R SPL-3 | 11 | −63 | 47 | 9 | 4.279 | −11.598 | 0.0583 | 9.651 | 0.1791 | 0.0065 |
| R IPL | 50 | −40 | 50 | 7 | 4.222 | −18.313 | 0.0007 | 4.567 | 0.3725 | 0.1262 |
| R IFG | 39 | 41 | 3 | 8 | 4.119 | −18.094 | 0.0002 | 1.479 | 0.7976 | 0.0744 |
| L SPL-2 | −24 | −55 | 53 | 8 | 4.080 | −13.731 | 0.0639 | 12.443 | 0.0310 | 0.0282 |
| PostCingG | 2 | −36 | 41 | 17 | 4.007 | −14.925 | 0.0133 | 9.303 | 0.0908 | 0.7321 |
| L ACC | −16 | 4 | 42 | 6 | 3.979 | −9.329 | 0.0451 | 18.250 | 0.0068 | 0.6027 |
| R PostcentralG | 48 | −22 | 38 | 7 | 3.728 | −11.573 | 0.1204 | 13.221 | 0.0263 | 0.6892 |
Δ ms/sd, change in RT per standard deviation of pretrial signal; ACC, anterior cingulate cortex; AI, anterior insula; IFG, inferior frontal gyrus; IPL, inferior parietal lobule; IPS, intraparietal sulcus; L, left; MFG, middle frontal gyrus; R, right; SFS, superior frontal sulcus; SPL, superior parietal lobule; SubThalNuc, subthalamic nucleus; SupCol, superior colliculus; Vox, voxels. Postcentral G, postcentral gyrus; PostCingG, posterior cingulate gyrus. Coordinates indicate center of mass in Talairach space.
An alternative “floor effect” interpretation holds that pretrial signal confers equal benefits upon all RTs, irrespective of task transition, but performance on the repeat trials has less room to improve because subjects already are responding quickly. However, although this account predicts modestly negative or negligible slopes on repeat trials, the slopes in all the regions were numerically positive (i.e., RTs became slower as pretrial signal increased). These positive slopes, which reached significance in a subset of the regions (Fig. 1B; Table 1), cannot be explained by a floor effect. Rather, the slowing of RT as pretrial signal increases (on repeat trials) is consistent with a previously articulated notion that cognitive flexibility trades off to some extent with cognitive stability (17–20). That is, in states of high flexibility, active task sets are not maintained rigidly across trials, thus reducing the benefits of task repetition.
Relationship to Task-Switch Network.
We next considered how the regions of interest (ROIs) identified in the current analysis relate to previous studies of flexibility. Although PFC, ACC, and PPC are regularly reported to participate in task switching (21), the role of subcortical brain regions, especially the BG, has been less understood. On one hand, neurodegenerative diseases impairing dopamine function in the BG have been associated with set-shifting deficits, as observed in Parkinson's and Huntington's diseases (22–25). Moreover, administration of dopaminergic drugs, particularly those acting on D2 receptors in the BG, can improve flexibility (17, 26, 27). On the other hand, the BG have not featured prominently in human neuroimaging studies of task switching (but see refs. 28 and 29). One possible key to explaining this puzzle is that the neuroimaging studies often have focused on identifying regions exhibiting greater transient increases in activity following the cue and target presentations on switch trials than on repeat trials, and this approach may overlook regions exerting sustained influences on flexibility (11). To examine whether the BG conform to this description, we carried out further analysis on the ROIs of the BG identified in our primary analysis (i.e., the putamen and the subthalamic nucleus). Specifically, we contrasted trial-evoked BOLD responses from switch versus repeat trials. For completeness, we also performed this analysis on the remaining ROIs identified in the primary analysis.
Results showed that neither the putamen nor the subthalamic nucleus ROIs exhibited a significant difference on switch versus repeat trials (P > 0.10 in both) (Fig. 1C; Table 1). In fact, fewer than half of the ROIs were significant; all these were contained within the PPC, lateral PFC, and ACC (Table 1). Given that most of the ROIs in this analysis were nonselective, these results confirm that brain areas can contribute to fluctuations in flexibility while maintaining insensitivity to the current degree of task-switching demands (11). Moreover, the results provide a parsimonious explanation for why the BG have not been prominent in prior neuroimaging studies of task switching.
Prediction from Distinct Sources of BOLD Variability.
The previous analysis established that the many ROIs in this study did not behave in uniform fashion, at least with respect to their trial-evoked responses. We next explored whether ROIs behaved similarly with respect to predicting cognitive flexibility. In particular, we sought to examine whether the BOLD variance from which the ROIs predicted flexibility could be distilled to a single source common to all regions or whether this variance was distributed across multiple independent sources represented to a greater or lesser degree in various subsets of regions.
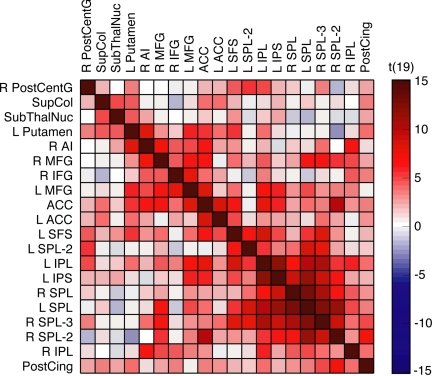
In an initial approach to this question, the samples of pretrial signals from each ROI were correlated with those of every other ROI. Because the single-source account stipulates that a common source of variance exists across all ROIs (from which flexibility can be predicted), it necessarily assumes that all ROIs should be correlated to some extent. However, although many regions did indeed correlate positively, several did not (Fig. 2). In particular, the subcortical ROIs (subthalamic nucleus, putamen, and SC) were uncorrelated with several ROIs of the PPC.
Fig. 2.
Cross-correlation results. ROI names correspond to those listed in Table 1.
We next evaluated the multiple-source account more directly. Using a data-driven approach consisting of 2 steps described in the following paragraphs, we were able to obtain evidence for at least 4 unique sources of variance from which flexibility could be predicted.
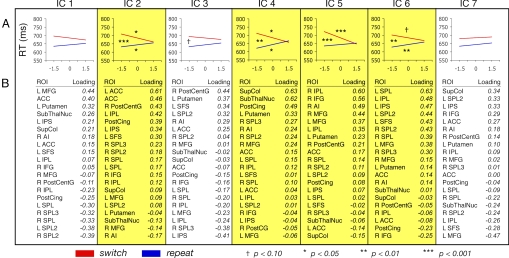
First, using independent component analysis (ICA), a technique that blindly separates uncorrelated and statistically independent sources of variance (30, 31), we extracted 7 independent components (ICs) contributing to the variance structure of the pretrial signal across the 20 ROIs. The degree to which the ICs correlated with the pretrial signal values from each of the ROIs (i.e., the summary of factor loadings) is shown in Fig. 3.
Fig. 3.
Components extracted from ICA, with ICs predicting cognitive flexibility highlighted. (A) Mean regression lines for switch and repeat conditions. Symbols above switch line and below repeat line denote significance of difference between slope value versus an hypothesized mean of 0. Symbols between switch and repeat lines denote significance of difference between the 2 slopes. (B) Summary of the ROIs' factor loadings.
After extracting the ICs, the second step was to test whether they actually predicted flexibility. To this end, using the estimated pretrial signals from each of the ICs, we carried out the primary regression analysis to determine whether these pretrial signals predicted flexibility (i.e., the convergence of RT on switch and repeat trials). Results revealed 4 of the ICs were reliably predictive (Fig. 3). Given that the components were uncorrelated and statistically independent, these results confirm that fluctuations in flexibility originated from multiple distinct sources.
Although additional research will be necessary to articulate each IC's unique contributions to flexibility, we offer some brief discussion for 3 of the sources. First, consider the component that is well characterized by the ACC, IC2. It has been proposed that the ACC monitors ongoing performance and provides internal feedback to the cognitive control system in response to changing task demands or recent behavioral success (32, 33). Thus, it is possible that this component reflects trial-to-trial evaluation-linked adjustments in flexibility. However, whether subjects adjust flexibility based on recent task demands is unclear. In this study, when preceded by switch and repeat trials, switch trial RTs were 685 ms and 684 ms, respectively, and repeat trial RTs were 642 ms and 642 ms, respectively. Thus, fluctuations of flexibility in task-switching studies may be evaluation invariant, a possibility that is consistent with reports that subjects largely are unable to adjust their flexibility willfully, despite detailed feedback and motivational payoff schemes (5). We currently are exploring alternative explanations for the role of the ACC.
The role of ICs 4 and 6 may be less controversial. Given the clustering of the putamen and subthalamic nucleus along IC4, and given further the previously established role of dopamine in cognitive control (17, 19, 20, 26), this source of flexibility may relate to moment-to-moment fluctuations of dopaminergic activity in the BG. Turning to IC6, it is notable that the ROIs best loading upon it—primarily in the PPC—also tended to show the largest differences in the conventional switch versus repeat analysis on the trial-evoked BOLD responses. Thus, this source of flexibility may relate to momentary readiness of the regions that carry out task-specific processing.§
Conclusions
The current work confirms that cognitive flexibility fluctuates from moment to moment and reveals that such fluctuations can be predicted from neural activity preceding knowledge of the upcoming task. That is, during periods when task-specific processing (7) presumably could not be implemented to benefit future performance, task-switching success still could be predicted. Recent advances in neuroimaging have granted researchers the tools to probe states of the mind in such a way that a myriad of subsequent behaviors may be predicted, including attentional control (16), memory (34, 35), motor responding (36), and problem solving (37). In studies like these, neural activity preceding task performance commonly is correlated with overall performance, allowing the interpretation that arousal, attention, or task engagement drives the variations in performance. The current study demonstrates that a single measure of neural activity can predict a more complex pattern of behavior that cannot be explained as arousal, because variations in arousal would modulate performance on both switch and repeat trials equally. In sum, further work examining normal fluctuations of mental activity in healthy adults promises to continue to provide new insights about the underlying architecture of cognitive control.
Methods
Participants.
Twenty-one neurologically intact individuals with normal or corrected-to-normal vision participated in exchange for monetary compensation. Twelve of the participants were female; all were right handed, and the mean age was 22.2 years (range = 18–32 years). Participation included an 8-min behavioral practice session followed by a 45-min scanning session. Informed consent was obtained, and the study protocol was approved by the Human Investigation Committee of the Yale School of Medicine.
Task and Stimuli.
Stimuli were generated with an Apple G4 computer, using MATLAB software (Mathworks) with Psychophysics Toolbox extensions (38, 39). During scanning, stimuli were displayed via a Mitsubishi XL30U LCD projector onto a screen mounted in the rear of the scanner bore, which participants viewed from a distance of 79 cm via a mirror attached to the head coil.
At the beginning of each run, a black outline square, which was filled in white, was presented in the center of the display (2.33° visual angle per side, outline stroke = 0.27°) on a gray background. This square remained displayed for the duration of the run. Five hundred milliseconds later, a black outline fixation circle appeared (0.82° diameter, stroke = 0.07°), centered inside the box.
Five hundred milliseconds before each trial, the fixation disappeared to signal that a trial was to begin. Upon the trial onset the fixation circle reappeared, now filled in with either red or green. This colored task cue remained for 200 ms and was followed by an 800-ms presentation of only the fixation circle, yielding a 1000-ms cue-target preparation interval. The target, a digit drawn randomly on each trial from the set of 2, 4, 7, and 9 (height = 1.64°, width = 0.27°, stroke = 0.14°), then was superimposed over the fixation circle for 200 ms and then was removed, leaving only the fixation circle.
The color of the task cue was mapped to 1 of 2 task types (counterbalanced between subjects). In the magnitude task, participants were to report whether the target was less than or greater than 5, responding with the left and right button presses, respectively. In the parity task, participants were to report whether the target was odd or even, responding with left and right buttons, respectively. Note that the chosen response mappings ensured that participants did not learn task-invariant responses (i.e., the correct response to all targets always was contingent on the cued task).
Responses were collected for up to 2000 ms following the target onset and were followed by an intertrial interval of 1.5 or 4.5 s, yielding trial-to-trial onset asynchronies, or trial spacings, of 4.5 or 7.5 s.
Each run consisted of 84 trials, including 2 filler trials at both the beginning and end. The first filler trial began 1.5 s into the run, and the remaining filler trials always were spaced 4.5 s after the previous trial onsets. Filler trial tasks were chosen randomly.
Three independent variables were factorially crossed to determine the trial conditions: 2 task types (magnitude and parity) × 2 trial spacings (4.5 s and 7.5 s) × 2 target responses (left button press and right button press). Each of the 8 trial conditions was presented 10 times to yield the 80 main trials per run.
The presentation order was randomized within each run of each subject with the constraint that half the trials of each task type were preceded by the same task (repeat trial) and half were preceded by a different task (switch trial). Target digits were chosen randomly on each trial so that they conformed to constraints of the trial's task type and target response (e.g., a magnitude task with a “left” response required that the target be either 2 or 4).
Participants were instructed to respond as quickly as possible and minimize errors. Left and right button-press responses were made using the index and middle fingers of the right hand, respectively. Responses were collected via a fiber-optic button box (Current Designs). No feedback was given.
fMRI Acquisition.
A Siemens Trio 3-Tesla scanner, equipped with a standard birdcage head coil, was used. After an initial anatomical localizer, a high-resolution T1-weighted anatomical image was acquired with a 3-dimensional magnetization prepared rapid acquisition gradient echo sequence. Then, 26 axial slices were defined (5-mm thickness, no gap), parallel to the anterior commissure-posterior commissure line, covering the whole brain, and a second anatomical image was acquired, using a T1 fast, low-angle shot sequence. Using the same 26-slice orientation, 3 functional runs collecting 336 successive volumes each then were carried out (TR = 1500 ms, TE = 25 ms, flip angle = 90°, 64 × 64 matrix with 3.5 mm × 3.5 mm in-plane resolution).
Behavioral Analysis.
RT data were trimmed on correct response trials at 3 SD above the mean, for each subject, within each task type (magnitude and parity) and each task transition (switch and repeat), leading to the removal of 1.7% of the trials. Additionally, error trials were excluded, as were trials following error trials (trials following errors cannot necessarily be categorized as switch or repeat). For all reported analyses, data were collapsed across task type.
fMRI Analysis.
Preprocessing.
Brain Voyager QX software (Brain Innovation) was used for preprocessing. For each subject, the first 7 volumes of each functional run were discarded, and the data subsequently were motion corrected, slice-time corrected, spatially smoothed with a 4-mm FWHM isotropic Gaussian kernel, and subjected to linear trend removal. Because we anticipated that fluctuations in flexibility might occur at slow temporal frequencies, we did not temporally high-pass filter the data. The data then were transformed to Talairach space (40) and resampled at a voxel size of 3 × 3 × 3 mm.
Whole-Brain Analysis: Predicting RT from Pretrial Signal.
By applying a mask covering the whole brain (but excluding the cerebellum and midbrain), the number of voxels was restricted to 62,980. The following described analyses were carried out independently for each subject, within each of the voxels.
Pretrial signal was obtained by extracting the fMRI signal value from the single time point collected before each trial. Trials beginning on the eighth volume acquisition of a run were excluded, because the pretrial volume (i.e., the seventh volume) was discarded during preprocessing. The remaining sample of pretrial signal values within each run then were z-transformed and pooled across all runs.
Within each task transition (switch versus repeat) and trial spacing (4.5 and 7.5 s), we carried out linear regression with pretrial signal as a predictor for current trial RT. It was essential to perform the regressions separately for each trial spacing because the close temporal proximity of trials ensured that the pretrial signal would be influenced by the still-unfolding hemodynamic response to the previous trial, and the degree of this influence might vary systematically as a function of the previous trial's onset time. (Note that this approach does not consider influences of spacings previous to the immediately preceding trial; this issue is addressed in the Alternative Analyses below.)
Four beta coefficients were obtained from the regressions (2 trial spacings × 2 task transitions), reflecting the relationship between pretrial signal and RT. We then collapsed across trial spacing to arrive at 2 mean coefficients (switch and repeat) per subject. These coefficients were treated as dependent measures for repeated-measures random effects analysis, where 2-tailed t tests were used to contrast the switch versus repeat coefficients. The resulting t values from each voxel were used to generate a whole-brain statistical parametric map.
To determine significant voxels, a single-voxel threshold was set at t (19) = 3.18, P < 0.005. By applying a spatial cluster threshold of 162 mm3, or 6 contiguous voxels, the family-wise (corrected) false probability rate was P < 0.025. This estimate was reached by carrying out 2000 Monte Carlo simulations of whole-brain statistical maps, using Brain Voyager's cluster threshold estimator (41).
Alternative Analyses to Predict RT from Pretrial Signal.
To address potential limitations of the primary analysis, 2 subsequent analyses were carried out on the ROIs yielded from the initial whole-brain results. Within each of these ROIs, the fMRI signal at each time point was averaged across all voxels, and the resulting mean values for each ROI were used.
1) Removing Contributions of Previous Evoked Responses from the Pretrial Signal.
Although the primary analysis was carried out separately for each trial spacing to avoid contamination of the pretrial signal by the mean evoked BOLD response to the previous trial, it is probable that earlier trial spacings also influenced the pretrial signal. This possibility was not viewed as a concern, because preliminary analysis showed that the earlier trial spacings did not affect current-trial RT. Nevertheless, we aimed to partial out contributions to the pretrial signal resulting from the mean trial-evoked responses preceding the current trial. This alternative analysis was done in a fashion similar to previous analyses of variations in RT as a function of variations in BOLD activity (16).
For each ROI of each subject, a finite impulse response model was used to estimate the mean trial-evoked response for both switch and repeat trials within each run (42). Eleven candlestick predictors were established for each task transition (switch and repeat), 1 for each time point following the trial onset; given a 1.5-s TR, a duration of 16.5 s following trial onset was thus modeled. Multiple regression then was used to partial out the variance in the fMRI signal explained by the 22 predictors and to remove contributions of the mean trial-evoked BOLD activity across each run.
Within each ROI, the primary regression analysis was recomputed using the residual signals to determine how RT could be predicted on switch and repeat trials. Results for these ROIs, shown in supporting information (SI) Table S1, were remarkably similar to those of the initial analysis, indicating that the mean trial-evoked responses before the immediately preceding trial had a negligible effect on the capacity for pretrial signal to predict flexibility.
2) Removing Contributions of Previous Trial RT from the Pretrial Signal.
It is possible that RT on the previous trial somehow might influence pretrial signal in such a way that it could account for the present results. To address this possibility, for each ROI, within each run of each subject, linear regression was used to partial out variance in the pretrial signal accounted for by previous trial RT. The primary regression analysis again was recomputed on the residuals for each ROI, and the results were virtually unchanged (Table S2).
Trial-Evoked Switch Versus Repeat Activity.
To examine the trial-evoked (postcue) BOLD activity on switch and repeat trials, both of these trial types were modeled with a 2-gamma hemodynamic response function and used as predictors in a multiple regression analysis carried out separately for each ROI for each subject. Resulting beta coefficients for subjects' switch and repeat trials subsequently were entered into paired-samples t tests, separately for each ROI.
Cross-Correlation Analysis.
Before correlations were computed, linear regression was used to partial out the following nuisance variables from the BOLD signal from each ROI, separately for each subject: (i) 6 parameters obtained from the motion correction procedure, (ii) BOLD signal measured from a region in deep white matter, and (iii) mean “global” BOLD signal, averaged across the 62,980 voxels in the whole-brain dataset. This was done to remove spurious sources of variance attributable to scanner and motion artifacts, respiration, and other factors deemed unrelated to neural activity (43, 44). Note that although others have also partialed out lateral ventricle activity, we did not because of the ventricles' spatial proximity to the BG. Also, we attempted to remove contributions of previous trial-evoked responses from the pretrial signal. Using the finite impulse response model described earlier in the Alternative Analyses (11 predictors each for switch and repeat trials), variance explained by these predictors was partialed out.
Next, the samples of pretrial signals were extracted from each of the ROIs and cross-correlated with each other, yielding 190 unique pairwise correlations, and the resulting correlation coefficients then were normalized, using Fischer's r-to-z transform. To evaluate the reliability of the pairwise correlations at the group level, the Fischer z values from each subject then were entered into t tests comparing each sample with a hypothesized mean of 0. Results were displayed in Fig. 3A via use of the “corrmap” function, which organizes variables using a K-nearest neighbor clustering algorithm (Eigenvector Research); minor modifications to this function allowed the display of t values in lieu of standard correlation coefficients. To determine significant correlations, the Holm-Bonferroni correction for multiple comparisons was used, yielding a critical t value of 4.351, P < 0.0004. Mean Fischer z values and corresponding t values for each pairwise correlation are reported in Fig. S1.
ICA.
A fixed-effects ICA was carried out to separate sources of variance across the 20 ROIs. First, within each ROI, the same samples of pretrial signal that were prepared for the cross-correlation analysis were concatenated across all runs of all subjects. Then principal component analysis was used to reduce the data to 7 components (using an eigenvalue cutoff of 1.0). Next, the FastICA algorithm was used to estimate the 7 ICs, using the deflation approach (30). Each of the ICs can be characterized in 2 ways. First, each of these sources has an estimated sample of pretrial values, much like each ROI. Second, each IC has contributions in varying degrees by pretrial activity from each ROI (i.e., the set of factor loadings).
To determine whether each IC predicted flexibility, the estimated samples of pretrial values were subjected to the primary regression analysis (i.e., pretrial signal was used as a predictor of RT on switch and repeat trials, and the resulting slope coefficients for each IC were compared via t tests).
Supplementary Material
Acknowledgments.
We thank J. Golomb for helpful comments and suggestions and T. Hickey and K. Martin for scanning assistance. This work was supported by National Institutes of Health Grants EY014193 and EY000785 (to M.M.C.), National Institutes of Health postdoctoral fellowship MH070115 (to A.B.L.), and a Natural Sciences and Engineering Research Council of Canada postgraduate scholarship (to N.B.T.-B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805423105/DCSupplemental.
An alternative account holds that these sources do not predict flexible switching between task rules but rather the degree of response priming, an effect that interacts with task switching (3). However, the relationship between pretrial signal and task-switch costs was not contingent on response repetition, thus ruling out this concern (Fig. S2).
References
- 1.Allport DA, Styles EA, Hsieh S. Shifting intentional set: Exploring the dynamic control of tasks. In: Umilta C, Moscovitch M, editors. Attention and Performance XV. Cambridge, MA: MIT Press; 1994. pp. 421–452. [Google Scholar]
- 2.Jersild AT. Mental set and shift. Archives of Psychology. 1927;(issue 89) [Google Scholar]
- 3.Rogers RD, Monsell S. Costs of a predictible switch between simple cognitive tasks. J Exp Psychol Gen. 1995;124:207–231. [Google Scholar]
- 4.De Jong R. An intention-activation account of residual switch costs. In: Monsell S, Driver J, editors. Control of Cognitive Processes: Attention and Performance XVIII. Cambridge, MA: MIT Press; 2000. pp. 357–376. [Google Scholar]
- 5.Nieuwenhuis S, Monsell S. Residual costs in task switching: Testing the failure-to-engage hypothesis. Psychon Bull Rev. 2002;9:86–92. doi: 10.3758/bf03196259. [DOI] [PubMed] [Google Scholar]
- 6.Bunge SA, Kahn I, Wallis JD, Miller EK, Wagner AD. Neural circuits subserving the retrieval and maintenance of abstract rules. J Neurophysiol. 2003;90:3419–3428. doi: 10.1152/jn.00910.2002. [DOI] [PubMed] [Google Scholar]
- 7.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 8.Brass M, von Cramon DY. Decomposing components of task preparation with functional magnetic resonance imaging. J Cognit Neurosci. 2004;16:609–620. doi: 10.1162/089892904323057335. [DOI] [PubMed] [Google Scholar]
- 9.Haynes JD, et al. Reading hidden intentions in the human brain. Curr Biol. 2007;17:323–328. doi: 10.1016/j.cub.2006.11.072. [DOI] [PubMed] [Google Scholar]
- 10.Sakai K, Passingham RE. Prefrontal interactions reflect future task operations. Nat Neurosci. 2003;6:75–81. doi: 10.1038/nn987. [DOI] [PubMed] [Google Scholar]
- 11.Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- 12.Gladwin TE, Lindsen JP, de Jong R. Pre-stimulus EEG effects related to response speed, task switching and upcoming response hand. Biol Psychol. 2006;72:15–34. doi: 10.1016/j.biopsycho.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Sakai K, Passingham RE. Prefrontal set activity predicts rule-specific neural processing during subsequent cognitive performance. J Neurosci. 2006;26:1211–1218. doi: 10.1523/JNEUROSCI.3887-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS. The role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci USA. 2000;97:13448–13453. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meiran N. Reconfiguration of processing mode prior to task performance. J Exp Psychol Learn Mem Cogn. 1996;22:1423–1442. [Google Scholar]
- 16.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 17.Cools R, Robbins TW. Chemistry of the adaptive mind. Philos Transact A: Math Phys Eng Sci. 2004;362:2871–2888. doi: 10.1098/rsta.2004.1468. [DOI] [PubMed] [Google Scholar]
- 18.Crofts HS, et al. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- 19.Dreisbach G, et al. Dopamine and cognitive control: The influence of spontaneous eye-blink rate and dopamine gene polymorphisms on perseveration and distractibility. Behav Neurosci. 2005;119:483–490. doi: 10.1037/0735-7044.119.2.483. [DOI] [PubMed] [Google Scholar]
- 20.Braver TS, Cohen JD. On the control of control: The role of dopamine in regulating prefrontal function and working memory. In: Monsell S, Driver J, editors. Control of Cognitive Processes: Attention and Performance XVIII. Cambridge, MA: MIT Press; 2000. pp. 713–737. [Google Scholar]
- 21.Dosenbach NUF, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowen FP, Kamienny RS, Burns MM, Yahr MD. Parkinsonism: Effects of levodopa treatment on concept formation. Neurology. 1975;25:701–704. doi: 10.1212/wnl.25.8.701. [DOI] [PubMed] [Google Scholar]
- 23.Cools AR, van den Bercken JH, Horstink MW, van Spaendonck KP, Berger HJ. Cognitive and motor shifting aptitude disorder in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1984;47:443–453. doi: 10.1136/jnnp.47.5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cools R, Barker RA, Sahakian BJ, Robbins TW. Mechanisms of cognitive set flexibility in Parkinsons disease. Brain. 2001;124:2503–2512. doi: 10.1093/brain/124.12.2503. [DOI] [PubMed] [Google Scholar]
- 25.Aron AR, et al. Task-set switching deficits in early-stage Huntington's Disease: Implications for basal ganglia function. J Cognit Neurosci. 2003;15:629–642. doi: 10.1162/089892903322307357. [DOI] [PubMed] [Google Scholar]
- 26.Frank MJ, O'Reilly RC. A mechanistic account of striatal dopamine function in human cognition: Psychopharmacological studies with cabergoline and haloperidol. Behav Neurosci. 2006;120:497–517. doi: 10.1037/0735-7044.120.3.497. [DOI] [PubMed] [Google Scholar]
- 27.Mehta MA, Manes FF, Magnolfi G, Sahakian BJ, Robbins TW. Impaired set-shifting and dissociable effects on tests of spatial working memory following the dopamine D 2 receptor antagonist sulpiride in human volunteers. Psychopharmacology (Berl) 2004;176:331–342. doi: 10.1007/s00213-004-1899-2. [DOI] [PubMed] [Google Scholar]
- 28.Cools R, Clark L, Robbins TW. Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. J Neurosci. 2004;24:1129–1135. doi: 10.1523/JNEUROSCI.4312-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crone EA, Wendelken C, Donohue SE, Bunge SA. Neural evidence for dissociable components of task-switching. Cereb Cortex. 2006;16:475–486. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- 30.Hyvarinen A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans Neural Netw. 1999;10:626–634. doi: 10.1109/72.761722. [DOI] [PubMed] [Google Scholar]
- 31.McKeown MJ, et al. Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp. 1998;6:160–188. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 33.Kerns JG, et al. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 34.Otten LJ, Quayle AH, Akram S, Ditewig TA, Rugg MD. Brain activity before an event predicts later recollection. Nat Neurosci. 2006;9:489–491. doi: 10.1038/nn1663. [DOI] [PubMed] [Google Scholar]
- 35.Turk-Browne NB, Yi DJ, Chun MM. Linking implicit and explicit memory: Common encoding factors and shared representations. Neuron. 2006;49:917–927. doi: 10.1016/j.neuron.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 36.Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 37.Kounios J, et al. The prepared mind: Neural activity prior to problem presentation predicts subsequent solution by sudden insight. Psychological Science. 2006;17:882–890. doi: 10.1111/j.1467-9280.2006.01798.x. [DOI] [PubMed] [Google Scholar]
- 38.Brainard DH. The psychophysics toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- 39.Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- 40.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme; 1988. [Google Scholar]
- 41.Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with Brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI II. Analysis. NeuroImage. 2001;13:218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- 43.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vincent JL, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.