Abstract
Photoinhibition, exacerbated by elevated temperatures, underlies coral bleaching, but sensitivity to photosynthetic loss differs among various phylotypes of Symbiodinium, their dinoflagellate symbionts. Symbiodinium is a common symbiont in many cnidarian species including corals, jellyfish, anemones, and giant clams. Here, we provide evidence that most members of clade A Symbiodinium, but not clades B–D or F, exhibit enhanced capabilities for alternative photosynthetic electron-transport pathways including cyclic electron transport (CET). Unlike other clades, clade A Symbiodinium also undergo pronounced light-induced dissociation of antenna complexes from photosystem II (PSII) reaction centers. We propose these attributes promote survival of most cnidarians with clade A symbionts at high light intensities and confer resistance to bleaching conditions that conspicuously impact deeper dwelling corals that harbor non-clade A Symbiodinium.
Keywords: coral bleaching, light-harvesting complexes, photoinhibition, Symbiodinium, cyclic electron transport
Corals and other cnidarians thrive in tropical, near-shore oceans due to intracellular symbioses with dinoflagellates of the genus Symbiodinium, which provide their hosts essential photosynthates (1, 2). Coral species distribution largely correlates with prevailing light and temperature gradients and likely is influenced by the differing photosynthetic and light tolerance properties of their symbionts coupled with host-symbiont specificity and biogeographical distribution (3–6). Hence, Symbiodinium phylotypes, predominantly clades A, B, and C and sometimes clade D and their respective hosts are found at characteristic depth ranges (4, 7). Clade A Symbiodinium are especially prevalent in shallow-water cnidarians including corals, jellyfish, and anemones in the Caribbean (4). Clade A isolates abundantly produce UV-protective mycosporine-like amino acids (MAAs) in culture whereas all clades, with a depth-dependent correlation, synthesize MAAs in hospice, especially mycosporine-glycine, which effectively absorbs harmful UV-B wavelengths (8). Little else is known about distinct physiological properties of Symbiodinium species and their susceptibilities to temperature and high light stress, although corals harboring clade D have been observed to be high-temperature tolerant (9).
Symbiodinium, like other photosynthetic organisms, are susceptible to photodamage of PSII (10). The resulting photoinhibition can lead to breakdown of symbioses and is an initial event in the onset of coral bleaching during episodes of elevated ocean temperatures (11). Differences in temperature sensitivity to photodamage (12) and rates of replenishment of the PSII D1 reaction center protein are documented in a few representatives of Symbiodinium in culture and in hospice (10, 12). Low degrees of fatty acid desaturation in thylakoid lipid membranes of cultured dinoflagellates have been correlated with elevated temperature tolerance and reduced production of reactive oxygen species under high irradiance, but there is not a strict correlation with heat sensitivity along Symbiodinium cladal lines (12).
Some corals bleach seasonally and others rarely (13), suggesting differential survival capabilities of the symbionts and their hosts when subject to environmental extremes. In the Caribbean, deeper water corals that commonly harbor clades B and C Symbiodinium are generally more susceptible to bleaching at elevated temperatures than are shallow-water corals in symbiosis with clade A (10), indicating that thermal and high irradiance tolerance can be interrelated. Photoprotection mechanisms identified in Symbiodinium include engagement of xanthophyll deepoxidation associated with nonphotochemical quenching (NPQ) within intrinsic light-harvesting complexes (LHCs) (14, 15) and longer-term photoacclimation processes that modulate the abundance and size of photosynthetic units and peripheral peridinin–chlorophyll-a–protein (PCP) antenna complexes (16, 17).
We show here that members of clade A Symbiodinium in culture and those inhabiting shallow-water cnidarians are conspicuously capable of PSII-independent CET. Evidence is also presented for the occurrence of chlororespiration, wherein oxygen serves as an alternative photosynthetic electron transport acceptor and is supplemental to CET. Both chlororespiration and CET can sustain ATP synthesis when PSII is inactivated (18). These studies employ a modified pulse amplitude modulated (PAM) fluorometry method that is easily applied in field studies. Clade A Symbiodinium are also found to readily undergo high light-induced dissociation of antenna complexes from PSII, particularly the “soluble” PCPs found only in dinoflagellates.
Conventional PAM fluorometry uses dark-adapted photosynthetic samples to assess PSII photosynthetic efficiency from minimal and maximal chlorophyll fluorescence levels. Emission from a weak modulated measuring light (ML) (F0) and maximal fluorescence (Fm) induced by a saturating light pulse estimates maximum quantum yield as Fv/Fm = Fm − F0. Typically, continuous actinic irradiation is subsequently imposed along with intermittent saturating flashes to monitor decreases of maximal fluorescence (Fm′) due to NPQ [(Fm − Fm′)/Fm′)]. We show a simple method to detect nonlinear photosynthetic pathways by measuring chlorophyll-a (chl-a) fluorescence in response to serial irradiation pulses (SIP) of 1-s duration without concurrent actinic irradiation.
Results and Discussion
Evidence for CET in clade A Symbiodinium by Using SIP Protocol.
When using the SIP method with dark-adapted symbiotic clade A Symbiodinium cells in culture and in hospice, incremental increases in the levels of F0 chl-a fluorescence are initially detected, reflecting progressive reduction of the electron acceptor pool for PSII, plastoquinone (PQ) (Fig. 1 A and C). SIP treatment clearly does not lead to reduction of PQ in clade B Symbiodinium (Fig. 1 B and D) indicating sustained linear electron transport activities between PSII and PSI in contrast to clade A cells. The lengthy duration of flashes and limited sensitivity of the instruments and detectors used enable only an approximation of Fm and Fm′ levels (19). However, the multiturnover excitation of PSII to drive water oxidation is beneficial to initiating and poising PQ in a reduced state as is apparent from clade A F0′ increases. As shown below, dissociation of PSII LHCs, particularly PCPs, lead to preferential PSI excitation in later phases of the clade A SIP response.
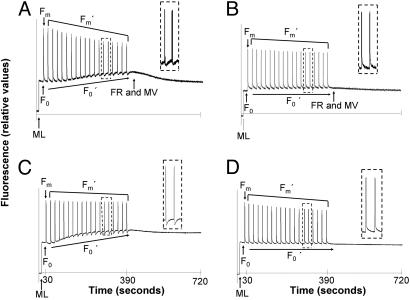
Fig. 1.
SIP detection of postillumination PQ reduction in clade A but not B Symbiodinium in culture and in hospice. Dark-adapted cultured cells and corals were subjected to saturating light pulses every 20 s after applying low-intensity ML. A progressive increase in F0′ levels reflect substantial reduction of the PQ pool in clade A3 cultured cells (A) but not in clade B1 cultured cells (B). (Insets) Light-induced PQ oxidation and dark rereduction between the 13th and 15th saturating pulses in clade A but not clade B phylotypes. (A and B) After clade A3 SIP treatment, 50 μM MV was used in combination with FR. (C and D) A Diving-PAM was used for SIP with dark-adapted corals. (C) Symbiodinium phylotype A4a in shallow-dwelling P. astreoides exhibits progressive PQ reduction and postillumination PQ reduction. (D) Fluorescence responses of M. faveolata harboring B1 Symbiodinium show no change with SIP.
When the PQ pool becomes significantly reduced in clade A samples at approximately the fifth flash and thereafter, abrupt F0′ decreases are then induced by each subsequent flash (Fig. 1A). These signals are attributed to transient PSI oxidation of the electron transport interchain, including PQ. During following dark phases, PQ reduction (F0′) then returns to levels above that of the prepulse signal with approximate half-times of ≈10 s in culture (Fig. 1A Inset) and 5 s in hospice (Fig. 1C Inset). A similar phenomenon has been described in a few other algae, but under conditions of limited electron acceptors and/or anaerobiosis (20). Such postillumination PQ reduction signals have also been observed after prolonged illumination of isolated spinach chloroplasts (21) and in leaves of C4 plants (22). The SIP response is significantly enhanced when clade A Symbiodinium are subjected to anaerobic conditions [supporting information (SI) Fig. S1]. Overall, the clade A fluorescence responses are ascribed to PSI-dependent plastoquinol oxidation followed by PQ rereduction by CET and/or chlororespiration (23, 24). The postillumination PQ reduction signal is likely to reflect only a small portion of CET activity that occurs during constant illumination.
Further evidence that PQ reduction/oxidation is PSI and CET-dependent in SIP-subjected clade A cells is that irradiation with far-red light (FR) and then administration of methyl viologen (MV), an artificial PSI acceptor, leads to decline of F0′ to the initial dark-adapted F0 level (Fig. 1A). Because MV interacts directly with PSI (17), it intercepts PSI electron transfer and downstream ferredoxin and NADPH-mediated processes, including ferredoxin-mediated CET through cytochrome b6/f and/or NAD(P)H-driven chlororespiration. The MV effect also indicates that PSII photodamage is not responsible for the SIP-induced F0′ increase.
Negative results of SIP treatments with clade B Symbiodinium certify the fluorescence phenomena in clade A are not an experimental artifact (Fig. 1 B and D). Also, measurements with clade A yield identical SIP responses when PAM fluorometry is used with settled cells in culture flasks (data not shown). Hence, the postillumination decrease and then increase of F0′ is not due to sample stirring and detector array configuration (25).
SIP Induced CET in Hospice.
The cultured clade A representative in Fig. 1A is of western Pacific origin (from the giant clam Tridacna gigas). Fig. 1C shows that clade A Symbiodinium from a Caribbean coral also exhibits diagnostic clade A SIP responses in hospice. Porites astreoides specimen were collected from shallow water (2 m), and their symbionts show an identical response to cultured clade A cells (Fig. 1A) when SIP-assayed with the submergible Diving-PAM (Fig. 1C). Subsequent genotyping by using PCR-denaturing gradient gel electrophoresis (DGGE) analysis of the rDNA ITS2 region (5) confirmed the predominance of subclade A4a symbionts. Symbionts of deeper dwelling P. astreoides consistently show no SIP-induced F0 fluctuations and harbor C1 Symbiodinium (4). Similarly, corals like Montastraea faveolata hosting subclade B1 Symbiodinium exhibit flat line F0′ and Fm′ when SIP-probed (Fig. 1D). We additionally surveyed many other species of cnidarians known to host clade A Symbiodinium in the Caribbean including corals Acropora cervicornis and Acropora palmata, anemones Aiptasia pallida and Bartholomea annulata, and the jellyfish Cassiopeia xamachana, as well as others known to host clades B and C. Employing SIP, only cnidarians harboring clade A Symbiodinium consistently display CET signals and flash-induced NPQ in hospice. Among cultured clade A, only subclade A2 isolates from a sea fan and a clam do not yield a CET SIP response (Table S1).
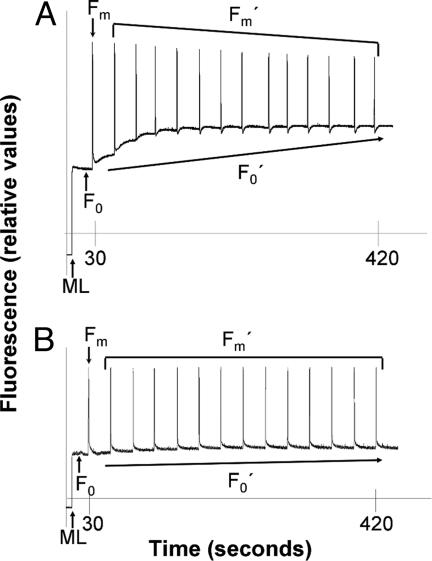
Porites furcata harbors multiple phylotypes of Symbiodinium in the Caribbean (4). A blind test was conducted by using SIP with P. furcata collected from a 1-m depth off Key Largo, Florida, revealing fluorescence patterns indicative of clade A, but not in this species when found at a 1.5-m depth (Fig. 2 A and B). Presence of the dominant phylotype A4a (in combination with B1) in the shallowest samples was confirmed by PCR-DGGE analysis of the rDNA ITS2 region. Thus, the SIP technique provides a noninvasive means for detecting the probable presence of clade A Symbiodinium and is potentially applicable to tracking symbiont genotypic and/or phenotypic fluctuations in the course of seasonal and long-term environmental change.
Fig. 2.
Detection of clade A Symbiodinium in coral hosts. SIP of dark-adapted P. furcata, from a 1-m depth, reveals a fluorescence pattern typical of clade A. (A) Genetic analysis by using DGGE revealed the presence of a combination of A4a and some B1 phylotypes of Symbiodinium. (B) Dark-adapted P. furcata from a 1.5-m depth, and found to harbor only B1 Symbiodinium, does not display a clade A specific fluorescence pattern SIP response.
SIP induces a marked decrease in Fm′ levels in clade A but not clade B Symbiodinium (Fig. 1 A and B) reflecting strong induction of NPQ and decreased light energy transfer to PSII. Analogous to model systems subjected to continuous illumination, this Fm′ decrease can follow from reduced cytochrome b6/f-dependent activation of chloroplast protein kinases to phospho rylate/dissociate PSII LHCs, conformational changes of LHCs by means of a large decrease of thylakoid lumen pH, activation of xanthophyll deepoxidase on lumen pH decline, or a combination of such excitation energy dissipation mechanisms (18). Dinoflagellates uniquely possess peripheral antennae PCP complexes, presumed to reside in the thylakoid lumen, that function to maximize green light energy transfer to PSII (26, 27). Shown below, dissociation of PCPs from PSII and possibly integral antennae complexes are conspicuous features of NPQ in clade A Symbiodinium.
Anaerobic Enhancement of Dark PQ Reduction and Engagement of NPQ in clade A Symbiodinium.
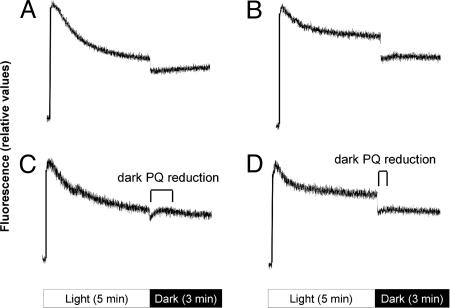
Clade A cells do not exhibit a transient PQ reduction/F0′ increase after termination of prolonged irradiation with moderate actinic light (250 μmol quanta m−2·s−1) and after NPQ approaches steady-state (Fig. 3A), likely due to activation of processes that divert PSI electron acceptors from CET such as carbon fixation, nitrate/sulfate reduction, the Mehler/ascorbate peroxidase pathways, or photorespiration. PSII-dependent oxygen evolution would promote the two latter pathways. We found that anaerobic conditions double the rate of SIP-induced PQ reduction (Fig. S1). Imposing anaerobic conditions (Fig. 3C), light-adapted clade A cells exhibit a postillumination F0′ increase with an overall half-time of 30 s. A similar signal, although weaker, is observed in clade B Symbiodinium but under both aerobic and anaerobic conditions (Fig. 3 B and D). Clearly, clade A Symbiodinium is capable of more complete engagement of NPQ as indicated by the fluorescence decrease in response to continuous illumination as compared with clade B cells. Additionally, substantial but gradual dark PQ reduction, likely due to partial engagement of a chlororespiration pathway, is discernable when the oxygen electron acceptor for photorespiration, chlororespiration, and/or pathways related to the Mehler reaction is diminished. Collapse of a transthylakoid pH gradient at the onset of darkness in clade B Symbiodinium and, partially in clade A cells, could also contribute to the F0′ postillumination increase.
Fig. 3.
Enhanced dark PQ reduction in anaerobic clade A Symbiodinium. After dark incubation for 10 min and supplementation with 10 mM bicarbonate to maximize carbon fixation, cultures of subclades A3 (A and C) and B2 (B and D) were bubbled with air (A and B) or argon (C and D) to impose aerobic and anaerobic conditions, respectively. Fluorescence decreases after onset of continuous actinic illumination reflect NPQ processes, which are almost complete after 5-min exposure to 250 μmol quanta m−2 s−1. During subsequent darkness, anaerobic clade A (C) and, to a lesser extent, both aerobic and anaerobic clade B exhibit PQ reduction (B and D).
Light-Induced Dissociation of PSII Antennae in clade A Symbiodinium.
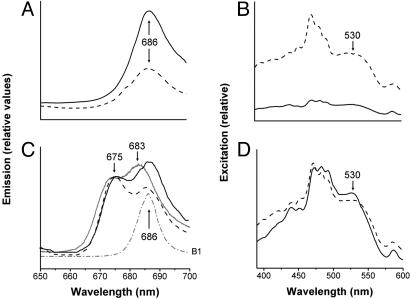
High light induction of NPQ is fairly well documented in Symbiodinium in culture and in hospice (11, 14, 15, 28). Only the initiation of xanthophyll deepoxidation to facilitate a NPQ thermal decay of excitation energy within integral LHC chl-a/c2 complexes has been documented (29). Other photoprotective processes akin to state transitions, in which LHCs dissociate from PSII, also have been theoretically deduced (30, 31). However, no definitive evidence for state transition-like mechanisms in Symbiodinium or other dinoflagellates have been documented. Fluorescence spectra at 77 K of dark-acclimated clade A Symbiodinium show that both intrinsic LHCs and extrinsic PCPs are fully connected to PSII as is apparent in the major fluorescence emission peak at 686 nm, presumably from the CP43 core antennae (Fig. 4A) (32). Excitation spectra of 686 nm show a strong contribution of PCP with novel peridinin absorption in the 530-nm region, along with the ≈400-nm Soret bands of chl (Fig. 4B). That PCPs are fully PSII-associated in dark-adapted cells is evidenced by minimal emission at 673 nm where a fluorescence shoulder from unassociated PCP chl-a would be selectively detected (Fig. 4B) (26, 33).
Fig. 4.
Light-Induced PCP and Intrinsic LHC Dissociation from PSII in clade A3 Symbiodinium. (A) The 77 K fluorescence emission spectra at excitation wavelengths 440 nm (solid line) and 530 nm (dashed line). (B) Excitation spectra of the same clade A3 cells detected at 673 nm (solid line) and 683 nm (dashed line) after 60-min dark adaptation. The dark-acclimated excitation spectrum, specifically the peridinin excitation shoulder at 530 nm, is higher at 683 nm than 673 nm as a result of the greater PSII connectivity of the PCP antenna complexes and resulting fluorescence. (C) Fluorescence emission spectra at excitation wavelengths of 440 nm (solid line) and 530 nm (dashed line) after exposure to 100 min of white light. The two emission peaks (675 and 686 nm) in both excitation spectra from white light-adapted clade A3 cells reflect partial dissociation of LHCs from PSII. Prolonged FR illumination (>690 nm) results in further loss of PSII 686 nm fluorescence, whereas 683-nm emission from PCPs and intrinsic LHCs is enhanced (solid line). No far-red-induced fluorescence shifts occur in clade B (dotted-dashed line). (D) Fluorescence excitation spectra of the same clade A3 cells as in C at detection wavelengths of 673 nm (solid line) and 683 nm (dashed line) after 100 min of actinic light exposure, showing a pronounced peridinin contribution to 675-nm emission by 530-nm excitation in comparison with that of dark-adapted cells in B.
After exposure to actinic white light (250 μmol m−2·s−1) for 100 min, fluorescence emission spectra of clade A cells exhibit dual peaks at 675 nm and 686 nm on preferential excitation of either chl (440 nm) or peridinin (530 nm) (Fig. 4C). The 675-nm peak is close to the PCP chl-a emission band of purified complexes (26, 27, 33). Peridinin, as a major contributor to the 675-nm emission peak, is further evidenced by an elevated 530 nm-induced excitation signal as compared with that obtained by the 440-nm excitation spectrum (Fig. 4D). We estimate from light/dark fluorescence spectral differences that almost half of the PCP population, perhaps together with integral LHCs dissociates from PSII as a major component of NPQ on prolonged light exposure in clade A Symbiodinium. No modifications of fluorescence emission spectra are detected in clade B and C Symbiodinium in response to these illumination conditions (Fig. S2).
Enhancement of fluorescence peaks at wavelengths longer than 700 nm would reflect intrinsic antenna translocation from PSII to PSI, the classical state transition earmark in green algal systems (34). In Symbiodinium, such a State 2 antenna association with PSI is not substantial (Fig. S2). When exposed to prolonged FR, the classical algal state transition response is antenna reversion from PSI to PSII connectivity (State 1) (35). Surprisingly, clade A cells respond to far-red irradiation differently, displaying dual fluorescence peaks at 675 nm and 683 nm, whereas 686-nm emission from PSII is minimized (Fig. 4C). The emergent 683-nm peak is best ascribed to disconnected intrinsic chl-a/c LHCs (33) antenna as evidenced by a chl-c contribution near 458 nm (27). The loss of 686-nm fluorescence could be due to diminished PSII integrity resulting from dissociation of core antenna CP43 in combination with the PSII disconnection of LHCs (Fig. 4 A and C). We attribute pronounced CET and cytochrome b6/f reduction as an underlying basis for the distinctive response of clade A Symbiodinium to far-red and high light illumination. Clades B and C exhibit no fluorescence changes as a consequence of far-red (Fig. 4C) or prolonged high light acclimation (Fig. S3).
Conclusions
Clade A is the most ancestral Symbiodinium phylotype (36). Most closely related subphylotypes found in shallow water appear to have evolutionarily retained constitutive CET/chlororespiration and PCP/LHC dissociation mechanisms. Moreover, when thermal perturbation of PSII photodamage and repair occurs, CET conceivably would sustain symbiont/host survival together with likely synergisms of xanthophyll deepoxidation contribution to NPQ and production of UV-protective MAAs.
Clades B, C, and D symbionts are analogous to shade plants because they are usually found in symbioses in deeper waters than clade A Symbiodinium and would benefit from enhanced light-harvesting capability. Some subclade B Symbiodinium are found in symbiosis with corals in shallow water but they likely employ photoprotection mechanisms other than antenna translocation under such circumstances. Symbiodinium clades B and C of deeper-dwelling corals, normally more susceptible to bleaching, can engage NPQ and varying degrees of chlororespiration (data not shown). However, kinetics and magnitude of their SIP-induced fluorescence patterns indicate they primarily sustain photosynthesis through linear photosynthetic electron transport and not CET.
Understanding Symbiodinium photoprotection and thermal tolerance mechanisms is crucial to predicting how coral-dinoflagellate symbioses will respond to increased ocean temperatures, irradiance conditions, and perhaps disease pressures. From the studies presented here, corals with clade A Symbiodinium are likely more resistant to combinations of high light and high temperature, conditions common at the end of summer (13), because of their enhanced and constitutive alternative photosynthetic electron pathways and photoprotection processes. CET and chlororespiration (37) may also serve to minimize oxygen evolution thereby decreasing the probability of the formation of and damage by reactive oxygen species. We propose that CET can sustain ATP synthesis and underlies clade A symbiont survival during warm conditions that can exacerbate PSII loss. Analogous to the case of PSII-less tobacco mutants (38), CET and/or chlororespiration could become dominant means for photosynthetic energy transduction during bleaching episodes. However, whether some non-clade A symbionts might conditionally develop CET and significant antenna dissociation capacity in response to elevated temperature deserves further study.
Materials and Methods
Symbiodinium Cultures and in Hospice.
Cultures of Symbiodinium were grown in ASP-8A medium (39) and maintained at 50 μmol quanta m−2·s−1 under cool white fluorescent lamps and at a constant temperature of 26°C under a 14:10-h light/dark photoperiod (16). Cells were harvested during exponential growth.
Coral samples gathered from Admiral Reef, Alligator Reef, or Little Grecian Reef in the northern Florida Keys included P. astreoides, M. faveolata, and P. furcata. Other samples were collected from Florida Bay directly offshore such as coral Siderastrea radians, anemones A. pallida and B. annulata and jellyfish C. xamachana (see Table 1 for a list of cnidarians analyzed). Specimen were immediately placed in fresh seawater and dark adapted for at least 60 min before SIP assays. Symbiodinium were genotyped by using PCR-DGGE analysis of the rDNA ITS2 region as described by LaJeunesse and Trench (40) and LaJeunesse (5).
SIP Fluorescence Analyses.
Chlorophyll fluorescence of cultured Symbiodinium was measured by using a 101/103 PAM fluorometer (Walz) with the ED-101US/MD optical unit and HPL-L470 blue light-emitting diode (LED) source for saturating flashes. A Hansatech LS2 halogen light source was used for actinic white light (250 μM quanta m−2·s−1) and when filtered, far-red irradiation (>690 nm). Culture samples were concentrated 10-fold by centrifugation, resuspended in fresh media, and dark-adapted for at least 20 min. Except for the comparison of anaerobic and aerobic experiments described below, cells were constantly stirred by using a cuvette mixer.
Cnidarian samples and cultured cells settled at the bottoms of culture flasks were assayed after dark-adaptation for at least 1-h (cnidarians) and 20 minutes (culture cells) with the submergible Diving-PAM (Walz), configured with a blue LED ML source. FR was not used for these samples but the fluorescence responses among clades were similar in cultures and in hospice specimens.
Baseline fluorescence (F0 and F0′) was monitored by a modulated ML (<1 μmol quanta m−2·s−1) to assess minimal fluorescence from PSII in dark periods. For SIP, maximal fluorescence (Fm and Fm′) was measured by administering a 1-s saturating flash of 470 nm of light (≈5000 μmol quanta m−2·s−1) every 20 s beginning at 30 s into the sampling period. When used, MV (1,1′-dimethyl-4,4′-bipyridium-dichloride) was added at a final concentration of 50 μM.
Anaerobic Measurements.
Cultures were incubated in the dark for 10 min in the presence of 10 mM bicarbonate to maximize carbon fixation rates during subsequent light treatments. The samples also were bubbled with air or argon without stirring to impose aerobic and anaerobic conditions, respectively, during the dark adaptation and fluorescence measurement periods.
Fluorescence Spectra.
Whole cell low-temperature (77 K) emission and excitation spectra were measured by using a SLM-Aminco SPF-500 spectrofluorometer. Cells were harvested and maintained for 100 min in darkness or 250 μM quanta m−2·s−1 actinic light. Immediately after treatment, samples were quickly suspended in 40% glycerol and frozen in liquid nitrogen in a custom glass Dewar. Emission spectra were measured with 440-nm or 530-nm excitation. Excitation spectra were measured at 673 nm or 683 nm and corrected for instrument response.
Supplementary Material
Acknowledgments.
We thank D. W. Kemp, D. J. Thornhill, and T. C. LaJeunesse for field and laboratory support and A. A. Reynolds for reviewing the manuscript. Research Funding was provided by National Science Foundation Grant OCE-0137007. J.M.R. is supported by a Dr. Nancy Foster Scholarship through the National Oceanic and Atmospheric Association.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805187105/DCSupplemental.
References
- 1.Stat M, Carter D, Hoegh-Guldberg O. The evolutionary history of Symbiodinium and scleractinian hosts - Symbiosis, diversity, and the effect of climate change. Perspect Plant Ecol Evol System. 2006;8:23–43. [Google Scholar]
- 2.Lesser MP. Experimental biology of coral reef ecosystems. J Exp Mar Biol Ecol. 2004;300:217–252. [Google Scholar]
- 3.Iglesias-Prieto R, et al. Different algal symbionts explain the vertical distribution of dominant reef corals in the eastern Pacific. Proc R Soc London Ser B. 2004;271:1757–1763. doi: 10.1098/rspb.2004.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LaJeunesse TC. Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar Biol. 2002;141:387–400. [Google Scholar]
- 5.LaJeunesse TC, et al. Low symbiont diversity in southern Great Barrier Reef corals, relative to those of the Caribbean. Limnol Oceanogr. 2003;48:2046–2054. [Google Scholar]
- 6.LaJeunesse TC, et al. Closely related Symbiodinium spp differ in relative dominance in coral reef host communities across environmental, latitudinal and biogeographic gradients. Mar Ecol Pro Ser. 2004;284:147–161. [Google Scholar]
- 7.Thornhill DJ, et al. Multi-year, seasonal genotypic surveys of coral-algal symbioses reveal prevalent stability or post-bleaching reversion. Mar Biol. 2006;148:711–722. [Google Scholar]
- 8.Banaszak AT, Santos MG, LaJeunesse TC, Lesser MP. The distribution of mycosporine-like amino acids (MAAs) and the phylogenetic identity of symbiotic dinoflagellates in cnidarian hosts from the Mexican Caribbean. J Exp Mar Biol Ecol. 2006;337:131–146. [Google Scholar]
- 9.Rowan R. Coral bleaching: Thermal adaptation in reef coral symbionts. Nature. 2004;430:742. doi: 10.1038/430742a. [DOI] [PubMed] [Google Scholar]
- 10.Warner ME, Fitt WK, Schmidt GW. Damage to photosystem II in symbiotic dinoflagellates: A determinant of coral bleaching. Proc Natl Acad Sci USA. 1999;96:8007–8012. doi: 10.1073/pnas.96.14.8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warner ME, Fitt WK, Schmidt GW. The effects of elevated temperature on the photosynthetic efficiency of zooxanthellae in hospite from four different species of reef coral: A novel approach. Plant Cell Environ. 1996;19:291–299. [Google Scholar]
- 12.Tchernov D, et al. Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc Natl Acad Sci USA. 2004;101:13531–13535. doi: 10.1073/pnas.0402907101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitt WK, McFarland FK, Warner ME, Chilcoat GC. Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnol Oceanogr. 2000;45:677–685. [Google Scholar]
- 14.Brown BE, et al. Diurnal changes in photochemical efficiency and xanthophyll concentrations in shallow water reef corals: Evidence for photoinhibition and photoprotection. Coral Reefs. 1999;18:99–105. [Google Scholar]
- 15.Warner ME, Berry-Lowe S. Differential xanthophyll cycling and photochemical activity in symbiotic dinoflagellates in multiple locations of three species of Caribbean coral. J Exp Mar Biol Ecol. 2006;339:86–95. [Google Scholar]
- 16.Iglesias-Prieto R, Trench RK. Acclimation and Adaptation to Irradiance in Symbiotic Dinoflagellates. I. Responses of the Photosynthetic Unit to Changes in Photon Flux-Density. Mar Ecol Prog Ser. 1994;113:163–175. [Google Scholar]
- 17.Schmidt GW, Matlin KS, Chua NH. A rapid procedure for selective enrichment of photosynthetic electron transport mutants. Proc Natl Acad Sci USA. 1977;74:610–614. doi: 10.1073/pnas.74.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niyogi KK. Photoprotection revisited: Genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:333–359. doi: 10.1146/annurev.arplant.50.1.333. [DOI] [PubMed] [Google Scholar]
- 19.Schreiber U, Endo T, Mi HL, Asada K. Quenching Analysis of Chlorophyll Fluorescence by the Saturation Pulse Method - Particular Aspects Relating to the Study of Eukaryotic Algae and Cyanobacteria. Plant Cell Physiol. 1995;36:873–882. [Google Scholar]
- 20.Xyländer M, Hagen C. ‘Low-waves’ in chlorophyll fluorescence kinetics indicate deprivation of bicarbonate. Photosynth Res. 2002;72:255–262. doi: 10.1023/A:1019864623049. [DOI] [PubMed] [Google Scholar]
- 21.Mano J, Miyaki C, Schreiber U. Photoactivation of the Electron Flow from NADPH to Plastoquinone in Spinach Chloroplasts. Plant Cell Physiol. 1995;36:1589–1598. [Google Scholar]
- 22.Takabayashi A, et al. Differential use of two cyclic electron flows around photosystem I for driving CO2-concentration mechanism in C4 photosynthesis. Proc Natl Acad Sci USA. 2005;102:16898–16903. doi: 10.1073/pnas.0507095102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larcher W, Neuner G. Cold-induced sudden reversible lowering of in vivo chlorophyll fluorescence after saturating light-pulses - a sensitive marker for chilling susceptibility. Plant Physiol. 1989;89:740–742. doi: 10.1104/pp.89.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuyama M, Shibata M, Kawazu T, Kobayashi Y. An analysis of the mechanism of the low-wave phenomenon of chlorophyll fluorescence. Photosynth Res. 2004;81:67–76. doi: 10.1023/B:PRES.0000028394.60328.b5. [DOI] [PubMed] [Google Scholar]
- 25.Cosgrove J, Borowitzka M. Applying Pulse Amplitude Modulation (PAM) fluorometry to microalgae suspensions: Stirring potentially impacts fluorescence. Photosynth Res. 2006;88:343–350. doi: 10.1007/s11120-006-9063-y. [DOI] [PubMed] [Google Scholar]
- 26.Iglesias-Prieto R, Govind NS, Trench RK. Isolation and characterization of three membrane-bound chlorophyll-protein complexes from four dinoflagellate species. Philos Trans R Soc London Ser B. 1993;340:381–392. [Google Scholar]
- 27.Polivka T, et al. Energy Transfer in the Major Intrinsic Light-Harvesting Complex from Amphidinium carterae. Biochemistry. 2006;45:8516–8526. doi: 10.1021/bi060265b. [DOI] [PubMed] [Google Scholar]
- 28.Robison JD, Warner ME. Differential impacts of photoacclimation and thermal stress on the photobiology of four different phylotypes of Symbiodinium (Pyrrhophyta) J Phycol. 2006;42:568–579. [Google Scholar]
- 29.Venn AA, et al. The impact of coral bleaching on the pigment profile of the symbiotic alga, Symbiodinium. Plant Cell Environ. 2006;29:2133–2142. doi: 10.1111/j.1365-3040.2006.001587.x. [DOI] [PubMed] [Google Scholar]
- 30.Hill R, Frankart C, Ralph PJ. Impact of bleaching conditions on the components of non-photochemical quenching in the zooxanthellae of a coral. J Exp Mar Biol Ecol. 2005;322:83–92. [Google Scholar]
- 31.Jones RJ, Hoegh-Guldberg O. Diurnal changes in the photochemical efficiency of the symbiotic dinoflagellates (Dinophyceae) of corals: Photoprotection, photoinactivation and the relationship to coral bleaching. Plant Cell Environ. 2001;24:89–99. [Google Scholar]
- 32.Shen G, Vermaas WF. Chlorophyll in a. Synechocystis sp PCC 6803 mutant without photosystem I and photosystem II core complexes Evidence for peripheral antenna chlorophylls in cyanobacteria. J Biol Chem. 1994;269:13904–13910. [PubMed] [Google Scholar]
- 33.Iglesias-Prieto R, Trench RK. Spectroscopic properties of chlorophyll. a in the water-soluble peridinin chlorophyll a protein complexes (PCP) from the symbiotic dinoflagellate Symbiodinium microadriaticum. J Plant Physiol. 1996;149:510–516. [Google Scholar]
- 34.Takahashi H, Iwai M, Takahashi Y, Minagawa J. Identification of the mobile light-harvesting complex II polypeptides for state transitions in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 2006;103:477–482. doi: 10.1073/pnas.0509952103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kargul J, Barber J. Photosynthetic acclimation: Structural reorganisation of light harvesting antenna - role of redox-dependent phosphorylation of major and minor chlorophyll a/b binding proteins. FEBS J. 2008;275:1056–1068. doi: 10.1111/j.1742-4658.2008.06262.x. [DOI] [PubMed] [Google Scholar]
- 36.LaJeunesse TC. Investigating the biodiversity, ecology, and phylogeny of endosymbiotic dinoflagellates in the genus. Symbiodinium using the ITS region: In search of a “species” level marker. J Phycol. 2001;37:866–880. [Google Scholar]
- 37.Peltier G, Schmidt GW. Chlororespiration: An adaptation to nitrogen deficiency in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 1991;88:4791–4795. doi: 10.1073/pnas.88.11.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baena-Gonzalez E, et al. Deletion of the tobacco plastid psbA gene triggers an upregulation of the thylakoid-associated NAD(P)H dehydrogenase complex and the plastid terminal oxidase (PTOX) Plant J. 2003;35:704–716. doi: 10.1046/j.1365-313x.2003.01842.x. [DOI] [PubMed] [Google Scholar]
- 39.Blank RJ. Presumed gametes of Symbiodinium: Feintings by a fungal parasite? Endocytobiosis Cell Res. 1987;4:297–304. [Google Scholar]
- 40.LaJeunesse TC, Trench RK. Biogeography of two species of Symbiodinium (Freudenthal) inhabiting the intertidal sea anemone Anthopleura elegantissima (Brandt) Biol Bull. 2000;199:126–134. doi: 10.2307/1542872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.