Abstract
A neuropeptide, calcitonin gene-related peptide (CGRP), is widely distributed in neuronal systems and exhibits numerous biological activities. Using CGRP-knockout mice (CGRP−/−), we examined whether or not endogenous CGRP facilitates angiogenesis indispensable to tumor growth. CGRP increased tube formation by endothelial cells in vitro and enhanced sponge-induced angiogenesis in vivo. Tumor growth and tumor-associated angiogenesis in CGRP−/− implanted with Lewis lung carcinoma (LLC) cells were significantly reduced compared with those in wild-type (WT) mice. A CGRP antagonist, CGRP8-37 or denervation of sciatic nerves (L1–5) suppressed LLC growth in the sites of denervation compared with vehicle infusion or sham operation. CGRP precursor mRNA levels in the dorsal root ganglion in LLC-bearing WT were increased compared with those in non-LLC-bearing mice. This increase was abolished by denervation. The expression of VEGF in tumor stroma was down-regulated in CGRP−/−. These results indicate that endogenous CGRP facilitates tumor-associated angiogenesis and tumor growth and suggest that relevant CGRP may be derived from neuronal systems including primary sensory neurons and may become a therapeutic target for cancers.
Keywords: knockout mice, neuropeptide, CGRP antagonist, sensory nerve
Functional and morphological relationships between primary afferent neurons and blood vessels during cancer development are poorly understood. However, recent evidence suggests that physical and biochemical interactions between these peripheral components are important to both tumor biology and cancer-associated pain (1). Postnatal neovascularization occurs through (i) capillary sprouting of resident endothelial cells (EC), angiogenesis; (ii) arteriogenesis; and (iii) vasculogenesis, a process thought to be peculiar to the embryo but possibly observable also in adults (2, 3). It is widely known that the neuronal system plays a fundamental role in the maturation of primitive embryonic vasculature. Mutations that disrupt peripheral sensory nerves or Schwann cells prevent proper arteriogenesis, whereas those that disorganize the nerves maintain the alignment of arteries with misrouted axons (4). It has also been reported that sensory neurons modulate the expression of arterial markers on ECs via the secretion of vascular endothelial growth factor (VEGF) 164/120. These data suggest that during development, peripheral nerves have a role in determining the organ-specific patterns of blood vessel branching and arterial maturation (5). Furthermore, some neuronal factors such as Notch and neuropeptide Y were reported to have roles in tumor-associated angiogenesis (6, 7).
Pain is the most disruptive influence on the quality of life (QOL) of cancer patients (1). Primary afferent sensory neurons exhibit a significant role in which sensory information from peripheral tissues is transmitted to the spinal cord and brain. The cell bodies of the sensory nerve fibers that innervate the head and body are located in the trigeminal ganglia and dorsal root ganglia and can be divided into two main categories: myelinated A-fibers, and thinner unmyelinated C-fibers. Thin sensory fibers, C-fibers and Aδ-fibers, are specialized sensory neurons known as nociceptors, the main function of which is to detect environmental stimuli. Nociceptors express a diverse repertoire of receptors and transduction molecules that can sense forms of noxious stimulation (thermal, mechanical, and chemical) with varying degrees of sensitivity (8).
In addition to cancer cells, tumors consist of stromal tissues including adjacent primary afferent nociceptors. Cancer cells and stromal cells release a variety of products, such as ATP, bradykinin, H+, nerve growth factor, prostaglandins, and VEGF, which either excite or sensitize the nociceptor (1). Painful stimuli are detected by the nociceptors, the cell bodies of which lie in the dorsal root ganglion (DRG), and are transmitted to neurons in the spinal cord. The signal is then transmitted to the higher centers of the brain. Nociceptor activation results in the release of neurotransmitters, such as calcitonin gene-related peptide (CGRP), endothelin, histamine, glutamate, and substance P (1). These sensory nerve-derived mediators have vasodilating actions, and as a result of these, the blood supply to the tumor tissues may be increased.
CGRP, a 37-amino acid neuropeptide, has various biological actions, including responses to sensory stimuli, cardiovascular regulation, and vasodilation (9). Recent studies using genetically engineered mice have shown that CGRP-knockout mice exhibit increased blood pressure (10). CGRP is synthesized by sensory C-fibers throughout the respiratory tree and potently constricts airway smooth muscle (11). The tissues contained considerably more CGRP than another neuropeptide, substance P (12, 13). We reported that CGRP also exhibited important roles in the gastrointestinal tract (14, 15). Maintenance of gastric mucosal integrity is highly dependent on the alarm systems that can rapidly sense the harmful chemical or mechanical stimuli to which the mucosa is exposed. The gastrointestinal tract is known to be rich in neuronal systems, among which afferent neurons of extrinsic origin are reported to operate as an emergency protective system (16). The functions of these afferents sensitive to chemicals are reported to be mediated by CGRP released in the gastric mucosa (16, 17). It was reported that a neuropeptide, substance P, had a proangiogenic activity (18), although the details of the mechanism of this proangiogenic activity were not studied precisely. It was reported that CGRP regulates the expression of VEGF in human HaCaT keratinocytes by activation of ERK1/2 MAPK (19) and that CGRP expression and neoangiogenesis are intensified in mixed invasive–preinvasive breast lesions of carcinoma (20). These suggested that CGRP may regulate tumor-associated angiogenesis.
In the present work, we first tested whether or not CGRP could enhance angiogenesis in vitro. To test the proangiogenic activity in vivo, we have developed a strain of knockout mice in which the genes for CGRP are disrupted (CGRP−/−) (10). The present work, using these mice, can therefore address the important roles of endogenous CGRP in enhancement of tumor-associated angiogenesis when CGRP release was stimulated. Further, we clarified whether or not CGRP released as a result of axon reflex of primary afferent neurons could enhance tumor-associated angiogenesis. The present work addresses the significance of CGRP as a target for solid tumor treatment and provides the concept that the blockade of primary afferent neurons may be of benefit not only in the prevention of cancer pain but also in the inhibition of tumor-associated angiogenesis that is indispensable to tumor growth.
Results and Discussions
Proangiogenic Activity of CGRP.
We tested first in the present study whether CGRP exhibits or not the proangiogenic activity in an in vitro culture model [supporting information (SI) Calcitonin Gene-Related Peptide CGRP Tube Formation and Fig. S1]. CGRP exhibits the tube formation activities when determined using human umbilical vein endothelial cells (HUVECs) cocultured with fibroblasts. We further verified with the surgical sponge model that CGRP has a significant role in angiogenesis in vivo (CGRP and Angiogenesis, in SI Text and Fig. S2). Sciatic unilateral and bilateral denervations suggested that the factors from neuronal systems including CGRP have a proangiogenic activity. Reduced angiogenesis in CGRP−/− suggested that endogenous CGRP has a significant role in enhancement of angiogenesis.
Reduced Tumor-Associated Angiogenesis and Tumor Growth in CGRP−/−.
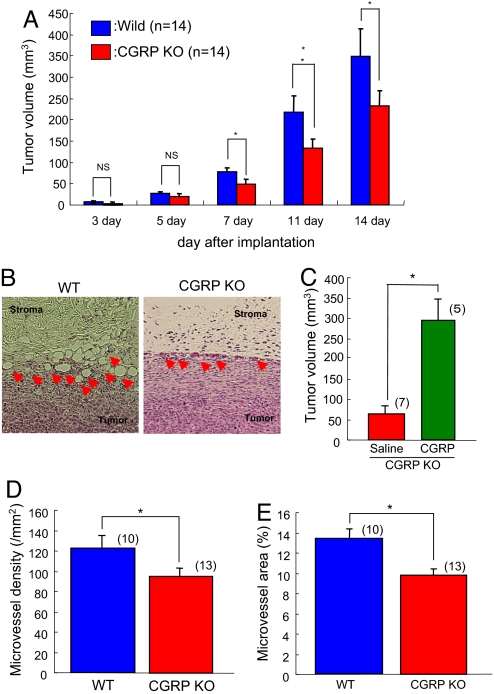
To elucidate the significance of roles of CGRP supplied from the host, Lewis lung carcinoma (LLC) cells were implanted into the dorsal s.c. tissues in wild-type (WT) and CGRP−/−. When tumor mass was determined (Fig. 1A), tumor growth in CGRP−/− after day 7 was significantly reduced compared with that in WT. The reductions in tumor volume in CGRP−/− were also seen 11 days and 14 days after implantation. Fourteen days after implantation, neovascularization in CGRP−/− assessed under histologic examination was suppressed compared with that in WT (Fig. 1B). When tumor growth in CGRP−/− mice infused with CGRP continuously (0.2 nmol/h) using miniosmotic pumps was estimated, CGRP supplementation certainly enhanced tumor growth (Fig. 1C). Quantitative analysis of microvessel density (MVD) and microvessel area (MVA) revealed that tumor-associated angiogenesis in CGRP−/− was significantly reduced in comparison with that in WT 14 days after implantation (Fig. 1 D and E). These results suggested that endogenous CGRP derived from the host has a significant role in tumor-associated angiogenesis and tumor growth.
Fig. 1.
Reduced tumor-associated angiogenesis and tumor growth in CGRP-knockout (KO) mice. (A) Tumor growth after implantation in CGRP-knockout mice and their WT counterparts. LLC cells were implanted s.c. Results were compared with growth in WT counterparts on the same day and are mean ± SEM from 14 animals. ANOVA was used. *, P < 0.05. NS, not significant. (B) Typical H&E staining of tumors (day 14) in CGRP-KO mice (Left) and their WT counterparts (Right). Arrowheads indicate newly formed blood vessels. (C) Tumor growth in CGRP−/− infused with CGRP continuously (0.1 nmol/h) by using miniosmotic pumps. LLC cells were implanted s.c. to the site of CGRP infusion. Results were compared with growth in vehicles-infused CGRP−/− on day 7 and are mean ± SEM from 5∼7 animals. Student's t test was used. *, P < 0.05. (D) In the histologic tumor samples isolated from the mice 14 days after implantation, microvessel density was determined. Results from the sham operation group (blue column) were compared with those of denervation (red column) and are mean ± SEM from 10∼13 animals. Student's t test was used. *, P < 0.05. (E) Microvessel area was determined in the same specimen as in C. Results from the CGRP-KO mice (red column) were compared with those of WT (blue column) and are mean ± SEM from 10∼13 animals. Student's t test was used. *, P < 0.05.
Effects of Continuous Infusion of a CGRP Antagonist on Tumor Growth and Tumor-Associated Angiogenesis.
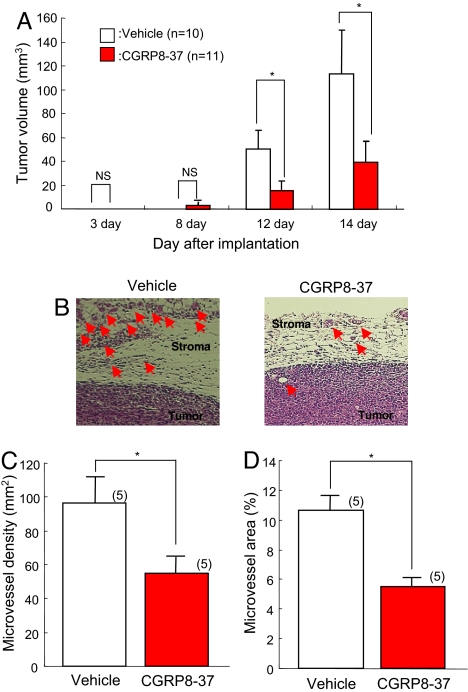
When LLC cells were implanted s.c. to WT, gradual tumor growth was observed, as shown in the open columns in Fig. 2A. Subcutaneous infusion of a CGRP antagonist, CGRP8-37, or vehicle solution with miniosmotic pumps from 1 day before tumor implantation suppressed tumor growth for 12 and 14 days after LLC implantation when substantial tumor growth was observed in vehicle-infused WT (Fig. 2A). Histologic examination 14 days after implantation revealed that neovascularization was observed predominantly in the stroma of the implanted tumors and that that in CGRP antagonist-treated mice was suppressed compared with vehicle-treated WT (Fig. 2B; arrowheads indicate newly formed blood vessels). In the histologic samples isolated from the tumors 14 days after implantation, the determined MVD and MVA were reduced in CGRP antagonist-treated WT compared with those in vehicle-infused WT (Fig. 2 C and D). These findings indicate that the results from CGRP−/− were essentially the same as those performed in the present experiment using a CGRP1 antagonist. CGRP1 signaling is known to activate adenylate cyclase to increase intracellular cAMP levels and enhances VEGF expression and angiogenesis in a sponge model (21). The intracellular cAMP levels may synergistically enhance the angiogenesis induced by prostaglandins because proangiogenic prostaglandin receptor signaling is closely related to the elevation of cAMP (21).
Fig. 2.
Effects of continuous s.c. infusion of CGRP antagonist on tumor growth and tumor-associated angiogenesis in an LLC implantation model. (A) Tumor growth after implantation. LLC cells were implanted s.c. into WT. Subcutaneous infusion of a CGRP antagonist, CGRP8-37, with miniosmotic pumps (2 nmol/h) suppressed tumor growth 12 and 14 days after implantation (red column). Results were compared with those from vehicle-infused mice and are mean ± SEM from 10∼11 animals. ANOVA was used. *, P < 0.05. NS, not significant. (B) Typical H&E staining of tumor receiving vehicle (Left) or CGRP antagonist (Right). Arrowheads indicate newly formed blood vessels. (C) In the histologic tumor samples isolated from the mice 14 days after implantation, microvessel density was determined. Results obtained with the CGRP antagonist (red column) were compared with those obtained with vehicle and are mean ± SEM from 5 animals. Student's t test was used. *, P < 0.05. (D) Microvessel area was determined in the same specimen as in C. Results from CGRP antagonist (red column) were compared with those from vehicle and are mean ± SEM from 5 animals. Student's t test was used. *, P < 0.05.
Effect of Sciatic Nerve Denervation on Tumor Growth and Tumor-Associated Angiogenesis.
Next we examined the source of endogenous CGRP that facilitates tumor-associated angiogenesis. To block release of the CGRP delivered by axonal transport from the peripheral neurones, we cut the sciatic nerves at the distal parts from the sciatic dorsal ganglions in WT. Seven days after the operation, LLC cells were implanted s.c. at the denervated site. Growth of the tumor in the denervated site (Fig. 3A) was suppressed compared with that in the sham-operated site. Fourteen days after implantation, neovascularization in denervated WT was suppressed compared with that in the sham-operated WT (Fig. 3B). Quantitative analysis of MVD and MVA as markers for angiogenesis revealed that tumor-associated angiogenesis in denervated WT was significantly reduced compared with that in sham-operated WT (Fig. 3 C and D). These results suggested that CGRP released from the peripheral nerve endings may stimulate the proangiogenic activities. Axon reflex is an event that was brought about by the passage of nerve impulses from sensory nerve endings to the effector organ along divisions of the nerve fibers without traversing a synapse (22). Axon reflex elicited by nociceptive stimulation may be closely related to the increased release of CGRP from nerve endings. It has been suggested that the axon reflex in human skin is a response neurally mediated by C-fiber nociceptors, which have been shown to stimulate vasodilatation via the release of CGRP and substance P (23). The present results suggested that axon reflex-mediated CGRP release may be a key event involved in facilitation in tumor-associated angiogenesis. The interesting phenotype of tumors seen during blockade of CGRP or CGRP release was the reduced formation of stromal tissues (Figs. 1B, 2B, and 3B). Because the stromal cells may be derived from bone marrow, it is plausible that CGRP regulates some signaling relevant to the recruitment of stromal cells. This may become a target for controlling tumor-associated angiogenesis.
Fig. 3.
Effect of sciatic nerve denervation on tumor growth and tumor-associated angiogenesis in an LLC implantation model. (A) Tumor growth after implantation. LLC cells were implanted s.c. into WT. Sciatic nerve denervation was performed as described in Materials and Methods. Results were compared with those from sham-operated mice and are mean ± SEM from 9∼11 animals. ANOVA was used. *, P < 0.05. NS, not significant. (B) Typical H&E staining of a tumor (day 14) after sham operation (Left) or denervation (Right). Arrowheads indicate newly formed blood vessels. (C) In the histologic tumor samples isolated from the mice 14 days after implantation, microvessel density was determined. Results from the sham-operation group (blue column) were compared with those after denervation (red column) and are mean ± SEM from 4∼6 animals. Student's t test was used. *, P < 0.05. (D) Microvessel area was determined in the same specimen as in C. Results from the sham operation group (blue column) were compared with those after denervation (red column) and are mean ± SEM from 4∼6 animals. Student's t test was used. *, P < 0.05. (E) Effects of sciatic nerve denervation on pro-CGRP mRNA levels in DRGs in tumor-bearing mice. Tumor implantation to the area innervated by L1–5 resulted in increased expression of pro-CGRP in sham-operated WT (blue column). Sciatic nerve denervation reduced expression of pro-CGRP (red column). Mean ± SEM from 4∼11 animals is shown. ANOVA was used. *, P < 0.05.
Effects of Sciatic Nerve Denervation on Pro-CGRP mRNA Levels in DRGs in Tumor-Bearing Mice.
To investigate increase in CGRP release during tumor growth, we determined the mRNA levels of pro-CGRP, a precursor of CGRP, in DRGs (L1–5) in tumor-bearing WT because CGRP was reported to be up-regulated, and the pro-CGRP mRNA levels in the DRGs rose in the case of peripheral inflammation (24, 25). As Fig. 3E shows, tumor implantation to the area innervated by L1–5 resulted in the increased expression of pro-CGRP in sham-operated WT compared with that in sham-operated WT without tumor implantation. By contrast, when sensory nerves were cut at the distal site of DRGs, tumor implantation did not increase the expression of pro-CGRP in DRGs even with LLC tumors. These findings suggested that tumor implantation up-regulated pro-CGRP in the DRGs innervating the area of implantation and increased CGRP release and that CGRP was synthesized in the neuronal systems and was delivered to the periphery of the nerves innervating the sites where tumors grow. When inflammation was induced, CGRP was reported to be up-regulated, and the pro-CGRP mRNA levels in the DRGs that innervate the sites of inflammation rose (24, 25). This may have been caused by the increased release of CGRP that was stimulated by the implanted tumors. The present findings suggested that tumor implantation up-regulated pro-CGRP in the DRGs that innervated the area of tumor implantation and that the element responsible for tumor angiogenesis may be primary afferent neurons that can sense the nociception.
Expressions of Growth Factors in Tumor Stromal Tissues in CGRP-Knockout Mice.
When LLC cells were implanted in WT, daily administration of VEGF receptor tyrosine kinase inhibitor ZD6474 for 2 weeks suppressed the growth of tumors markedly (Fig. 4A), suggesting that the LLC tumor growth observed in the present study was highly dependent on the VEGF expressed. The same was true in tumor-associated angiogenesis (Fig. 4B). Real-time PCR analysis on VEGF in tumor tissues including stroma revealed the significant reduction in VEGF mRNA levels in CGRP−/− (Fig. 4C). The expressions in bFGF, CTGF, and TGF-β were not significantly reduced in CGRP-knockout mice (Fig. 4 D–F). Immunohistochemical localization of VEGF shows that the expression of VEGF in the surrounding stromal tissues isolated 2 weeks after implantation was markedly suppressed in CGRP−/− (Fig. 4G), although that in tumor cells was not different between WT and CGRP−/−. These results taken together suggested that the downstream molecule relevant to CGRP-dependent enhancement of angiogenesis is VEGF.
Fig. 4.
Expressions of growth factors in tumor stromal tissues in CGRP-knockout mice. (A) VEGF dependence in LLC tumor growth used in the present work. Daily administration of the VEGF receptor tyrosine kinase inhibitor ZD6474 for 2 weeks suppressed the growth of LLC tumors. Results were compared with vehicle-treated mice and are mean ± SEM from 6∼10 animals. Student's t test was used. **, P < 0.05. (B) VEGF dependence in angiogenesis in LLC tumors. Daily administration of ZD6474 for 2 weeks suppressed angiogenesis. Results were compared with vehicle-treated mice and are mean ± SEM from 6∼10 animals. Student's t test was used. **, P < 0.05. (C) Reduced expressions of VEGF in tumor stromal tissues in CGRP-knockout mice 2 weeks after LLC implantation. Real-time PCR was performed as described in Materials and Methods. Results were compared with WT mice and are mean ± SEM from 7∼9 animals. Student's t test was used. **, P < 0.05. (D) Expression of bFGF in tumor stromal tissues. Experimental conditions were the same as those in C. NS, not significant. (E) Expression of CTGF in tumor stromal tissues. Experimental conditions were the same as those in C. (F) Expression of TGF-β in tumor stromal tissues. Experimental conditions were the same as those in C. (G) Immunohistochemical localization of VEGF in tumor tissues including stroma. The samples were isolated from CGRP−/− and their WT counterparts.
CGRP, a 37-amino acid neuropeptide, has various biological actions, including responses to sensory stimuli, cardiovascular regulation, and vasodilation (9). Recent studies using genetically engineered mice have shown that CGRP-knockout mice exhibit increased blood pressure (10). CGRP is synthesized by sensory C-fibers throughout the respiratory tree and potently constricts airway smooth muscle (11). The tissues contained considerably more CGRP compared with substance P (12, 13). We reported that CGRP also exhibited important roles in the gastrointestinal tract (14, 15). Maintenance of gastric mucosal integrity is highly dependent on the alarm systems that can rapidly sense the harmful chemical or mechanical stimuli to which the mucosa is exposed (16). The gastrointestinal tract is known to be rich in neuronal systems, among which afferent neurons of extrinsic origin are reported to operate as an emergency protective system (16). The functions of these afferents sensitive to chemicals are reported to be mediated by CGRP released in the gastric mucosa (16).
It was reported that a neuropeptide, substance P, had a proangiogenic activity (18). We showed here that another neuropeptide, CGRP, certainly has tube-forming activity in coculture systems, although the potency of CGRP was less than that of VEGF, the most potent inducer of angiogenesis (Fig. S1A). Because VEGF-neutralizing antibody suppresses CGRP-induced tube formation (Fig. S1A), VEGF may work as a downstream molecule. These results were consistent with the previous reports using human HaCaT keratinocytes (19) and breast cancer cells (20). In the present experiment, we can also show that CGRP exerts a crucial action in enhancement of angiogenesis (Figs. 1 and 2). Further, CGRP relevant to tumor-associated angiogenesis may be derived from the neuronal systems judging from the results from denervation experiments (Fig. 3).
Cancer pain is a critical determinant of the patient's QOL. The negative impact that cancer pain has on QOL cannot be overestimated. Because advances in cancer detection and therapy are extending the life expectancy of cancer patients, there is an increasing focus on improving patients' QOL. For many patients, pain is the first sign of cancer, and 30–50% of all cancer patients will experience moderate to severe pain (26). Cancer can cause pain at any time during its course, but the frequency and intensity of pain tend to increase during the advanced stages. In fact, 75–95% of patients with metastatic or advanced-stage cancer will experience significant amounts of cancer-induced pain (27). Neuronal system-derived CGRP was believed to promote tumor growth by vasodilatation to increase the supply of nutrients for tumors (1). But, as described in the present work, CGRP may increase the density of the newly formed blood vessels as a result of angiogenesis, which may be an activity of the neuropeptide CGRP.
In conclusion, we demonstrated that CGRP exerts a crucial action in enhancement of angiogenesis. We found that the tumor growth and tumor-associated angiogenesis of implanted LLC cells in CGRP-knockout mice were significantly reduced compared with WT mice. CGRP8-37 can block the tumor-associated angiogenesis and tumor growth, indicating that the development of CGRP receptor antagonists is highly promising. CGRP relevant to tumor-associated angiogenesis may be derived from the neuronal systems judging from the results from denervation experiments in which the sciatic nerves and the femoral nerves were cut. VEGF expression was up-regulated in the tumor implantation models in a CGRP-dependent manner, suggesting that VEGF is a downstream molecule of CGRP signaling. These results taken together indicate that CGRP released from the neuronal systems facilitates tumor-associated angiogenesis and tumor growth and that CGRP together with neuronal system blockade may become a therapeutic target for cancers. If the present results can be extrapolated to the human cancers, some drugs against pain or anesthesia therapy on cancer patients certainly decrease tumor growth in vivo via suppression of CGRP system. This possibility will provide a conceptional advance in cancer treatment.
Materials and Methods
Estimation of Angiogenesis in Tumor Implantation Models.
LLC cells (1 × 106 cells per mouse) were implanted in CGRP−/− and WT mice as reported (28). A CGRP antagonist, CGRP8-37 (Peptide Institute) in the physiological saline was infused into (2 nmol/h) the s.c. tissues of the backs of mice by using osmotic pumps (Alzat). The delivery rate was 0.25 μl/h, and the mice received an antagonist every few days for 14 days. One day after the operation, LLC cells were implanted in the vicinity of the osmotic pumps. In some CGRP−/−, CGRP was infused with the same pumps (0.2 nmol/h). The left sciatic nerve distal to the DRG was removed as described above. Seven days after the unilateral axotomy, LLC cells were implanted, and the growth and angiogenesis were observed as stated above.
Denervation of Sciatic Nerves.
Unilateral or bilateral denervation of the sciatic nerve was performed in mice under pentobarbital sodium anesthesia (25 mg/kg). The sciatic nerve was exposed through a gluteus muscle incision, and 5 mm of the sciatic nerve distal to the DRG was removed. The operated region was marked with a polypropylene suture (6-0; Ethicon) with the aid of an operating microscope. Sham-operated mice were prepared as controls with the same operation but without the sciatic nerve axotomy.
Determination of Pro-CGRP mRNA Levels in DRGs in Real-Time PCR.
The medulla spinalis was removed, and the DRGs of the L1–5 levels were isolated. DRG tissues were homogenized in 1 ml of TRIzol reagent (GIBCO). A sample of RNA was extracted from the tissue according to the manufacturer's instructions. Real-time PCR was performed as reported (28). The oligonucleotide primers were as follows: for pro-CGRP, 5′-CCCCAGAATGAAGGTTACACA-3′ (sense) and 5′-TGTCAAAGGGAGAAGGGTTTT-3′ (antisense); for GAPDH, 5′-CCCTTCATTGACCTCAACTACAATGGT-3′ (sense) and 5′-GAGGGGCCATCCACAGTCTTCTG-3′ (antisense).
Determination of MVD.
MVD in the most intensely neovascularized areas (hot spots) was assessed as a parameter of tumor-associated angiogenesis according to the established methods described in ref. 28.
Determination of mRNA Levels of Growth Factors in Tumor Stromal Tissues by Using Real-Time PCR.
The sample tissues were taken from mice killed with an excess dose of ether. The tissues were homogenized in 1 ml of TRIzol reagent. Real-time PCR was performed as described above. The oligonucleotide primers were as follows: for VEGF, 5′-CCCCAGAATGAAGGTTACACA-3′ (sense) and 5′-TGTCAAAGGGAGAAGGGTTTT-3′ (antisense); for bFGF, 5′-CCCCAGAAAATGAAGGTTACACA-3′ (sense) and 5′-TGTCAAACCGAAGGAGAAGGGTTTT-3′ (antisense); for CTGF, 5′-CCCCAGAATGAGGAGGTTACACA-3′ (sense) and 5′-TGTCAAAGGGAGAAAGGGTTTT-3′ (antisense); for TGF-β, 5′-CCCCAGAATGAAGGGTTACACA-3′ (sense) and 5′-TGTCAAAGGGAGAAAGGGTTTT-3′ (antisense); and for GAPDH, 5′-CCCTTCATTGACCTCCAACTACAATGGT-3′ (sense) and 5′-GAGGGGCCATCCACACGTCTTCTG-3′ (antisense).
H&E Staining and Immunohistochemistry.
Tumor tissues including stroma were immediately fixed with 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.4), dehydrated with a graded series of ethanol solutions, and embedded in paraffin. For immunostaining, the sections were first exposed to dilute normal horse serum and then incubated with rabbit antiserum to mouse VEGF (Santa Cruz Biotechnology). Immune complexes were detected with a Vectastain ABC kit (Vector Laboratories).
Statistical Analysis.
Data are shown as mean ± SEM. The statistical difference between the two groups was examined by using Student's unpaired t test after confirming that the variance of data was not heterogeneous. Multiple comparisons were performed by using one-way ANOVA with Bonferroni's correction. P < 0.05 was considered to be statistically significant.
Supplementary Material
Acknowledgments.
We thank Michiko Ogino, Akira Nara, Kyoko Yoshikawa, and Osamu Katsumata for their technical assistance. We are grateful to Mr. C. W. P. Reynolds for linguistic assistance in the preparation of the manuscript. This work was supported by Research Grants 12470529 and 12670094 and by a High-tech Research Center grant from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and by an Integrative Research Program of the Graduate School of Medical Science, Kitasato University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800767105/DCSupplemental.
References
- 1.Patrick W, et al. Molecular mechanisms of cancer pain. Nat Rev Cancer. 2002;2:2001–2209. doi: 10.1038/nrc747. [DOI] [PubMed] [Google Scholar]
- 2.Direkze NC, et al. Bone marrow-derived stromal cells express lineage-related messenger RNA species. Cancer Res. 2006;66:1265–1269. doi: 10.1158/0008-5472.CAN-05-3202. [DOI] [PubMed] [Google Scholar]
- 3.Rao G, et al. Facilitating role of preprotachykinin-I gene in the integration of breast cancer cells within the stromal compartment of the bone marrow: A model of early cancer progression. Cancer Res. 2004;1564:2874–2881. doi: 10.1158/0008-5472.can-03-3121. [DOI] [PubMed] [Google Scholar]
- 4.Mukouyama YS, Shin D, Brittisch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- 5.Miller G. Developmental biology: Nerves tell arteries to make like a tree. Science. 2002;296:2121–2123. doi: 10.1126/science.296.5576.2121a. [DOI] [PubMed] [Google Scholar]
- 6.Bolos V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr Rev. 2007;28:339–363. doi: 10.1210/er.2006-0046. [DOI] [PubMed] [Google Scholar]
- 7.Ekstrand AJ, et al. Deletion of neuropeptide Y (NPY) 2 receptor in mice results in blockage of NPY-induced angiogenesis and delayed wound healing. Proc Natl Acad Sci USA. 2003;100:6033–6038. doi: 10.1073/pnas.1135965100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 9.Amara SG, Jonas V, Rosenfeld MG, Ong ES, Evans RM. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298:240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- 10.Oh-hashi Y, et al. Elevated sympathetic nervous activity in mice deficient in α-CGRP. Circ Res. 2001;89:983–990. doi: 10.1161/hh2301.100812. [DOI] [PubMed] [Google Scholar]
- 11.Cadieux A, Monast NP, Pomerleau F, Fournier A, Lanoue C. Bronchoprotector properties of calcitonin-gene related peptide in guinea pig and human airways. Am J Respir Crit Care Med. 1999;159:235–243. doi: 10.1164/ajrccm.159.1.9711031. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi H, et al. Transient prevention of ethanol-induced gastric lesion by capsaicin due to release of endogenous calcitonin gene-related peptide in rats. Jpn J Pharmacol. 2001;86:351–354. doi: 10.1254/jjp.86.351. [DOI] [PubMed] [Google Scholar]
- 13.Mizuguchi S, et al. Calcitonin gene-related peptide released by capsaicin suppresses myoelectrical activity of gastric smooth muscle. J Gastroenterol Hepatol. 2005;20:611–618. doi: 10.1111/j.1440-1746.2004.03764.x. [DOI] [PubMed] [Google Scholar]
- 14.Boku K, et al. Adaptive cytoprotection mediated by prostaglandin I(2) is attributable to sensitization of CRGP-containing sensory nerves. Gastroenterology. 2001;120:134–143. doi: 10.1053/gast.2001.20916. [DOI] [PubMed] [Google Scholar]
- 15.Arai K, et al. Endogenous prostaglandin I2 regulates the neural emergency system through release of calcitonin-gene related peptide. Gut. 2003;52:1242–1249. doi: 10.1136/gut.52.9.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holzer P. Neural emergency system in the stomach. Gastroenterology. 1998;114:823–839. doi: 10.1016/s0016-5085(98)70597-9. [DOI] [PubMed] [Google Scholar]
- 17.Saeki T, et al. Mild irritant prevents ethanol-induced gastric mucosal microcirculatory disturbances through actions of calcitonin gene-related peptide and PGI2 in rats. Am J Physiol. 2004;286:G68–G75. doi: 10.1152/ajpgi.00538.2002. [DOI] [PubMed] [Google Scholar]
- 18.Fan TP, Hu DE, Guard S, Gresham GA, Watling KJ. Stimulation of angiogenesis by substance P and interleukin-1 in the rat and its inhibition by NK1 or interleukin-1 receptor antagonists. Br J Pharmacol. 1993;110:43–49. doi: 10.1111/j.1476-5381.1993.tb13769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu XJ, Li CY, Wang KY, Dai HY. Calcitonin gene-related peptide regulates the expression of vascular endothelial growth factor in human HaCaT keratinocytes by activation of ERK1/2 MAPK. Regul Pept. 2006;137:134–139. doi: 10.1016/j.regpep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Papantoniou V, et al. The potential role of calcitonin gene-related peptide (CGRP) in breast carcinogenesis and its correlation with 99mTc-(V)DMSA scintimammography. Am J Clin Oncol. 2007;30:420–427. doi: 10.1097/COC.0b013e3180337792. [DOI] [PubMed] [Google Scholar]
- 21.Amano H, et al. Adenylate cyclase/protein kinase A signaling pathway enhances angiogenesis through induction of vascular endothelial growth factor in vivo. Jpn J Pharmacol. 2001;87:181–188. doi: 10.1254/jjp.87.181. [DOI] [PubMed] [Google Scholar]
- 22.Lynn B. Neurogenic inflammation. Skin Pharmacol. 1988;4:217–224. doi: 10.1159/000210778. [DOI] [PubMed] [Google Scholar]
- 23.Gee MD, Lynn B, Cotsell B. The relationship between cutaneous C fibre type and antidromic vasodilatation in the rabbit and the rat. J Physiol (London) 1997;15:31–44. doi: 10.1111/j.1469-7793.1997.031bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weihe E, et al. Calcitonin gene-related peptide gene expression in collagen-induced arthritis. Can J Physiol Pharmacol. 1995;73:1015–1019. doi: 10.1139/y95-142. [DOI] [PubMed] [Google Scholar]
- 25.Xu P, Van Slambrouck C, Berti-Mattera L, Hall AK. Activin induces tactile allodynia and increases calcitonin gene-related peptide after peripheral inflammation. J Neurosci. 2005;25:9227–9235. doi: 10.1523/JNEUROSCI.3051-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Portenoy RK, Lesage P. Management of cancer pain. Lancet. 1999;353:1695–1700. doi: 10.1016/S0140-6736(99)01310-0. [DOI] [PubMed] [Google Scholar]
- 27.Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: Characteristics and impact in patients with cancer pain. Pain. 1999;81:129–134. doi: 10.1016/s0304-3959(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 28.Amano H, et al. Host prostaglandin E(2)-EP3 signaling regulates tumor-associated angiogenesis and tumor growth. J Exp Med. 2003;197:221–232. doi: 10.1084/jem.20021408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.