Abstract
Refractory coeliac disease (RCD) is a very rare and dangerous form of CD, in which gluten-free diet loses its therapeutic effect and the damage of intestinal mucosa persists. Because of the adherence to the diet, serological markers of CD [immunoglobulin A (IgA) antibodies against gliadin, tissue transglutaminase (tTG) and endomysium] are often missing in RCD patients. We found substantially elevated levels of IgA anti-calreticulin (CRT) antibodies in the sera of almost all RCD patients tested. These sera were negative for IgA antibodies to gliadin and tTG and only some of them showed IgA antibodies to enterocytes. Analysis of patients' IgA reactivity to CRT fragments (quarters and halves) by Western blotting revealed differences in the specificity of IgA antibodies between RCD and CD patients. We therefore used the Pepscan technique with synthetic overlapping decapeptides of CRT to characterize antigenic epitopes recognized by serum IgA antibodies of RCD patients. Employing this method we demonstrated several dominant antigenic epitopes recognized by IgA antibodies of RCD patients on the CRT molecule. Epitope GVTKAAEKQMKD was recognized predominantly by serum IgA of RCD patients. Our results suggest that testing for serum IgA antibodies against CRT and its selected peptide could be a very useful tool in RCD differential diagnosis.
Keywords: antibody specificity, calreticulin, ELISA, Pepscan, refractory coeliac disease
Introduction
Coeliac disease (CD), or gluten-sensitive enteropathy, is an inflammatory disorder of small intestine induced in genetically susceptible individuals by food containing wheat gluten and related prolamins. Histological analysis of small bowel biopsy reveals a characteristic mucosal lesion that impairs nutrient absorption. Prompt improvement of nutrient absorption and healing of the mucosal lesion is seen upon withdrawal of dietary gluten [1–3].
However, in a small subgroup of adult-onset CD patients (2–5%) serious complications develop in the form of refractoriness or the development of pre- and malignant complication [4]. Patients with CD may be regarded as suffering from refractory CD (RCD) when symptoms persist, or recur after a former good response, despite a strict adherence to gluten-free diet (GFD). RCD is defined as villous atrophy with crypt hyperplasia and increased number of T lymphocytes persisting for more than 12 months in spite of a strict GFD [5,6]. Immunologically, two types of RCD are defined depending on the presence of aberrant intraepithelial lymphocytes in the small bowel mucosa – RCD I and II. Patients with RCD I have a normal phenotype of intraepithelial T lymphocytes (IELs) in small bowel biopsy. They often develop concomitant autoimmune diseases, infectious and thromboembolic complications. These patients have a low mortality rate, which is not different from that of the general population [7]. RCD II is associated with an aberrant phenotype of intraepithelial T cells characterized by the expression of intracytoplasmic CD3e, surface CD103 and lack of classical surface T cell markers such as CD4, CD8 and T cell receptors (TCR)-α and β. Furthermore, the aberrant IELs phenotype is associated with clonal TCR gene rearrangement. Patients with RCD II, in contrast to RCD I, are known to be at a greater risk of developing malignancy, particularly enteropathy-associated T cell lymphoma (EATL) [5,8–11].
Elevated levels of immunoglobulin A (IgA) and/or IgG antibodies against gliadin (a hydrophobic component of gluten inducing the disease), and autoantibodies to tissue transglutaminase (anti-tTG) and endomysial antibodies (EMA), are serological hallmarks of florid CD [1]. However, data concerning the occurrence of IgA anti-gliadin, anti-endomysium and anti-tTG antibodies in sera of RCD are very limited and incoherent and no reliable serological markers of RCD exist. It appears likely that, because of the adherence to a GFD both in CD and RCD patients, some of these characteristic serological markers are lacking [12,13]. However, in a case report, a patient with RCD was positive for serum IgA antibodies against isolated enterocytes and other autoantigens [14].
Calreticulin (CRT) is a ubiquitous cellular calcium-binding protein and molecular chaperone with many biological activities. CRT is a phylogenetically conserved, acidic (pI 4·65) protein that migrates at about Mr 60–65 kDa on sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), although the molecular mass deduced from the amino acid sequence is 46 kDa [15–17]. The molecule consists of three structurally and functionally different domains: N, P and C. The N domain (amino acid residues 1–180) is predicted to form a globular structure comprising eight anti-parallel beta-strands. The P-domain (181–290) contains a proline-rich region and forms an extended-arm structure. The C-terminal domain (291–400) is highly acidic and binds Ca2+ with high capacity [18,19].
We found high titres of IgA and IgG antibodies to CRT in sera of almost all patients with active CD. Our test of anti-CRT antibodies achieved sensitivity for CD 92% (IgA and IgG) and specificity of 85·5% (IgA) and 79·7% (IgG). In all CD patients keeping to a GFD, the levels of IgA anti-CRT antibodies, as well as those of anti-gliadin and anti-tTG antibodies, decreased to the levels detected in healthy individuals [20]. Additional testing of the cohort of 50 CD patients, adhering strictly to a GFD for 6 months and longer, confirmed our previous finding, i.e. the significant decrease in the reactivity to CRT and other CD serological markers. Furthermore, we characterized the fine specificity of serum IgA antibodies of patients with CD using the Pepscan technique with overlapping synthetic decapeptides of CRT [21].
In this paper we documented high levels of serum IgA anti-CRT antibodies in almost all RCD patients (seronegative for coeliac markers because of GFD adherence) detected by enzyme-linked immunosorbent assay (ELISA) test using human recombinant CRT as an antigen. Moreover, we characterized immunodominant B cell epitopes of CRT recognized predominantly by serum IgA antibodies of RCD patients employing the Pepscan technique, and Western blot analysis with recombinant fragments of CRT corresponding to its quarters and halves.
Materials and methods
Patients
Refractory CD
Serological profiles of 13 patients were analysed. The baseline characteristics of these patients are shown in Table 1. The patients were diagnosed initially as coeliacs on the basis of European Society of Pediatric Gastroenterology and Nutrition criteria [22] and were positive for IgA antibodies to gliadin, tTG and EMA at the time of histological analysis of small intestinal biopsy according to the Marsh classification [23]. Diagnosis of RCD was based on persisting malabsorptive symptoms such as diarrhoea, weight loss, abdominal pain and biopsy-proven villous atrophy and hyperplasia of crypts of small intestine mucosa (Marsh III), after excluding syndromes mimicking CD: autoimmune enteropathy, radiation enteritis, bacterial overgrowth, common variable immunodeficiency syndrome, amyloidosis, intestinal lymphangiectasia, immunoproliferative small intestinal disease, Whipple's disease, hypogammaglobulinaemia, eosinophilic gastroenteritis, acquired immune deficiency syndrome, inflammatory bowel disease, exocrine pancreatitis/insufficiency, hyperthyroid disease, collagenous sprue, tropical sprue, giardiasis, post-infectious diarrhoea, hypergastrinaemia, intestinal lymphoma, protein intolerance and tuberculosis [6,24]. Worsening of the disease symptoms in patients appeared despite their keeping to a strict GFD for at least 1 year. Patients no. 5 and 9 were suspicious for RCD, but they did not initially adhere appropriately to GFD. The time span between blood-taking was 6 months. The diagnosis of RCD was established as type II when > 20% aberrant T cells were present [25–27]. Patients with RCD I were treated by standard immunosuppressive therapy comprising corticosteroids (prednisone). Cytotoxic chemotherapy (cladribine, cyclophosphamide, doxorubicin) was used in the treatment of patients with RCD II and EATL.
Table 1.
The baseline characteristics of refractory coeliac patients.
| Patients | Diagnosis | Sex | Age | Marsh |
|---|---|---|---|---|
| 1 | RCD I | M | 70 | III |
| 2 | RCD I | F | 53 | III |
| 3 | RCD I | M | 67 | III |
| 4 | RCD I | F | 49 | III |
| 5 | RCD I | M | 63 | III |
| 6 | RCD I | F | 51 | III |
| 7 | RCD II | F | 73 | III |
| 8 | RCD II | F | 72 | III |
| 9 | RCD II | M | 79 | III |
| 10 | RCD II, EATL | M | 63 | III |
| 11 | RCD II, EATL | F | 46 | III |
| 12 | RCD II, EATL | M | 67 | III |
| 13 | RCD II, EATL | M | 59 | III |
RCD, refractory coeliac disease; Marsh, Marsh classification of the disease; EATL, enteropathy-associated T cell lymphoma.
Coeliac disease patients and healthy controls
Sera of biopsy-confirmed CD patients (n = 14) positive for EMA and anti-tTG, anti-gliadin and anti-CRT antibodies tested in our previous study [21] were used for comparison of antibody specificity of RCD patients. The sera of healthy donors (n = 14) were used as controls.
The study was approved by a local Ethics Committee.
Expression and purification of recombinant CRT and its fragments
DNA coding for human CRT and its fragments was obtained by polymerase chain reaction (PCR) amplification using the full-length human CRT cDNA (GenBank™ Accession no. M32294). The oligonucleotide primer pairs used for amplification of the nucleotide sequence encoding full-length CRT (1–400 amino acids) were 5′-GGA ATT GAA TTCCAT ATG GAC CCT GCC CTG TAC TTC AAG-3′ and 5′-GGG ATT GAA TTC CTA [GTG GTG GTG GTG GTG GTG] CAG CTC GTC CTT GGC CTG-3′; for the first quarter of CRT (1–103 amino acids): 5′-GGA ATT GAA TTCCAT ATG GAC CCT GCC CTG TAC TTC AAG-3′ and 5′-G CGC GAA TTC CTA [GTG GTG GTG GTG GTG GTG] GCT TGT CTG GTC CAA ACT ATT AGG AAA CAG-3′; for the second quarter (104–206 amino acids): 5′-GGG ATT GAA TTCCAT ATG CAC GGA GAC TCA GAA TAC AAC3′ and 5′-CGC GAA TTC CTA [GTG GTG GTG GTG GTG GTG] CGG TTT TGA AGC ATC AGG ATC CTT TAT C-3′; for the third quarter (207–309 amino acids): 5′-GGG ATT GAA TTCCAT ATG GAA GAC TGG GAT GAG CGG GCC-3′ and 5′- GGG ATT GAA TTC CTA [GTG GTG GTG GTG GTG GTG] GCC CAG CAG CGG AAA GTT ATC-3′; and for the fourth quarter (310–400 amino acids): 5′-GGG ATT GAA TTCCAT ATG CTG GAC CTC TGG CAG GTC AAG-3′) and 5′-GGG ATT GAA TTC CTA [GTG GTG GTG GTG GTG GTG] CAG CTC GTC CTT GGC CTG-3′. The primers used for the first half (1–206 amino acids): 5′-GGA ATT GAA TTCCAT ATG GAC CCT GCC CTG TAC TTC AAG-3′ and 5′-CGC GAA TTC CTA [GTG GTG GTG GTG GTG GTG] CGG TTT TGA AGC ATC AGG ATC CTT TAT C-3′, and for the second half (207–400 amino acids): 5′-GGG ATT GAA TTCCAT ATG GAA GAC TGG GAT GAG CGG GCC-3′ and 5′-GGG ATT GAA TTC CTA [GTG GTG GTG GTG GTG GTG] CAG CTC GTC CTT GGC CTG-3′). Italic type indicates an EcoRI cleavage site, bold type a NdeI site and methionine-encoding ATG are underlined. The nucleotide sequences of 3′-primers in brackets in front of the stop codon encode the 6xHis tag. PCR products were cut with NdeI/EcoRI and subcloned into the NdeI/EcoRI site of the expression vector pET-28a (Novagen, Madison, WI, USA).
Recombinant proteins were expressed in liquid cultures of Escherichia coli BL21 (DE3) after induction of protein synthesis with isopropyl-D-thiogalactoside (0·5 mM). The recombinant proteins were purified by affinity chromatography on a nickel–nitrilotriacetic acid resin column as described previously [21].
Western blot analysis
Four micrograms of isolated fragments of CRT and a complete molecule of CRT were subjected to SDS-PAGE (12·5% gel) under reducing conditions [28]. After separation, the proteins were electroblotted to nitrocellulose membrane (Hybond-C pure, Amersham International, Aylesbury, UK). The membranes were blocked with 4% low-fat milk in phosphate-buffered saline (PBS)–Tween (PBS-T, 0·1%) for 1 h at room temperature (RT) and then incubated with human sera (1/100) or anti-CRT antibody (ABR, Golden, CO, USA) diluted in blocking solution (1/1000) for 2 h at RT. After washing with PBS and PBS-T, anti-human IgA antibody peroxidase conjugate (The Binding Site, Birmingham, UK) or anti-rabbit antibody peroxidase conjugate (The Binding Site) diluted in blocking solution (1/1000) was applied to the membrane for 1 h at RT. Chemiluminescence reagents (SuperSignal® West Pico Trial Kit, Rockford, IL, USA) and X-ray film (X-Omat RA, Kodak, Chalons/Saône, France) were used for visualizing the binding of antibody specific for CRT. Various exposure times of the X-ray films were used to evaluate the reactivity of IgA antibody with CRT or its recombinant fragments.
Enzyme-linked immunosorbent assay for determining serum levels of antibodies to CRT, gliadin, tTG and enterocytes
Enzyme-linked immunosorbent assay for IgA antibodies to CRT, gliadin, tTG and rat enterocytes was performed as described in our previous study [20,21]. Results of the ELISA test are expressed as arbitrary units (AU) referring to the optical density of internal standard serum (100%). Cut-off values were calculated as the mean value plus two standard deviations from the data for 90 control sera, according to our previous study. Cut-off value was 60 AU for IgA antibodies against CRT (mean ± standard deviation, 33·6 ± 13·2, n = 90), 55 AU for IgA antibodies to enterocytes (31·5 ± 12·1) and 30 AU for both IgA antibodies to gliadin and tTG (15·2 ± 7·4, 13·8 ± 8·1). The presence of serum IgA anti-tTG antibodies was verified by a commercially available ELISA test using human recombinant tTG activated by gliadin (BioSystems, Barcelona, Spain). The ELISA was performed according to the manufacturer's instructions.
Pepscan analysis
The Pepscan experiment was performed in accordance with Sánchez et al.[21]. Two hundred and one decapeptides, each overlapping by eight amino acid residues, covering the sequence of human CRT were synthesized and bound covalently to cellulose membrane (Abimed, Langenfeld, Germany). CRT amino acid sequence was derived from Michalak [29]. Amino acid sequences of CRT peptides were identical to our previous study [21].
The membranes were blocked with 4% low-fat milk in Tris (Serva, Heidelbery, Germany)-buffered saline (TBS) with Tween (0·1%, TBS-T) for 1 h at RT and then incubated with human sera (1/100) diluted in blocking solution for 2 h at RT. After washing with TBS and TBS-T, anti-human IgA antibody peroxidase conjugate diluted in blocking solution (1/1000) was applied to the membrane for 1 h at RT. Chemoluminescence was employed to visualize the binding of antibody to individual peptides. Development time of chemiluminescence was 2 min for each sample.
The binding of serum IgA antibodies of individuals from the tested groups (CD, n = 14; RCD, n = 12; healthy donors, n = 14) to CRT peptides was evaluated qualitatively as positive or negative. In RCD patients, when blood-taking was performed repeatedly, the last sample was used for Pepscan analysis. The data are expressed as the percentage of patients/controls in the tested group that recognized an individual peptide by IgA antibody.
Enzyme-linked immunosorbent assay for determinationserum levels of IgA antibodies to CRT peptides
Nunc (Roskilde, Denmark) maxisorp microtitre 96-well immunoplates were coated with 50 µl/well peptide GVTKAAEKQMKD or IFDNFLITND diluted in PBS (100 µg/ml) overnight at 4°C. Unoccupied absorption sites were blocked by 1% bovine serum albumin for 2 h at RT. After threefold washing with PBS and PBS-T, sera of patients and controls diluted 1/20 in blocking solution were added to the wells (50 µl/well) in triplicate for 3 h at RT. Anti-human IgA antibodies conjugated with peroxidase (The Binding Site) diluted in blocking solution at 1/750 for 1 h at RT were incubated in the wells (50 µl/well) after repeated washing. Then, the plates were washed and enzyme reaction was developed by adding a solution containing 1,2-o-phenylenediamine (0·7 mg/ml) in 0·1 M sodium citrate (pH 4·2). The reaction was stopped by 2 M H2SO4 and optical density was read on a spectrophotometer at 402 nm (Titertek Multiscan MCC/340; Flow Laboratory, Irvine, UK).
Statistical methods
The non-parametric Mann–Whitney U-test was used for comparison of serum levels of IgA antibodies to CRT peptides GVTKAAEKQMKD or IFDNFLITND among patients with RCD, CD, CD patients on a GFD and healthy controls tested by the ELISA.
Results
Refractory coeliac patients show elevated IgA anti-CRT antibody levels
Immunoglobulin A anti-CRT antibodies were present in high titres in the sera of all patients with RCD tested keeping a GFD (n = 13), except patient 6. The level of IgA anti-CRT antibodies in the majority of patients exceeded the cut-off value two to three times. Nine of 13 patients were also positive for anti-enterocyte IgA antibodies; however, positivity for these antibodies varied between individual blood-taking (patients 8 and 11). Serum levels of CD markers (IgA antibodies against tTG, gliadin) were tested for comparison (Table 2). IgA anti-tTG and anti-gliadin antibodies were below cut-off values in all RCD patients tested. Moreover, we demonstrated changes in IgA antibody levels in patients 5 and 9. These patients, diagnosed initially as coeliac (blood-taking 1, or 1 and 2), developed a resistance to the GFD and were diagnosed finally as RCD. We observed decreased serum levels of anti-gliadin and anti-tTG IgA antibodies, but a persistently high level of IgA antibodies to CRT in these patients after 6 months of adherence to the GFD (blood-taking 2 and 3 respectively).
Table 2.
Serum levels of immunoglobulin A (IgA) antibodies to gliadin, tissue transglutaminase, enterocytes and human recombinant calreticulin in patients with refractory coeliac disease.
| Patient | Gliadin | IgA antibody to | CRT | |
|---|---|---|---|---|
| tTG | Enterocytes | |||
| 1 | 24·6 | 10·7 | 65·1 | 122·1 |
| 2 | 8·2 | 8·6 | 37 | 125·8 |
| 3 | 4·6 | 5·1 | 161·1 | 86·2 |
| 4 | ||||
| 1st b.t. | 11·7 | 18·7 | 128·5 | 140·9 |
| 2nd b.t. | 11·8 | 16·8 | 117·1 | 129·9 |
| 5 | ||||
| 1st b.t.* | 141·1 | 85·6 | 173·2 | 366·7 |
| 2nd b.t. | 28·8 | 17·7 | 107·3 | 327·2 |
| 6 | 13·1 | 18·5 | 22·9 | 45·2 |
| 7 | ||||
| 1st b.t. | 15·4 | 18·4 | 92·1 | 139·2 |
| 2nd b.t. | 17·7 | 16·3 | 77·9 | 159·2 |
| 8 | ||||
| 1st b.t. | 25·1 | 19·2 | 60·7 | 222·8 |
| 2nd b.t. | 14·4 | 26·1 | 49·1 | 103·9 |
| 9 | ||||
| 1st b.t.* | 61 | 62·3 | 152·5 | 225·7 |
| 2nd b.t.* | 55 | 56·3 | 141 | 189·9 |
| 3rd b.t. | 26·5 | 29·1 | 129·7 | 188·9 |
| 10 | 15·4 | 18·4 | 92·1 | 139·2 |
| 11 | ||||
| 1st b.t. | 17·7 | 16·3 | 41·9 | 61·1 |
| 2nd b.t. | 20·9 | 11·9 | 77·9 | 159·2 |
| 12 | 10·2 | 13·7 | 125 | 67 |
| 13 | 6·3 | 15·7 | 54·4 | 142·2 |
The level of IgA antibodies is expressed in arbitrary units (AU). Cut-off values for IgA antibodies against: gliadin (30 AU), tTG (30 AU), enterocytes (55 AU) and CRT (60 AU). Tissue transglutaminase (tTG), calreticulin (CRT), blood-taking (b.t., 6-month periods).
Patients non-adhering to gluten-free diet.
In an attempt to explain the low level of IgA anti-CRT antibodies in serum of RCD patient 6 treated by prednisone and budenoside, the total serum IgA level was determined by routine immunoturbidimetry using Roche™ (Basel, Switzerland) reagents from Agilab Ltd (Prague, Czech Republic). A decreased level of total IgA was found in this patient (0·99 g/l; the reference interval for the laboratory is 1·96–4·36 g/l).
The fine specificity of anti-CRT IgA antibody reactivity differs between refractory coeliac and coeliac patients
Western blot analysis of patients' sera reactivity with humanrecombinant CRT and its fragments
Our findings led us to analyse the specificity of anti-CRT antibodies in RCD patients. Western blot analysis with recombinant CRT and its fragments corresponding to its quarters and halves prepared as fusion proteins with 6xHis tag is documented in Fig. 1. Polyclonal rabbit anti-CRT antibodies recognized all CRT fragments. Figure 2 documents the reactivity of serum IgA antibodies of individual patients suffering from RCD and CD with recombinant CRT and its fragments, while the binding of antibodies of healthy donors was not observed. IgA antibodies of all patients recognized full-length CRT on Western blot analysis. However, the recognition pattern of IgA antibodies with CRT fragments differed both between patients' groups and in patients of one group. These findings led us to use the Pepscan to analyse specificity of serum IgA anti-CRT antibodies in RCD patients.
Fig. 1.
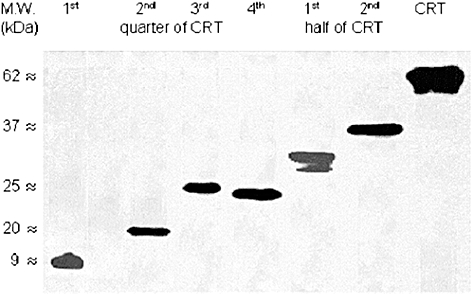
Recombinant calreticulin (CRT) fragments are recognized by a rabbit anti-CRT anti-serum. Nitrocellulose-blotted recombinant 6xHis-tagged quarters and halves of human CRT and, for control purposes, recombinant full length CRT were exposed to a polyclonal rabbit anti-CRT anti-serum. Specific antibody binding was detected with peroxidase-labeled anti-rabbit antibodies and visualized using chemiluminescence detection system by autoradiography. Molecular weights are indicated in the left margin. The individual quarters of CRT corresponded with 1–103 amino acids, 104–206 amino acids, 207–309 amino acids and 310–400 amino acids; corresponding halves of CRT corresponded with 1–206 and 207–400 amino acids.
Fig. 2.
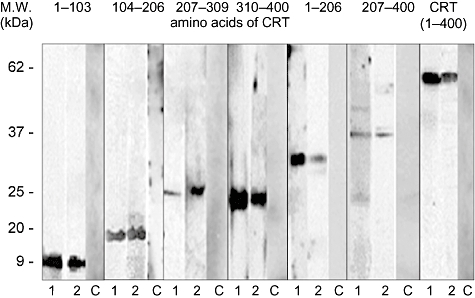
Illustrative depiction of immunoglobulin A (IgA) antibody reactivity with human calreticulin (CRT) fragments in refractory coeliac disease (RCD) and coeliac (CD) patients. Nitrocellulose-blotted recombinant 6xHis-tagged quarters and halves of human CRT and recombinant full-length CRT were incubated with sera from CD (lane 1) and RCD (lane 2) patients and, for control purposes, with sera from healthy individuals (lane C). Specific IgA antibody binding was detected with peroxidase-labelled anti-human IgA antibodies and visualized using chemiluminescence detection system by autoradiography. Molecular weights are indicated in the left margin.
Identification of CRT epitopes predominantly recognizedby IgA antibodies of patients with RCD
The fine specificity of IgA antibodies to CRT in RCD patients was analysed by Pepscan technique using a set of synthetic decapeptides covering the whole sequence of CRT molecule. An example of reactivity of IgA antibodies of RCD patients with CRT peptides is shown in Fig. 3. The reactivity of IgA antibodies of untreated CD patients was indicated for comparison. The recognition profile of IgA antibodies of RCD and CD patients (expressed as the frequency of individual peptide recognition) is documented in Fig. 4. The reactivity of IgA antibodies of RCD patients is distributed over all N-, P- and C-CRT domains. The most frequently recognized antigenic epitopes were detected in the CRT C-domain. Moreover, in this domain the spectrum of CRT peptides recognized by IgA antibodies of RCD and CD patients differs significantly. The most frequently recognized CRT peptides by RCD (on GFD) and active (non-treated by GFD) CD patients are listed in Table 3. The ELISA test with peptide GVTKAAEKQMKD, recognized predominantly by IgA antibodies of RCD patients on Pepscan experiments, was developed to estimate the level of IgA antibodies in sera of RCD patients. These levels were then compared with the levels in active CD patients, in patients on a GFD and in healthy controls. The peptide IFDNFLITND, recognized mainly by IgA antibodies of both RCD and CD patients, was used as a control. The results are summarized in Table 4. Significantly (P < 0·001) increased levels of IgA antibodies against peptide GVTKAAEKQMKD were documented in patients with RCD in comparison with CD patients on a GFD (P < 0·001) and to healthy controls (P < 0·001).
Fig. 3.
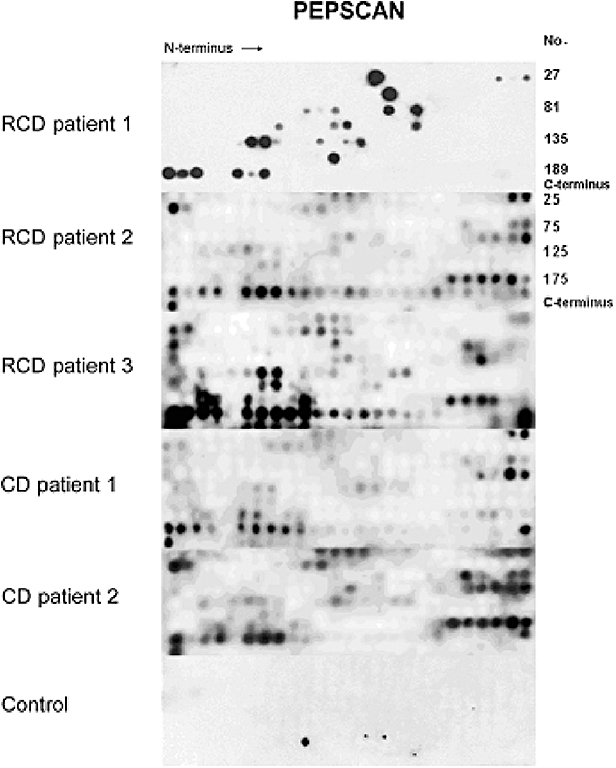
Illustrative picture of serum immunoglobulin A (IgA) antibody reactivity of patients with refractory coeliac disease (RCD) and active coeliac disease (CD) and control with calreticulin (CRT) decapeptides using Pepscan membranes. The membranes differed in the number of CRT peptides per line: 27 peptides (RCD patient 1) or 25 peptides for all others. No. (CRT peptide number).
Fig. 4.
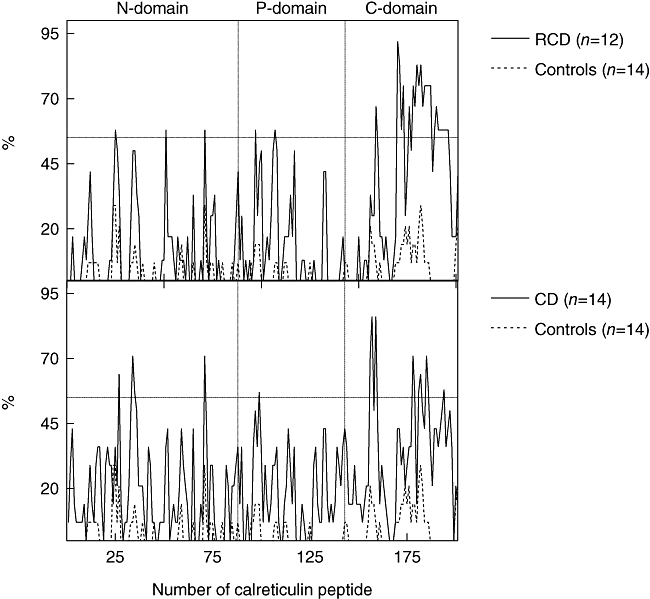
Recognition profiles of serum immunoglobulin A (IgA) antibodies against calreticulin (CRT) decapeptides (each overlapping by eight amino acids) in refractory coeliac disease (RCD, n = 12), coeliac disease (CD, n = 14) and healthy controls (n = 14). These profiles are cumulative data (% of sera reactive with individual CRT peptides) relative to each group size and values shown on the right. The synthetic peptides 1–88, 89–143 and 144–201 correspond to N-, P- and C-domains of the CRT molecule.
Table 3.
Frequently recognized decapeptides of calreticulin by immunoglobulin A (IgA) antibodies of patients with refractory coeliac disease (RCD) and coeliac disease (CD) in Pepscan experiment.
| Domain | No. | RCD | Ratio* | No. | CD | Ratio* |
|---|---|---|---|---|---|---|
| N | 25 | EKDKGLQTSQ | 7/12 | |||
| 27 | GLQTSQDARF | 9/14 | ||||
| 34 | ASFAEAPFSN | 10/14 | ||||
| 35 | FAEAPFSNKG | 8/14 | ||||
| 51 | KLFPNSLDQT | 7/12 | ||||
| 71 | KGKNVLINKD | 7/12 | 71 | KGKNVLINKD | 10/14 | |
| P | 97 | LPPKKIKDPD | 7/12 | |||
| 99 | KIKDPDASKP | 8/14 | ||||
| 107 | AKIDDPTDSK | 7/12 | ||||
| C | 156 | QVKSGTIFDN | 10/14 | |||
| 157 | KSGTIFDNFL | 12/14 | ||||
| 159 | IFDNFLITND | 8/12 | 159 | IFDNFLITND | 12/14 | |
| 170 | GVTKAAEKQM | 11/12 | ||||
| 171 | TKAAEKQMKD | 10/12 | ||||
| 172 | AAEKQMKDKQ | 7/12 | ||||
| 173 | EKQMKDKQDE | 9/12 | ||||
| 176 | KQDEEQRLKE | 8/12 | ||||
| 178 | EQRLKEEEED | 9/12 | 178 | EQRLKEEEED | 10/14 | |
| 179 | RLKEEEEDKK | 8/12 | 179 | RLKEEEEDKK | 8/14 | |
| 180 | KEEEEDKKRK | 10/12 | ||||
| 181 | EEEDKKRKEE | 9/12 | 181 | EEEDKKRKEE | 8/14 | |
| 182 | EDKKRKEEEE | 10/12 | 182 | EDKKRKEEEE | 9/14 | |
| 184 | RKEEEEAEDK | 9/12 | ||||
| 185 | EEEEAEDKED | 9/12 | 185 | EEEEAEDKED | 10/14 | |
| 186 | EEAEDKEDDA | 9/12 | 186 | EEAEDKEDDA | 8/14 | |
| 187 | AEDKEDDAED | 9/12 | ||||
| 189 | EDDAEDKDED | 7/12 | ||||
| 190 | DAEDKDEDEE | 8/12 | ||||
| 191 | EDKDEDEEDE | 7/12 | ||||
| 192 | KDEDEEDEED | 7/12 | ||||
| 193 | EDEEDEEDKE | 7/12 | ||||
| 194 | EEDEEDKEED | 7/12 | 194 | EEDEEDKEED | 8/14 | |
| 195 | DEEDKEEDEE | 7/12 | ||||
| 196 | EDKEEDEEED | 7/12 |
No, decapeptide number;
ratio of patients reacting positively with peptides/the number of patients in the group.
Table 4.
The levels of immunoglobulin A (IgA) antibodies to calreticulin peptides in patients with active coeliac disease (CD), CD patients on a gluten-free diet (CD-GFD), patients with refractory coeliac disease (RCD-GFD) and healthy controls.
| Peptides | Cohorts/numbers | |||
|---|---|---|---|---|
| CD/12 | CD-GFD/10 | RCD-GFD/12 | Healthy controls/12 | |
| GVTKAAEKQMKD | 334.7 ± 247.6* | 83.6 ± 72.6 | 595.2 ± 385.7*** | 131.4 ± 93.9 |
| IFDNFLITND | 238 ± 229.2** | 60.4 ± 58.4 | 327.3 ± 315.5** | 181.2 ± 102.1** |
Data are expressed as mean ± standard deviation of optical density.
P < 0·001, RCD-GFD versus healthy controls, CD-GFD patients.
P < 0·01, CD, RCD-GFD versus CD-GFD, healthy controls versus CD-GFD patients.
P < 0·05, CD versus CD-GFD patients.
Discussion
Our study represents the first finding of persistent serum IgA antibodies against CRT in most patients with RCD, where other serological markers of CD are missing. IgA antibodies against CRT were described in our previous study to be present in sera of active coeliac patients. Similarly to other serological markers of the disease (anti-gliadin, EMA and anti-tTG antibodies), they decreased after GFD treatment [20]. Based on these results, it was of interest to analyse the fine specificity of IgA anti-CRT antibodies persisting in RCD patients and to compare it with the specificity of IgA antibodies detected in active coeliac patients.
The comparison of IgA antibody reactivity pattern with CRT peptides using Pepscan revealed a limited number of recognized peptides in patients with RCD in contrastto patients with active CD, probably because of the GFD adherence of RCD patients. This suggestion is supported by the low reactivity of IgA antibodies of RCD patients with CRT peptide QVKSGTIFDNL, which is structurally similar to gliadin. Interestingly, this peptide inhibited the binding of the anti-gliadin antibodies of CD patients to CRT [30].
In addition, the peptide KGKNVLINKD, which we identified in our previous study as a common immunodominant peptide recognized by IgA antibodies of patients with CD, autoimmune hepatitis, primary biliary cirrhosis and alcoholic liver cirrhosis, was recognized only by antibodies of some RCD patients. The recognition profile of IgA antibody reactivity with decapeptides of CRT in RCD was characterized by a peak corresponding to the peptide GVTKAAEKQM, which was recognized by antibodies of almost all patients with RCD tested and was not immunodominant for patients with CD and patients with liver diseases [21].
Interestingly, IgA antibodies of RCD patients recognize a number of neighbouring, overlapping peptides in the C-domain of CRT, indicating the prevalence of sequential antigenic epitopes in this domain, which was shown to be sensitive to enzymatic digestion [31]. In contrast, IgAantibodies of RCD recognized only few individual antigenicpeptides in the N- and P-domains of CRT, both resistant to proteolytic cleavage. N- and C- domains of CRT (similar to highly homologous calnexin) were suggested to form a folded globular structure [18,31,32]. These data indicate that immunodominant peptides of the N- and P-domains could be part of conformational antigenic epitopes.
The presence of IgA antibodies to CRT in patients with RCD could also be a consequence of gut mucosa damage. Progressive apoptosis in small intestine of RCD patients could increase the release of CRT and other intracellular proteins from dying cells to the extracellular spaces [33–36]. CRT is an intracellular protein located in endoplasmic reticulum, hidden from the immune system; when appearing extracellularly, i.e. under stress (inflammatory) conditions, necrosis or apoptosis, it could elicit autoimmune reaction [18,37]. Recently, the translocation of CRT to the cell surface of tumour cells treated by doxorubicin was shown to induce immunogenic cell death [38]. Doxorubicin was used therapeutically in patients suffering from RCD together with EATL and could, therefore, elicit immune response to CRT in these cases. However, the increased levels of IgA anti-CRT antibodies were also detected in RCD patients cured with corticosteroids.
Basu and Srivastava [39] proposed that CRT might not be an autoantigen per se. A characteristic feature of CRT is its ability to interact with many molecules, including the molecules of extracellular matrix. The associated molecules can either be immunogenic on their own or their immunogenicity could be increased in the molecular complex with CRT [40,41].
Taken together, the antibodies persisting in the sera of RCD patients adhering strictly to GFD were IgA antibodies to CRT, while IgA antibodies against gliadin and tTG were below cut-off values. We found that the reactivity profile of serum IgA antibodies with overlapping decapeptides of CRT detected by Pepscan differed in RCD and CD patients. Based on these data we suppose that ELISA for anti-CRT antibodies with CRT molecule and/or disease specific peptide selected by Pepscan would be a promising tool for RCD serological diagnosis. However, the persistence of anti-CRT antibodies in RCD patients and changes in IgA antibody fine specificity need further analysis.
Acknowledgments
This work was supported by grants 310/03/H147, NPVII637, A 500200801 (from Academy of Sciences) and 310/07/0414 from the Grant Agency of Czech Republic, by grants S500200572 and A500200709 from the Academy of Sciences of the Czech Republic, by grants B5020407 and 2B06155 from the Ministry of Education of Czech Republic and by Institutional Research Concept Grant AVOZ50200510. We thank Professor Valenta from University of Vienna for support and help with CRT fragments preparation.
References
- 1.Schuppan D. Current concepts of celiac disease pathogenesis. Gastroenterology. 2000;119:234–42. doi: 10.1053/gast.2000.8521. [DOI] [PubMed] [Google Scholar]
- 2.Green PH, Jabri B. Coeliac disease. Lancet. 2003;362:383–91. doi: 10.1016/S0140-6736(03)14027-5. [DOI] [PubMed] [Google Scholar]
- 3.Londei M, Ciacci C, Ricciardelli I, Vacca L, Quaratino S, Maiuri L. Gliadin as a stimulator of innate response in celiac disease. Mol Immunol. 2005;42:913–18. doi: 10.1016/j.molimm.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Daum S, Cellier C, Mulder CJJ. Refractory coeliac disease. Best Pract Res Clin Gastroenterol. 2005;19:413–24. doi: 10.1016/j.bpg.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Cellier C, Delabesse E, Helmer C, et al. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet. 2000;356:203–8. doi: 10.1016/s0140-6736(00)02481-8. [DOI] [PubMed] [Google Scholar]
- 6.Biagi F, Corazza GR. Defining gluten refractory enteropathy. Eur J Gastroenterol Hepatol. 2001;13:561–5. doi: 10.1097/00042737-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Al-Toma A, Verbeek WHM, Hadithi M, von Blomberg BME, Mulder CJJ. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut. 2007;56:1373–8. doi: 10.1136/gut.2006.114512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gale J, Simmonds PD, Mead GM, Sweetenham JW, Wright DH. Enteropathy-type intestinal T-cell lymphoma: clinical features and treatment of 31 patients in a single center. J Clin Oncol. 2000;18:795–803. doi: 10.1200/JCO.2000.18.4.795. [DOI] [PubMed] [Google Scholar]
- 9.Howdle PD, Jalal PK, Holmes GKT, Houlston RS. Primary small-bowel malignancy in the UK and its association with coeliac disease. Q J Med. 2003;96:345–53. doi: 10.1093/qjmed/hcg058. [DOI] [PubMed] [Google Scholar]
- 10.Cellier C, Brousse N, Cerf-Bensussan N. Classification and outcome of refractory sprue. In: Cerf-Bensussan N, Brousse N, Caillat-Zucman S, Cellier C, Schmitz J, editors. Coeliac disease. Montrouge: John Libbey Eurotext Publishers; 2003. pp. 215–23. [Google Scholar]
- 11.Meijer JW, Mulder CJJ, Goerres MG, Boot H, Schweizer JJ. Coeliac disease and (extra)intestinal T-cell lymphomas: definition, diagnosis and treatment. Scand J Gastroenterol Suppl. 2004;241:78–84. doi: 10.1080/00855920410014605. [DOI] [PubMed] [Google Scholar]
- 12.Di Domenico MR, Annaluisa S, Pluvio R, Iovine C, Rea F. The role of anti-endomysium and anti-transglutaminase antibodies in the diagnosis and follow-up of celiac disease. Pediatr Med Chir. 2002;24:208–12. [PubMed] [Google Scholar]
- 13.Wahab PJ, Meijer JW, Mulder CJJ. Histologic follow-up of people with celiac disease on a gluten-free diet: slow and incomplete recovery. Am J Clin Pathol. 2002;118:459–63. doi: 10.1309/EVXT-851X-WHLC-RLX9. [DOI] [PubMed] [Google Scholar]
- 14.Rolny P, Sigurjonsdottir HA, Remotti H, et al. Role of immunosuppressive therapy in refractory sprue-like disease. Am J Gastroenterol. 1999;94:219–25. doi: 10.1111/j.1572-0241.1999.00799.x. [DOI] [PubMed] [Google Scholar]
- 15.Fliegel L, Burns K, MacLennan DH, Reithmeier RAF, Michalak M. Molecular cloning of the high affinity calcium-binding protein (calreticulin) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1989;264:21522–8. [PubMed] [Google Scholar]
- 16.Smith MJ, Koch GL. Multiple zones in the sequence of calreticulin (CRP55, calregulin, HACBP), a major calcium binding protein ER/SR protein. EMBO J. 1989;8:3581–6. doi: 10.1002/j.1460-2075.1989.tb08530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krause KH, Michalak M. Calreticulin. Cell. 1997;88:439–43. doi: 10.1016/s0092-8674(00)81884-x. [DOI] [PubMed] [Google Scholar]
- 18.Michalak M, Corbett EF, Mesaeli N, Nakamura K, Opas M. Calreticulin: one protein, one gene, many functions. Biochem J. 1999;344:281–92. [PMC free article] [PubMed] [Google Scholar]
- 19.Gelebart P, Opas M, Michalak M. Calreticulin, a Ca2+-binding chaperone of the endoplasmic reticulum. Int J Biochem Cell Biol. 2000;37:260–6. doi: 10.1016/j.biocel.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 20.Sánchez D, Tučková L, Šebo P, et al. Occurrence of IgA and IgG autoantibodies to calreticulin in coeliac disease and various autoimmune diseases. J Autoimmun. 2000;15:441–9. doi: 10.1006/jaut.2000.0452. [DOI] [PubMed] [Google Scholar]
- 21.Sánchez D, Tučková L, Mothes T, Kreisel W, Beneš Z, Tlaskalová-Hogenová H. Epitopes of calreticulin recognised by IgA autoantibodies from patients with hepatic and coeliac disease. J Autoimmun. 2003;21:383–92. doi: 10.1016/s0896-8411(03)00137-9. [DOI] [PubMed] [Google Scholar]
- 22.Walker-Smith JA, Guadalini S, Schmitz J, Shmerling DH, Visakorpi JK. Revised criteria for diagnosis of coeliac disease. Report of working group of European Society of Pediatric Gastroenterology and. Nutrition. Arch Dis Child. 1990;65:909–11. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–94. doi: 10.1097/00042737-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Al-Toma A, Verbeek WHM, Mulder CJJ. Update on the management of refractory coeliac disease. J Gastroitestin Liver Dis. 2007;16:57–63. [PubMed] [Google Scholar]
- 25.Cellier C, Patey N, Mauvieux L, et al. Abnormal intestinal intraepithelial lymphocytes in refractory sprue. Gastroenterology. 1998;114:471–81. doi: 10.1016/s0016-5085(98)70530-x. [DOI] [PubMed] [Google Scholar]
- 26.Daum S, Hummel H, Weiss D, et al. Refractory sprue syndrome with clonal intraepithelial lymphocytes evolving into overt enteropathy-type intestinal T-cell lymphoma. Digestion. 2000;62:60–5. doi: 10.1159/000007779. [DOI] [PubMed] [Google Scholar]
- 27.Rongey C, Micallef I, Smyrk T, Murray J. Successful treatment of enteropathy-associated T-cell lymphoma with autologous stem cell transplant. Dig Dis Sci. 2006;51:1082–86. doi: 10.1007/s10620-006-8013-z. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli UK, Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973;80:575–99. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- 29.Michalak M. Calreticulin. Austin: R. G. Landes Company; 1996. [Google Scholar]
- 30.Krupičková S, Tučková L, Flegelová Z, et al. Identification of common epitopes on gliadin, enterocytes, and calreticulin recognised by antigliadin antibodies of patients with coeliac disease. Gut. 1999;44:168–73. doi: 10.1136/gut.44.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Højrup P, Roepstorff P, Houen G. Human placental calreticulin characterization of domain structure and post-translational modifications. Eur J Biochem. 2001;268:2558–65. doi: 10.1046/j.1432-1327.2001.02138.x. [DOI] [PubMed] [Google Scholar]
- 32.Tan Y, Chen M, Li Z, Mabuchi K, Bouvier M. The calcium- and zinc-responsive regions of calreticulin reside strictly in the N-/C-domain. Biochim Biophys Acta. 2006;1760:745–53. doi: 10.1016/j.bbagen.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Ogden CA, deCathelineau A, Hoffmann PR, et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–95. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pittoni V, Valesini G. The clearance of apoptotic cells: implications for autoimmunity. Autoimmun Rev. 2002;1:154–61. doi: 10.1016/s1568-9972(02)00032-0. [DOI] [PubMed] [Google Scholar]
- 35.Ghebrehiwet B, Peerschke EI. cC1q-R (calreticulin) and gC1q-R/p33: ubiquitously expressed multi-ligand binding cellular proteins involved in inflammation and infection. Mol Immunol. 2004;41:173–83. doi: 10.1016/j.molimm.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Diosdado B, van Oort E, Wijmenga C. Coelionomics’: towards understanding the molecular pathology of coeliac disease. Clin Chem Lab Med. 2005;43:685–95. doi: 10.1515/CCLM.2005.117. [DOI] [PubMed] [Google Scholar]
- 37.Eggleton P, Llewellyn DH. Pathophysiological roles of calreticulin in autoimmune disease. Scand J Immunol. 1999;49:466–73. doi: 10.1046/j.1365-3083.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- 38.Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 39.Basu S, Srivastava PK. Calreticulin, a peptide-binding chaperone of the endoplasmic reticulum, elicits tumor- and peptide-specific immunity. J Exp Med. 1999;189:797–802. doi: 10.1084/jem.189.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eggleton P. Stress protein–polypeptide complexes acting as autoimmune triggers. Clin Exp Immunol. 2003;134:6–8. doi: 10.1046/j.1365-2249.2003.02263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staikou EV, Routsias JG, Makri AA, et al. Calreticulin binds preferentially with B cell linear epitopes of Ro60 kD autoantigen, enhancing recognition by anti-Ro60 kD autoantibodies. Clin Exp Immunol. 2003;134:143–50. doi: 10.1046/j.1365-2249.2003.02246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


