Abstract
Aquaporin 5 (AQP5) is one of the water channel proteins which participate in a wide array of physiological processes and are primary determinants of membrane osmotic water permeability. The AQP5 gene is located in human chromosome 12q, the same region as the location of the major asthma susceptibility loci. In this study we try to determine whether the AQP5 knock-out has some effect on allergen-induced asthma. With a mouse asthma model induced by ovalbumin (OVA), we found that deletion of AQP5 reduced some major characteristic features of asthma, such as less inflammation cell infiltration in lung tissues, lower cytokine expression and fewer inflammation cells in bronchoalveolar lavage fluids compared with those from wild-type (WT) mice. Because it was found that mice injected intratracheally with OVA-pulsed dendritic cells (DCs), the AQP5 gene knock-out (AQP5−/−) ones presented fewer inflammation cells. Because DCs are major antigen-presenting cells that play an important role in antigen-induced asthma, we also probed into the possible effect of gene knock-out on DCs. Surprisingly, reverse transcription–polymerase chain reaction and fluorescence activated cell sorter analysis showed high levels of AQP5 on the surface of DCs from in vivo or bone marrow monocyte-derived DCs (mDC) in vitro. Immature mDC from AQP5 knock-out mice (AQP5−/−) showed decreased expression of CD80 and CD86 and endocytosis ability compared with that from WT, but the difference disappeared after mDCs matured with lipopolysaccharide. AQP5-mediated water transmembrane may play some role in the function of DCs. However, the mechanism of the effect of AQP5 on the DCs' function needs to be investigated further.
Keywords: antigen-presenting function, aquaporin, asthma, dendritic cell, inflammation
Introduction
Aquaporins (water channel proteins) participate in a wide array of physiological processes and are the primary determinants of membrane osmotic water permeability [1]. Aaquaporin 5 (AQP5) also has different functions ex vivo; for example, silencing AQP5 reduces mucin secretion, especially MUC5AC [2], which prompted the postulation that deletion of AQP5 was related to hypersecretory respiratory diseases, such as chronic obstructive pulmonary disease and asthma. The airway of AQP5 ablation mice was hyperresponsive to cholinergic stimulation compared with that of wild-type (WT) mice [3]. Previous genetic studies have identified major asthma-susceptibility loci on human chromosome 12q, the same region as the location of the AQP5 gene [4,5], which may point to some potential functional relation between the two genes. β-adrenergic agonist, a type of bronchodilator used in asthma, could produce increased abundance and redistribution of AQP5 on the apical membrane of mouse lung epithelial cells (MLE-12) through the cyclic adenosine-5′-monophosphate–protein kinase A-dependent pathway [6]. Expression of AQP3 and AQP7 in dendritic cells (DCs) and the role of these AQPs in volume regulation have been reported previously [7], and the findings of AQP1, AQP3 and AQP5 expression in lymphocytes and AQP3 and AQP5 expression in DCs suggests that these AQPs may play a similar role in volume regulation of lymphocytes and DCs [8]. That AQPs are also expressed in activated lymphocytes and immature DCs suggests a possible role of AQPs in volume regulation during the proliferation and activation of these cells, and also an opportunity to be a special activation or differentiation marker [7,8]. The organomercurial sulphydrylreactive reagent, p-chloromercuribenzenesulphonate, can inhibit fluid phase reversibly but not receptor-mediated endocytosis, indicating that AQPs play an essential role in the process of antigen uptake and concentration via fluid phase macropinocytosis.
Between antigen capture and antigen presentation to T cells, DCs undergo a maturation programme that governs their capacity to generate the major histocompatibility complex (MHC)–peptide complexes, migrate toward lymph nodes and stimulate T cells [9]. DCs direct the differentiation of CD4+ T cells into either interferon (IFN)-γ-producing T helper type 1 (Th1) cells or interleukin (IL)-4-producing Th2 cells [10,11]. Because DCs are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice [3], repeated allergen exposure would lead to progressive decreases in airway hyperresponsiveness and allergic inflammation through decrease in myeloid DC numbers and DC function [12].
Study of AQP1 has indicated that it has multiple biological functions and plays an important role in angiogenesis and metastasis, mainly because normal water movment can influence the formation of the cytoskeleton. It was then postulated that AQP5 expression on the surface of DCs may play some role. It was also reported that AQP5 played an important role in ovalbumin (OVA)-induced airway inflammation, and provided some direct evidence that AQPs had a multiple biological function.
Materials and methods
Animals
AQP5−/− mice (gift from Dr A. S. Verkmann, Califonia University) were generated as described previously [13] and back-crossed to a C57BL/6 background for six to 10 generations. C57BL/6 or BALB/c mice were used as controls. Mice transgenic for the OVA323–339-specific and I-Ad-restricted DO11·10 T cell receptor αβ on a BALB/c genetic background were provided by the National Resource Center for Mutant Mice Model Research Center in Nanjin University. All studied mice were age- and sex-matched and bred in a specific pathogen-free environment. Our experiments were conducted following the guidelines of the Fudan University, Institutional Animal Care and Use Committee.
Mouse models of asthma induced by systemic intraperitoneal sensitization
All mice (n = 12) were sensitized to OVA by intraperitoneal (i.p.) injection of OVA/alum (10 µg OVA grade V adsorbed to 1 mg aluminium hydroxide; Sigma-Aldrich, St. Louis, MO, USA) on days 0, 7 and 15 and were subjected to OVA aerosol challenges (grade III) on days 26–28; aerosol challenges were dispensed from a jet nebulizer delivering 1% OVA in phosphate-buffered saline (PBS) for 30 min. Sham-sensitized animals received PBS i.p. Twenty-four hours after the last OVA exposure, bronchoalveolar lavage (BAL) was performed and lungs and lymph nodes were resected and digested using collagenase/DNase [14]. The mice used only for proliferation were subjected to OVA by i.p. injection.
Bronchoalveolar lavage
On day 28 of the experiment, 24 h after the last aerosol challenge, mice were killed by sodium pentobarbital overdose (60 mg/kg body weight). BAL was performed with 3 × 1 ml of Ca2+ and Mg2+ free Hank's balanced salt solution (HBSS) supplemented with 0·05 mM sodium ethylenediamine tetraacetic acid (EDTA) [3]. The BAL fluid was centrifuged (10 min, 4°C, 700 g), and supernatant was collected and stored at −80°C until cytokine content was analysed. After resuspension in HBSS, cells were counted in a haemocytometer (Coulter Counter, Hertfordshire, UK). Differential cell counts were performed on cytospin preparations (Cytospin 2; Shandon, Cheshire, UK) stained with May–Grünwald–Giemsa by classification of 300 cells on standard morphological criteria. The cytokine content in unconcentrated BAL fluid was determined using commercially available cytokine enzyme-linked immunosorbent assay (ELISA) kits. The ELISA test for determination of murine IFN-γ had a sensitivity of 2 pg/ml (R&D Systems, Abingdon, UK). The ELISA assay for determination of murine IL-4 had a sensitivity of 5 pg/ml (Biotrak; Life Science, Amersham, UK).
Flow cytometric analysis of BAL fluid cells
Monoclonal antibodies conjugated to phycoerythrin (PE) or fluorescein isothiocyanate (FITC) were purchased from Pharmingen (San Diego, CA, USA). All reactions were performed on ice in staining buffer [PBS, 1% bovine serum albumin and 0·02% sodium azide]. Aliquots of 2 × 105 BAL cells were incubated for 15 min with 2·4G2 monoclonal antibodies (mAb) at 5 mg/ml to reduce non-specific binding via FcγRII (CD32). After washing in staining buffer, cells were stained with anti-CD3-PE (clone 145-2C11) and anti-CD4-FITC (clone H129·19), anti-CD8α-FITC (clone 53–6·7) or anti-B cell mAb B-220-FITC (CD45R, clone RA3-6B2) at 1 µg of mAb/106 cells for 30 min. Controls were generated by staining cells under identical conditions with fluorochrome-conjugated irrelevant myeloma immunoglobulin G (IgG). Cells were fixed in 1% paraformaldehyde in PBS and analysed on a fluorescence activated cell sorter (FACS)-Vantage flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA). Data were acquired in list mode on 1 × 104 cells and analysed.
Lung tissue pathology
Some mice (six mice in each group) were killed and saline was perfused through the right ventricle to remove blood. Total lung from the trachea were taken and fixative (4% paraformaldehyde in PBS) was infused gently through the lavage catheter by a continuous-release pump under pressure- and volume-controlled conditions. The lungs were resected and fixed for an additional 4 h. After routine paraffin embedding, 4 µm sections were stained with May–Grünwald–Giemsa and haematoxylin and eosin and examined by light microscopy for histological changes. Assay of the goblet cells was performed according to the study by Grünig et al.[15]). Namely, periodic acid-Schiff (PAS)-stained positive lung sections were examined at 200× magnification. Twenty to 50 consecutive airways from WT and AQP5−/− mice sensitized and challenged with OVA were categorized according to the abundance of PAS+ goblet cells and assigned numerical scores (0: 5% goblet cells; 1: 5–25%; 2: 25–50%; 3: 50–75%; 4: 75%). All airway scores from each lung were divided by the airway number examined for the score of goblet cells (expressed as arbitrary units: U) [15].
Measurement of OVA-specific IgE
Ovalbumin-specific IgE was determined by coating plates overnight with 10 µg/ml OVA grade V (Sigma-Aldrich). Serial dilutions of serum were applied, followed by biotin-conjugated anti-mouse IgE. A serum pool of OVA-sensitized mice was used as internal laboratory standard. A 1 : 100 dilution of this pool was chosen as U. The lower detection limit of this assay is 0·00165 U/ml.
Preparation of monocyte-derived DCs, splenic monolymphocytes, cell isolation and co-culture studies
Mouse femur was flushed aseptically with RPMI-1460 medium. Bone marrow cells recovered were incubated in recombinant mouse granulocyte–macrophage colony-stimulating factor (10 ng/ml) and IL-4 (1 ng/ml) containing medium for 7 days according to our laboratory procedure. At day 3, the media were changed by half; at day 5, lipopolysaccharide (LPS) (100 ng/ml) was added to the media for 24 h; and at day 7, some of the cells were purified further by anti-CD11c-coated magnetic affinity cell sorting beads (Miltenyi Biotec, Auburn, CA, USA) and part of the cultured cells were used for assay the expression of surface molecular. The cells thus cultured were > 95% CD11c+ by flow cytometry monocyte-derived DCs (mDCs), which were used for endocytosis assay.
The spleens were cut into small fragments, and then digested with collagenase D (1 mg/ml; Sigma-Aldrich) and DNase I (0·02 mg/ml; Roche Diagnostics Corporation, Indianapolis, IN, USA) in RPMI-1640 medium supplemented with 5% fetal calf serum (FCS) for 20 min at 37°C. Digested fragments were washed twice in PBS/2% FCS/5 mM EDTA, and single-cell suspensions were made. For the functional studies, splenic T cells were purified using anti-CD4 magnetic beads (Miltenyi Biotec) (purity > 95%). For the DC proliferation assays, whole DCs were stimulated with the following I-Ab-binding peptides: OVA323–339 (ISQAVHAAHAEINEAGR), synthesized at the Shanghai Biochem Corporation Ltd (Shanghai, China). For the T cell–antigen-presenting cell (APC) co-culture study, splenic T cells (1 × 105/ml) purified with anti-CD4 beads and bone marrow-derived DCs (5 × 104/ml, or 1 × 105/m) pulsed with OVA (1000 nM) peptide were co-cultured for 24–108 h, and the T cell response was ascertained by measurement of thymidine incorporation, as described previously. In another experiment, T cells from corresponding genotype mice were used for assaying the function of antigen-presenting DCs. In addition, IFN-γ and IL-4 production was assessed using commercially available kits (R&D Systems).
Mannose receptor-mediated endocytosis
Mannose receptor-mediated endocytosis was measured as the cellular uptake of FITC–OVA and quantified by flow cytometry. Approximately 2 × 105 cells per sample were incubated in medium containing 10 µg/ml FITC–OVA for 0, 10, 20, 30 and 60 min. After incubation, cells were washed with PBS and fixed in cold 1% formalin. A minimum 1 × 105 cells per sample were used to analyse the uptake of FITC–OVA by the cells.
Reverse transcriptase–polymerase chain reaction
To detect mRNAs for selected AQP5 proteins in mDC, primers were designed from murine sequences provided by the National Center for Biotechnology Information (Bethesda, MD, USA) (GenBank no. NM_009701). The primers were synthesized by Shanghai Sangon Bioengineering Corporation Ltd (Shanghai, China). Total RNA from purified bone marrow DCs was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA). The RNA was cleaned by treatment with RNase-free DNase I (Ambion, Austin, TX, USA). Reverse transcriptase–polymerase chain reaction (RT–PCR) was performed using the ProSTAR HF single-tube RT–PCR System (Stratagene, La Jolla, CA, USA). Briefly, 100 ng of total RNA and Moloney murine leukaemia virus (MMLV) reverse transcriptase were used to reverse-transcribe cDNA at 42°C for 15 min. After inactivation of the reverse transcriptase at 95°C for 1 min, the cDNA fragments were amplified by 40 cycles of PCR (denaturing at 95°C for 30 s, annealing at 60°C for 30 s, and extension at 68°C for 2 min) using the GeneAmp PCR System 9700 (PerkinElmer, Norwalk, CT, USA). PCR products were visualized by ethidium bromide agarose gel electrophoresis and ultraviolet transillumination (Fluor-S Multi-Imager; Bio-Rad Laboratories, Philadelphia, PA, USA). A negative control without RT was included in each reaction. The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as control.
Flow cytometric analysis surface molecular and antibodies
Flow cytometric analysis was performed as per our laboratory routine. Briefly, cells were first blocked with staining medium (PBS, 5% horse serum, 0·05% azide) containing 10% normal rabbit serum. Cells were then stained on ice with optimal amounts of FITC-, PE- or biotin-conjugated primary antibodies diluted in staining medium for 30 min. The following dye-coupled antibodies were obtained from BD Biosciences Pharmingen (Mountain View, CA, USA) or eBioscience (San Diego, CA, USA), and used at pretitrated dilutions: CD11c (HL3), CD40 (3/23), CD4 (RM4-5), CD80/B7-1 (16-10A1) and CD86/B7-2 (GL1), I-Ab. Whereas the antibodies to CD11c were used to define different cell subsets, the levels of CD80, CD86, CD40 and I-Ab were used to gauge the activation/maturation status of these cells. Cell staining was analysed using a FACScan (BD Biosciences). All isotype-control staining yielded median fluorescence intensities of less than 60 units. Dead cells were excluded on the basis of scatter characteristics, and 20 000–50 000 events were acquired per sample.
Statistical analysis
Ovalbumin-specific IgE levels were log-transformed before calculation of the mean ± standard error of the mean. Comparison of means between different groups was performed with the Mann–Whitney U-test for unpaired data using the Spreadware Statistics (Spreadware, Palm Desert, CA, USA) statistical package. Differences were considered significant if P < 0·05.
Results
Aquaporin 5 depletion in sensitized animals abolishes the cardinal features of asthma
AQP5−/− mice and WT littermates were sensitized to OVA on days 0 and 15 by administering an i.p. injection of OVA, and were challenged subsequently with five OVA aerosols during days 26–30. In AQP5−/− mice histological analysis did not show peribronchial and perivascular inflammatory infiltrates as seen in WT mice (Fig. 1), the same as seen in the control mice sensitized by PBS and challenged with OVA aerosols.
Fig. 1.
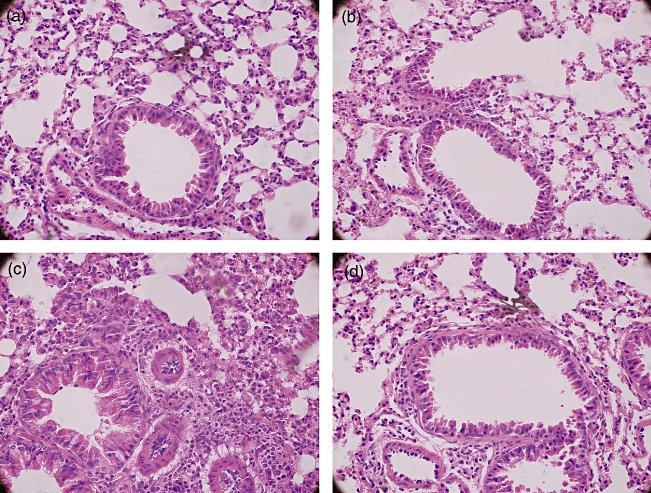
Haematoxylin and eosin (H&E) staining of the mice lung tissue. Knock-out aquaporin 5 (AQP5) reduced inflammation infiltration. (a) Wild-type (WT) mice, control; (b) AQP5−/− mice, control; (c) WT mice, asthma; (d) AQP5−/− mice, asthma (a, b, c, d: 400×). Representative H&E-stained sections are shown.
To investigate the difference of alcian blue (AB)/PAS of the goblet cells between the WT and AQP5−/− mice, we first scored the goblet cells according to Grünig's methods [15], and the results showed a lower score in AQP5−/− mice than in WT mice (Fig. 2). Then, using a semi-quantitive assay, we probed into the possible difference of AB/PAS+ staining cells between the two types of animal model. There was a lower score of AB/PAS+ staining cells in AQP5−/− mice than those of WT mice (1·333 versus 2·667, P < 0·05).
Fig. 2.
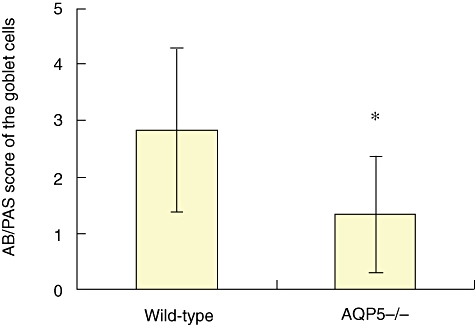
Knock-out aquaporin 5 (AQP5) attenuated the density of alcian blue/periodic acid-Schiff staining. PAS-stained positive lung sections were examined at 200× magnification. Twenty to 50 consecutive airways from wild-type (WT) and AQP5−/− mice sensitized and challenged with ovalbumin (OVA) were categorized according to the abundance of PAS+ goblet cells and assigned numerical scores (0: 5% goblet cells; 1: 5–25%; 2: 25–50%; 3: 50–75%; 4: 75%). The sum of the airway scores from each lung was divided by the number of airways examined for the histological goblet cell score (expressed as arbitrary units; U) (*P < 0·05 versus WT). Note: no data are shown from mice that were not sensitized and challenged, as the figure approximated to zero.
Analysis of BALF cells from the asthma model in AQP5−/− mice revealed a significantly lower number of total BALF cells and eosinophils compared with WT mice (Fig. 3). There was 1·87 ± 107/ml versus 7·1 ± 106/ml (P < 0·01) of total cells and 2·6 × 106/ml versus 5·3 × 105/ml (P < 0·01) of eosinophils in BALF from WT or AQP5−/− mice respectively.
Fig. 3.
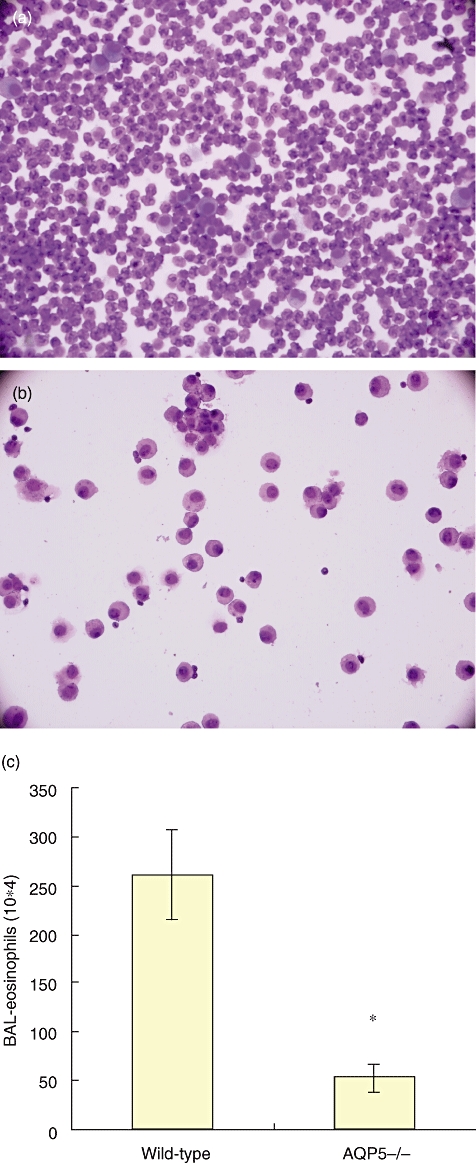
Wild-type (WT) mice show typical, whereas aquaporin 5-negative (AQP5−/−) mice show less, inflammation in the lung tissues of asthma. (a) Cells from bronchoalveolar lavage fluid (BALF) of WT mice asthma model; (b) cells from BALF of AQP5−/− mice asthma model; (c) total numbers of the eosinophils from WT and AQP5−/− mice was calculated by differential stained BALF cytospins. Bars represent the mean (± standard error of the mean) number of cells per group of mice (n = 8, *P < 0·01).
The levels of IL-4 and IFN-γ in supernatants of BALF from each group were different from each other. AQP5 deletion reduced IL-4 but promoted IFN-γ expression in BALF of the asthma animal model (Fig. 4). The concentration of IFN-γ in BALF from AQP5−/− mice was higher than in WT mice (P < 0·01), and the concentration of IL-4 in BALF from AQP5−/− mice was also lower than in WT mice (P < 0·05).
Fig. 4.
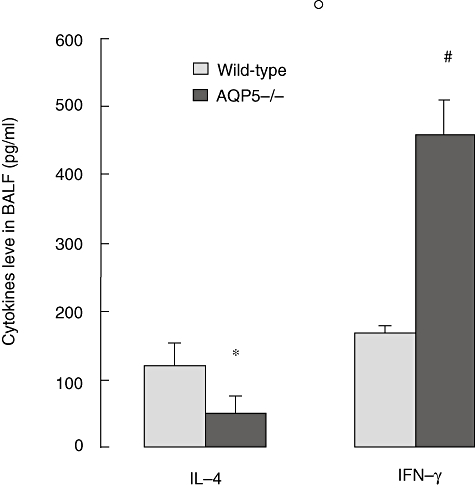
Aquaporin 5 (AQP5) deletion reduced interleukin (IL)-4 but promoted interferon (IFN)-γ expression in bronchoalveolar lavage fluid (BALF) of asthma. After the last aerosol exposure, all mice were exanguinated and lavaged with 1 ml phosphate-buffered saline repeatedly three times; the BALF was then assayed for IL-4 and IFN-γ. Results represent means ± standard error of the mean (*P < 0·05; #P < 0·01).
Also, AQP5 knock-out reduced the levels of sera OVA-specific IgE. OVA-specific IgE levels in sera from WT mice were higher than those from AQP5−/− asthma models (P < 0·01) (see Fig. 5).
Fig. 5.
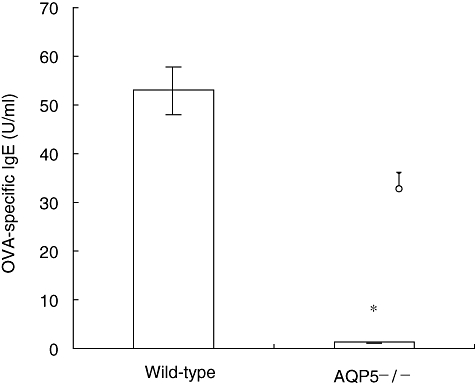
Aquaporin 5 (AQP5) knock-out reduced the level of sera ovalbumin (OVA)-specific immunoglobulin E (IgE) (*P < 0·01). OVA-specific IgE levels in sera from wild-type mice were higher than those from AQP5−/− asthma models. Coating plates overnight with 10 µg/ml OVA grade, serial dilutions of sera were applied, followed by biotin-conjugated anti-mouse IgE. A serum pool of OVA-sensitized mice was used according to the internal laboratory standard.
Effect of AQP5 on the viability and phenotye of mDC
At day 5 of in vitro culture, mDC from both the WT and AQP5−/− mice showed > 95% viability judged by trypan blue exclusion test, and an immature phenotype characterized by CD11C+ CD1a+ CD83lowIab+. Expression of inducible co-stimulator ligand (ICOSL), CD40, CD80 and CD86 from both WT and AQP5−/− mice was analysed. With RT–RCR and FACS, it showed high level of AQP5 on the surface of DCs from WT (Fig. 6). DCs from AQP5−/− mice expressed lower levels of CD80 and CD86 than those of WT DCs (Fig. 8) (CD80: 53·23% in WT versus 39·22% in AQP5−/− mice; CD86: 32·79% in WT versus 21·01% in AQP5−/− mice). After stimulation with 100 ng/ml LPS for 24 h, however, both sources of DCs showed similar levels of CD80 and CD86. There was also no difference in CD40 expression between DCs from WT and AQP5−/− mice matured or not with LPS. However, in the WT DCs, there was lower-level expression of ICOSL than in the AQP5−/− (0·75% versus 11·73%). If unstimulated with LPS (100 ng/ml) for 24 h, the expression of ICOSL on the surface of WT mice increased significantly and achieved 15·38%, which was similar to the level of DCs from AQP5−/− mice after stimulation with LPS.
Fig. 6.
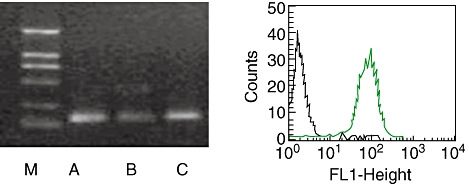
Aquaporin 5 (AQP5) expressed in dendritic cells (DCs). Left: reverse transcription–polymerase chain reaction analysis for AQP5 in lung tissue (a), spleen (b) and purified monocyte-derived DCs (mDCs) (c). DCs are purified from in vitro 5-day culture of wild-type (WT) mouse bone marrow in RPMI-1640 containing granulocyte–macrophage colony-stimulating factor and interleukin-4. One representative result of three independent experiments is shown. Expression of AQP5 is observed in lung tissue, spleen and purified DCs. The molecular weight markers (M) are also shown. Right: expression of AQP5 on immature DCs detected by flow cytometry.
Fig. 8.
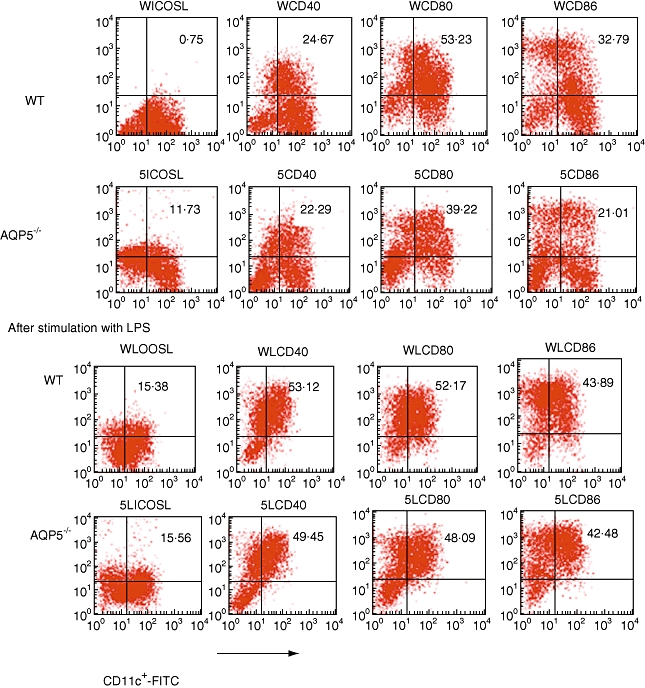
Cell surface molecule expression of bone marrow-derived dendritic cells. Columns represent binding of individual monoclonal antibodies to cultured cells observed in wild-type and aquaporin 5-negative mice. All test were repeated three times.
Effect of AQP5 on the endocytosis of DCs and mixed lymphocyte reaction
With OVA–FITC, endocytosis of DCs were investigated. After co-incubation with OVA–FITC for 10, 20, 30 and 60 min, DCs were washed and detected by FACS. The result indicated that the uptake ability of AQP5−/− DCs was lower than that of the WT DCs at each time-point (Fig. 7, P < 0·05).
Fig. 7.
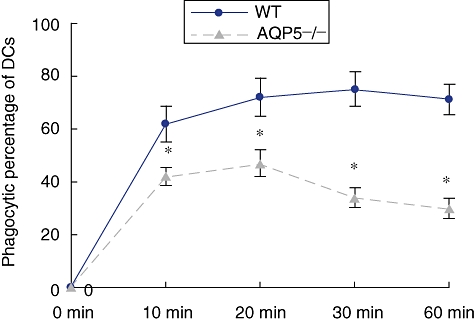
Endocytosis ability of dendritic cells (DCs) from wild-type (WT) and aquaporin 5-negative (AQP5−/−) mice. DCs were co-cultured with 100 µg/ml ovalbumin-fluoresein isothiocyanate for different times and then measured by fluorescence acticated cell sorter analysis. Enhanced endocytosis of AQP5-mediated water permeability by DCs. Results are expressed as mean ± standard deviation endocytic percentage (six mice, six controls). A significant enhancement of particle endocytosis by DCs from WT mice in 10, 20, 30 and 60 min was observed compared with AQP5−/− (*P < 0·05).
Next, we wanted to determine whether the reduced endocytosis function played a role in the allo- and autostimulatory capacity. To address this question, DC-induced primary mixed lymphocyte reaction and OVA-specific T cell activation were tested in this study. DCs from WT mice had increased allostimulatory capacity compared with AQP5−/− DCs at ratios of 5 : 1 or 10 : 1 (Fig. 9, effector cells to stimulatory cells). If the effector cells were replaced with OVA-specific CD4+ T cells isolated from F1 generation of DO11.10 and C57 mice, the [3H]-TdR incorporation (counts per minute) of the WT DCs was higher than that of AQP5−/− DC.
Fig. 9.
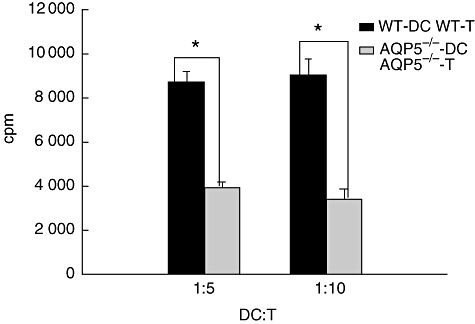
Expression of aquaporin 5 (AQP5) in dendritic cells (DCs) related to the change of stimulating function: AQP5 knock-out reduced the stimulation index by incubating DCs and antigen-specific T cells. Shown here are the different ratios of stimulators and targets. *Compared with AQP5−/−, P < 0·05.
Discussion
Previously published studies have addressed functional questions limited to the AQP5 in type I cells in fluid clearance and airspace-capillary osmotic water permeability [16,17]. However, Krane [3]et al. have suggested that deletion of AQP5 increase the airway hyperresponsiveness of mouse to cholinergic stimulation, which indicates that AQP5 might play some novel roles in those diseases, such as asthma, resulting from airway hyperresponsiveness. To confirm this presumption, a mouse asthma model was induced in AQP5−/− mice or their littermates, and AQP5−/− mice showed less inflammatory cell infiltration, fewer total cells in BALF and lower OVA-specific IgE in sera compared with those from WT littermates. Therefore, it may be concluded that deletion of AQP5 in mice abrogated the characteristic features of asthma.
Allergic asthma is a chronic inflammatory disease of the airways associated with a predominant Th2 response to inhaled allergens, leading to airway infiltration by eosinophils and mast goblet cell hyperplasia and bronchial hyperactivity. In this process, in animals or humans, DCs play a very important role. DCs are important APCs in the immune system, recognized mainly for their extraordinary capacity to induce primary immune responses [18]. Airway DCs from a network in the bronchial epithelium capture inhaled antigen, and migrate to the mediastinal lymphoid nodes where antigen is presented to recirculating naive CD4+ and CD8+ T cells [19]. Not surprisingly, antigen presentation by airway DCs is the basis of the Th2 sensitization process that occurs in patients with allergy and animals exposed to OVA antigen [20]. It is also known that the number and maturation state of lung DCs is elevated during secondary immune challenge with allergens and during chronic airway inflammation [21]. It has been shown that DCs are involved functionally in presenting allergens to T cells and, thus, control airway inflammation [20,22]. We wondered whether or not DCs from AQP5−/− mice are deficient in some functions.
Dendritic cells express the aquaporins AQP3 and AQP7 [8]. Immature DCs express these aquaporins, but down-regulate their expression concomitantly with maturation and the down-regulation of constitutive macropinocytosis. Blockade of the aquaporins reduced fluid-phase, but not receptor-mediated, endocytosis [8]. Thus, DCs are highly specialized to internalize large volumes of solute. In this study AQP5 was expressed in DCs, which was investigated with FACS and RT–PCR assay in both the activated and non-activated DCs. We also examined the expression of AQP3 in mice DCs, which showed positive results (data not shown). However, Moon et al.[7] provided data suggesting that the expression of AQP3 and AQP5 was detected in DCs, not in non-activated but in activated DCs. This difference, we assume, was due to the sensitivity of the assay used.
Verkmann concluded that aquaporins have some unexpected cellular roles more than just water channels [23]. Whether in humans or animals, it is known that abnormality of the expression of AQPs impairs cell motility [24–28]. In humans, AQP9 expressed in neutrophils [24] regulated lamellipodium formation and neutrophil motility if anti-AQP9 antibodies, HgCl2 or tetraethyl ammonium were added, which inhibit the function of AQP9, and then motility-related shape changes were blocked. As is known, the ability of neutrophils to sense and move to sites of infection is essential for human defence against pathogens, so inhibition of AQP9 would impair the human defence function. Loitto and Magnusson [25] found that Hg2+ affected host defence, in general, and neutrophil functions. Exposure of human neutrophils to HgCl2 impairs chemoattractant-stimulated motility dose-dependently through altering membrane permeability by affecting the bidirectional flux through the leucocyte AQP9. AQP7 [26] was expressed at the tail of spermatids and spermatozoa in the human testis. Some infertile patients lacked AQP7 expression in ejaculated sperm, although all fertile men expressed AQP7 protein. The motility rate of AQP7-negative sperm was significantly lower than that of AQP7-positive sperm, while sperm concentration was not different between AQP7-positive and -negative subjects. Aquaglyceroporin (lmAQP1) in Leishmania modulates drug uptake and drug resistance [27], whereas glucose-driven, AQP-mediated localized water influx is involved in the membrane protrusion during Cryptosporidium parvum cellular invasion [28], phenomena that may also be relevant to the mechanism of cell membrane protrusion in general. Therefore, we presume that deficiency of AQP5 in DCs impairs the ability of water transport across the membrane; the cells cannot then regulate their volume and cannot protrude into the membrane. Cell migration involves a series of cellular events, including actin polymerization and depolymerization, and the generation of cell protrusions such as lamella, lamellipodia and blebs [29]. We and other authors have proposed that AQP-facilitated water permeability in cell protrusions enhances their formation and thus the rate of cell migration [23,30]. In previous reports [30–32], polarization of various AQP-expressing cells would form lamellipodia and increase number/size of lamellipodia, which was also found in the DCs in our study. Local actin depolymerization, which creates new osmoles, might provide the osmotic driving force responsible for water influx into cell protrusions, as might transmembrane solute transport and/or altered solute osmotic coefficients in submembrane cytoplasm [29,33]. Thus, it is predicted that cell membrane water permeability at its migrating surface may be an important determinant of tumour cell migration, which would depend upon membrane lipid composition, the presence and membrane polarization of AQPs and the biophysical properties of submembrane cytoplasm.
Our study indicates that AQP5−/− DCs reduce endocytosis of antigen, which indicates that uptake of DCs is related to crossing the water transmembrane. For this reason, if plasmids expressing AQP5 were transfected to the AQP5−/− DCs, endocytosis ability is reversed (unpublished data from our laboratory). This phenomenon partly interpreted the impaired phagocytosis function as being due to the deficiency of AQP5 in DCs. It is indicated that AQP5−/− DCs have less expression of CD80 and CD86 than WT DCs before being stimulated with LPS. Other work has been conducted showing that reduced endocytosis had a possible relation to the function of antigen-presenting DCs (these data not published here). First, DCs isolated from AQP5−/− and WT mice, pulsed with OVA, were administered intratracheally into WT mice. The following day, all these mice were challenged with 1% OVA. It was found that the lung infiltration showed to a lesser extent from mice that received AQP5−/− DCs than those that received WT DCs. This result provides evidence that AQP5 deficiency may have a relation to its function. Further research on DCs function and AQP5 ablation needs to be done.
The B7 family members, B7-1 (CD80) and B7-2 (CD86), bind to specific ligand CD28 or cytotoxic T lymphocyte antigen-4 on the T cell surface, in which procedure signals generated may regulate the activation of memory or effector T cells. B7-2 (CD86) regulates survival, phenotype and function of APCs [34]. A significant reduction in phagocytic ability was observed in both splenic and pancreatic lymph node-associated DCs in B7-2 knock-out mice. DCs from B7-2 KO mice exhibited enhanced susceptibility to death, which was reflected by their reduced total cell numbers. CD28 induces immunostimulatory signals in DCs via CD80 and CD86; the production of IL-6 required B7-1 (CD80), B7-2 (CD86) and p38 mitogen-activated protein kinase and prevented IFN-γ-driven expression of immunosuppressive tryptophan catabolism [35]. That DC function is impaired is related to the lesser inflammatory cell infiltration in lungs from AQP5−/− mice that suffered from OVA-induced asthma.
In addition to this, the ability of DCs to migrate to the draining lymph node has a very important impact on lymphocyte trafficking and priming [36]. Having greater migration efficiency to the draining lymph node, CCR7+/+ DCs induced a rapidly increased cellularity in the lymph node, which was observed before the onset of T cell proliferation. Many researchers have found that AQPs mediate rapid water-transport across the cell membrane [30], which promotes the membrane protrusion and facilitates migration of the cells. Therefore, we postulate that deletion of AQP5 would impair the mobility of DCs. Whether this procedure is via actin remodelling or not requires further research.
Acknowledgments
This work was supported by the Shanghai Leading Academic Discipline Project, Project no. B115.
References
- 1.King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 2004;5:687–98. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z, Wang X, Gao L, Li B, Zhu R, Bai C. Regulation of MUC5AC mucin secretion by depletion of AQP5 in SPC-A1 cells. Biochem Bioph Res Commun. 2006;342:775–81. doi: 10.1016/j.bbrc.2006.01.103. [DOI] [PubMed] [Google Scholar]
- 3.Krane CM, Fortner CN, Hand AR, et al. Aquaporin 5-deficient mouse lungs are hyperresponsive to cholinergic stimulation. Proc Natl Acad Sci USA. 2001;98:14114–19. doi: 10.1073/pnas.231273398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee MD, Bhakta KY, Raina S, et al. The human aquaporin-5 gene: molecular characterization and chromosomal localization. J Biol Chem. 1996;271:8599–604. doi: 10.1074/jbc.271.15.8599. [DOI] [PubMed] [Google Scholar]
- 5.Malerba G, Lauciello MC, Scherpbier T, et al. Linkage analysis of chromosome 12 markers in Italian families with atopic asthmatic children. Am J Respir Crit Care Med. 2000;162:1587–90. doi: 10.1164/ajrccm.162.4.9909031. [DOI] [PubMed] [Google Scholar]
- 6.Sidnaye V, Hoffert JD, King LS. cAMP has distinct acute and chronic effects on aquaporin-5 in lung epithelial cell. J Biol Chem. 2005:3590–6. doi: 10.1074/jbc.M411038200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Baey A, Lanzavecchia A. The role of aquaporins in dendritic cell macropinocytosis. J Exp Med. 2000;191:743–8. doi: 10.1084/jem.191.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon C, Rousseau R, Soria J-C, et al. Aquaporin expression in human lymphocytes and dendritic cells. Am J Hematol. 2004;75:128–33. doi: 10.1002/ajh.10476. [DOI] [PubMed] [Google Scholar]
- 9.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 10.Pulendran B, Smith JL, Caspary G, et al. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA. 1999;96:1036–41. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maldonado-Lopez R, De Smedt T, Michel P, et al. CD8alpha+ and CD8alpha− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–92. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koya T, Kodama T, Takeda K, et al. Importance of myeloid dendritic cells in persistent airway disease after repeated allergen exposure. Am J Respir Crit Care Med. 2006;173:42–55. doi: 10.1164/rccm.200505-783OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem. 1999;274:20071–4. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- 14.Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med. 2001;193:51–60. doi: 10.1084/jem.193.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grünig G, Warnock M, Wakil AE, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–3. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma T, Fukuda N, Song Y, Matthay MA, Verkman AS. Lung fluid transport in aquaporin 5 knockout mice. J Clin Invest. 2000;105:93–100. doi: 10.1172/JCI8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song Y, Yang B, Matthay MA, Ma T, Verkman AS. Role of aquaporin water channels in pleural fluid dynamics. Am J Physiol Cell Physiol. 2000;279:C1744–50. doi: 10.1152/ajpcell.2000.279.6.C1744. [DOI] [PubMed] [Google Scholar]
- 18.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 19.Lambrecht BN, Pauwels RA, Fazekas B, De St Groth ••. Induction of rapid T cell activation, division, and recirculation by intratracheal injection of dendritic cells in a TCR transgenic model. J Immunol. 2000;164:2937–46. doi: 10.4049/jimmunol.164.6.2937. [DOI] [PubMed] [Google Scholar]
- 20.Lambtecht BN, Hammad H. Taking our breath away: dendritic cells in the pathogenesis of asthma. Nat Rev Immunol. 2003;3:994–1003. doi: 10.1038/nri1249. [DOI] [PubMed] [Google Scholar]
- 21.Huh JC, Strickland DH, Jahnsen FL, et al. Bidirectional interactions between antigen-bearing respiratory tract dendritic cells (DCs) and T cells precede the late phase reaction in experimental asthma: DC activation occurs in the airway mucosa but not in the lung parenchyma. J Exp Med. 2003;198:19–30. doi: 10.1084/jem.20021328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jahnsen FL, Moloney ED, Hogan T, Upham JW, Burke CM, Holt PG. Rapid dendritic cell recruitment to the bronchial mucosa of patients with atopic asthma in response to local allergen challenge. Thorax. 2001;56:823–6. doi: 10.1136/thorax.56.11.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verkman AS. More than just water channels: unexpected cellular roles of aquaporins. J Cell Sci. 2005;118:3225–32. doi: 10.1242/jcs.02519. [DOI] [PubMed] [Google Scholar]
- 24.Loitto V-M, Forslund T, Sundqvist T, Magnusson K-E, Gustafsson M. Neutrophil leukocyte motility requires directed water influx. J Leukoc Biol. 2002;71:212–22. [PubMed] [Google Scholar]
- 25.Loitto VM, Magnusson KE. Hg2+ and small-sized polyethylene glycols have inverse effects on membrane permeability, while both impair neutrophil cell motility. Biochem Biophys Res Commun. 2004;316:370–8. doi: 10.1016/j.bbrc.2004.02.056. [DOI] [PubMed] [Google Scholar]
- 26.Saito K, Kageyama Y, Okada Y, et al. Localization of aquaporin-7 in human testis and ejaculated sperm: possible involvement in maintenance of sperm quality. J Urol. 2004;172:2073–6. doi: 10.1097/01.ju.0000141499.08650.ab. [DOI] [PubMed] [Google Scholar]
- 27.Gourbal B, Sonuc N, Bhattacharjee H, et al. Drug uptake and modulation of drug resistance in leishmania by an aquaglyceroporin. J Biol Chem. 2004;279:31010–17. doi: 10.1074/jbc.M403959200. [DOI] [PubMed] [Google Scholar]
- 28.Chen XM, O'Hara SP, Huang BQ, Splinter PL, Nelson JB, LaRusso NF. Localized glucose and water influx facilitates Cryptosporidium parvum cellular invasion by means of modulation of host-cell membrane protrusion. Proc Natl Acad Sci USA. 2005;102:6338–43. doi: 10.1073/pnas.0408563102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–69. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 30.Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–92. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- 31.Hu J, Verkman AS. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J. 2006;20:1892–4. doi: 10.1096/fj.06-5930fje. [DOI] [PubMed] [Google Scholar]
- 32.Hara-Chikuma M, Verkman AS. Aquaporin-1 facilitates migration of epithelial cells in kidney proximal tubule. J Am Soc Nephrol. 2006;17:39–45. doi: 10.1681/ASN.2005080846. [DOI] [PubMed] [Google Scholar]
- 33.Schwab A. Function and spatial distribution of ion channels and transporters in cell migration. Am J Physiol. 2001;280:F739–47. doi: 10.1152/ajprenal.2001.280.5.F739. [DOI] [PubMed] [Google Scholar]
- 34.Yadav D, Sarvetnick N. B7-2 regulates survival, phenotype, and function of APCs. J Immunol. 2007;178:6236–41. doi: 10.4049/jimmunol.178.10.6236. [DOI] [PubMed] [Google Scholar]
- 35.Orabona C, Grohmann U, Belladonna ML, et al. CD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86. Nat Immunol. 2004;5:1134–42. doi: 10.1038/ni1124. [DOI] [PubMed] [Google Scholar]
- 36.Martín-Fontecha A, Sebastiani S, Höpken UE, et al. Regulation of dendritic cell migration to the draining lymph node: impact on the lymphocyte traffic and priming. J Exp Med. 2003;198:615–21. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]


