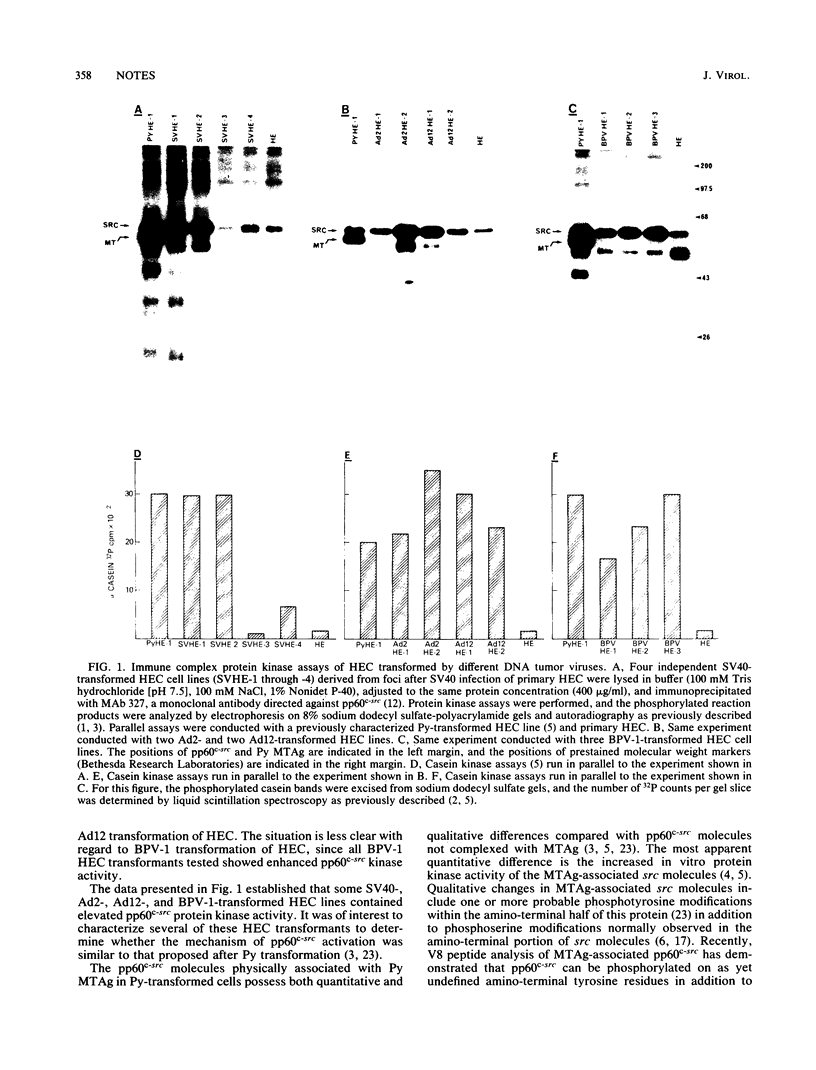
Abstract
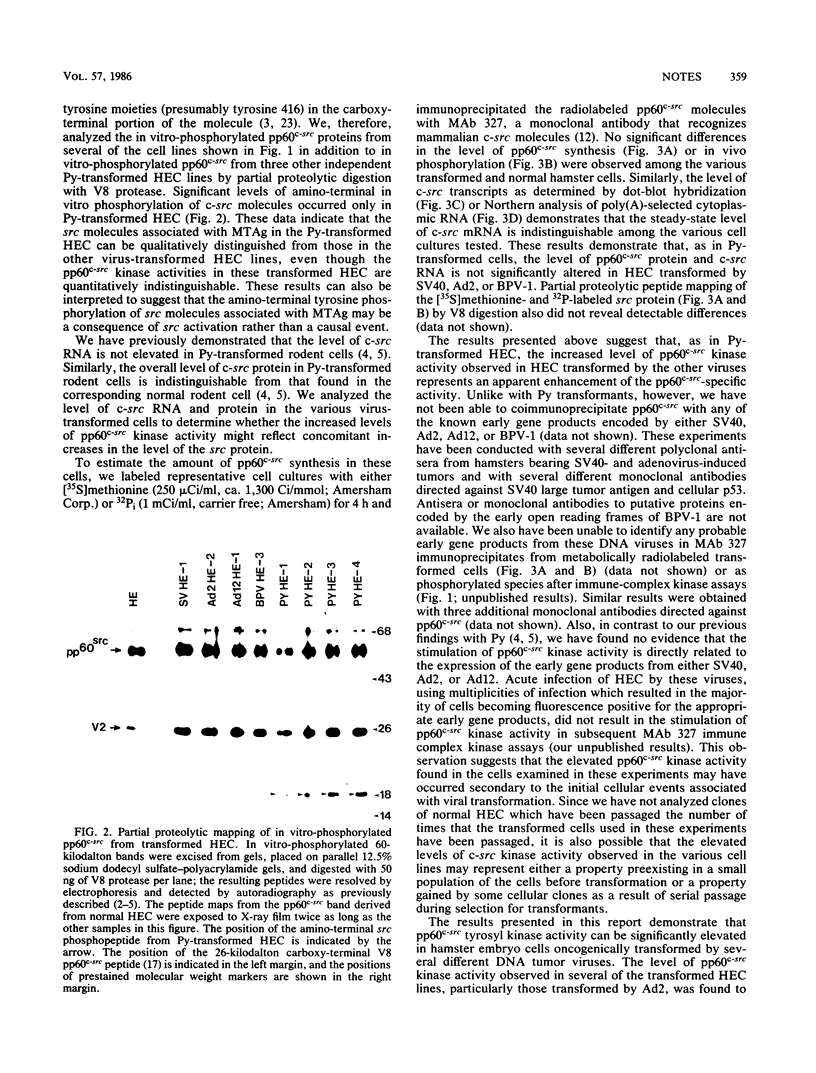
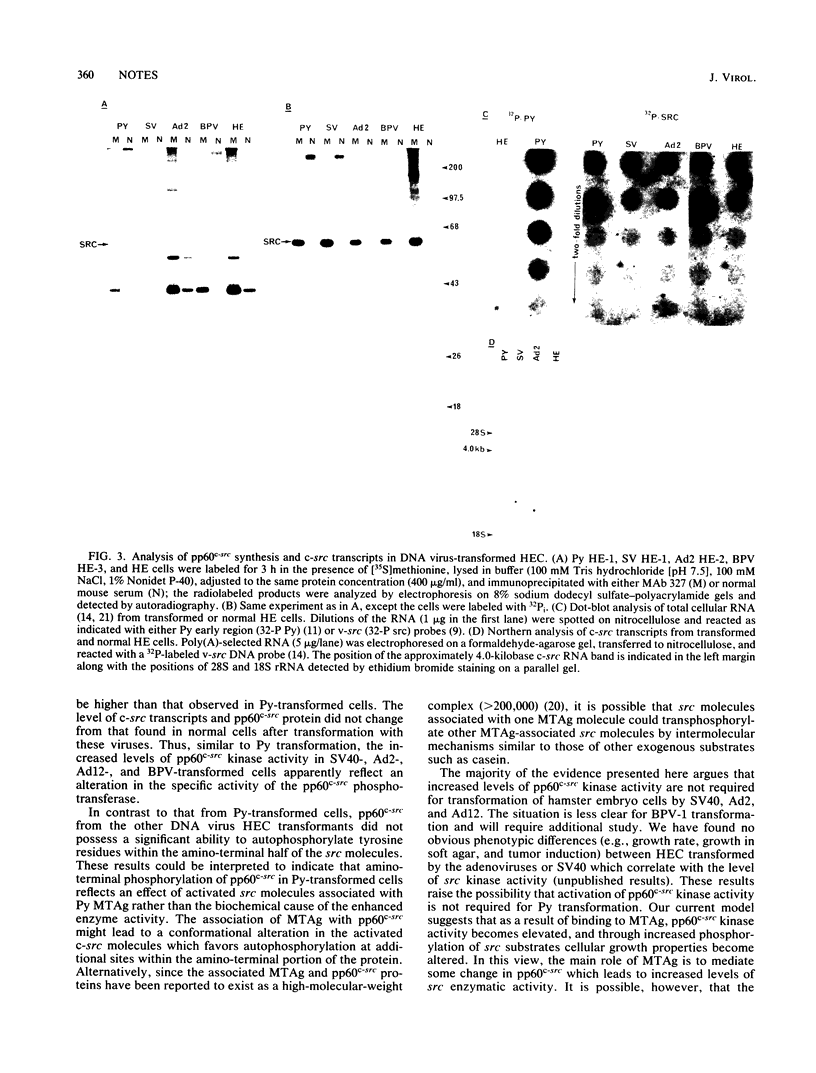
We have examined the effect of DNA tumor virus transformation of primary hamster embryo cells on the tyrosyl kinase activity of pp60c-src. Our present study demonstrates that some clones of hamster embryo cells transformed by simian virus 40, adenovirus type 2, adenovirus type 12, or bovine papillomavirus 1 can possess elevated pp60c-src kinase activity when compared with normal hamster embryo cells. However, other clones of hamster embryo cells transformed by these same viruses were found to have normal levels of pp60c-src kinase activity. In those clones of transformed cells where pp60c-src kinase activity was elevated, the increased levels of kinase activity were the result of an apparent increase in the specific activity of the pp60c-src phosphotransferase rather than an increase in the amount of the src gene product. Additionally, pp60c-src was not found to be physically associated with tumor antigens known to be encoded by these viruses. These results indicate that elevated levels of pp60c-src kinase activity can be found in hamster embryo cells transformed by several different DNA tumor viruses and suggest that the molecular mechanism by which pp60c-src kinase activity is elevated may differ from that previously observed in polyomavirus-transformed cells. These results also imply that elevation of pp60c-src kinase activity is not required for the transformation of hamster cells by these viruses.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolen J. B., Israel M. A. In vitro association and phosphorylation of polyoma virus middle T antigen by cellular tyrosyl kinase activity. J Biol Chem. 1984 Oct 10;259(19):11686–11694. [PubMed] [Google Scholar]
- Bolen J. B., Israel M. A. Inhibition of polyoma virus middle T antigen-associated tyrosyl kinase activity by N-ethylmaleimide. J Biol Chem. 1983 Dec 25;258(24):15135–15140. [PubMed] [Google Scholar]
- Bolen J. B., Israel M. A. Middle tumor antigen of polyomavirus transformation-defective mutant NG59 is associated with pp60c-src. J Virol. 1985 Jan;53(1):114–119. doi: 10.1128/jvi.53.1.114-119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Lewis A. M., Jr, Israel M. A. Stimulation of pp60c-src tyrosyl kinase activity in polyoma virus-infected mouse cells is closely associated with polyoma middle tumor antigen synthesis. J Cell Biochem. 1985;27(2):157–167. doi: 10.1002/jcb.240270209. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Thiele C. J., Israel M. A., Yonemoto W., Lipsich L. A., Brugge J. S. Enhancement of cellular src gene product associated tyrosyl kinase activity following polyoma virus infection and transformation. Cell. 1984 Oct;38(3):767–777. doi: 10.1016/0092-8674(84)90272-1. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Erikson R. L. Structural analysis of the avian sarcoma virus transforming protein: sites of phosphorylation. J Virol. 1979 Feb;29(2):770–781. doi: 10.1128/jvi.29.2.770-781.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983 Jun 2;303(5916):435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. The complex of polyoma virus middle-T antigen and pp60c-src. EMBO J. 1984 Mar;3(3):585–591. doi: 10.1002/j.1460-2075.1984.tb01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart W., Hutchinson M. A., Hunter T. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell. 1979 Dec;18(4):925–933. doi: 10.1016/0092-8674(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Israel M. A., Chan H. W., Rowe W. P., Martin M. A. Molecular cloning of polyoma virus DNA in Escherichia coli: plasmid vector system. Science. 1979 Mar 2;203(4383):883–887. doi: 10.1126/science.217087. [DOI] [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C., Baltimore D. Immunoglobulin gene transcription is activated by downstream sequence elements. Cell. 1983 Jul;33(3):741–748. doi: 10.1016/0092-8674(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. S., Benjamin T. L. Phosphorylation of polyoma T antigens. Cell. 1979 Dec;18(4):935–946. doi: 10.1016/0092-8674(79)90206-x. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. Transforming genes and gene products of polyoma and SV40. CRC Crit Rev Biochem. 1982;13(3):215–286. doi: 10.3109/10409238209114230. [DOI] [PubMed] [Google Scholar]
- Smart J. E., Oppermann H., Czernilofsky A. P., Purchio A. F., Erikson R. L., Bishop J. M. Characterization of sites for tyrosine phosphorylation in the transforming protein of Rous sarcoma virus (pp60v-src) and its normal cellular homologue (pp60c-src). Proc Natl Acad Sci U S A. 1981 Oct;78(10):6013–6017. doi: 10.1073/pnas.78.10.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. E., Smith R., Griffin B., Fried M. Protein kinase activity associated with polyoma virus middle T antigen in vitro. Cell. 1979 Dec;18(4):915–924. doi: 10.1016/0092-8674(79)90204-6. [DOI] [PubMed] [Google Scholar]
- Walter G., Hutchinson M. A., Hunter T., Eckhart W. Purification of polyoma virus medium-size tumor antigen by immunoaffinity chromatography. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4025–4029. doi: 10.1073/pnas.79.13.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M., Kaplan D. R., Schaffhausen B., Cantley L., Roberts T. M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985 May 16;315(6016):239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- Yonemoto W., Jarvis-Morar M., Brugge J. S., Bolen J. B., Israel M. A. Tyrosine phosphorylation within the amino-terminal domain of pp60c-src molecules associated with polyoma virus middle-sized tumor antigen. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4568–4572. doi: 10.1073/pnas.82.14.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]