Abstract
It is known that the same antigen can induce different immune responses, depending upon the way that it is presented to the immune system. The objective of this study was to compare cytokine responses of peripheral blood mononuclear cells (PBMC) from cutaneous leishmaniasis patients and subjects immunized with a first-generation candidate vaccine composed of killed Leishmania amazonensis promastigotes to a whole-cell promastigote antigen extract (La) and to the recombinant protein LACK (Leishmania analogue receptor for activated C kinase), both from L. amazonensis. Thirty-two patients, 35 vaccinees and 13 healthy subjects without exposure to Leishmania, were studied. Cytokine production was assessed by enzyme-linked immunosorbent assay and enzyme-linked immunospot assay. The interferon (IFN)-γ levels stimulated by La were significantly higher and the levels of interleukin (IL)-10 significantly lower than those stimulated by LACK in the patient group, while LACK induced a significantly higher IFN-γ production and a significantly lower IL-10 production compared with those induced by La in the vaccinated group. LACK also induced a significantly higher frequency of IFN-γ-producing cells than did La in the vaccinated group. The contrast in the cytokine responses stimulated by LACK and La in PBMC cultures from vaccinated subjects versus patients indicates that the human immune response to crude and defined Leishmania antigens as a consequence of immunization differs from that induced by natural infection.
Keywords: American cutaneous leishmaniasis, cytokines, LACK antigen, Leishmania amazonensis, vaccine
Introduction
Leishmaniasis is caused by protozoan parasites of the genus Leishmania, which possess two main stages in their life cycle: amastigotes inside phagocytic mononuclear cells in the mammalian host and motile promastigotes in the gut of the sandfly vector. There are several different clinical forms of leishmaniasis, which can be broadly classified as visceral and tegumentary (the latter encompassing cutaneous and mucosal). Leishmaniasis affects 88 countries, and a population of 350 million is at risk [1]. The burden of this disease as a global health problem is increasing because of the low effectiveness of current control strategies [2].
Vaccination represents a reasonable perspective for leishmaniasis control [3]. First-generation candidate vaccines consist of crude extracts of killed Leishmania promastigotes [4]. A first-generation candidate vaccine, composed of a whole-cell extract of L. amazonensis promastigotes, has been developed in Brazil and tested in several clinical trials. Although a recent study did not demonstrate a protective effect of this vaccine in Colombia [5], its high immunogenicity has been well established [5–8]. On the other hand, several parasite molecules have been identified as candidate antigens for genetically constructed second-generation vaccines [9], including the Leishmania analogue receptor for activated C kinase (LACK) [10].
There is solid evidence that the same antigen can induce different and even contrasting types of immune responses, depending upon how it is presented to the immune system. After infection of BALB/c mice with L. major, the LACK antigen induces early production of interleukin (IL)-4 within CD4+ T cells expressing Vβ4 Vα8 T cell receptors (TCRs), which initiates the development of an aberrant T helper type 2 (Th2) response leading to progressive disease [11]. In contrast, a 24-kD portion of the same antigen protected BALB/c susceptible mice against L. major infection when administered as a vaccine with IL-12 before infection [12]. DNA encoding LACK also confers protective immunity to BALB/c mice against L. major infection [13]. We used whole-cell promastigote extract and the recombinant protein LACK, both from L. amazonensis, to analyse and compare human cytokine responses primed by natural leishmanial infection and by immunization with killed promastigote antigens.
Materials and methods
Subjects
Thirty-two patients with active cutaneous leishmaniasis from endemic areas of Rio de Janeiro state were studied before treatment. Diagnosis was made based upon immunological and parasitological criteria, as described elsewhere [14]. A second group included 35 subjects immunized in former clinical trials performed approximately 1 year before this study with a first-generation candidate vaccine composed of killed L. amazonensis promastigotes, manufactured as described previously [6,8]. The vaccination schedule consisted of two doses of 1·5 ml (360 µg protein nitrogen per ml) injected intramuscularly with a 21-day interval [6,8]. A third group of 13 healthy subjects from non-endemic areas and without evidence of previous exposure to Leishmania (naive subjects) was also studied. Informed consent was obtained from all participants and the Ethics Review Committee of Fundação Oswaldo Cruz, Brazilian Ministry of Health, approved the research protocol.
Leishmania antigens
The L. amazonensis (IFLA/BR/67/PH8) promastigote whole-cell extract (La) was prepared as described previously [15].
The LACK gene cloning and recombinant protein expression were performed as follows. Total RNA was extracted from stationary phase promastigotes using standard methods [16]. Double-stranded cDNA molecules were synthesized by polymerase chain reaction (PCR) with oligonucleotides based on the L. infantum homologue gene sequence, named L1 (5′-GGGGGGATCCATGAACTACGAGGGTCACCTG-3′) and L2 (5′-GGGGAAGCTTTTACTCGGCGTCGGAGATGGAC-3′). Amplification reactions were performed in a GeneAmp PCR System 2400 thermocycler (Perkin Elmer, Norwalk, CT, USA) under the following conditions: initial denaturation at 94°C for 5 min followed by 30 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min, and a final extension at 72°C for 5 min. The amplicon was purified with the ‘Wizard Gel and PCR Clean-Up System’ (Promega, Madison, WI, USA). Both the purified amplicon and the expression vector pQE30 (Qiagen, Hilden, Germany) were digested with BamHI and HindIII, incubated with T4 ligase, and used subsequently for transformation of Escherichia coli strain DH5α cells by electroporation. The positive clones were selected by PCR and double digestion with BamHI and HindIII to confirm the presence of the inserts 939 base pairs. The LACK encoding sequence was confirmed by DNA sequencing [17] with T3, T7 and internal primers, using the Sequenase kit (United States Biochemical Co., Cleveland, OH, USA). DNA sequence analysis was performed with the dnastar (University of Wisconsin, Madison, WI, USA) and gcg (Genetics Computer Group, San Diego, CA, USA) software packages. The L. amazonensis LACK encoding sequence was deposited in the GenBank database (Accession no.: AY053464).
The pQE30 vector (Qiagen) carrying the LACK gene was used for expression of the recombinant protein in E. coli M15 strain. Bacteria were transformed with the recombinant plasmid and the expression of the LACK protein was induced subsequently with 1 mM isopropyl-β-D-thiogalactopyranoside according to standard methods [16]. Bacterial cells were harvested and stored at −70°C until use. The pellet was resuspended in lysis buffer containing 10 mM Tris pH 8, 0·1% Triton, 0·5 mM phenylmethylsulphonyl sulphide, 1 % lysozyme and 5 mM imidazole. Cells were sonicated in an ice bath throughout four cycles of 15 s at 200 W with intervals of 15 s and subsequently centrifuged. The recombinant protein was purified by chromatography using a Ni–NTA super flow column (Qiagen) according to the manufacturer's instructions. The protein was eluted in 8 M urea salt buffer with 60 mM imidazole and dialysed in phosphate-buffered saline (PBS) at pH 7·4. Further, the adsorbed recombinant protein was eluted from the chromatography affinity column through nine washes in 100 mM Tris pH 8, 300 mM NaCl, 4 M urea and increasing molarities of imidazole (i.e. 0, 5 and 20 mM). The eluted protein was pooled and dialysed in PBS at pH 7·4.
The protein extract was analysed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) 12·5% and blotted onto nitrocellulose membranes. Bands were detected by staining with Ponceau S and Ni2+–NTA conjugate (Qiagen) specific for the hexahistidine tag, present in the recombinant protein. The purified protein was also analysed by SDS-PAGE and silver nitrate staining, which indicated absence of protein contaminants (Fig. 1). The predicted amino acid sequence of the L. amazonensis LACK protein was confirmed by mass spectrometry using a matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF)/TOF 4700 Proteomics Analyzer (Applied Biosystems, Foster City, CA, USA). The levels of bacterial lipopolysaccharide (LPS) contamination in the recombinant proteins were determined using the Limulus amoebocyte lysate (LAL) test (BioWhittaker, Walkersville, MD, USA) according to the manufacturer's recommendations. LPS contamination of 0·2 µg per mg of protein was detected in the LACK preparation. Before use of the LACK samples in further experiments, their LPS content was removed using a polymyxin B-agarose column (Sigma, St. Louis, MO, USA) following the manufacturer's instructions. After this procedure, no LPS contamination was detected by the LAL assay in the recombinant protein samples.
Fig. 1.
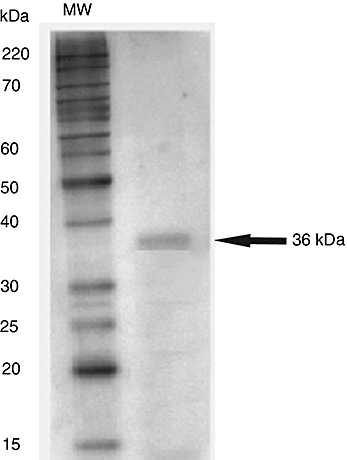
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of the Leishmania amazonensis LACK protein. The recombinant protein was expressed in Escherichia coli and purified by a one-step Ni-NTA column procedure. Six micrograms of the purified protein were submitted to 12% SDS-PAGE under reducing conditions and stained with silver nitrate. The left lane represents the molecular weight standards (MW). The right lane shows a 36 kDa band corresponding to the LACK protein in the absence of contaminants.
Both L. amazonensis antigen preparations (La and LACK) were suspended in PBS, aliquoted and stored at −70°C until use.
Cell culture and cytokine assays
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized venous blood by Ficoll–Hypaque gradient centrifugation (Sigma). After being washed three times in phosphate sodium saline, the PBMC were resuspended in RPMI-1640 medium (Sigma) supplemented with human antibody serum, 10 mM Hepes, 1·5 mM l-glutamine, mM 2- mercaptoethanol and antibiotics (200 IU/ml penicillin and 200 mg/ml streptomycin) (all purchased from Sigma). Cells were adjusted to 106 cells/ml, placed in 24-well plates and stimulated with La or LACK antigens (10 µg/ml), or left unstimulated as controls. Cultures of PBMC were incubated for 120 h at 37°C and 5% CO2. The levels of interferon (IFN)-γ, IL-5, IL-10 and tumour necrosis factor (TNF)-α in supernatants from PBMC cultures were determined by enzyme-linked immunosorbent assay (ELISA) sandwich methods, as described previously [18]. The cytokine levels shown represent the differences between stimulated and unstimulated control cultures (background). Enzyme-linked immunospot (ELISPOT) assays were performed to determine the frequency of IFN-γ- and IL-4-producing cells after antigen stimulation. Polyvinylidene fluoride plates with 96 wells (Millipore, Bedford, MA, USA) were coated with 0·5 µg mouse anti-human IFN-γ (BD Pharmingen, San Diego, CA, USA) or IL-4 monoclonal antibody (BD Pharmingen) per well. After overnight incubation at 4°C, the wells were washed with PBS and occupied sites were blocked with RPMI-1640 medium (Sigma) supplemented with 10% fetal bovine serum (Invitrogen Gibco, Grand Island, NY, USA), 1 mmol/l l-glutamine (Sigma) and 1% penicillin/streptomycin (Sigma). PBMC (2 × 105 cells/well) were preincubated in the presence or absence of the antigens (10 µg/ml) in a final volume of 200 µl/well for 2 h at 37°C in a 5% CO2 atmosphere. The cells were then transferred into the precoated polyvinylidene fluoride plates. After 40 h incubation at 37° C in a 5% CO2 atmosphere, the cells were removed, and 100 µl of biotinylated anti-IFN-γ or IL-4 monoclonal antibody (BD Pharmingen) per well were added (1 µg/ml). Four hours later, the plates were washed, and 100 µl of streptavidin–alkaline phosphatase (BD Pharmingen), diluted at 1 : 500, were added to each well. After 1 h at 37°C, the plates were washed again and incubated with 100 µl Tris buffer substrate (5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium) (Sigma) for 5–20 min at 25°C. Dark violet spots on plate membranes were counted using an ImmunoSpot® image analyser (CTL, Cleveland, OH, USA).
Statistical analysis
The Wilcoxon matched-pairs test was used to analyse differences between the results obtained with the different antigen preparations in each study group. The following tests were used to compare the study groups with regard to demographic characteristics: Kruskal–Wallis test, followed by Dunn's multiple comparison test and Fisher's exact test. Values of P < 0·05 were considered significant.
Results
The following subjects donated cells for the determination of cytokine levels by ELISA in PBMC culture supernatants: 16 patients (10 males and six females), 23 vaccinees (nine males and 14 females) and 13 naive subjects (three males and 10 females). Age median and ranges for the groups of patients, vaccinees and naive subjects were, respectively, 32 (13–69), 33 (12–73) and 42 (22–60) years. No statistical difference was found among these groups with respect to gender or age.
In the patient group, IFN-γ levels measured in the supernatants of PBMC cultures stimulated with La were significantly higher than those induced by stimulation with LACK (P < 0·05), as shown in Fig. 2a. In contrast with the patient group, LACK induced significantly higher levels of IFN-γ in the PBMC cultures from the vaccinated subjects (Fig. 2b) when compared with La (P = 0·01). No difference was observed between La and LACK with regard to IFN-γ production in the group of naive subjects.
Fig. 2.
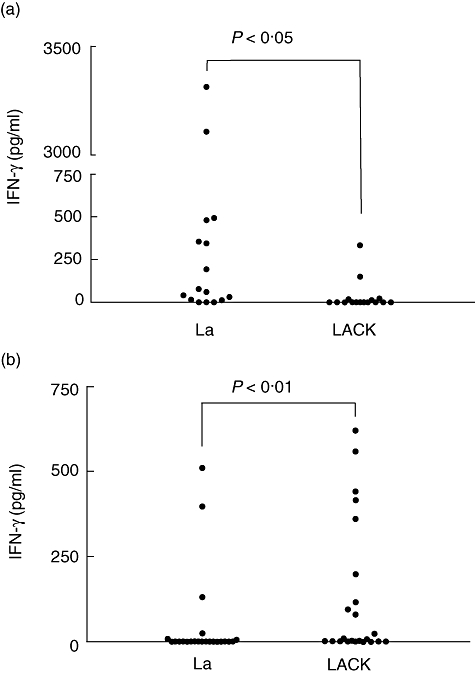
Interferon-γ production measured by enzyme-linked immunosorbent assay in supernatants of peripheral blood mononuclear cell cultures (106 cells per well in a final volume of 1 ml) of cutaneous leishmaniasis patients (a) and vaccinated subjects (b) in response to stimulation with 10 µg/ml of Leishmania amazonensis crude antigen extract (La) and LACK for 5 days. Dots indicate individual values for each study participant.
In the patient group, LACK induced significantly higher levels (P < 0·005) of IL-10 than those stimulated by La (Fig. 3a). Among the vaccinated subjects (Fig. 3b), in contrast to what was found among the patients, La was a more potent inducer of IL-10 production than LACK (P < 0·05). Finally, in the naive group (Fig. 3c), similar to what was seen in the patient group, LACK was responsible for significantly higher levels of IL-10 compared with La (P = 0·0005). Again, in the naive group, LACK stimulated higher levels of TNF-α (median 134·7, range 0–1242·84 pg/ml) than did La (median 21·82, range 0–667·6 pg/ml) (P < 0·001, data not shown). Using ELISA, no other differences were seen between the antigens with regard to their capacity of inducing TNF-α or IL-5 production in any group.
Fig. 3.

Interleukin (IL)-10 levels measured by enzyme-linked immunosorbent assay in supernatants of peripheral blood mononuclear cells cultures (106 cells per well in a final volume of 1 ml) of cutaneous leishmaniasis patients (a), vaccinated subjects (b) and healthy subjects without exposure to Leishmania (c) in response to stimulation with 10 µg/ml of L. amazonensis crude antigen extract (La) and LACK for 3 days. Dots indicate individual values for each study participant.
ELISPOT assays were performed with cells from 18 patients (15 males and three females), 12 vaccinees (seven males and five females) and nine naive subjects (one male and eight females) with median ages of 41 years (range 18–78 years), 35 years (range 12–60 years) and 45 years (range 23–60) respectively. The composition of the naive group was significantly different from that of the patient group with regard to gender (P < 0·001). No other difference was found between the groups with regard to gender and age. Using ELISPOT (Fig. 4), we found in the vaccinated group that the number of cells producing IFN-γ in response to LACK was significantly higher (P < 0·05) than that after La stimulation, in agreement with the results obtained with ELISA. There was no difference between the frequencies of IFN-γ-producing cells induced by stimulation with La and LACK in the other two groups. No differences between the frequencies of cells producing IL-4 in response to La and LACK were seen in any group.
Fig. 4.
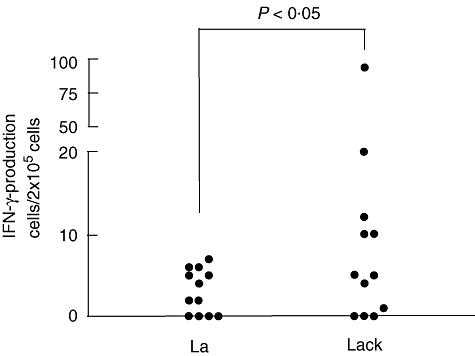
Frequencies of interferon-γ-producing cells assayed by enzyme-linked immunospot assay in peripheral blood mononuclear cell cultures (2 × 105 cells per microwell in a final volume of 200 µl) from vaccinated subjects stimulated during 48 h with 10 µg/ml of Leishmania amazonensis crude antigen extract (La) and LACK. Dots indicate individual values for each subject.
Discussion
In the murine infection with L. major, resistance is associated with the development of a Th1 response, characterized by the production of IFN-γ, while susceptibility correlates with the expansion of a Th2 population that produces IL-4 [19]. This model and the so-called Th1/Th2 paradigm have been used extensively for the selection of parasite molecules as vaccine candidates. In experimental studies, significant protection has been achieved with several defined Leishmania proteins [9]. However, translation of data from animal models to human disease remains a major challenge for the development of an effective human vaccine [20]. Characterization of human immune response to the parasite molecules considered to be vaccine candidates has been performed usually with cells from leishmaniasis patients, either with active disease or cured [21–23]. However, studies on mice and humans have uncovered important differences between immune responses primed by infection versus immunization.
During the past decade, the Th1/Th2 paradigm has guided the strategy for antigen selection in leishmaniasis vaccine research. Thus, leishmanial antigens that stimulate predominantly Th1 responses in cells from mice or human patients have been considered commonly as promising vaccine candidates. Conversely, antigens that stimulate predominantly a Th2 response have been considered as disease-associated antigens and disregarded as vaccine candidates. Paradoxically, many leishmanial antigens that stimulate a Th1 immune response during active disease or after cure have shown no protective effect, while other Th2-associated antigens have been found to be highly protective if a Th1 response is generated before infection. Considering these facts, Campos-Neto proposed that finding antigens that stimulate disease-associated Th2 responses during infection and inducing a Th1 immune response to them using defined vaccination protocols would be a useful approach for the discovery of a leishmaniasis vaccine [24]. Experimental studies using the LACK antigen in the murine model of L. major infection have provided support for this speculation [11–13].
Recent evidence indicates that the LACK molecule is important for effective mammalian infection with Leishmania[25], and it has been shown that this protein is able to inhibit IFN-γ production induced by a soluble Leishmania antigen extract in cultures of PBMC from tegumentary leishmaniasis patients [26]. An exaggerated type 1 response may be the immunopathological basis for the development of mucosal leishmaniasis, a severe clinical form characterized by destructive lesions of the nasal and oral mucosa, which often responds poorly to chemotherapy [27]. Based on its ability to downmodulate the inflammatory response of cells from tegumentary leishmaniasis patients, it has been proposed that LACK would be a suitable candidate antigen for immunotherapy [26].
To our knowledge, this is the first description of LACK-induced cytokine responses in humans immunized with killed Leishmania antigens, although previous studies with this molecule have been performed with cells from tegumentary leishmaniasis patients [26,28] and healthy naive subjects [29]. In agreement with our results, others have shown that LACK induces more IL-10 and less IFN-γ than crude Leishmania extracts in cells from American cutaneous leishmaniasis patients [26,28]. It has been determined by flow cytometry that PBMC from cutaneous leishmaniasis patients that produce IFN-γ in response to a soluble Leishmania extract belong to several different cell phenotypes [28]. We are currently investigating the phenotype of cells that produce IFN-γ in LACK-stimulated cultures of PBMC from vaccinated subjects.
There are very few studies comparing immune responses in leishmaniasis patients versus subjects immunized with Leishmania antigens. We have shown that the majority of PBMC from subjects vaccinated with a first-generation candidate vaccine composed of killed promastigotes that respond to a whole-cell promastigote antigen extract were CD8+ T cells, in contrast to the response of patients with active lesions of tegumentary leishmaniasis, in which the responding cells were predominantly of the CD4+ T cell phenotype [30]. A recent report revealed differences in the expression of Vβ chains of TCRs during cutaneous leishmaniasis and after immunization with the same vaccine preparation used in our study. Modulation in the TCR Vβ repertoire was observed during active disease, with or without antigen stimulation, represented by a significant decrease in the frequency of expression of certain Vβ TCRs in the PBMC from patients. Vaccination, however, led to a broad expansion of various Vβ TCRs [31].
In conclusion, we found clear differences between immune responses to Leishmania antigens primed by natural infection versus immunization that could be exploited for the identification of Leishmania molecules with immunoprotective potential for humans.
Acknowledgments
We are grateful to Dr. Elezer Monteblanco Lemes and Natália Plinio de Souza for the purification of the LACK antigen, Jair Cecílio de Paula for invaluable help in the fieldwork, Dr Alex Chapeaurouge for sequencing the LACK protein and Dr Laila A. Nahum for critical reading of the manuscript. This study was supported by Fundação Oswaldo Cruz (PDTIS and PAPES 3), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil. Armando Schubach, Wilson Mayrink and Sergio C. F. Mendonça are investigators of CNPq.
References
- 1.Desjeux P. Leishmaniasis. Nat Rev Microbiol. 2004;2:692. doi: 10.1038/nrmicro981. [DOI] [PubMed] [Google Scholar]
- 2.Desjeux P. The increase in risk factors for the leishmaniasis worldwide. Trans R Soc Trop Med Hyg. 2001;95:239–43. doi: 10.1016/s0035-9203(01)90223-8. [DOI] [PubMed] [Google Scholar]
- 3.Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366:1561–77. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 4.Khamesipour A, Rafati S, Davoudi N, Maboudi F, Modabber F. Leishmaniasis vaccine candidates for development: a global overview. Indian J Med Res. 2006;123:423–38. [PubMed] [Google Scholar]
- 5.Vélez ID, Gilchrist K, Arbelaez MP, et al. Failure of a killed Leishmania amazonensis vaccine against American cutaneous leishmaniasis in Colombia. Trans R Soc Trop Med Hyg. 2005;99:593–8. doi: 10.1016/j.trstmh.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 6.De Luca PM, Mayrink W, Alves CR, et al. Evaluation of the stability and immunogenicity of autoclaved and non-autoclaved preparations of a vaccine against American tegumentary leishmaniasis. Vaccine. 1999;17:1179–85. doi: 10.1016/s0264-410x(98)00338-7. [DOI] [PubMed] [Google Scholar]
- 7.Velez ID, del Pilar Agudelo S, Arbelaez MP, et al. Safety and immunogenicity of a killed Leishmania (L.) amazonensis vaccine against cutaneous leishmaniasis in Colombia: a randomized controlled trial. Trans R Soc Trop Med Hyg. 2000;94:698–703. doi: 10.1016/s0035-9203(00)90239-6. [DOI] [PubMed] [Google Scholar]
- 8.De Luca PM, Mayrink W, Santiago MA, et al. A randomized double-blind placebo-controlled trial to evaluate the immunogenicity of a candidate vaccine against American tegumentary leishmaniasis. Acta Trop. 2001;80:251–60. doi: 10.1016/s0001-706x(01)00181-4. [DOI] [PubMed] [Google Scholar]
- 9.Coler RN, Reed SG. Second-generation vaccines against leishmaniasis. Trends Parasitol. 2005;21:244–9. doi: 10.1016/j.pt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Jimenez E, Kochan G, Gherardi MM, Esteban M. MVA-LACK as a safe and efficient vector for vaccination against leishmaniasis. Microbes Infect. 2006;8:810–22. doi: 10.1016/j.micinf.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Launois P, Maillard I, Pingel S, et al. IL-4 rapidly produced by V beta 4 V alpha 8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity. 1997;6:541–49. doi: 10.1016/s1074-7613(00)80342-8. [DOI] [PubMed] [Google Scholar]
- 12.Mougneau E, Altare F, Wakil AE, et al. Expression cloning of a protective Leishmania antigen. Science. 1995;268:563–6. doi: 10.1126/science.7725103. [DOI] [PubMed] [Google Scholar]
- 13.Gurunathan S, Sacks DL, Brown DR, et al. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J Exp Med. 1997;186:1137–47. doi: 10.1084/jem.186.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendonça SC, Coutinho SG, Amendoeira RR, Marzochi MC, Pirmez C. Human American cutaneous leishmaniasis (Leishmania b. braziliensis) in Brazil: lymphoproliferative responses and influence of therapy. Clin Exp Immunol. 1986;64:269–76. [PMC free article] [PubMed] [Google Scholar]
- 15.Telino E, De Luca PM, Matos DC, et al. In vitro responses of human peripheral blood mononuclear cells to whole-cell, particulate and soluble extracts of Leishmania promastigotes. Clin Exp Immunol. 2006;143:338–44. doi: 10.1111/j.1365-2249.2006.02995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sambrook J, Fritsh EF, Maniatis T. Molecular cloning: a laboratory manual. 2. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 17.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–7. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matos DS, Azeredo-Coutinho RB, Schubach A, et al. Differential interferon-gamma production characterizes the cytokine responses to Leishmania and Mycobacterium leprae antigens in concomitant mucocutaneous leishmaniasis and lepromatous leprosy. Clin Infect Dis. 2005;40:e5–12. doi: 10.1086/427069. [DOI] [PubMed] [Google Scholar]
- 19.Locksley RM, Heinzel FP, Sadick MD, Holaday BJ, Gardner KD., Jr Murine cutaneous leishmaniasis: susceptibility correlates with differential expansion of helper T cell subsets. Ann Inst Pasteur Immunol. 1987;138:744–9. doi: 10.1016/s0769-2625(87)80030-2. [DOI] [PubMed] [Google Scholar]
- 20.Kedzierski L, Zhu Y, Handman E. Leishmania vaccines: progress and problems. Parasitology. 2006;133(Suppl):S87–112. doi: 10.1017/S0031182006001831. [DOI] [PubMed] [Google Scholar]
- 21.Mendonça SC, Russell DG, Coutinho SG. Analysis of the human T cell responsiveness to purified antigens of Leishmania: lipophosphoglycan (LPG) and glycoprotein 63 (gp 63) Clin Exp Immunol. 1991;83:472–8. doi: 10.1111/j.1365-2249.1991.tb05663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Carvalho LP, Soto M, Jeronimo S, et al. Characterization of the immune response to Leishmania infantum recombinant antigens. Microbes Infect. 2003;5:7–12. doi: 10.1016/s1286-4579(02)00051-5. [DOI] [PubMed] [Google Scholar]
- 23.Farajnia S, Mahboudi F, Ajdari S, Reiner NE, Kariminia A, Alimohammadian MH. Mononuclear cells from patients recovered from cutaneous leishmaniasis respond to Leishmania major amastigote class I nuclease with a predominant Th1-like response. Clin Exp Immunol. 2005;139:498–505. doi: 10.1111/j.1365-2249.2004.02702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campos-Neto A. What about Th1/Th2 in cutaneous leishmaniasis vaccine discovery? Braz J Med Biol Res. 2005;38:979–84. doi: 10.1590/s0100-879x2005000700001. [DOI] [PubMed] [Google Scholar]
- 25.Kelly BL, Stetson DB, Locksley RM. Leishmania major LACK antigen is required for efficient vertebrate parasitization. J Exp Med. 2003;198:1689–98. doi: 10.1084/jem.20031162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carvalho LP, Passos S, Dutra WO, et al. Effect of LACK and KMP11 on IFN-gamma production by peripheral blood mononuclear cells from cutaneous and mucosal leishmaniasis patients. Scand J Immunol. 2005;61:337–42. doi: 10.1111/j.1365-3083.2005.01581.x. [DOI] [PubMed] [Google Scholar]
- 27.Bacellar O, Lessa H, Schriefer A, et al. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun. 2002;70:6734–40. doi: 10.1128/IAI.70.12.6734-6740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bottrel RL, Dutra WO, Martins FA, et al. Flow cytometric determination of cellular sources and frequencies of key cytokine-producing lymphocytes directed against recombinant LACK and soluble Leishmania antigen in human cutaneous leishmaniasis. Infect Immun. 2001;69:3232–9. doi: 10.1128/IAI.69.5.3232-3239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bourreau E, Collet M, Prévot G, et al. CD45RA+CD8+ and IL-10-producing CD45RA-CD4+ T cells generated in response to LACK in naive subjects never exposed to Leishmania. Eur J Immunol. 2002;32:510–20. doi: 10.1002/1521-4141(200202)32:2<510::AID-IMMU510>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Mendonça SC, De Luca PM, Mayrink W, et al. Characterization of human T lymphocyte-mediated immune responses induced by a vaccine against American tegumentary leishmaniasis. Am J Trop Med Hyg. 1995;53:195–201. doi: 10.4269/ajtmh.1995.53.195. [DOI] [PubMed] [Google Scholar]
- 31.Clarencio J, de Oliveira CI, Bomfim G, et al. Characterization of the T-cell receptor Vbeta repertoire in the human immune response against Leishmania parasites. Infect Immun. 2006;74:4757–65. doi: 10.1128/IAI.00265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


