Abstract
Streptococcus suis serotype 2 sequence type 7 strains emerged in 1996 and caused a streptococcal toxic shock-like syndrome in 1998 and 2005 in China. Evidence indicated that the virulence of S. suis sequence type 7 had increased, but the mechanism was unknown. The sequence type 7 strain SC84, isolated from a patient with streptococcal toxic shock-like syndrome during the Sichuan outbreak, and the sequence type 1 strain 31533, a typical highly pathogenic strain isolated from a diseased pig, were used in comparative studies. In this study we show the mechanisms underlying cytokine production differed between the two types of strains. The S. suis sequence type 7 strain SC84 possesses a stronger capacity to stimulate T cells, naive T cells and peripheral blood mononuclear cell proliferation than does S. suis sequence type 1 strain 31533. The T cell response to both strains was dependent upon the presence of antigen-presenting cells. Histo-incompatible antigen-presenting cells were sufficient to provide the accessory signals to naive T cell stimulated by the two strains, indicating that both sequence type 7 and 1 strains possess mitogens; however, the mitogenic effect was different. Therefore, we propose that the difference in the mitogenic effect of sequence type 7 strain SC84 compared with the sequence type 1 strain 31533 of S. suis may be associated with the clinical, epidemiological and microbiological difference, where the ST 7 strains have a larger mitogenic effect.
Keywords: Mitogenic effect, sequence type 7, streptococcal toxic shock-like syndrome, Streptococcus suis, virulence
Introduction
Streptococcus suis serotype 2 is an important swine pathogen that primarily causes meningitis in humans and occasionally causes other infections such as endocarditis, arthritis and pneumonia [1]. The majority of human infections are associated with occupational exposure to pigs and result in meningitis, often associated with hearing loss. However, a large outbreak of 215 human cases emerged in the summer of 2005 in Sichuan Province, China, with 61 cases of streptococcal toxic shock-like syndrome that was not observed previously [2–4].
Of the 35 serotypes described, serotype 2 is the serotype associated most frequently with the disease. Using multi-locus sequence typing (MLST), the causative pathogen of the Sichuan outbreak was identified as serotype 2 sequence type 7 (ST7), a member of the ST1 complex, that is associated strongly with most human infection septicaemias and meningitis worldwide [4,5]. S. suis ST7 appeared for the first time infecting a patient with sepsis in Hong Kong in 1996, then emerged to cause a small outbreak in Jiangsu in 1998 and spread to cause the largest-ever outbreak in 2005. This strain has so far been isolated exclusively in China.
Streptococcus suis ST7 was assumed to have acquired putative virulence factors responsible for the so-called streptococcal toxic shock-like syndrome. Streptococcal toxic shock syndrome is associated predominantly with Group A streptococcal infections that are toxin-mediated, associated with superantigens. However, when the genomes of the S. suis isolates associated with the outbreaks were sequenced, no putative superantigen or homologous genes were identified, indicating that a unique mechanism was used for the pathogenesis of this strain [6].
Here, we demonstrate that S. suis ST7 strain SC84 isolated from a patient with streptococcal toxic shock-like syndrome during the 2005 outbreak has a higher capacity to induce the production of cytokines than S. suis ST1 strain 31533. The mechanisms contributing to this difference were investigated.
Materials and methods
Bacterial strains and growth conditions
The strains used in this study included S. suis 31533 and SC84, which were typed as ST1 and ST7 respectively [4]. Strain 31533 is a typical European strain isolated from a diseased pig. It was used previously for cytokine induction studies with murine and human cells [7–11]. SC84 was isolated from a patient with streptococcal toxic shock-like syndrome during the Sichuan outbreak in 2005.
The S. suis strains were grown overnight on Columbia blood base agar plates (Guangzhou Detgerm Microbiological Science, PR China) at 37°C and the isolated colonies were used as the inocula into 10 ml of Todd–Hewitt broth (THB, Oxoid Ltd, London, UK). This culture was incubated for 8 h at 37°C with agitation (100 rpm). Working cultures were prepared by transferring 300 µl of 8-h cultures into 30 ml of THB and incubated stationary for 15 h at 37°C with 5% CO2. The bacteria were washed twice in phosphate-buffered saline (PBS; pH 7·4, Gibco, Invitrogen, Carlsbad, CA, USA). The pellet was then resuspended in PBS. Serial dilutions of the suspension were plated onto blood agar to determine the colony-forming units (CFU)/ml before use.
Preparation of killed bacteria
Bacterial cells were prepared as indicated above, resuspended in PBS and heat-killed by incubating the organisms at 56°C for 60 min, as described previously [9,12]. In selected experiments, bacteria were treated at 121°C for 20 min. The killed cultures were subcultured onto Columbia blood base agar plates at 37°C for 24 h to determine if viable organisms remained. The killed bacterial preparations were stored at 4°C for no longer than 1 week before use.
In vivo cytokine production
C57BL/6 mice (6 weeks old, female) were injected intraperitoneally (i.p.) with 1 × 109 CFU of the heat-killed strains or 1 × 106 CFU of the live strains in 1 ml PBS. Each group contained five mice. At 8 h (live strains) or 6 h (heat-killed strains) post-infection the mice were killed and peripheral blood was collected. These experiments were repeated twice on different days. All serum samples were diluted at least four times before testing using a Luminex kit (R&D Systems, Inc., Minneapolis, MN, USA), as recommended by the manufacturer.
Determination of viable bacteria in organs
At 8 h post-infection, the peripheral blood, liver and spleen of three infected mice from each strain were obtained aseptically. The organs were rubbed in 1 ml PBS after accurate weighing. The homogenates of organ and blood were diluted with PBS. One hundred µl of dilutions was plated onto blood agar plates. Colonies were counted and expressed as CFU/0·1 g for organ samples or CFU/ml for blood samples.
Isolation of mononuclear cells
Peripheral blood was obtained from the Red Cross of China, Beijing Branch. Peripheral blood mononuclear cells (PBMC) were isolated by centrifugation (400 g for 25 min) using Ficoll-Paque (GE Healthcare Biosciences, Uppsala, Sweden). PBMC were harvested, washed three times and then resuspended in white blood cells complete medium (GenMed Scientifics, Shanghai, China). Viable cells were counted using trypan blue exclusion as visualized with light microscopy.
To isolate T lymphocytes, the PBMC were processed using a MagCellect* human CD3+ T cell isolation kit (R&D Systems, Inc.), according to the protocol provided in the kit. To obtain either naive T cells or memory T cells, the total T cells suspension was processed with anti-human CD45RA particles (BD Biosciences, Minneapolis, MN, USA) or anti-human CD45RO particles (BD Biosciences), according to the protocol provided in the kit.
Preparation of antigen-presenting cells and determination of major histocompatibility complex restriction
Mitomycin C-treated PBMC preparations were used as the source of antigen-presenting cells (APCs) [12]. The total PBMCs (at 1 × 106/ml), purified as described above, were treated with mitomycin C (Calbiochem, Gibbstown, NJ, USA) at 25 µg/ml for 30 min at 37°C. These APCs were then co-cultured with T cells at a ratio of 1:1 for the T cell proliferation studies. The procedure for proliferation is described below.
HLA typing was performed to ensure that the donors were allogeneic. The HLA-ABDR SSP typing kit (ROSE Europe GmbH, Frankfurt/main, Germany) was used. Three different donor combinations were used. The first combination of donors was A* 02 A* 30, B*13 B*40, DRB* 07 DRB* 09; the second combination was A* 01 A* 26, B*40 B*49, DRB* 13 DRB* 14; and the third combination was A* 11 A* 30, B*13 B*81, DRB* 07 DRB* 12.
Lymphocyte proliferation assays
Peripheral blood mononuclear cell and T cell proliferation was measured using an enzyme-linked immunosorbent assay (ELISA) based on bromo-2′-deoxyuridine (BrdU) incorporation (Chemicon International, Inc., Billerca, MA, USA). We have demonstrated previously that both strains are toxic for PBMC at incubation times as short as 4 h [4]. Thus, these high levels of cytotoxicity render long incubation times impossible to be performed with live bacteria (such as those required for proliferation studies). Thus, heat-killed (56°C for 60 min) S. suis (5 × 106 organisms/well) was added to the different APC : T cell combinations or total PBMC (1 × 105 cells/well) prepared as described above and incubated for 66 h in 96-well flat-bottomed plates (Falcon, Becton Dickinson, Franklin Lakes, NJ, USA). At 18 h before the end of the incubation, 20 µl BrdU (dilute 500 times) was added. Concanavalin A (ConA), a T cell mitogen, was used as positive control at a concentration of 20 µg/ml. The plates were then centrifuged for 10 min at 200 g. The supernatant was removed and the cell proliferation [optical density (OD) values] was determined following the protocol provided in the kit.
Statistics
The cytokine values are expressed as the median pg/ml values and the BrdU OD values are expressed as the mean ± standard error of the means. Each proliferation experiment was performed with different donors on different days. Statistical analysis of bacterial CFU counts and proliferation data were performed using Student's unpaired t test and analysis of variance test. Statistical analysis of the cytokine data was performed using Wilcoxon's two-sample test. For these tests, a P-value < 0·05 was considered significant.
Results
Production of cytokines varies with the S. suis ST phenotype
Cytokine production in mice after 8 h of infection with live S. suis using the i.p. route varied among the different cytokines. Production of proinflammatory cytokines tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, chemoattractant (KC) and monocyte chemoattractant protein 1 (MCP-1), as well as IL-10 in the group of mice infected with live S. suis SC84, was statistically higher than that observed in mice infected with live 31533 strain. There was no statistical difference in the serum level of interferon (IFN)-γ between mice infected with SC84 and 31533 (Fig. 1).
Fig. 1.
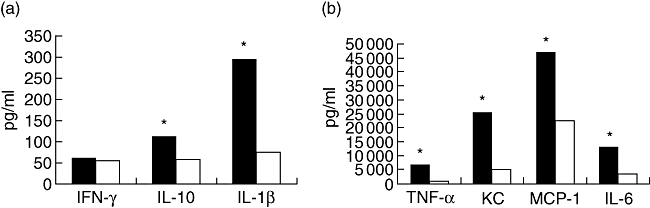
Median cytokine levels in sera of mice infected with live Streptococcus suis strains. ▪: SC84, □: 31533. *Statistically significant differences in cytokine levels between mice infected with S. suis strain SC84 and mice infected with strain 31533. The experiment was repeated twice with similar results. Statistical analysis of the cytokine data was performed using the Wilcoxon two-sample test. P < 0·05 was considered significant.
Bacterial counts in organs of infected mice do not differ
In order to rule out the possibility that the differences in induction of cytokines after i.p. injection of mice between the two strains could be due to differences in bacterial replication, we counted the bacterial in the blood, liver and spleen of infected mice at 8 h post-infection. Bacterial counts in the blood exceeded 1 × 107 CFU/ml in all tested samples. Bacterial counts in the organs exceeded 1 × 106 CFU/ml in all tested samples. The bacterial counts in blood, liver and spleen were not statistically different between SC84 and 31533 (Fig. 2).
Fig. 2.
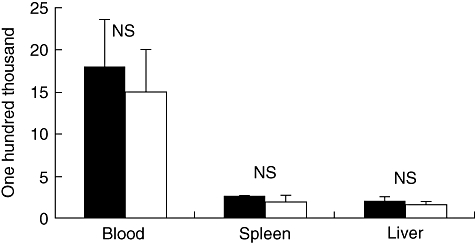
Bacterial counts in different organs from mice infected intraperitoneally with live Streptococcus suis strains. ▪: SC84; □: 31533. Bacterial loads in the liver and spleen were expressed as colony-forming units (CFU)/0·1 g of tissue and in the blood as CFU/ml. Results represent mean ± standard error of the mean values. Statistical analysis of the data was performed using Student's unpaired t-test; n.s.: bacterial counts comparing the groups of mice inoculated with S. suis strain SC84 with the groups of mice inoculated with 31533 were not statistically significant. P < 0·05 was considered significant.
Mechanism for inducing cytokine production in vivo is different between SC84 and 31533
In order to investigate whether some possible superantigen-mediated effects contribute to the increasing capacity of SC84 to induce cytokine production, we used two temperature ranges to killed S. suis. Treatment at 56°C for 60 min did not affect the cytokine-inducing capacity, as described previously [7], whereas treatment at 121°C for 20 min was used to kill strains and, at the same time, denatured potential superantigen [13]. Our previous result showed that TNF-α, one of the most important host mediators in the pathogenesis of septic shock, could not be induced unless the heat-killed S. suis strains exceeded 1 × 109 CFU. The production of cytokine in mice after infection with heat-killed S. suis showed a high but transitory peak at 6 h post-infection (p.i.) and a drastic return to basal levels after 9 h (data not shown). Hence, 6 h and 1 × 109 CFU were used to analyse the production of cytokine in mice infected with heat-killed S. suis.
The serum levels of cytokine in the group of mice injected i.p. with S. suis ST7 strain SC84 heat-killed at 56°C for 60 min was significantly higher than the same strainheat-killed at 121°C for 20 min. However, there were no significant differences between the group of mice injected i.p. with S. suis ST1strain 31533 heat-killed at 56°C for 60 min and the group injected with 31533 heat-killed at 121°C for 20 min, even though cytokine levels of mice injected with 31533 heat-killed at 56°C for 60 min was slightly higher than those injected with heat-killed at 121°C for 20 min (Fig. 3).
Fig. 3.
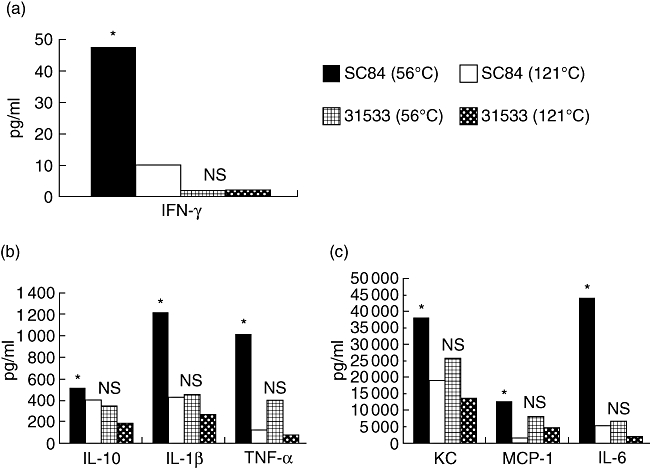
Median cytokine levels in sera of mice stimulated using Streptococcus suis cells heat-treated at 56°C for 60 min or 121°C for 20 min. *Cytokine levels comparing the groups of mice inoculated with S. suis strain SC84 treated at 56°C for 60 min with the groups of mice inoculated with SC84 treated at 121°C for 20 min were statistically significant; n.s.: cytokine levels comparing the groups of mice inoculated with S. suis strain 31533 treated at 56°C for 60 min with the groups of mice inoculated with 31533 treated at 121°C for 20 min were not statistically significant. The experiment was repeated twice with similar results. Statistical analysis of the cytokine data was performed using the Wilcoxon two-sample test. P < 0·05 was considered significant. OD, optical density.
Peripheral blood mononuclear cell proliferate in response to S. suis ST7
Proliferation studies with total PBMC showed that S. suis strain SC84 (heat-killed 56°C for 60 min) had more capacity to stimulate PBMC proliferation than strain 31533 (heat-killed 56°C for 60 min) (Fig. 4). As a control, ConA was used to confirm that the PBMC were able to proliferate.
Fig. 4.
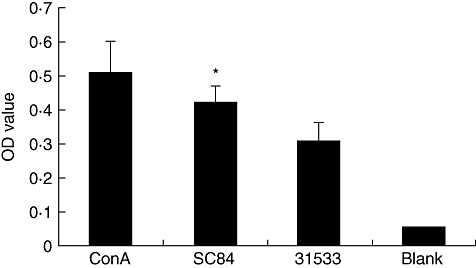
Peripheral blood mononuclear cells (PBMC) proliferate in response to Streptococcus suis. The concentration of PBMC was 1 × 105 cells per well. PBMC were cultured with concanavalin A (ConA) (20 µg/ml) and heat-killed (56°C for 60 min) S. suis ST7 SC84 or ST1 31533 (5 × 106/well); or medium only (blank). The experiment was repeated three times with similar results. *P < 0·05 calculated by Student's unpaired t test comparing the optical density (OD) values of S. suis ST7 SC84 with the OD values of S. suis ST1 31533.
Requirement of APC
It is possible that S. suis contains superantigens. If this were the case, T cell proliferation would be independent of APC. By contrast, both mitogens and recall antigens required APC signals to stimulate T lymphocytes to proliferate [14,15]. To determine whether the CD3+T cell response to heat-killed (56°C for 60 min) S. suis required APC, purified CD3+T cells were treated with heat-killed S. suis ST7 strain SC84 and S. suis ST1 strain 31533 in the presence and absence of autologous APC. The data showed that both strains failed to stimulate significant proliferation of purified CD3+T cells without autologous APC. Nevertheless, S. suis ST7 strain SC84 had a higher capacity to stimulate purified CD3+T cells in the presence of autologous APC than did strain 31533 (Fig. 5). Thus, CD3+T cells required APC when stimulated by both strains of S. suis where the ST7 representative strain SC84 showed higher proliferative-inducing capacity, as observed with the total PBMC (Fig. 4).
Fig. 5.
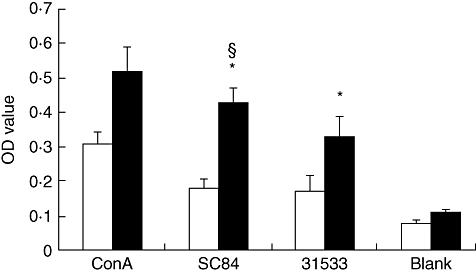
Purified T cells require antigen-presenting cells (APC) to proliferate in response to Streptococcus suis. □: CD3+T cells without autologous APC (1 × 105 cells/ well); ▪: CD3+T cells with autologous APC (1 × 105 cells/well for each kind of cell).*P < 0·05 calculated by analysis of variance (anova) comparing the optical density (OD) value of CD3+T cells with autologous APC stimulated by heat-killed S. suis with the OD value of CD3+ T cell without autologous APC stimulated by heat-killed S. suis. §P < 0·05 calculated by anovacomparing the OD value of CD3+ T cells with autologous APC stimulated by heat-killed SC84 with those stimulated by heat-killed 31533.
Naive and memory T cells proliferate in response to S. suis
CD45RA is expressed by naive T cells, while CD45RO is expressed by primed/memory T cells [16–18]. ConA, a T cell mitogen, can induce proliferation of both CD45RA and CD45RO populations. Our data show that both CD45RA+T cells and CD45RO+T cells proliferated in response to S. suis (Fig. 6). We did not observe significant differences between strain SC84 and 31533 using CD45RO+T cells.
Fig. 6.
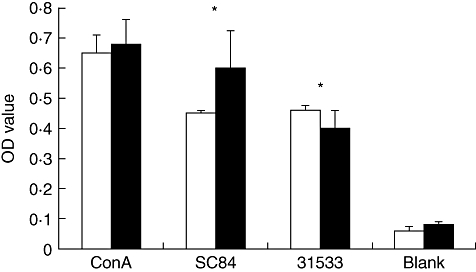
CD45RA+ (naive) T cells and CD45RO+ (memory) T cells response to Streptococcus suis. □: CD45RO+ T cells with autologous antigen-presenting cells (APC) (1 × 105 cells/well for each kind of cell); ▪: CD45RA+ T cells with autologous APC (1 × 105 cells/well for each kind of cell). The experiment was repeated three times with similar results.*P < 0·05 calculated by analysis of variance comparing the optical density (OD) value of CD45RA+ T cell or CD45RO+ T with autologous APC stimulated by heat-killed S. suis with those of the unstimulated group (blank). ConA, concanavalin A.
T lymphocyte proliferation in response to S. suis is not major histocompatibility complex-restricted
We observed that purified T cells needed APC for proliferation in response to S. suis; however, it is important to determine whether the response was major histocompatibility complex (MHC)-restricted. Mitogens can stimulate naive T cell proliferation in the presence of allogeneic APC [19,20]. There was significant naive T lymphocyte cell proliferation in the presence of allogeneic cells, indicating that the T lymphocyte cell response to heat-killed S. suis was not MHC-restricted. Furthermore, the capacity of S. suis ST7 strain SC84 to stimulate naive T cells to proliferate was higher than observed with S. suis ST1 strain 31533 (Fig. 7).
Fig. 7.
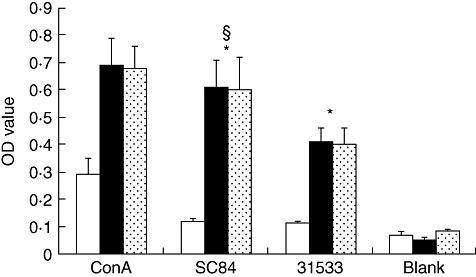
CD45RA+ (naive) T cells response to Streptococcus suis. □: CD45RA+ T cells without antigen-presenting cells (APC) (1 × 105/well), ▪: CD45RA+ T cells with autologous APC (1 × 105cells/well for each kind of cell),  : CD45RA+ T cells with allogeneic APC (1 × 105 cells/well for each kind of cell). The experiment was repeated three times with similar results. *P < 0·05 calculated by analysis of variance (anova) comparing the optical density (OD) value of CD45RA+ T cells with APC stimulated by heat-killed S. suis with the OD value of CD45RA+ T cells without APC stimulated by heat-killed S. suis. §P < 0·05 calculated by anovacomparing the OD value of CD45RA+ T cells with APC (autologous or allogeneic) stimulated by heat-killed SC84 with the OD value of groups stimulated by heat-killed 31533. ConA, concanavalin A.
: CD45RA+ T cells with allogeneic APC (1 × 105 cells/well for each kind of cell). The experiment was repeated three times with similar results. *P < 0·05 calculated by analysis of variance (anova) comparing the optical density (OD) value of CD45RA+ T cells with APC stimulated by heat-killed S. suis with the OD value of CD45RA+ T cells without APC stimulated by heat-killed S. suis. §P < 0·05 calculated by anovacomparing the OD value of CD45RA+ T cells with APC (autologous or allogeneic) stimulated by heat-killed SC84 with the OD value of groups stimulated by heat-killed 31533. ConA, concanavalin A.
Discussion
A striking feature of the Sichuan outbreak was the unusually high rate of mortality with a streptococcal toxic shock-like syndrome as the major clinical manifestation [2,3]. This increased severity of S. suis infection in humans was represented by a shorter incubation period, rapid disease onset and progression and higher mortality. Thus, our major goal is to increase understanding of the factors associated with the pathogenesis of S. suis ST7, the causative agent of the Sichuan outbreak [4]. ST7 may be only derived very recently from ST1, as they share six of seven housekeeping loci used in the MLST typing and have one locus, thyA, differing only in a single nucleotide [5]. Our previous study suggested that the virulence of S. suis ST7 has increased. Indeed, live S. suis ST7 strain SC84 was significantly more toxic to PBMC than live S. suis ST1 strain 31533 [4].
Because streptococcal toxic shock-like syndrome was the major clinical feature of the Sichuan outbreak, a possible superantigen involvement was assumed a priori. However, when the genome of S. suis responsible for the Sichuan outbreak was sequenced, it was demonstrated that superantigen-coding genes were absent [6]. It has been suggested that S. suis infections are associated with the overproduction of cytokines such as TNF-α, IL-1β and IL-6. These cytokines are believed to mediate reactions associated with clinical deterioration, multi-organ system failure and death during toxic shock [21]. Dominguez-Punaro et al. demonstrated recently that S. suis ST1 strain 31533 could stimulate experimental mice to produce high levels of serum cytokines, i.e. TNF-α, IL-6, IL-12, IFN-γ, MCP-1, KC and regulated upon activation normal T cell expressed and secreted (RANTES), that may be responsible in part for the sudden death of 20% of the infected animals [22]. Faulkner et al. found that early burst of TNF-α is attributed crucially to lethality during toxic shock [23]. In this regard, we demonstrated here that the live SC84 strain induced higher cytokine production in vivo than the 31533 strain. This higher cytokine burst may be responsible for and resembles the so-called streptococcal toxic shock-like syndrome observed in the Sichuan outbreak [2,4]. It should be noted that the differences observed in serum cytokine levels are not related to differences in bacterial replication, as similar high levels of S. suis organisms were observed after infection with either strain. Similarly, the susceptibility of different inbred strains of mice to S. suis septic shock was related to cytokine levels rather than to bacterial loads in blood (our unpublished observations).
Thus, to determine the possible pathogenic mechanism(s) used by S. suis ST7 to induce streptococcal toxic shock-like syndrome, we investigated further the differences in cytokine production in vivo and T cell-proliferation capacities of representative ST7 and ST1 strains.
We found that heat treatment (56°C versus 121°C) significantly decreased the levels of serum cytokines in experimental mice injected with S. suis ST7 strain SC84, indicating a possible heat-sensitive component involved. In contrast, heat treatments (121°C for 20 min) had no effect on the capacity of S. suis ST1 strain 31533 to induce serum cytokine production in experimental mice. Segura et al. found that the relatively heat-stable cell-associated components of S. suis ST1 31533 are probably responsible for most of the cytokine stimulation observed in murine macrophages in vitro[9]. They may include the surface or cell wall-associated components, i.e. capsular polysaccharide, peptidoglycan or lipoteichoic acid, that are demonstrated to be potent cytokine inducers which resist heat treatment in various Gram-positive cocci [13,24,25]. It should be noted that previous studies showed that heat-killed S. suis 31533 induced similar levels of cytokine release by human monocytes to live bacteria in vitro[7]. Furthermore, experiments with cytochalasin-treated macrophages showed that the stimulation of cytokine production was phagocytosis-independent [9].
Thus, our findings suggest that although both ST1 and ST7 strains of S. suis could induce high levels of serum cytokines in experimental mice, which may be responsible for the shock syndrome; however, they do so by possibly different components.
We next measured the T cell proliferation-stimulating capacity of the two sequence types of S. suis using an ELISA based on BrdU incorporation. Messele et al. reported BrdU incorporation can be used as an alternative to [3H]-thymidine ([3H]-TdR) incorporation to measure in vitro T cell proliferation [26]. Using this test, we found that: (i) S. suis ST7 strain SC84 possesses a stronger capacity to stimulate T cell and PBMC proliferation than ST1 strain 31533; (ii) the T cell response to both ST1 and ST7 strains of S. suis (SC84 and 31533) requires APC; and (iii) naive T cells (CD45RA+T cells) could be stimulated to proliferate by both S. suis ST7 SC84 and ST1 31533 in the presence of allogenic APC. However, strain SC84 systematically induced a stronger proliferative response.
In vitro lymphocyte proliferation in response to recall antigens can be detected only in T cells that have undergone prior clonal expansion, thus only CD45RO+cells proliferate [19,20]. In contrast, mitogens stimulate a large percentage of the T cells regardless of whether or not they have undergone prior expansion, and thus both CD45RO+and CD45RA+cells proliferate [19,20]. Our studies demonstrate that S.suis can stimulate CD45RA+T cells to proliferate in the presence of APC.
As mentioned above, our data show that CD45RA+T cells can proliferate in response to heat-killed S. suis in the presence of either allogenic APC or autologous APC. The APC requirement for mitogens and recall antigens are distinct. Recall antigens are taken up by antigen-processing cells, processed by lysosomal digestion and placed in the binding groove of an MHC molecule. The MHC–peptide complex is then transported to the surface of the APC, where it is available for recognition by T cells bearing histocompatible T cell receptors. T cells recognize the combination of the epitope and histotope, and therefore the response is MHC-restricted. By contrast, mitogens bind to the surface of T cells and APC, resulting in receptor ligation, signalling and entry of the T cells into the cell cycle. The receptor ligation on the T cells does not recognize the unique sequences of the epitope–histotope combination and the response is not MHC-restricted. Our data here show the histo-incompatible APC are sufficient to provide accessory signals to CD45RA+T cells; and therefore, the response is not MHC-restricted. This suggests that S. suis possesses mitogenic capacities.
Mody et al. found that Cryptococcus neoformans possesses mitogens for human T lymphocytes in addition to potent recall antigens. Because both mitogens and recall antigens can have profound effects on host defence, it was proposed that both mechanisms contribute to lymphocyte proliferation in vitro. When naive lymphocytes and allogeneic APC are present, then the only possible mechanism of lymphocyte activation is a mitogenic response. When efficient antigen presentation is present, then a recall antigen may predominate [19]. We believe that both mechanisms occurred during the immune response to S. suis in vivo and in vitro. Although S. suis ST1 strain 31533 possesses mitogens as shown here, the ability to stimulate naive T cells to proliferate was much weaker than for the ST7 S. suis SC84. This may suggest that the mitogenic effect is predominant during the response to S. suis ST7 SC84 and that the recall antigenic effect is predominant during the immune response to S. suis ST1 31533.
Bacterial mitogens can induce the stimulation of large populations of T cells. These stimulated T cells can produce toxic concentrations of cytokines that have major effects on the host [27]. The difference in the mitogenic effects between S. suis ST7 SC84 and ST1 31533 may be responsible for the higher level of cytokines observed in vivo after inoculation with SC84 strain.
In conclusion, our data suggest that mitogenic effects might contribute to the increased virulence of and the toxic shock-like syndrome induced by S. suis ST7 strain SC84. However, further studies are needed to identify the possible component(s) related to the differences in the mitogenic effects observed between S.suis ST1 and ST7 strains.
Acknowledgments
This work was supported by grants (2008DFA31830 2005CB522904 and 200802016 to JGX) from the Ministry of Science and Technology, PR China (to J. G. X.); by the international research programme of the Ministry of Science and Technology, PR China in conjunction with the Ministère du Développement économique, de l’Innovation et de l’Exportation (MDEIE), Québec, Canada (to J. G. X., M. G. and M. S.); and by Natural Sciences and Engineering Research Council of Canada Grant no. 342150-07 (to M. S.). M. S. is the recipient of a FRSQ Junior 1 Career Award.
References
- 1.Gottschalk M, Segura M, Xu J. Streptococcus suis infections in humans: the Chinese experience and the situation in North America. Anim Health Res Rev. 2007;8:29–45. doi: 10.1017/S1466252307001247. [DOI] [PubMed] [Google Scholar]
- 2.Yu H, Jing H, Chen Z, et al. Human Streptococcus suis outbreak, Sichuan, China. Emerg Infect Dis. 2006;12:914–20. doi: 10.3201/eid1206.051194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang J, Wang C, Feng Y, et al. Streptococcal toxic shock syndrome caused by Streptococcus suis serotype 2. PLoS Med. 2006;3:e151. doi: 10.1371/journal.pmed.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye C, Zhu X, Jing H, et al. Streptococcus suis sequence type 7 outbreak, Sichuan, China. Emerg Infect Dis. 2006;12:1203–8. doi: 10.3201/eid1208.060232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King SJ, Leigh JA, Heath PJ, et al. Development of a multilocus sequence typing scheme for the pig pathogen Streptococcus suis: identification of virulent clones and potential capsular serotype exchange. J Clin Microbiol. 2002;40:3671–80. doi: 10.1128/JCM.40.10.3671-3680.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C, Tang J, Dong W, et al. A glimpse of streptococcal toxic shock syndrome from comparative genomics of S. suis 2 Chinese isolates. PLoS One. 2007;2:e315. doi: 10.1371/journal.pone.0000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segura M, Vadeboncoeur N, Gottschalk M. CD14-dependent and -independent cytokine and chemokine production by human THP-1 monocytes stimulated by Streptococcus suis capsular type 2. Clin Exp Immunol. 2002;127:243–54. doi: 10.1046/j.1365-2249.2002.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vadeboncoeur N, Segura M, Al-Numani D, Vanier G, Gottschalk M. Pro-inflammatory cytokine and chemokine release by human brain microvascular endothelial cells stimulated by Streptococcus suis serotype 2. FEMS Immunol Med Microbiol. 2003;35:49–58. doi: 10.1111/j.1574-695X.2003.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 9.Segura M, Stankova J, Gottschalk M. Heat-killed Streptococcus suis capsular type 2 strains stimulate tumor necrosis factor alpha and interleukin-6 production by murine macrophages. Infect Immun. 1999;67:4646–54. doi: 10.1128/iai.67.9.4646-4654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Gottschalk M, Esgleas M, et al. Immunization with recombinant Sao protein confers protection against Streptococcus suis infection. Clin Vaccine Immunol. 2007;14:937–43. doi: 10.1128/CVI.00046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segura M, Vanier G, Al-Numani D, Lacouture S, Olivier M, Gottschalk M. Proinflammatory cytokine and chemokine modulation by Streptococcus suis in a whole-blood culture system. FEMS Immunol Med Microbiol. 2006;47:92–106. doi: 10.1111/j.1574-695X.2006.00067.x. [DOI] [PubMed] [Google Scholar]
- 12.Ponchio L, Duma L, Oliviero B, Gibelli N, Pedrazzoli P, Robustelli della CG. Mitomycin C as an alternative to irradiation to inhibit the feeder layer growth in long-term culture assays. Cytotherapy. 2000;2:281–6. doi: 10.1080/146532400539215. [DOI] [PubMed] [Google Scholar]
- 13.von HC, Totolian A, Alfarone G, et al. Soluble antigens from group B streptococci induce cytokine production in human blood cultures. Infect Immun. 1997;65:4017–21. doi: 10.1128/iai.65.10.4017-4021.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sopori ML, Hurt YL, Cherian S, Kaplan AM, Diamantstein T. Differential requirement for accessory cells in polyclonal T-cell activation. Cell Immunol. 1987;105:174–86. doi: 10.1016/0008-8749(87)90066-9. [DOI] [PubMed] [Google Scholar]
- 15.Licastro F, Davis LJ, Morini MC. Lectins and superantigens: membrane interactions of these compounds with T lymphocytes affect immune responses. Int J Biochem. 1993;25:845–52. doi: 10.1016/0020-711x(93)90239-b. [DOI] [PubMed] [Google Scholar]
- 16.Prince HE, York J, Jensen ER. Phenotypic comparison of the three populations of human lymphocytes defined by CD45RO and CD45RA expression. Cell Immunol. 1992;145:254–62. doi: 10.1016/0008-8749(92)90329-n. [DOI] [PubMed] [Google Scholar]
- 17.Merkenschlager M, Terry L, Edwards R, Beverley PC. Limiting dilution analysis of proliferative responses in human lymphocyte populations defined by the monoclonal antibody UCHL1: implications for differential CD45 expression in T cell memory formation. Eur J Immunol. 1988;18:1653–61. doi: 10.1002/eji.1830181102. [DOI] [PubMed] [Google Scholar]
- 18.Byrne JA, Butler JL, Cooper MD. Differential activation requirements for virgin and memory T cells. J Immunol. 1988;141:3249–57. [PubMed] [Google Scholar]
- 19.Mody CH, Wood CJ, Syme RM, Spurrell JC. The cell wall and membrane of Cryptococcus neoformans possess a mitogen for human T lymphocytes. Infect Immun. 1999;67:936–41. doi: 10.1128/iai.67.2.936-941.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plebanski M, Saunders M, Burtles SS, Crowe S, Hooper DC. Primary and secondary human in vitro T-cell responses to soluble antigens are mediated by subsets bearing different CD45 isoforms. Immunology. 1992;75:86–91. [PMC free article] [PubMed] [Google Scholar]
- 21.Cerami A. Inflammatory cytokines. Clin Immunol Immunopathol. 1992;62:S3, 10. doi: 10.1016/0090-1229(92)90035-m. [DOI] [PubMed] [Google Scholar]
- 22.Dominguez-Punaro MC, Segura M, Plante MM, Lacouture S, Rivest S, Gottschalk M. Streptococcus suis serotype 2, an important swine and human pathogen, induces strong systemic and cerebral inflammatory responses in a mouse model of infection. J Immunol. 2007;179:1842–54. doi: 10.4049/jimmunol.179.3.1842. [DOI] [PubMed] [Google Scholar]
- 23.Faulkner L, Cooper A, Fantino C, Altmann DM, Sriskandan S. The mechanism of superantigen-mediated toxic shock: not a simple Th1 cytokine storm. J Immunol. 2005;175:6870–7. doi: 10.4049/jimmunol.175.10.6870. [DOI] [PubMed] [Google Scholar]
- 24.Heumann D, Barras C, Severin A, Glauser MP, Tomasz A. Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect Immun. 1994;62:2715–21. doi: 10.1128/iai.62.7.2715-2721.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Timmerman CP, Mattsson E, Martinez-Martinez L, et al. Induction of release of tumor necrosis factor from human monocytes by staphylococci and staphylococcal peptidoglycans. Infect Immun. 1993;61:4167–72. doi: 10.1128/iai.61.10.4167-4172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Messele T, Roos MT, Hamann D, et al. Nonradioactive techniques for measurement of in vitro T-cell proliferation: alternatives to the [(3)H]thymidine incorporation assay. Clin Diagn Lab Immunol. 2000;7:687–92. doi: 10.1128/cdli.7.4.687-692.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallace FM, Mach AS, Keller AM, Lindsay JA. Evidence for Clostridium perfringens enterotoxin (CPE) inducing a mitogenic and cytokine response in vitro and a cytokine response in vivo. Curr Microbiol. 1999;38:96–100. doi: 10.1007/s002849900410. [DOI] [PubMed] [Google Scholar]


