Abstract
We have added constitutively active MAP kinase/ERK kinase (MEK), an activator of the mitogen-activated protein kinase (MAPK) signaling pathway, to cycling Xenopus egg extracts at various times during the cell cycle. p42MAPK activation during entry into M-phase arrested the cell cycle in metaphase, as has been shown previously. Unexpectedly, p42MAPK activation during interphase inhibited entry into M-phase. In these interphase-arrested extracts, H1 kinase activity remained low, Cdc2 was tyrosine phosphorylated, and nuclei continued to enlarge. The interphase arrest was overcome by recombinant cyclin B. In other experiments, p42MAPK activation by MEK or by Mos inhibited Cdc2 activation by cyclin B. PD098059, a specific inhibitor of MEK, blocked the effects of MEK(QP) and Mos. Mos-induced activation of p42MAPK did not inhibit DNA replication. These results indicate that, in addition to the established role of p42MAPK activation in M-phase arrest, the inappropriate activation of p42MAPK during interphase prevents normal entry into M-phase.
INTRODUCTION
The activation of the mitogen-activated protein kinase (MAPK) signaling pathway in response to extracellular signals that stimulate cell proliferation, such as growth factors, has been well established (for review, see Cobb and Goldsmith, 1995; Waskiewicz and Cooper, 1995). Recent evidence suggests that components of this signaling pathway may also have recurring roles during the cell cycle after cells have been stimulated to proliferate. For example, Raf, a critical protein kinase directly upstream of MAPK/extracellular signal regulated kinase (ERK) kinase (MEK) in the MAPK signaling cascade, is activated at M-phase in proliferating tissue culture cells (Laird et al., 1995; Lovric and Moelling, 1996). In addition, the MAPK signaling pathway is involved in a cell cycle checkpoint that detects defects in mitotic spindle assembly (for review, see Wells, 1996), suggesting that components of externally stimulated signaling pathways may affect cell cycle progression by responding to intracellular signals.
The p42MAPK signaling pathway is activated in response to extracellular stimuli that reinitiate the cell cycle of Xenopus oocytes, and microinjection of activators of the MAPK pathway stimulate cell cycle reentry (for review, see Ruderman, 1993). p42MAPK is also activated in cycling extracts of Xenopus eggs in response to intracellular signals that detect improper spindle formation, resulting in arrest of the cell cycle in M-phase (Minshull et al., 1994; Takenaka et al., 1997). Recently, the involvement of p42MAPK in this checkpoint was demonstrated in vivo by using Xenopus tadpole cells (Wang et al., 1997). Ras and Mos both activate the p42MAPK signaling pathway after microinjection into Xenopus oocytes (Hattori et al., 1992; Shibuya et al., 1992b; Nebreda and Hunt, 1993; Posada et al., 1993) and arrest the cell cycle of early embryonic cells at M-phase (Sagata et al., 1989; Daar et al., 1991). Furthermore, constitutively activated p42MAPK has the same effects (Haccard et al., 1993, 1995). Therefore, it appears that p42MAPK has at least two important roles in Xenopus oocytes: one in mediating the response of oocytes to extracellular “meiogens” and the other in the spindle assembly checkpoint.
Herein, we have added constitutively active MEK1 to cycling Xenopus egg extracts at various points in the cell cycle to examine the effect of p42MAPK activation on cell cycle progression. When p42MAPK was activated during entry into M-phase, the cell cycle arrested at metaphase. In contrast, p42MAPK activation during interphase inhibited the entry into M-phase. The arrest in interphase was mediated at the level of Cdc2 activation, because the level of cyclin B synthesis was not affected and the tyrosine-phosphorylated form of Cdc2 accumulated. MEK also inhibited the activation of Cdc2 by recombinant cyclin B in extracts devoid of endogenous cyclins. Even though inactive cyclin/Cdc2 complexes accumulate in interphase-arrested extracts, the extracts can be driven into M-phase by the addition of recombinant cyclin B, indicating that the arrest is reversible. These results indicate that the activation of p42MAPK during interphase can affect cell cycle progression by inhibiting the activation of mitotic cyclin/Cdc2 complexes and preventing entry into M-phase. Therefore, depending upon when it is activated, the p42MAPK signaling pathway can have three distinct effects on cell cycle progression: stimulation of reentry into the cell cycle, arrest of the cell cycle in M-phase, or as shown herein, arrest of the cell cycle in interphase.
MATERIALS AND METHODS
Preparation of Xenopus Egg Extracts
Cycling egg extracts that undergo repeated cell cycles in vitro were prepared from dejellied Xenopus eggs as described previously (Murray and Kirschner, 1989; Murray, 1991) with minor modifications. Briefly, electrically activated eggs were incubated for 10 to 12 min, washed three times with modified extraction buffer (EB = 50 mM sucrose, 100 mM KCl, 5 mM MgCl2, 0.1 mM CaCl2, 10 mM HEPES-KOH, pH 7.7), then two times with EB plus 10 μg/ml leupeptin, 10 μg/ml chymostatin, and 10 μg/ml pepstatin, before transferring to 3.0-ml Ultraclear tubes (Beckman, Mississauga, Ontario, Canada) containing 0.6 ml of silicon oil (Versalube F-50) overlain with 0.6 ml of EB (plus protease inhibitors and 100 μg/ml cytochalasin B). At 17–18 min after activation, eggs were packed by centrifugation at 1080 × g for 1 min at 4°C and excess buffer and oil were removed, and 21–23 min after activation, eggs were crushed by centrifugation at 10,200 × g for 15 min at 4°C. The lysate between the lipid cap and yolk pellet was recentrifuged. The cleared lysate (extract) was removed and a freshly made ATP-regenerating system (40 mM ATP, 0.4 M creatine phosphate, 2 mg/ml creatine kinase) was added immediately (1 volume of ATP-regenerating system:37 volumes of lysate). These supplemented extracts were kept on ice for up to 2 h before use.
Extracts from activated eggs devoid of endogenous mitotic cyclin proteins (CHX extracts) were prepared as described previously (Shibuya et al., 1992b). Unactivated dejellied eggs were treated with 100 μg/ml cycloheximide in 20% Steinberg’s solution (made fresh) for 15 min followed by activation by electrical shock. Eggs were continuously incubated in the above solution for 45 min and then processed as described above for cycling egg extracts, except all solutions contained 100 μg/ml cycloheximide. CHX extracts for DNA replication studies were made in the same way, except eggs were incubated for 20 min after electrical shock prior to processing.
Preparation of Sperm Nuclei
Demembranated sperm nuclei were prepared as described previously (Vigers and Lohka, 1991) and frozen in aliquots (6.0 × 106 sperm/μl) at −80°C. Sperm preparations were thawed on ice, diluted, and added to reactions at a final concentration of 100 sperm/μl (Murray, 1991). For DNA replications assays, sperm were added to a final concentration of 600 sperm/μl.
Recombinant Protein Preparations
Recombinant wild-type Mos and a kinase-inactive mutant were expressed as fusion proteins with an N-terminal maltose binding protein epitope in Escherichia coli and purified as previously described (Yew et al., 1992; Shibuya and Ruderman, 1993). Recombinant N-terminal oligohistidine-tagged kinase-inactive ERK2 (in which the lysine at position 52 has been mutated to arginine = ERK2KR; Robbins et al., 1993), and N-terminal oligohistidine-tagged kinase-inactive Xenopus p42MAPK [in which the lysine at position 52 is mutated to methionine = p42MAPK(KM); Lange-Carter and Johnson, 1995] were expressed in E. coli and purified as described previously. To produce recombinant rat MEK1 proteins with an N-terminal glutathione S-transferase (GST) epitope, we subcloned the BamHI–NotI fragment of cDNA encoding the wild-type kinase-inactive mutant in which the lysine at position 97 is mutated to a methionine [MEK(KM); Lange-Carter et al., 1993], and a constitutively active mutant MEK1 in which Gln-56 is mutated to a Pro [MEK (QP); Bottorff et al., 1995] into the vector pGEX4T1 (Pharmacia, Baie d’Urfe, Quebec, Canada) cut with the same enzymes. Proteins were expressed in E. coli [BLR(DE3)pLysS cells (Novagen, Madison, WI)] and then purified by glutathione-Sepharose chromatography (Smith and Johnson, 1988). GST-MEK1 proteins were concentrated to 0.8–1.0 mg/ml.
To produce a recombinant C-terminal oligohistidine-tagged Xenopus Cdc25C for antiserum production, we subcloned the NdeI–HindIII fragment of pET3a-xCdc25(1) (Kumagai and Dunphy, 1992) into the vector pET21b (Novagen, Madison, WI), digested with the same enzymes. Oligohistidine-tagged-xCdc25C was produced in E. coli [BLR(DE3), Novagen] and used for generation of antiserum (see below).
Assays using Egg Extracts
For cycling extract experiments, an individual reaction was made by transferring 66 μl of supplemented lysate into a 1.5-ml microcentrifuge tube on ice, adding 0.5 μl of sperm suspension, followed by the addition of 3.5 μl of buffer or protein preparation (final volume of 70 μl) just prior to starting incubation at 21–23°C. Samples for analysis by immunoblotting, phosphorylation of histone H1, and cytology were taken from each reaction at 10-min intervals as follows. Two microliters of reaction were mixed with 20 μl of ice-chilled H1 kinase dilution buffer [50 mM sucrose, 100 mM KCl, 5 mM MgCl2, 10 mM NaF, 5 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 10 mM HEPES-KOH, pH 7.7]. Immediately, 17 μl of this sample was withdrawn and mixed with 20 μl of 2× sample buffer (Laemmli, 1970), boiled 2–5 min, frozen in liquid N2, and stored at −80°C (this sample was used for immunoblot analysis). The remaining sample diluted in H1 kinase dilution buffer (5 μl) was frozen in liquid N2 and stored at −80°C. Then, a sample for cytology was taken by removing 2.5 μl of reaction and mixing gently with 10 μl of EGS fixative. EGS fixative was made by combining 1 volume of 0.2 M EGS [ethylene glycol-bis(succinic acid N-hydrosysuccinimide ester), freshly dissolved in dimethyl sulfoxide (DMSO)]; 19 volumes of 100 mM KCl, 5 mM MgCl2, 2.5% glycerol, and 20 mM HEPES-KOH, pH 7.5; and Hoechst 33342 added to 2 μg/ml. With these extracts, we found that the lengths of the first cell cycle (from the start of incubation until the peak of histone H1 kinase activity) varied from 40 to 60 min, and the second cell cycle varied from 40 to 50 min. The lengths of these cell cycles are comparable with those observed previously (Murray and Kirschner, 1989; Murray, 1991).
Assays using CHX extracts were done in the same way, with the following exceptions. MEK(QP) was added to reaction tubes containing supplemented CHX extracts and sperm and then incubated for 20 min. During this time, the sperm decondensed and formed nuclear envelopes equally well in both buffer and MEK(QP)-added samples. At 20 min of incubation, samples were taken (zero time point), then cyclin BΔ90 protein was added to buffer or MEK(QP) reactions, and reactions were incubated at 21–23°C. Samples were then taken at 20-min intervals up to 120 min and analyzed as described above for cycling egg extract assays. The same procedure was used for experiments in which Mos was added to CHX extracts, except that the reactions were incubated for 45 min prior to the addition of cyclin BΔ90 protein. For experiments using the PD098059 (PD), the inhibitor was added to lysates on ice at a final concentration of 250 μM (diluted from a stock solution of 50 mM in DMSO) prior to the addition of MEK or Mos.
Antibodies and Immunoblotting
Immunoblotting with p42MAPK antiserum (antiERK1 691; Boulton and Cobb, 1991) and phosphotyrosine antibodies (Druker et al., 1989) was as previously described (Shibuya et al., 1992a,b, 1996; Shibuya and Ruderman, 1993), except that 12.5% gels were used. Affinity-purified antibodies raised against Xenopus Cdc25C protein (a generous gift from A. Kumagai, California Institute of Technology; Kumagai and Dunphy, 1992) were used for all experiments unless otherwise indicated (see below). For anti-cyclin B2 immunoblots, we used an affinity-purified polyclonal antibody raised against the N-terminal peptide of cyclin B2 (van der Velden and Lohka, 1994; Lohka, unpublished results). Antiserum raised to a synthetic peptide (HPYFDDLDKSSLPDNQIRN) corresponding to the C-terminal 19 amino acids of Xenopus Cdc2 was used for Cdc2 immunoblots (Lohka, unpublished results). p90rsks were identified by immunoblotting with a commercially obtained antibody (product sc-231; Santa Cruz Biotechnology, Santa Cruz, CA). Detection of primary antibody binding was by alkaline phosphatase-conjugated secondary antibody by following the manufacturer’s instructions (Promega, Madison, WI).
We also produced our own affinity-purified Xenopus Cdc25C antibodies that were used for the experiments shown in Figures 2A and 7. C-terminal oligohistidine-tagged Xenopus Cdc25C protein was expressed in E. coli, purified, and used to raise polyclonal antiserum in rabbits. Affinity-purified antibodies were isolated according to standard methods (Harlow and Lane, 1988).
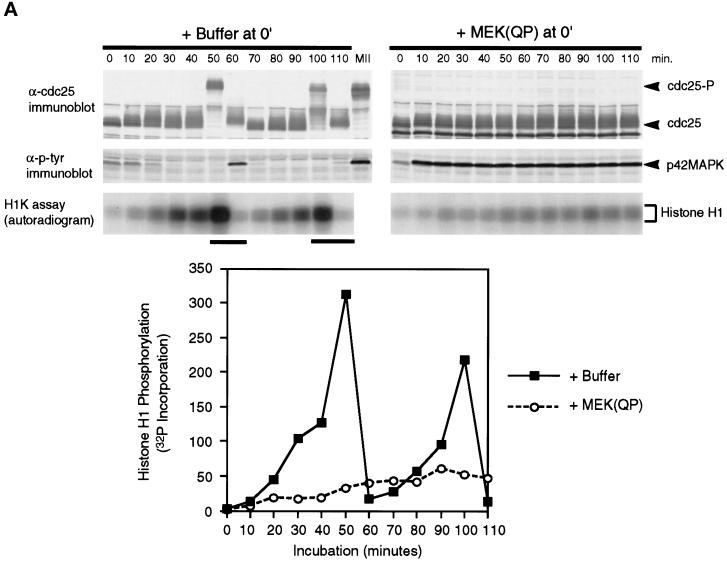
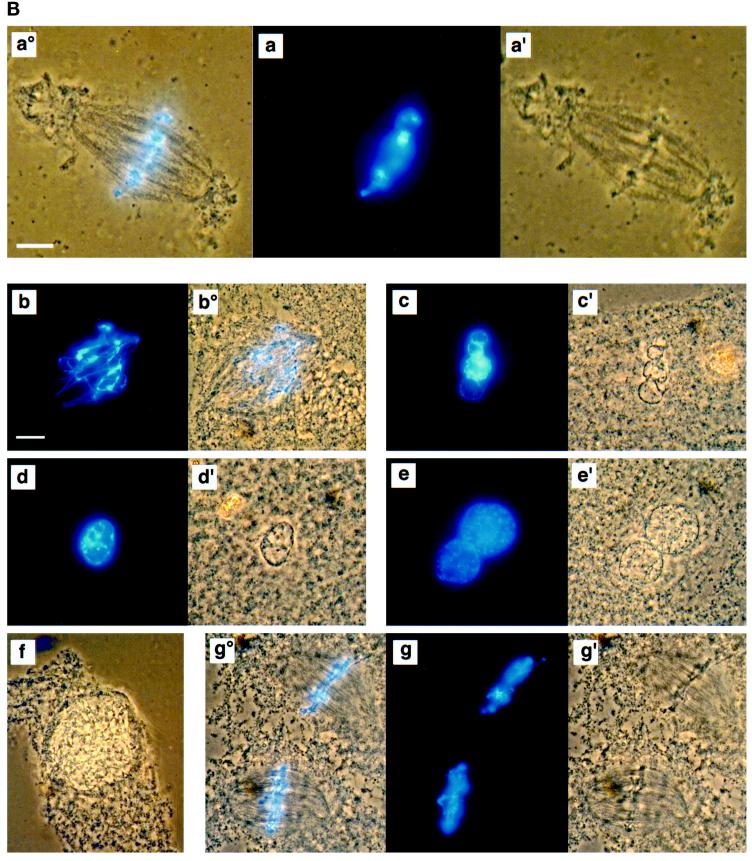
Figure 2.
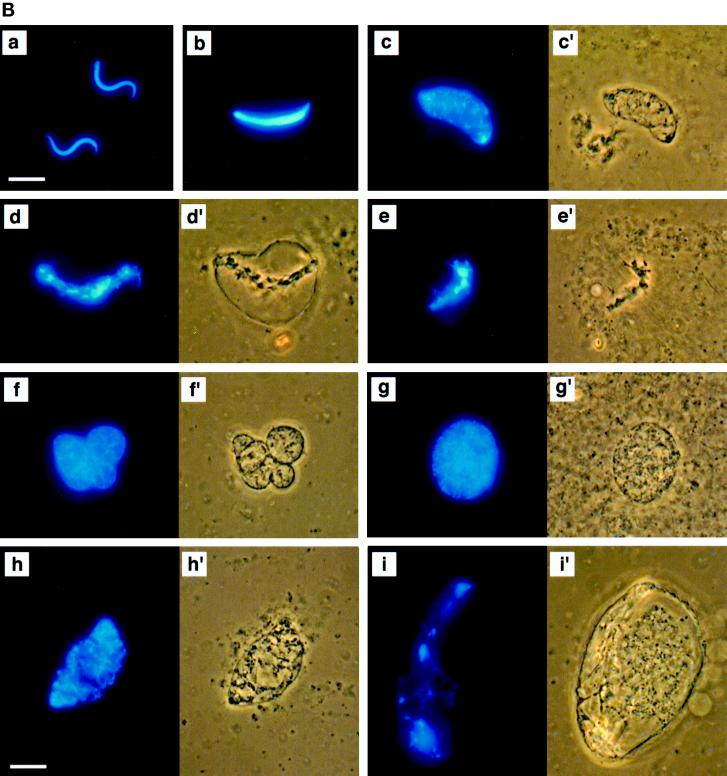
MEK(QP) Activates p42MAPK and inhibits entry into M-phase in cycling Xenopus egg extracts. (A) Cycling egg extracts were prepared and buffer or constitutively active MEK(QP) was added. Reactions were incubated at room temperature and samples were taken for analysis by immunoblotting with Cdc25C antibodies (top) or anti-phosphotyrosine antibodies (middle) and for assay of histone H1 kinase activity (bottom). Bold lines under H1 kinase assays indicate time points when NEBD and chromosome condensation (CC), markers of M-phase, were observed. Bottom, quantitation of H1 kinase activity. (B) Samples were taken for cytology from control cycling extracts or extracts to which MEK(QP) had been added at the start of incubation. Bars, 10 μm. In double adjoining panels, the left image is Hoechst fluorescence and the right image, denoted with ′, is a phase-contrast image. (a–g) Control cycling extracts. (a) Sperm preparation. (b) Sperm nucleus at 10 min. (c and c′) Decondensed nucleus at 20 min. (d and d′) Nucleus just before M-phase at 40 min. (e and e′) NEBD and CC at 50 min. (f and f′) Multiple decondensing nuclei at 70 min. (g and g′) Decondensed nucleus with nuclear envelope at 70. (h and i) Extracts inhibited from entering M-phase by p42MAPK activation in interphase. (h and h′) Decondensed nucleus at 30 min. (i and i′) Nucleus with enlarged intact nuclear envelope at 90 min.
Figure 7.
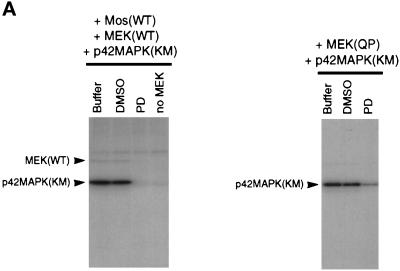
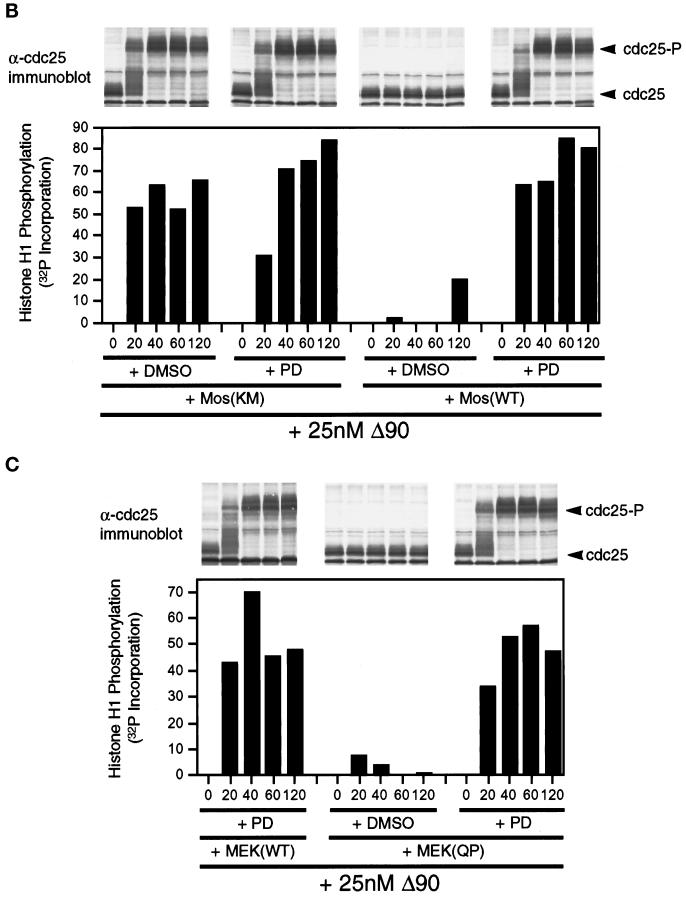
MEK(QP) and Mos-induced inhibition of Cdc2 activation occurs by specific activation of the p42MAPK signaling pathway. (A) PD inhibits phosphorylation of p42MAPK by MEK(WT) and MEK(QP) in vitro. Left, MEK(WT) was preincubated with buffer, DMSO, or PD (100 μM), and then activity was assayed in vitro by using recombinantXenopus Mos as an activator and recombinant kinase-inactive Xenopus p42MAPK as a substrate. Right, MEK(QP) was preincubated with buffer, DMSO, or PD (100 μM), and then activity was assayed in vitro by using the p42MAPK protein used above as a substrate (see MATERIALS AND METHODS for details). (B) PD prevents the Mos-induced inhibition of Cdc2 activation by cyclin B. DMSO or PD (250 μM) was added to aliquots of CHX extracts on ice, then Mos(WT) or Mos(KM) were added, and reactions were incubated at room temperature for 45 min before the addition of cyclin BΔ90 to 25 nM. Samples were taken for analysis by immunoblotting and H1 kinase assay. (C) PD prevents the MEK(QP)-induced inhibition of Cdc2 activation by cyclin B. DMSO or PD (250 μM) was added to aliquots of CHX extracts on ice, MEK(WT) or MEK(QP) were added, and then reactions were incubated at room temperature for 20 min prior to the addition of cyclin BΔ90 to 25 nM. Samples were taken for analysis by immunoblotting and H1 kinase assay.
Kinase Assays
MEK proteins were tested for kinase activity in vitro by using recombinant kinase-inactive N-terminal oligohistidine-tagged rat p42MAPK (ERK2KR; Figure 1; Robbins et al., 1993; Shibuya et al., 1996) or Xenopus MAPK [p42MAPK(KM), see Figure 7A] as a substrate (Lange-Carter et al., 1993). Five microliters of GST-MEK protein (10 ng/μl in the final reaction) was added to 6.5 μl of kinase mixture containing 10 mM MgCl2, 5 mM EGTA, 30 μM unlabeled ATP, [γ-32P]ATP (0.5 μCi/μl), 20 mM HEPES-NaOH, pH 7.5, and 100 ng/μl ERK2KR or 100 ng/μl Xenopus p42MAPK(KM), and then the reactions were incubated at 30°C for 30 min. Mos(WT) was incubated in oocyte lysates for 60 min, immunoprecipitated with anti-maltose binding protein (MBP) antibodies and then assayed in vitro with MEK(WT) and p42MAPK(KM) under the same conditions as the MEK(QP) reactions except that reactions were incubated for 60 min at 21–23°C. For inhibition studies, MEK(WT or QP) proteins were incubated in 100 μM PD (a MEK-specific inhibitor; Alessi et al., 1995) for 15 min at 21–23°C before addition to kinase assays, which contained 100 μM PD. Reactions were stopped by the addition of 11.5 μl of double-strength sample buffer and then samples were electrophoresed on 15% polyacrylamide gels and processed for autoradiography. 32P incorporation into proteins was quantified by phosphorimage analysis using the MacBase (1.0) software program.
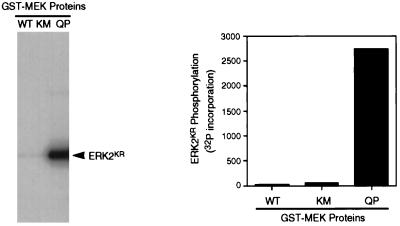
Figure 1.
Recombinant constitutively active MEK1 protein phosphorylates ERK2 in vitro. Left, recombinant GST-MEK fusion proteins (wild-type = WT; kinase mutant = KM; and a constitutively active mutant in which Gln-56 was mutated to Pro = QP) were produced in E. coli and purified as described in MATERIALS AND METHODS. These proteins were then tested for activity in an in vitro kinase assay with [γ-32P]ATP and a recombinant kinase-inactive ERK2 (ERK2KR) as a substrate. Right, quantitation of ERK2KR phosphorylation in the above assay by phosphorimage analysis.
Histone H1 kinase assays were carried out as previously described with minor modifications (Shibuya et al., 1992b). Five-microliter samples were thawed on ice. Three microliters of kinase mixture (10 mM MgCl2, 5 mM EGTA, 0.2 mg/ml histone H1 [Life Technologies-BRL, Gaithersburg, MD], 30 μM unlabeled ATP, 20 μM protein kinase A inhibitor peptide (PKI) [Sigma], 10 μM calmidazolium [Sigma], γ-32P-ATP [0.5 μCi/μl], and 20 mM HEPES-NaOH, pH 7.5) was added to each sample, and reactions were incubated for 20 min at 21–23°C, followed by quenching the reaction with 8 μl of double-strength sample buffer. Samples were then processed for autoradiography and quantified as described above.
Because kinase reactions were done at different times with radioisotope of various ages, 32P incorporation into substrate proteins is expressed in arbitrary units.
DNA Replication Assays
DNA replication assays were done essentially as previously described (Fang and Newport, 1991), with the following modifications. Reactions were prepared on ice by mixing 27 μl of CHX extract (see above); 1.2 μl of ATP-regenerating system; and 1.5 μl of buffer, Mos(KM), or Mos(WT) and then incubating at room temperature for 40 min. Demembranated sperm nuclei (600 nuclei/μl) and [α-32P]dATP (0.2 μCi/μl) were added to each reaction, and samples were taken at the time of these additions (0 h) and at 0.5, 1, and 2 h of further incubation. Samples were electrophoresed on 0.8% agarose gels cast on glass plates, and gels were dried and processed for autoradiography. p42MAPK activation was determined by immunoblotting as described above.
RESULTS
Activation of p42MAPK Inhibits Entry into M-phase in Cycling Xenopus Egg Extracts
Three forms of recombinant GST-MEK1 fusion proteins (wild-type = WT; a kinase-inactive mutant with Lys-97 mutated to Met = KM; and a constitutively active mutant with Gln-56 mutated to Pro = QP; Bottorff et al., 1995) were expressed in E. coli, purified, and tested for activity in an in vitro kinase assay using recombinant kinase-inactive p42MAPK (Lys- 52 mutated to Arg = ERK2KR; Robbins et al., 1993) as a substrate. Only the MEK(QP) was active in this assay (Figure 1) and in the rapid activation of p42MAPK in oocyte extracts (our unpublished results). MEK proteins were added at various times to cycling Xenopus egg extracts (Murray and Kirschner, 1989) to examine the effects of p42MAPK activation on cell cycle progression. These extracts are particularly well-suited for such detailed studies, because they undergo cell cycles with complete synchrony in vitro.
Buffer or MEK(QP) was added to egg extracts before the start of the incubation and samples were taken every 10 min for analysis of 1) Cdc25C phosphorylation, 2) p42MAPK tyrosine phosphorylation, 3) histone H1 kinase activity, and 4) nuclear morphology. Extracts with added buffer underwent two cell cycles as judged by H1 kinase activity, the tyrosine phosphorylation state of p42MAPK, and nuclear morphology (Figure 2, A, left, and B, a–g), as had been seen previously (Murray and Kirschner, 1989; Izumi et al., 1992; van der Velden and Lohka, 1993; Minshull et al., 1994). In addition, the hyperphosphorylation of Cdc25C, which coincided with peaks of H1 kinase activity, nuclear envelope breakdown (NEBD), and chromosome condensation, was used as a marker for M-phase (Izumi et al., 1992; Kumagai and Dunphy, 1992). In extracts receiving MEK(QP), endogenous p42MAPK was activated fully within 10 min [judged by anti-phosphotyrosine antibody immunoblotting (Figure 2A) and the complete shift of p42MAPK to a slower migrating form (Shibuya et al., 1992a,b, 1996; Shibuya and Ruderman, 1993; our unpublished results)]. However, extracts containing MEK(QP) did not cycle: levels of histone H1 kinase activity remained low, Cdc25C did not become hyperphosphorylated (Figure 2A, right), and nuclei became greatly enlarged with condensed chromatin and intact nuclear envelopes (Figure 2B, h and i). Except for their large size, the nuclei in the MEK(QP)-inhibited extract resembled closely nuclei seen in cycling extracts just prior to entry into M-phase (Figure 2B compare d and d′ with i and i′). MEK(WT) or MEK(KM) had no effect on the cell cycles in egg extracts (our unpublished results). Extracts with MEK(QP)-activated p42MAPK did not enter M-phase for at least 120 min, whereas control extracts [buffer, MEK(WT) or MEK(KM)] had completed two cell cycles by this time. The MEK(QP)-induced interphase arrest ocurred in the absence of added sperm (our unpublished results), indicating that MEK(QP) was not acting directly on the nuclei.
These data indicate that activation of p42MAPK in interphase of the cell cycle inhibited entry into M-phase.
Activation of p42MAPK during Entry into M-Phase Arrests the Cell Cycle at Metaphase
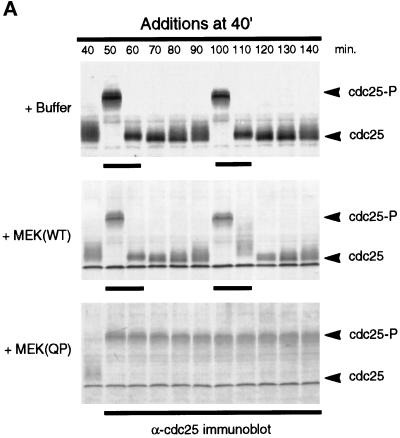
The results described above appeared to be inconsistent with those reported previously in which p42MAPK activation led to M-phase arrest both in vivo (for review, see Sagata, 1996) and in vitro (Minshull et al., 1994; Takenaka et al., 1997). To determine whether the timing of MEK(QP) addition influenced the phase at which the cell cycle arrested, we added buffer, MEK(WT), or MEK(QP) at 40 min of incubation, so that the endogenous p42MAPK would be fully activated just as the extract was entering M-phase. Samples were analyzed for the phosphorylation state of Cdc25C by immunoblotting and for nuclear morphology by fluorescence microscopy. Extracts to which buffer or MEK(WT) had been added at 40 min continued to cycle normally (Figure 3A, top and middle). In contrast, addition of MEK(QP) at 40 min led to arrest of the cell cycle in M-phase with hyperphosphorylated Cdc25C (Figure 3A, lower) and metaphase spindles (Figure 3B, a°, a, and a′). The M-phase arrest was sustained for at least 100 min after the addition of MEK(QP) (Figure 3B, g°, g, and g′). However, addition of Ca2+ to the M-phase-arrested extracts initiated reentry into interphase (Figure 3B, b–f) as has been previously observed (Minshull et al., 1994). Histone H1 kinase activity disappeared, cyclin B2 was degraded and Cdc25 was dephosphorylated after Ca2+ addition, without the inactivation of p42MAPK (our unpublished results).
Figure 3.
MEK(QP)-induced p42MAPK activation during entry into M-phase in cycling egg extracts leads to a Ca2+-sensitive metaphase arrest. (A) Cycling egg extracts were incubated for 40 min before buffer, MEK(WT), or MEK(QP) was added. Reactions were incubated further and samples were taken for analysis as in Figure 2A. Bold lines under blots indicate time points when M-phase (NEBD and CC) was observed. (B) Samples were taken for cytology as described in Figure 2B after MEK(QP) protein had been added to extracts at 40 min of incubation to activate p42MAPK during entry into M-phase. Extracts arrested in metaphase by MEK(QP) induced activation of p42MAPK during entry into M-phase (sample from A above). (a°, a, and a′) Metaphase spindle at 100 min [60 min after MEK(QP) addition]. Bar, 10 μm. Extracts were arrested in M-phase by the addition of MEK(QP) protein as described above. Thirty minutes later, samples were taken, then Ca2+ (b–f) or double distilled H2O (g) was added, and samples were taken for cytology during further incubation. The samples noted below indicate the duration of incubation after the addition of Ca2+ or double distilled H2O. Bar, 10 μm. Letter alone denotes fluorescence image (Hoechst staining); ′ denotes phase/contrast image; ° denotes fluorescence/phase double exposure. (b and b°) Anaphase at 10 min. (c and c′) Decondensing nuclei at 30 min. (d and d′) Decondensed nucleus at 30 min. (e and e′) Decondensed nuclei at 40 min. (f) Enlarging nucleus at 70 min. (g°, g, and g′) Metaphase spindles at 70 min.
In further experiments (our unpublished results), it was found that the addition of MEK(QP) during the first 30 min of incubation consistently inhibited the entry into M-phase. The cell cycle phase at which the extract arrested was dependent upon the timing of p42MAPK activation relative to the peak of endogenous H1 kinase activity. Activation of p42MAPK before the activation of the M-phase histone H1 kinase activity inhibited entry into M-phase, whereas addition of MEK(QP) to extracts within 10 min of the peak of H1 kinase activity (p42MAPK was fully activated within 10 min) arrested the cell cycle at metaphase. Addition of MEK(QP) at the time of the peak of H1 kinase activity (p42MAPK was fully activated after the extract had exited from M-phase) failed to arrest the cell cycle in M-phase but instead led to interphase arrest in the second cell cycle.
The simplest interpretation of these data is that MEK(QP) arrests extracts in M-phase only when p42MAPK activation occurs coincident with the peak of M-phase H1 kinase activity, but activation of p42MAPK before or after this point leads to an interphase arrest.
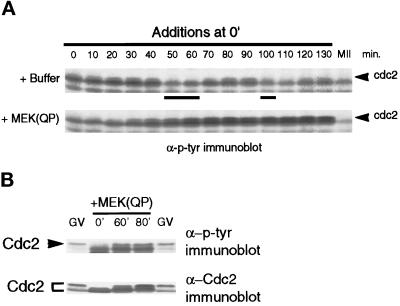
p42MAPK Activation in Interphase Induces the Accumulation of Tyrosine-phosphorylated Cdc2
Because MAPK activation during interphase kept levels of H1 kinase activity low and inhibited entry into M-phase, we examined the tyrosine phosphorylation of Cdc2 in the extracts. Cdc2 is known to undergo a sequence of phosphorylations after binding to cyclin B, including inhibitory phosphorylations on Tyr-15 and Thr-14 (for review, see Coleman and Dunphy, 1994). The tyrosine phosphorylation on Cdc2 can be detected by anti-phosphotyrosine antibodies and is an accurate measure of inactive Cdc2/cyclin B complexes (Kumagai and Dunphy, 1991; Shibuya et al., 1992b; Shibuya and Ruderman, 1993). In extracts with added buffer, tyrosine-phosphorylated Cdc2 was detected during interphase of the first and second cell cycles, but disappeared in M-phase (Figure 4A, top). However, in interphase-arrested extracts, tyrosine-phosphorylated Cdc2 accumulated (Figure 4A, bottom). The tyrosine-phosphorylated slower-mobility form of Cdc2 in MEK(QP) interphase-arrested extracts comigrated with the tyrosine-phosphorylated inactive form of Cdc2 found in extracts of G2-arrested oocytes (Figure 4B, top). The oocyte Cdc2 is phosphorylated on both Thr-14 and Tyr-15 (Kumagai and Dunphy, 1991). The slower-mobility tyrosine-phosphorylated protein was identified as Cdc2 by immunoblotting with antiserum specific for the C terminus of Xenopus Cdc2 (Figure 4B, bottom).
Figure 4.
Tyrosine phosphorylation of Cdc2 increases in extracts inhibited from entering M-phase by MEK(QP). (A) Buffer or MEK(QP) was added to cycling extracts, and then samples were taken during incubation for analysis by immunoblotting with anti-phosphotyrosine antibodies. (B) MEK(QP) was added to cycling extracts, and then samples were taken at 0, 60, or 80 min of incubation and immunoblotted with anti-phosphotyrosine (top) and anti-Cdc2 antibodies (bottom) along with samples from G2-arrested germinal vesicle stage oocytes (GV).
These data show that MEK(QP) inhibited the entry into M-phase by preventing the dephosphorylation of tyrosine from Cdc2, thereby keeping the protein kinase activity of cyclin/Cdc2 complexes inactive.
Addition of Cyclin BΔ90 to Interphase-arrested Egg Extracts Triggers Entry into M-Phase and Activation of Endogenous Cdc2
To determine whether the interphase-arrested extracts could be driven into M-phase, exogenous recombinant sea urchin cyclin B protein (cyclin BΔ90) was added to the extracts. The addition of cyclin proteins to oocyte extracts has been shown to bind to and activate uncomplexed Cdc2 and to activate endogenous inactive cyclin B/Cdc2 complexes (Izumi et al., 1992; Shibuya et al., 1992b; Hoffman et al., 1993). It is likely that the excess exogenous cyclins activate Cdc2 by bypassing the endogenous inhibitory mechanisms that keep cyclin B/Cdc2 complexes inactive: once some cyclin/Cdc2 complexes are activated, they hyperphosphorylate and activate Cdc25C, leading to the activation of inactive cyclin B/Cdc2 (Izumi and Maller, 1993). It has been proposed (Kumagai and Dunphy, 1992) that this is the basis for the cyclin B/Cdc2 [M-phase-promoting factor (MPF)] autoamplification mechanism originally described by Masui and Markert (1971).
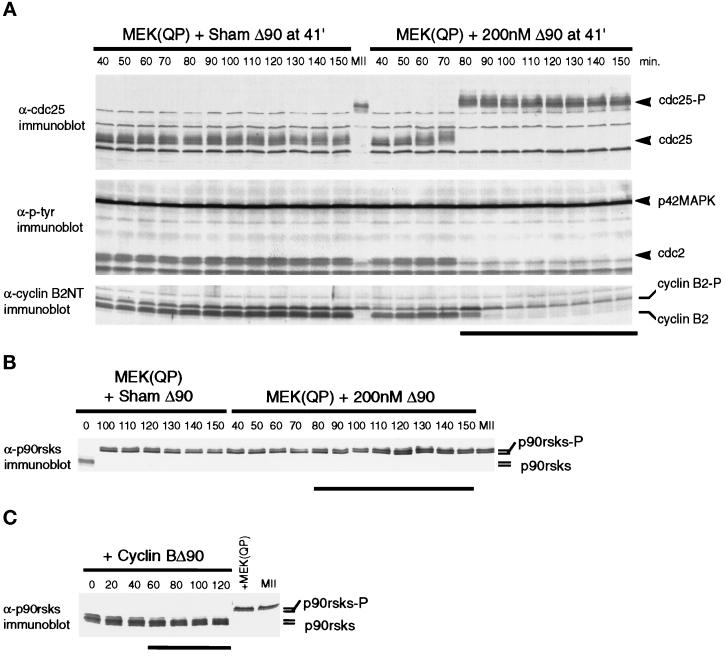
Extracts were incubated with MEK(QP) for 40 min, at which time buffer or cyclin BΔ90 was added. Samples were taken immediately before additions and at 10-min intervals afterward. As was seen in the experiments described above, interphase-arrested extracts did not cycle: Cdc25C remained hypophosphorylated, the unphosphorylated form of cyclin B2 accumulated, and nuclei did not undergo NEBD (Figure 5A, left). In contrast, the addition of 200 nM cyclin BΔ90 to interphase-arrested extracts induced M-phase after 40 min as judged by NEBD and Cdc25C hyperphosphorylation (Figure 5, right). In addition, the abrupt shift of endogenous cyclin B2 to a slower mobility form (Izumi and Maller, 1991; Minshull et al., 1994) and the loss of tyrosine phosphorylation on Cdc2 indicates that the endogenous cyclin B2/Cdc2 complexes were activated (Figure 5A, right, middle and bottom). After the shift to a slower-mobility form, cyclin B2 levels declined because the cyclin destruction machinery was turned on (Murray et al., 1989; Luca et al., 1991; Minshull et al., 1994).
Figure 5.
Activation of Cdc2 by exogenous cyclin BΔ90 triggers entry into M-phase in MEK(QP) interphase extracts. (A) MEK(QP) was added to cycling extracts, and then reactions were incubated at room temperature. At 40 min, samples were taken, then 1 min later a buffer or cyclin BΔ90 was added, and samples were taken during further incubation. Samples were analyzed by immunoblotting with anti-Cdc25 antibodies (top), anti-phosphotyrosine antibodies (middle), and anti-cyclin B2 antibodies (bottom). Bold line indicates time points when NEBD and CC were observed. (B) Samples from the experiment in A were immunoblotted with anti-p90rsk antibodies. Bold line indicates time points when NEBD and CC were observed. (C) Extracts were prepared from cycloheximide-treated activated eggs that lack mitotic cyclins (see MATERIALS AND METHODS), cyclin BΔ90 protein was added, and then samples were taken during further incubation and immunoblotted with anti-p90rsk antibodies. Bold line indicates time points when NEBD and CC were observed.
To confirm that p42MAPK was not inactivated after the addition of cyclin BΔ90 and the subsequent activation of Cdc2, samples from Figure 5A were immunoblotted with antibodies that recognize the 90-kDa ribosomal S6 kinases or p90rsks. p90rsks are known targets of MAPKs (for review, see Erikson, 1991) and undergo hyperphosphorylation during maturation of Xenopus oocytes (Erikson and Maller, 1989) and as a consequence of p42MAPK activation in Xenopus oocyte extracts (Shibuya and Ruderman, 1993; Shibuya et al., 1996). p90rsks were hyperphosphorylated in MEK(QP)-induced interphase-arrested egg extracts and were maintained in a hyperphosphorylated state after the addition of cyclin BΔ90 and entry into M-phase (Figure 5B). However, active cyclin BΔ90/Cdc2 complexes alone did not induce p90rsks hyperphosphorylation (Figure 5C). Thus, these results indicate that p42MAPK in these interphase-arrested extracts was not inactivated by cyclin BΔ90/Cdc2.
These results demonstrate that the interphase arrest caused by MEK(QP) is reversible and that the inactive cyclin/Cdc2 complexes can be activated when an endogenous inhibitory mechanism is bypassed or overcome by exogenous cyclin B.
MEK(QP) Inhibits the Activation of Cdc2 by Cyclin B
In the interphase-arrested extracts, the level of cyclin B2 continued to increase even though H1 kinase activity was not activated. This suggested that MEK may be suppressing the mechanisms which activate cyclin/Cdc2 complexes. To test this directly, we made use of egg extracts that are devoid of endogenous cyclins. These extracts were prepared in the same manner as the cycling extracts except that eggs were first incubated in the protein synthesis inhibitor cycloheximide (CHX), then activated to initiate cyclin degradation, and incubated in the presence of CHX until the mitotic cyclins were completely destroyed (see MATERIALS AND METHODS for details). Because these extracts (CHX extracts) lack endogenous mitotic cyclins, the activation of Cdc2 can be achieved only by exogenous cyclin protein. Increasing concentrations of cyclin BΔ90 protein were added to CHX extracts after the endogenous p42MAPK had been fully activated by MEK(QP) (see MATERIALS AND METHODS). Samples were analyzed as described above for cycling egg extracts.
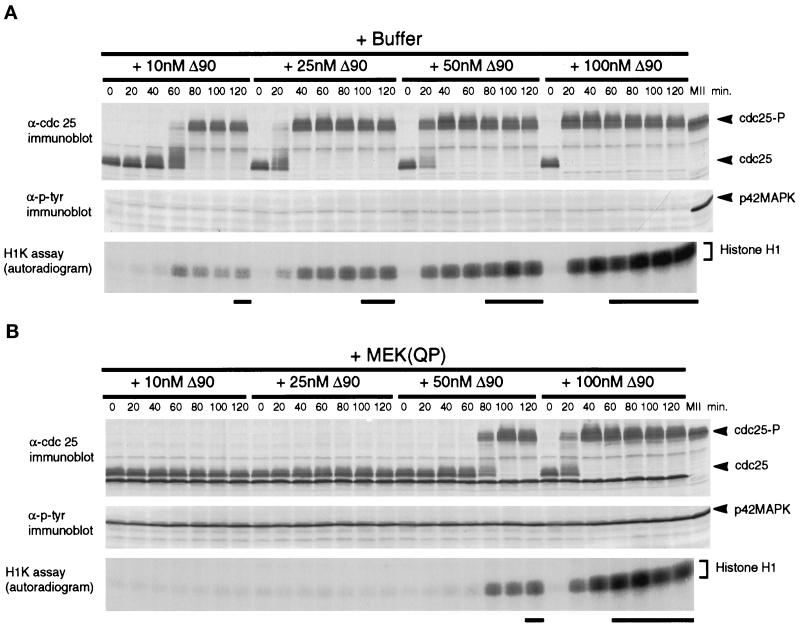
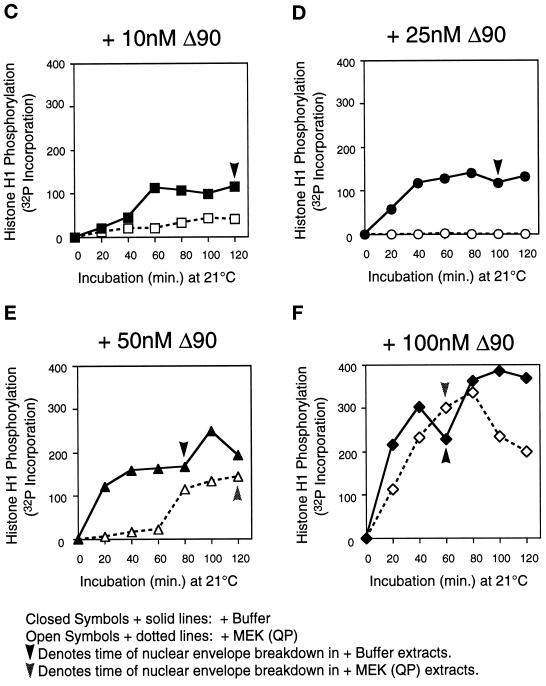
In extracts preincubated with buffer or with MEK(QP), nuclear envelopes had formed around the decondensed sperm chromatin by the time cyclin BΔ90 was added. After the addition of cyclin BΔ90 protein to buffer-treated extracts, Cdc2 was activated, Cdc25C was hyperphosphorylated, and nuclear envelope disassembly was induced at all concentrations of cyclin BΔ90 (Figure 6A). As expected, the entry into M-phase was accelerated with increasing concentrations of cyclin BΔ90 (Figure 6A, see bold lines below H1K assay autoradiogram). In MEK(QP)-treated extracts, only the highest concentration of cyclin BΔ90 activated Cdc2 and induced entry into M-phase with the same efficiency as control extracts. At lower cyclin BΔ90 concentrations, the activation of Cdc2 and entry into M-phase was either inhibited completely (10 and 25 nM) or delayed significantly (50 nM) compared with controls (Figure 6, A and B). Quantitation of the H1 kinase assay showed clearly that Cdc2 activation was inhibited at 10 and 25 nM cyclin BΔ90 (Figure 6, C and D) and delayed at 50 mM cyclin BΔ90 (Figure 6E). At 100 nM cyclin BΔ90, the kinetics of Cdc2 activation and levels of Cdc2 activity were similar to control reactions (Figure 6F). In extracts where Cdc2 was not activated by cyclin BΔ90 in the presence of MEK(QP), the tyrosine-phosphorylated form of Cdc2 accumulated, indicating that cyclin BΔ90 could bind to Cdc2 (our unpublished results).
Figure 6.
MEK(QP) inhibits activation of Cdc2 by cyclin BΔ90 in CHX extracts. Extracts were prepared from cycloheximide-treated activated eggs that lack endogenous mitotic cyclins (see MATERIALS AND METHODS). Either buffer or MEK(QP) was added to extracts, after incubation for 20 min. Samples were taken, then increasing concentrations of recombinant cyclin BΔ90 protein were added, and samples were taken as described above. Samples were analyzed by immunoblotting with anti-Cdc25C antibodies (top) and anti-phosphotyrosine antibodies (middle) and assayed for histone H1 kinase activity (bottom). Bold lines below H1 kinase data indicate the time points when NEBD and CC were observed. (A) Sham. Buffer added to reactions, then incubated for 20 min before cyclin BΔ90 addition. (B) MEK(QP) was added to reactions and then incubated for 20 min before cyclin BΔ90 addition. (C–F) Phosphorimager quantitation of histone H1 kinase assay data in A and B above.
These experiments clearly demonstrate that MEK(QP) inhibits the cyclin B-dependent activation of Cdc2, providing further support for the conclusion that the MEK-induced interphase arrest in cycling extracts occurs by suppression of Cdc2 activation.
Inhibition of Cdc2 Activation by MEK(QP) and Mos Occurs by Specific Activation of the p42MAPK Signaling Pathway
To date, p42MAPK is the only substrate that is known for MEK1 in oocytes. Throughout our experiments, we used the minimum concentration of MEK(QP) that would fully activate the endogenous p42MAPK within 10 min. There was, however, the possibility that MEK(QP) was inhibiting Cdc2 activation by a mechanism other than the MAPK pathway. This issue was addressed by repeating the assays by adding to CHX extracts recombinant Xenopus Mos, which activates the p42MAPK signaling pathway (Nebreda and Hunt, 1993; Posada et al., 1993; Shibuya and Ruderman, 1993) by directly phosphorylating and activating MEK (Posada et al., 1993). We reasoned that by using an activator of the p42MAPK signaling pathway directly upstream of the endogenous MEK, we could eliminate the possibility that MEK(QP) was inhibiting Cdc2 activation due to a different substrate specificity than endogenous MEK1. Moreover, inhibition of Cdc2 activation by Mos would show that endogenous levels of MEK (and p42MAPK) activity could mediate an interphase arrest. In some reactions, we examined the effects of PD, a specific inhibitor of MEK1 (Alessi et al., 1995), on the inhibition of Cdc2 activation by MEK(QP) and Mos. If the inhibition of Cdc2 activation by Mos could be blocked by PD, then it is highly probable that Mos is affecting Cdc2 activation through a mechanism that is dependent on MEK activation. In pilot experiments (our unpublished results), PD completely inhibited the transient p42MAPK activation in cycling extracts but had no discernible effect on any of the cell cycle parameters that were assayed.
Initially, PD was tested in kinase assays using purified recombinant components. PD inhibited the in vitro phosphorylation of p42MAPK by MEK(WT) that had been activated by Mos (Figure 7A, left). The phosphorylation of p42MAPK by MEK(QP) was also blocked by PD (Figure 7A, right). In other experiments, PD or DMSO (vehicle control) was added to CHX extracts with Xenopus Mos(WT) or Mos(KM = kinase inactive mutant; Yu et al., 1992) to determine whether specific inhibition of endogenous MEK would prevent the inhibition of Cdc2 activation. Activation of Cdc2 by added cyclin BΔ90 (25 nM) and entry into M-phase (nuclear envelope disassembly and chromosome condensation at 80 min of incubation) were unaffected by the addition of Mos(KM) to extracts (Figure 7B). In contrast, Mos(WT) addition to control extracts activated p42MAPK (our unpublished results) and inhibited Cdc2 activation by cyclin BΔ90, Cdc25C hyperphosphorylation, and entry into M-phase (Figure 7B). However, in the presence of PD, the Mos(WT)-induced activation of p42MAPK was abolished, and Cdc2 activation and the timing of entry into M-phase were indistinguishable from that seen in extracts containing Mos(KM) (Figure 7B). Consistent with these data, the effects of MEK(QP) on p42MAPK activation and on Cdc2 activation were blocked by PD (Figure 7C).
These results indicate that Mos, an upstream direct activator of endogenous MEK, can also inhibit Cdc2 activation. Because the effects of Mos on both Cdc2 activation and p42MAPK activation are both inhibited by PD, the effect of Mos on Cdc2 activation is most likely mediated by stimulation of the p42MAPK signaling pathway. Further, these results demonstrate that endogenous MEK, when stimulated, is sufficient to prevent activation of Cdc2, arguing that our results with MEK(QP) were not due to artificially high levels of MEK activity or a different substrate specificity than that of endogenous MEK. If different target proteins were involved in the inhibition of Cdc2 activation, they would have to be substrates common to both MEK(QP) and endogenous MEK. To date, other substrates for MEK have not been identified in Xenopus. Hence, MEK(QP) and endogenous MEK are likely activating the same substrates and signaling pathways to prevent the activation of Cdc2.
DNA Replication Is Not Inhibited by Mos-induced p42MAPK Activation
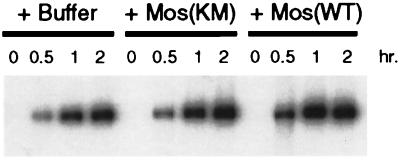
To further determine the phase of interphase cell cycle arrest induced by activation of the p42MAPK pathway, we assayed DNA replication in Xenopus egg extracts in the presence and absence of activated p42MAPK by using a method previously described by Fang and Newport (1991). Buffer, Mos(KM), or Mos(WT) were added to CHX egg extracts (see MATERIALS AND METHODS) and incubated for 40 min at room temperature, and then demembranated sperm nuclei and α-32P-dATP were added to each reaction. Samples were taken at the time of these additions (0 h) and at 0.5, 1, and 2 h of further incubation, followed by agarose gel electrophoresis and autoradiography. As seen in Figure 8, DNA replication occurred equally well in all reactions. Immunoblotting confirmed that p42MAPK was fully-activated at the time of sperm and [α-32P]dATP addition and during the course of the labeling period. These results suggest that the interphase arrest induced by the inappropriate activation of p42MAPK occurs after DNA replication in G2-phase.
Figure 8.
Mos-induced activation of p42MAPK does not inhibit DNA replication in Xenopus egg extracts. Buffer, Mos(KM), or Mos(WT) was added to CHX extracts and then incubated at room temperature for 40 min. Demembranated sperm nuclei (600 nuclei/μl) and [α-32P]dATP (0.2 μCi/μl) were added to each reaction, and samples taken at 0, 0.5, 1, and 2 h of incubation at room temperature. Samples were analyzed by electrophoresis on 0.8% agarose gels (see MATERIALS AND METHODS).
DISCUSSION
p42MAPK Activation Inhibits Entry into M-phase in Cycling Egg Extracts
Previous experiments in a number of different cell systems have shown that MAPK activation is stimulated by both extracellular and intracellular signals (for review, see Cobb et al., 1991). Activators of the MAPK pathway have previously been shown to inhibit the cell cycle in M-phase and to be a critical component of the spindle assembly checkpoint in intact cells and in cycling egg extracts (Minshull et al., 1994; Takenaka et al., 1997; Wang et al., 1997). We have used cycling Xenopus egg extracts and recombinant constitutively active proteins to examine the consequences of stimulating the p42MAPK pathway on cell cycle progression. Because these extracts undergo cell cycle transitions without the problems of asynchrony encountered in vivo, they are particularly well-suited to detailed studies of cell cycle regulation. As in other studies, we have observed an M-phase arrest of the cell cycle when the MAPK pathway was inappropriately stimulated. However, an M-phase arrest is not the only consequence of MAPK activation. Extracts were arrested in interphase when p42MAPK was fully activated prior to the entry into M-phase. In the interphase-arrested extracts, H1 kinase activity remained low, the tyrosine-phosphorylated form of Cdc2 accumulated, cyclin B2 remained hypophosphorylated, Cdc25C did not undergo hyperphosphorylation, and nuclei continued to enlarge. In addition, M-phase-specific phosphoproteins were not detected with the MPM-2 antibody (Davis et al., 1989; Kuang et al., 1994; our unpublished results), providing further support that these extracts remained in interphase. The interphase arrest persisted for at least 130 min, even though control extracts had completed at least two cell cycles by this time. The point in the cell cycle that is sensitive to p42MAPK inhibition was just prior to entry into M-phase. The Mos-induced activation of p42MAPK did not inhibit DNA replication. In the starfish, Tachibana et al. (1997) have found that active MAPK inhibits DNA replication. We do not know why our results differ from those of Tachibana et al., 1997, but DNA replication clearly is not inhibited by active p42MAPK in Xenopus egg extracts. Because the additions of MEK(QP) to cycling egg extracts progressively later during the cell cycle lead to interphase arrest up until just prior to entry into M-phase and p42MAPK activation does not inhibit DNA replication, our results suggest that the stage of cell cycle arrest is in G2-phase.
Activation of the p42MAPK Signaling Pathway Inhibits Cyclin B-induced Cdc2 Activation
When the mechanism of interphase arrest was examined, we found that cyclin B2 continued to be synthesized and that the tyrosine-phosphorylated form of Cdc2 accumulated (indicating cyclin/Cdc2 complexes were formed) but that the increase in histone H1 kinase activity normally associated with entry into mitosis did not occur. These observations indicated that stimulation of the p42MAPK pathway downstream of MEK altered the regulation of cyclin B/Cdc2 activation.
The effect of an activated p42MAPK pathway on Cdc2 activation by exogenous cyclin BΔ90 was tested directly in extracts devoid of mitotic cyclins. Low concentrations of cyclin BΔ90 (10 nM and 25 nM) were able to activate Cdc2 and induce M-phase in control extracts but not when the p42MAPK pathway was first activated by Mos or MEK(QP). Increasing the concentration of cyclin B overcame the effects of an activated p42MAPK pathway. At 50 nM cyclin BΔ90, Cdc2 activation was delayed, and at 100 nM cyclin BΔ90, Cdc2 activation occurred as rapidly in most of the p42MAPK-activated extracts as in control extracts, although in some p42MAPK-activated extracts Cdc2 activation was still inhibited at these high concentrations of cyclin BΔ90. The rapid activation of Cdc2 in the presence of these high cyclin BΔ90 concentrations was most likely due to the direct activation of Cdc25C by active cyclin B/Cdc2 complexes, which, in turn, triggers the autoamplification loop (Hoffman et al., 1993; Izumi and Maller, 1993). Because the kinetics of Cdc2-associated histone H1 kinase activation was the same at higher cyclin B concentrations, activation of the p42MAPK pathway does not inhibit autoamplification.
The simplest explanations for the accumulation of the tyrosine-phosphorylated form of Cdc2 in interphase-arrested extracts is that the endogenous Wee1-like kinase activity is elevated or that the activation of Cdc25C phosphatase is inhibited. Wee1 phosphorylates Tyr-15 of Cdc2, preventing Cdc2 activation, whereas Cdc25C removes the inhibitory phosphate from this site. However, Wee1-like kinase and Cdc25C phosphatase may not be the direct targets for the p42MAPK pathway. For example, a Cdc25C-activating kinase has been identified as the Xenopus homologue of the Polo-like kinase (Plx; Kumagai and Dunphy, 1996). Preventing the activation of this kinase would also be expected to inhibit the activation Cdc25C. Alternatively, other upstream regulators of Wee1-like kinase or of Cdc25C could be targets for the activated p42MAPK pathway (Mueller et al., 1995). Recently, it has been found that the Chk1 protein kinase can phosphorylate both Wee-1 (O’Connell et al., 1997) and Cdc25 (Peng et al., 1997; Sanchez et al., 1997). Moreover, the site phosphorylated on Cdc25 by Chk1 appears to be involved in the inhibition of Cdc25 phosphatase activity (Peng et al., 1997). Therefore, Chk1 could be another candidate for regulation by the p42MAPK pathway.
There is evidence that the activation of the MAPK signaling pathway in intact tissue culture cells during interphase may inhibit entry into M-phase. Treatment of synchronized HeLa cells with epidermal growth factor (EGF) or phorbol ester arrests cells in G2-phase, with inactive Cdc2 and hypophosphorylated Cdc25C (Barth et al., 1996). The MAPK signaling pathway is one of the multiple pathways known to be activated by EGF and phorbol esters in a variety of cell types (for review, see Cobb et al., 1991). Therefore, our observations suggest that the G2-phase arrest induced in HeLa cells by EGF and phorbol ester may be due to the specific activation of the MAPK signaling pathway.
In Vivo Versus In Vitro Inhibition of Cell Cycle Progression by p42MAPK Activation
We have observed two separate reproducible effects of p42MAPK activation in cycling Xenopus egg extracts: the inhibition of entry into M-phase by the activation of the p42MAPK pathway during interphase and an M-phase arrest by activation of the p42MAPK pathway during entry into M-phase. In vivo, however, microinjections of cytoplasmic preparations containing cytostatic factor or of activators of the p42MAPK pathway, including Ras, Mos, and constitutively active p42MAPK, have been shown to arrest the cell cycle in M-phase in the majority of cases (Masui and Markert, 1971; Shibuya and Masui, 1988; Sagata et al., 1989; Daar et al., 1991; Yew et al., 1992; Haccard et al., 1993). In none of these studies was an interphase arrest predominant. It is unlikely, however, that activators of the p42MAPK pathway were injected so that the endogenous MAPK was activated only during the brief interval before M-phase, which would lead to an M-phase arrest. Why then has an interphase arrest not been seen previously? The failure to detect an interphase arrest is probably due to several differences between the in vivo and the in vitro assays.
1) In microinjection studies, the time required for proteins to be synthesized from injected mRNA or for the diffusion of proteins from the site of microinjection may delay activation of p42MAPK until just before entry into M-phase. Alternatively, the intracellular concentration of injected proteins may not fully activate p42MAPK until just prior to entry into M-phase. In our experiments with egg extracts, we have identified the optimal concentration of MEK(QP) as that which should be added to fully activate p42MAPK within 10 min. At lower concentrations of MEK(QP), the activation of p42MAPK is slower. In addition, the optimal concentration of MEK(QP) required for full activation of p42MAPK within 10 min leading to interphase arrest is higher than that required for an M-phase arrest. This is not surprising, given that p42MAPK is activated transiently in M-phase of cycling egg extracts (Minshull et al., 1994; Takenaka et al., 1997; Figure 2A), although this activation has not been observed in vivo (Ferrell et al., 1991; Shibuya et al., 1992a). We have also seen that Mos-induced activation of p42MAPK in cycling extracts was slower and less complete during interphase than during M-phase, suggesting that negative regulators of p42MAPK activation, such as MKP-1, a MAPK-specific phosphatase (Sun et al., 1993), or inhibitors of Mos activation are more active in interphase than in M-phase (our unpublished results).
2) In the cell-free extracts, we are able to monitor changes in regulatory proteins more precisely than in intact cells because cell cycle changes occur synchronously throughout the entire volume of the reaction, whereas these changes are less synchronous in a group of microinjected cells. Moreover, in extracts, samples can be taken at shorter time intervals than are realistically possible for microinjected cells.
3) With prolonged incubation of interphase-arrested extracts (greater than 2.5 h), we have observed that some extracts spontaneously entered M-phase. When this occurred, the extracts abruptly entered M-phase coincident with a sudden large spike of H1 kinase activity, which was three to four times the maximum normally seen in control cycling extracts during entry into M-phase. These data argue that the continued accumulation of cyclin B/Cdc2 complexes eventually exceeds the capacity of the extract to keep the complexes inhibited. As a result, a small amount of active Cdc2 triggers the autoamplification loop, resulting in M-phase arrest. In microinjection experiments, the cell cycle phase of the injected cell is usually assayed several hours after injection, when uninjected cells have already divided many times (note that the early cell cycles of Xenopus embryos are approximately 30 min long). This delay in evaluating the cell cycle arrest point could have allowed the injected cells to “break through” the interphase arrest, leading to arrest in M-phase.
Cycling egg extracts have permitted us to identify a novel effect of the activation of p42MAPK signaling pathway on cell cycle progression that was not observed previously when similar experiments were carried out with intact cells. p42MAPK is already recognized as an essential component of the spindle assembly checkpoint. When mitotic spindles fail to assemble properly, such as in the presence of nocodozole, p42MAPK is activated in response to an, as yet, unidentified signal. As a result, the cell cycle is delayed in M-phase until the spindle defect is corrected. It is tempting to speculate that the observations we have made are also involved in the regulation of cell cycle progression. By analogy, activation of the p42MAPK signaling pathway in interphase by a signal different than the one involved in the spindle assembly checkpoint would prevent entry into M-phase. If this were the case, the arrest point would be in G2-phase of the cell cycle, before the activation of cyclin B/Cdc2 complexes. However intriguing the idea, the involvement of the p42MAPK signaling pathway in an interphase checkpoint has yet to be demonstrated.
ACKNOWLEDGMENTS
We thank James Stone for the constitutively active rat MEK1(Q56P) cDNA, Gary Johnson for constructs encoding rat MEK1 (wild-type, K97 M) and Xenopus p42MAPK fusion proteins (wild-type, KM), A. Kumagai for affinity-purified Cdc25C antibodies and the pET3a-xCdc25 construct, George Vande Woude for MBP-mos fusion protein constructs, Dr. M. Carpenter and R. Dahl for MBP-cyclin BΔ90, Melanie Cobb for the HisERK2KR construct and ERK antibodies, and Brian Druker for phosphotyrosine antibodies. This work was supported by Medical Research Council Operating Grants to M.J.L. and E.K.S. and an Alberta Heritage Foundation for Medical Research Establishment Grant to E.K.S. E.K.S. is a Scholar of the Medical Research Council and the Alberta Heritage Foundation for Medical Research.
REFERENCES
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Barth H, Hoffmann I, Klein S, Kaszkin M, Richards J, Kinzel V. Role of Cdc25-C phosphatase in the immediate G2 delay induced by the exogenous factors epidermal growth factor and phorbol ester. J Cell Physiol. 1996;168:589–599. doi: 10.1002/(SICI)1097-4652(199609)168:3<589::AID-JCP11>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Boulton TG, Cobb MH. Identification of multiple extracellular signal-regulated kinases (ERKs) with anti-peptide antibodies. Cell Reg. 1991;2:357–371. doi: 10.1091/mbc.2.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottorff D, Stang S, Agellon S, Stone JC. RAS signalling is abnormal in c-raf MEK1 double mutant. Mol Cell Biol. 1995;5:5113–5122. doi: 10.1128/mcb.15.9.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb MH, Boulton TG, Robbins DJ. Extracellular signal-regulated kinases: ERKs in progress. Cell Reg. 1991;2:965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb MH, Goldsmith EJ. How MAP kinases are regulated. J Biol Chem. 1995;271:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- Coleman TR, Dunphy WG. Cdc2 regulatory factors. Curr Opin Cell Biol. 1994;6:877–882. doi: 10.1016/0955-0674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Daar I, Nebreda AR, Yew N, Sass P, Paules R, Santos E, Wigler M, Vande Woude GF. The ras oncoprotein and M-Phase activity. Science. 1991;253:74–76. doi: 10.1126/science.1829549. [DOI] [PubMed] [Google Scholar]
- Davis FM, Wright DA, Penkala E, Rao PN. Mitosis-specific monoclonal antibodies block cleavage in amphibian embryos. Cell Struct Funct. 1989;14:271–277. doi: 10.1247/csf.14.271. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Manon HJ, Roberts TM. Oncogenes, growth factors, and signal transduction. N Engl J Med. 1989;321:1383–1389. doi: 10.1056/NEJM198911163212007. [DOI] [PubMed] [Google Scholar]
- Erikson E, Maller JL. In vivo phosphorylation and activation of ribosomal protein S6 kinases during Xenopus oocyte maturation. J Biol Chem. 1989;264:13711–13717. [PubMed] [Google Scholar]
- Erikson RL. Structure, expression and regulation of protein kinases involved in the phosphorylation of ribosomal protein S6. J Biol Chem. 1991;266:6007–6010. [PubMed] [Google Scholar]
- Fang F, Newport JW. Evidence that the G1–S and G2–M transitions are controlled by different cdc2 proteins in higher eukaryotes. Cell. 1991;66:731–742. doi: 10.1016/0092-8674(91)90117-h. [DOI] [PubMed] [Google Scholar]
- Ferrell JE, Wu M, Gerhart JC, Martin GS. Cell cycle tyrosine phosphorylation of p34Cdc2 and a MAP-kinase homolog in Xenopus oocytes and eggs. Mol Cell Biol. 1991;11:1965–1971. doi: 10.1128/mcb.11.4.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haccard O, Lewellyn A, Hartley R, Erikson E, Maller J. Induction of Xenopus oocyte meiotic maturation by MAP kinase. Dev Biol. 1995;168:677–682. doi: 10.1006/dbio.1995.1112. [DOI] [PubMed] [Google Scholar]
- Haccard O, Sarcevic B, Lewellyn A, Hartley R, Roy L, Izumi T, Erikson E, Maller JL. Induction of metaphase arrest in cleaving Xenopus embryos by MAP kinase. Science. 1993;262:1262–1265. doi: 10.1126/science.8235656. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies. A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Laboratory Press; 1988. [Google Scholar]
- Hattori S, Fukuda M, Yamashita T, Nakamura S, Gotoh Y, Nishida E. Activation of mitogen-activated protein kinase and its activator by ras in intact cells and in a cell-free system. J Biol Chem. 1992;267:20346–20351. [PubMed] [Google Scholar]
- Hoffman I, Clarke PR, Marcote MJ, Karsenti E, Draetta G. Phosphorylation and activation of human Cdc25-C by Cdc2-cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J. 1993;12:53–63. doi: 10.1002/j.1460-2075.1993.tb05631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Maller JL. Phosphorylation of Xenopus cyclins B1 and B2 is not required for cell cycle transitions. Mol Cell Biol. 1991;11:3860–3867. doi: 10.1128/mcb.11.8.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Maller JL. Elimination of cdc2 phosphorylation sites in the cdc25 phosphatase clocks initiation of M-phase. Mol Biol Cell. 1993;4:1337–1350. doi: 10.1091/mbc.4.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Walker DH, Maller JL. Periodic changes in phosphorylation of the Xenopus Cdc25 phosphatase regulate its activity. Mol Biol Cell. 1992;3:927–939. doi: 10.1091/mbc.3.8.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang J, Ashorn CL, Gonzalez-Kuyvenhoven M, Penkala JE. Cdc25 is one of the MPM-2 antigens involved in the activation of maturation-promoting factor. Mol Biol Cell. 1994;5:135–145. doi: 10.1091/mbc.5.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. The Cdc25 protein controls tyrosine dephosphorylation of the Cdc2 protein in a cell-free system. Cell. 1991;64:903–914. doi: 10.1016/0092-8674(91)90315-p. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Regulation of the Cdc25 protein during the cell cycle in Xenopus extracts. Cell. 1992;70:139–151. doi: 10.1016/0092-8674(92)90540-s. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science. 1996;273:1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laird AD, Taylor SJ, Oberst M, Shalloway D. Raf-1 is activated during mitosis. J Biol Chem. 1995;270:26742–25745. doi: 10.1074/jbc.270.45.26742. [DOI] [PubMed] [Google Scholar]
- Lange-Carter CA, Johnson GL. Assay of MEK kinases. Methods Enzymol. 1995;255:290–301. doi: 10.1016/s0076-6879(95)55032-1. [DOI] [PubMed] [Google Scholar]
- Lange-Carter CA, Leiman CM, Gardner AM, Blumer KJ, Johnson GL. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993;260:315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- Lovric J, Moelling K. Activation of Mil/Raf protein kinases in mitotic cells. Oncogene. 1996;12:1109–1116. [PubMed] [Google Scholar]
- Luca FC, Shibuya EK, Dohrmann CE, Ruderman JV. Both cyclin AΔ60 and BΔ97 are stable and arrest cells in M-phase, but only cyclin BΔ97 turns on cyclin destruction. EMBO J. 1991;10:4311–4320. doi: 10.1002/j.1460-2075.1991.tb05009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui Y, Markert CL. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool. 1971;177:129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- Minshull J, Sun H, Tonks NK, Murray AW. A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell. 1994;79:475–486. doi: 10.1016/0092-8674(94)90256-9. [DOI] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Dunphy WG. Cell cycle regulation of a Xenopus wee1-like kinase. Mol Biol Cell. 1995;6:110–134. doi: 10.1091/mbc.6.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AW. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- Murray AW, Kirschner MW. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989;339:275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Murray AW, Solomon MJ, Kirschner MW. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989;339:280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Nebreda A, Hunt T. The c-mos protooncogene protein kinase turns on and maintains the activity of MAP kinase, but not MPF, in cell-free extracts of Xenopus oocytes and eggs. EMBO J. 1993;12:1979–1986. doi: 10.1002/j.1460-2075.1993.tb05847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell MJ, Raleigh JM, Verkade HM, Nurse P. Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. EMBO J. 1997;16:545–554. doi: 10.1093/emboj/16.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H. Mitotic and G2 checkpoint control: Regulation of 14–3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- Posada J, Yew N, Ahn NG, Vande Woude GF, Cooper JA. Mos stimulates MAP kinase in Xenopus oocytes and activates a MAP kinase kinase in vitro. Mol Cell Biol. 1993;13:2546–2553. doi: 10.1128/mcb.13.4.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins DJ, Zhen E, Owaki H, Vanderbilt CA, Ebert D, Geppert TD, Cobb MH. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2 in vitro. J Biol Chem. 1993;268:5097–5106. [PubMed] [Google Scholar]
- Ruderman JV. MAP kinase and the activation of quiescent cells. Curr Opin Cell Biol. 1993;5:207–213. doi: 10.1016/0955-0674(93)90104-x. [DOI] [PubMed] [Google Scholar]
- Sagata N. Meiotic metaphase arrest in animal oocytes: its mechanisms and biological significance. Trends Cell Biol. 1996;6:22–28. doi: 10.1016/0962-8924(96)81034-8. [DOI] [PubMed] [Google Scholar]
- Sagata N, Watanabe N, Vande Woude GF, Ikawa Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature. 1989;342:512–518. doi: 10.1038/342512a0. [DOI] [PubMed] [Google Scholar]
- Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, Elledge SJ. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- Shibuya EK, Boulton TG, Cobb MH, Ruderman JV. Activation of p42 MAP kinase and the release of oocytes from cell cycle arrest. EMBO J. 1992a;11:3963–3975. doi: 10.1002/j.1460-2075.1992.tb05490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya EK, Masui Y. Stabilization and enhancement of primary cytostatic factor (CSF) by ATP and NaF in amphibian egg cytosols. Dev Biol. 1988;129:253–264. doi: 10.1016/0012-1606(88)90179-0. [DOI] [PubMed] [Google Scholar]
- Shibuya EK, Morris J, Rapp UR, Ruderman JV. Activation of the Xenopus oocyte mitogen-activated protein kinase pathway by Mos is independent of Raf. Cell Growth Diff. 1996;7:235–241. [PubMed] [Google Scholar]
- Shibuya E, Polverino AJ, Chang E, Wigler M, Ruderman JV. Oncogenic Ras triggers the activation of 42-kDa mitogen activated protein kinase in extracts of Xenopus oocytes. Proc Natl Acad Sci USA. 1992b;89:9831–9835. doi: 10.1073/pnas.89.20.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya EK, Ruderman JV. Mos induces the in vitro activation of MAP kinases in lysates of frog oocytes and mammalian somatic cells. Mol Biol Cell. 1993;3:781–790. doi: 10.1091/mbc.4.8.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DB, Johnson KS. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Sun H, Charles CH, Lau LF, Tonks NK. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Machida T, Nomura Y, Kishimoto T. MAP kinase links the fertilization signal transduction pathway to the G1/S-phase transition in starfish eggs. EMBO J. 1977;16:4333–4339. doi: 10.1093/emboj/16.14.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka K, Gotoh Y, Nishida E. MAP kinase is required for the spindle assembly checkpoint but is dispensable for the normal M phase entry and exit in Xenopus egg cell cycle extracts. J Cell Biol. 1997;136:1091–1097. doi: 10.1083/jcb.136.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velden HMW, Lohka MJ. Mitotic arrest caused by the amino terminus of Xenopus cyclin B2. Mol Cell Biol. 1993;13:1480–1488. doi: 10.1128/mcb.13.3.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velden HMW, Lohka MJ. Cell cycle-regulated degradation of Xenopus cyclin B2 requires binding to p34Cdc2. Mol Biol Cell. 1994;5:713–724. doi: 10.1091/mbc.5.7.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigers GP, Lohka MJ. A distinct vesicle population targets membranes and pore complexes to the nuclear envelope in Xenopus eggs. J Cell Biol. 1991;112:545–556. doi: 10.1083/jcb.112.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XM, Zhai Y, Ferrell JE., Jr A role for mitogen-activated protein kinase in the spindle assembly checkpoint in XTC cells. J Cell Biol. 1997;137:433–443. doi: 10.1083/jcb.137.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskiewicz AJ, Cooper JA. Mitogen and stress response pathways: MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr Opin Cell Biol. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- Wells WA. The spindle-assembly checkpoint: aiming for a perfect mitosis, every time. Trends Cell Biol. 1996;6:228–234. doi: 10.1016/0962-8924(96)10018-0. [DOI] [PubMed] [Google Scholar]
- Yew N, Mellini ML, Vande Woude GF. Meiotic initiation by the Mos protein in Xenopus. Nature. 1992;355:649–652. doi: 10.1038/355649a0. [DOI] [PubMed] [Google Scholar]