Abstract
Mechanical stress is one of the risk factors believed to influence intervertebral disc degeneration. Animal models have shown that certain regimes of compressive loading can induce a cascade of biological effects that ultimately results in cellular and structural changes in the disc. It has been proposed that both cell-mediated breakdown of collagen and the compromised stability of collagen with loss of anular tension could result in degradation of lamellae in the anulus fibrosus (AF). To determine whether this may be important in the AF, we subjected entire rings of de-cellularized AF tissue to MMP-1 digestion with or without tension. Biomechanical testing found trends of decreasing strength and stiffness when tissues were digested without tension compared with those with tension. To determine the physiologic significance of tissue level tension in the AF, we used an established in vivo murine model to apply a disc compression insult known to cause degeneration. Afterward, that motion segment was placed in fixed-angle bending to impose tissue level tension on part of the AF and compression on the contralateral side. We found that the AF on the convex side of bending retained a healthy lamellar appearance, while the AF on the concave side resembled tissues that had undergone degeneration by loading alone. Varying the time of onset and duration of bending revealed that even a brief duration applied immediately after cessation of compression was beneficial to AF structure on the convex side of bending. Our results suggest that both cell-mediated events and cell-independent mechanisms may contribute to the protective effect of tissue level tension in the AF.
Keywords: Intervertebral disc, Mechanical stress, Anulus fibrosus, Biomechanics, Animal model
Introduction
Intervertebral disc degeneration is characterized by the gradual breakdown of the disc’s tissue architecture [1], leading to compromised biomechanics of the affected motion segment and, consequently, of the spine [32, 51, 55]. Directly or indirectly, these changes may lead to back pain and secondary complications [1]. While the physical and molecular mechanisms that govern the initiation and progression of disc degeneration are currently not well understood, several interacting risk factors are believed to be involved.
Among these risk factors, mechanical stress has been demonstrated to play a significant role in governing disc health. In rodent tail disc loading models, structural changes in the extracellular matrix (ECM) occur in response to static or cyclic compression [7, 20, 53], resulting in altered disc biomechanics [7, 15, 20, 37]. The observation that static bending of healthy discs was found to produce adverse effects only on the concave (compressive) side of bending [9] indicates that these changes are closely linked to tissue deformations. Conversely, dynamically applied tension has been found to reverse the effects of compression-induced changes in rabbit anulus fibrosus (AF) [18].
Such animal studies have also provided insight into specific cellular and cell-mediated changes induced by loading. In mice, supraphysiologic static compression induces apoptosis [20], the degree of which is quantitatively related to the dose and duration of load [19]. At the same time, regional specificity in expression of ECM proteins is lost [20], and catabolic processes are elevated [14, 38]. Such changes are likely responsible for degenerate disc morphology, such as the breakdown of the anular lamellar structure. As expected, certain frequency–magnitude combinations of cyclic loading attenuated adverse responses brought about by static compression, but discs still lacked proper spatial patterns in gene expression [53]. In rats, a comprehensive set of studies that recently examined the transient changes in gene expression during cyclic compression paralleled these observations [22–24]. Notably, long periods of continuous loading upregulated genes encoding both anabolic and catabolic molecules indiscriminately in both nucleus pulposus (NP) and AF.
The consequences of these changes remain unclear, as matrix deposition and cleavage depend on several other regulatory steps. For example, translated and secreted proteins must be properly incorporated into the existing matrix; the presence of certain molecules for bridging collagen is often required [6], and excessive enzymatic glycosylation of secreted proteins and existing matrix can also affect deposition [10]. In terms of matrix breakdown, catalytic cleavage of the metalloproteinase pro-domain (both MMPs and ADAMs) is required to transform the inactive latent form to an active zymogen [31]. Inhibitory proteins such as tissue inhibitors of metalloproteinase (TIMPs) present in the matrix or produced by cells further complicate the modulation of metalloproteinase activity by binding to their active sites [5]. Recent findings have additionally suggested that tension, itself, enhances the stability of other collagenous tissues against enzymatic cleavage [30, 43]. However, it is not known whether tension would have a similar effect in the AF.
To address this question, we conducted a two part study. The first sought to determine whether tissue level AF tension could reduce the effects of MMP digestion in vitro. We hypothesized that, consistent with other tissues, tension would mitigate MMP-mediated changes in AF material properties. The second part of the study examined the potential physiologic impact of tension by placing discs in bending after an adverse loading event in vivo, such that the AF on one side of the disc receives tensional loading. Since various groups have proposed that degeneration may be precipitated by loss of tension in anular lamellae, we hypothesized that applying tension to part of the AF would help preserve the tissue architecture. Following an initial trial to demonstrate the efficacy of tension, the bulk of the study focused on comparing the timing and amount of tension to gain an understanding of potential mechanisms involved. Motion segment bending was employed to place a portion of the AF in tension, since that is a physiologically relevant motion. The transient involvement of MMPs during loading led us to hypothesize that tissue level tension would have a protective effect only if applied soon after loading. Overall, results of our study provide evidence that enzymatic digestion of the AF is less effective when the tissue is loaded in tension, and that immediate application of bending is able to preserve AF architecture in the face of adverse biological and biochemical environments.
Materials and methods
MMP-1 digestion of slack and tension-loaded discs
Five 12-week-old male Swiss-Webster mice were obtained and sacrificed by CO2 asphyxiation followed by cervical dislocation, as approved by the Committee on Animal Research (CAR) at the University of California, San Francisco (UCSF). Three motion segments from each tail were isolated. A pin whose diameter is smaller than that of the NP was used to drill a small hole through the center of the motion segment to denucleate the disc and facilitate diffusion into the inner anulus. Using other test specimens, we examined Safranin-O stained paraffin sections to confirm that this procedure maintained the integrity of the AF (not shown).
Immediately after denucleation, motion segments were subjected to four cycles of flash freezing on dry ice and thawing in PBS to de-cellularize specimens. Perpendicular pairs of stainless steel pins were then inserted transversely through the diameters of vertebral bodies, the skin of the tail removed, tendons carefully cut circumferentially superior to the disc, and specimens frozen once more. Motion segments were then thawed in PBS and randomly assigned to one of three groups.
Untreated discs were tested immediately after thawing (n = 5).
Tension-loaded motion segments were placed in a miniature Ilizarov-type apparatus by means of the perpendicular pins to distract the disc to approximately 33% strain as assessed by radiographs (n = 5).
Slack discs were placed in similar apparatuses with no tension applied (n = 5).
Loaded and slack specimens were immersed in a common bath of DMEM containing 100 ng/ml human MMP-1 (Sigma-Aldrich, St Louis, MO, USA). Specimens were divided into two separate trials, due to volume constraints to achieve desired MMP concentrations. The bath was placed in a vacuum chamber for 5 min to remove air bubbles, and then incubated at 37 C for 24 h.
Biomechanical testing
Following incubation in MMP-1 solution, all specimens were placed in a bath containing 10 mM EDTA and other non-specific protease inhibitors to terminate digestion. Vertebral bodies including pins were potted in dental cement and mounted onto a materials testing system (MTS Systems Corp., Eden Prarie, MN, USA). Three preconditioning cycles were performed prior to each tensile load-to-failure test (all at slow strain rates of 0.003 mm/s). Ultimate tensile force (UTF) and stiffness were measured.
Surgical procedures
Twenty-five 12-week-old Swiss-Webster mice were obtained and subjected to surgical procedures approved by CAR at UCSF in order to initiate degeneration in caudal discs as described previously [14]. Briefly, mice were anesthetized with a cocktail of ketamine (120 mg/kg mouse) and xylazine (10 mg/kg mouse), and pairs of stainless steel pins were percutaneously inserted cross-wise in the medial-lateral and dorsal-ventral directions through the 9th and 10th caudal vertebrae to ensure uniform compressive loading. A static compressive stress of approximately 1.3 MPa was applied to the intervening disc continuously for 7 days by four pre-calibrated elastic bands placed across pins in the adjacent vertebrae (Fig. 1a). This loading protocol was selected based on its consistency for inducing degenerative changes in the disc as assessed 3 weeks after removal of compression [20]. Such changes include extensive nuclear cell death and disorganization of the AF and are highly reproducible. We used an adjacent disc as sham control by inserting a third pair of pins through the 8th caudal vertebra. Buprenorphine (0.1 mg/kg) was administered for analgesia.
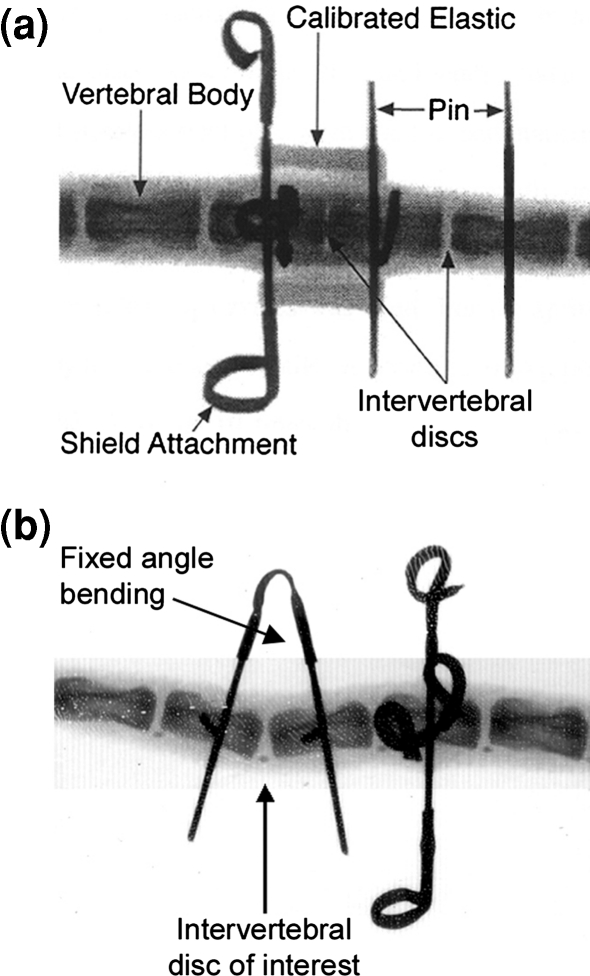
Fig. 1.
Radiographic image of a mouse tail placed under a static uniform compression and b fixed-angle bending after compression loading. For compression (a), the center disc spanned by elastics is subjected to load while the adjacent sham disc (right) is not. During bending (b), an angled tube is crimped onto the pins to hold the motion segment at a fixed angle. Attachment of a polypropylene shield prevented tampering by the mouse. No observable change in bending angle was detected between the times of surgery and sacrifice
Experimental groups
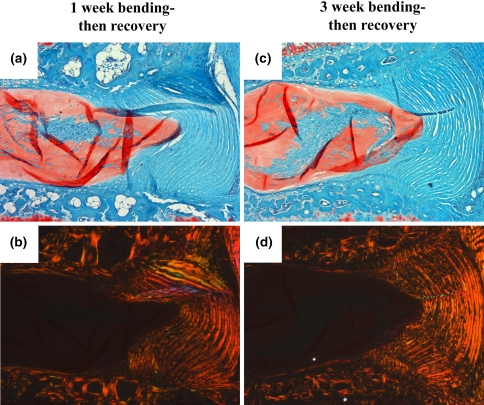
In a first set of experiments, four mice were used to determine whether bending would have any effect over the usual 3-week assessment after 7 days of 1.3 MPa loading [20]. Following compression, mice were anesthetized and elastics were removed. Loaded motion segments for three mice were placed in fixed-angle bending of 25 in dorsal rotation. The remaining mouse served as a positive control where the disc was allowed to recover without bending. Only one control specimen was included because this was only to confirm the same changes in disc morphology that had previously been characterized extensively [20]. In all tails, adjacent sham control discs remained unmanipulated. To apply fixed-angle bending, spacers were first placed on the lateral aspects to hold pins at their original distance. Hypodermic tubing was bent to a specific angle and then crimped over the two adjacent pins on the dorsal aspect of the tail, with the spacers acting as a fulcrum (Fig. 1b). It was noted that angular fixation was robust, and normal mouse activity would likely not perturb bending angle. No direct measurements were made, but there was no observable change of the applied angle during or after the experiment.
Because of the dramatic results we observed initially, a second, more comprehensive, experiment was performed. The remaining 21 mice were used to investigate the timing of bending application, since we have found load-induced increases in MMP-2 activation to be transient [14]. After all mice were subjected to 7 days 1.3 MPa static compression, they were divided into six experimental groups defined according to treatment after loading. All post-compression treatments spanned a 6-week duration to allow protocols that include both three total weeks of recovery and three total weeks of bending:
Group 1–6 weeks recovery (n = 3)
Group 2–3 weeks recovery followed by 3 weeks bending (n = 3)
Group 3–1-week recovery, 3 weeks bending, then recovery for the final 2 weeks (n = 3)
Group 4–1-week bending followed by 5 weeks recovery (n = 4)
Group 5–3 weeks bending followed by 3 weeks recovery (n = 4)
Group 6–3 weeks of low angle bending followed by 3 weeks recovery (n = 4)
Bending angles of 23.2 ± 1.8° and 15.3 ± 1.1° were imposed for high and low angle bending, respectively, using the method described above. For recovery periods before and after bending, all elastics, spacers, and bending instrumentation were removed. Two mice, one from Group 1 and one from Group 2, died during the 6-week long experiment and were excluded. Although this resulted in specimen numbers that are not optimal, this was counterbalanced by the dramatic differences in observed disc morphologies among groups. At the time points of interest, mice were sacrificed by CO2 asphyxiation followed by cervical dislocation. Sham and experimental motion segments were excised from the tail and the side of the disc in tension marked with tissue marking dye.
Qualitative measures of disc morphology—histology and polarized light microscopy
Biomechanically tested and in vivo loaded tissues were fixed overnight in 10% phosphate buffered formalin, decalcified in a 9% (w/v) EDTA 2Na, 10% (w/v) EDTA 4Na solution for at least 1 week, and processed in graded ethanol, xylene, and paraffin infiltrations sequentially. Paraffin-embedded samples were cut sagittally into 6 μm sections. Sections near the mid-sagittal plane were used for all analyses. For histology, sections were stained using Safranin-O/Fast-green and hematoxylin, then imaged with an Olympus IX-81 inverted microscope (Olympus America Inc., Center Valley, PA, USA) with CCD camera under brightfield for histology and polarized light for collagen birefringence.
Quantitative measures of disc morphology—second harmonic generation imaging
Some deparaffinized unstained mid-sagittal sections obtained from the same tissue specimens used for histology were set aside for polarization-modulated second harmonic generation imaging (PM-SHGI) [48, 49]. This procedure has been recently applied to study intervertebral disc structure. Detailed information on instrumentation used to perform the imaging can be found in Reiser et al. [41]. The data were then subjected to a signal processing algorithm performed in Matlab (Mathworks, IL, USA) to yield information about both the magnitude and the pattern of disorder in the tissue as described previously [48, 49]. A single disorder index (DI) was computed using the following equation, detailed by Reiser et al. [40] and averaged across the inner and middle portions of the AF among specimens:
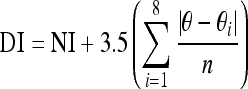 |
where NI is total number of nonparallel neighbors, excluding those that are part of a boundary, θ is the angle of the index pixel expressed in radians, θi is the angle of the ith nearest neighbor pixel in radians, i is the ith neighboring pixel in the 3 × 3 neighborhood and n is the number of nonexcluded nearest neighbors.
For these analyses, we excluded the nucleus and the outer one-third of the anulus, which did not possess observable degradative changes for any discs subjected to load-induced degeneration.
Statistical analyses
Statistical analyses were performed to compare biomechanical testing measurements. One-way analysis of variance (ANOVA) was performed with Tukey’s HSD post hoc tests using SPSS (v14.0, SPSS, Inc., Chicago, IL, USA) with critical significance values of α = 0.05.
Results
Effects of tension on AF material properties after MMP-1 digestion
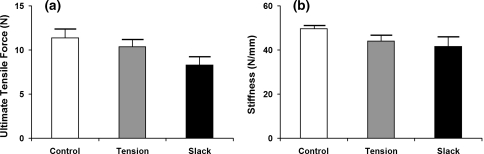
Mechanical testing of loaded and slack anular ring specimens incubated in human MMP-1 suggest that tissue level tension inhibits changes in material properties of the AF brought about by enzymatic digestion. We observed a trend in which control motion segments possessed the greatest UTF and stiffness followed by loaded specimens and then slack specimens incubated in MMP-1 (Fig. 2). The differences were, however, not statistically significant (P < 0.1).
Fig. 2.
Biomechanical testing results of denucleated, de-cellularized motion segments subjected to 24 h MMP-1 digestion while placed in tension or remaining slack. Ultimate tensile force (a) and stiffness (b) decreased for specimens that were digested in MMP-1 solution compared with controls. Slack specimens exhibited trends of greater decreases than tension specimens. Data are represented as mean ± SEM
Anular tension inhibits degenerative changes after mechanical overload in vivo
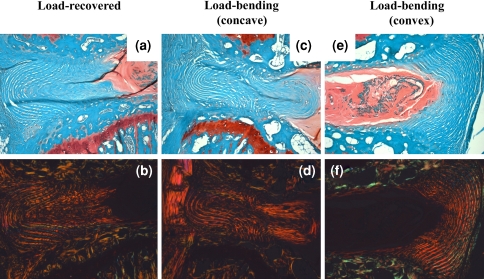
The first set of experiments demonstrated that placing discs in bending for 3 weeks protected the AF from the degenerative changes observed in discs after injurious compression. As described in previous studies [20], the control disc-static compression followed by 3 weeks of unloaded recovery-possessed severe disorganization of anular lamellae (Fig. 3a). Under polarized light, these sections demonstrated a qualitative loss of collagen birefringence, notably in the inner anulus (Fig. 3b). For all mice used in this study, adjacent sham controls possessed healthy disc architectures, cell morphologies, and cell populations (not shown). For discs placed in bending, the AF on the concave side of bending possessed a similar appearance with that in loaded-recovery discs both under brightfield (Fig. 3c) and polarized light microscopy (Fig. 3d). In contrast, the lamellae on the convex side of bending appeared intact (Fig. 3e, f).
Fig. 3.
Effects of 1 week uniform 1.3 MPa mechanical compression followed by a, b 3 weeks of unloaded recovery or 3 weeks of fixed-angle bending: concave (c, d) and convex side (e, f). Safranin-O/Fast-green stained sections were visualized under brightfield microscopy (a, c, e), and the same sections were then imaged under polarized light microscopy (b, d, f). The AF on the concave side of bending resembled that of unloaded recovery discs, but with inward anular bulging (c). In contrast, the AF on the convex side of bending possessed organized lamellae similar to that of healthy AF, as indicated by the strong birefringence of collagen (f)
Immediate application of tension maximizes protection from degradation
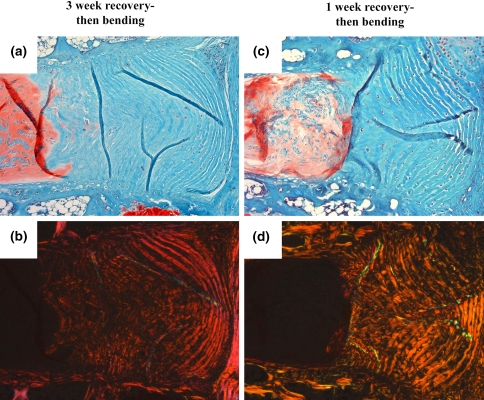
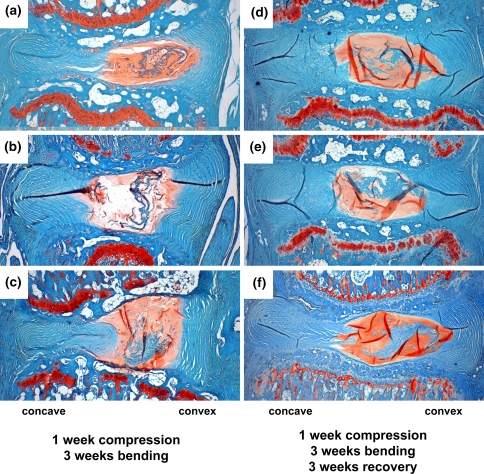
The second set of experiments examined the role of timing and duration of bending, and determined that tension allows the AF to circumvent the degradative process, not simply delay it. For Group 1 specimens disc morphology at 6 weeks recovery was not appreciably worse under brightfield or polarized light microscopy compared with 3-week recovery specimens (not shown). In line with the first experiment, the AF on the concave side of bending in all Groups 2–6 specimens appeared similar to Group 1 discs. In discs that were first allowed to recover for 3 weeks (Group 2; Fig. 4a, b) or 1 week (Group 3; Fig. 4c, d) the AF on the convex side of bending did not exhibit improvement, demonstrating that anular lamellar architecture was not restored simply by placing discs in bending after degenerative changes.
Fig. 4.
Effects of varying the amount of time before initiation of bending during a 6-week period after loading. The protective effects found in the anulus on the convex side of bending were not observed when discs were subjected to unloaded recovery for 3 weeks (a, b) or 1 week (c, d) before being subjected to 3 weeks of bending. Safranin-O/Fast-green stained sections viewed under both brightfield microscopy (a, c) and polarized light microscopy (b, d) showed a lamellar outer annulus and a clearly disorganized inner anulus
Discs that were placed under immediate bending, however, did exhibit striking differences in the anulus. Under brightfield microscopy, anular lamellae are clearly evident on the convex side of bending in Groups 4 and 5; this was confirmed in polarized light micrographs, with 3-week bending discs (Fig. 5c, d) exhibiting somewhat more defined architectural characteristics and collagen birefringence than 1-week bending discs (Fig. 5a, b). Group 6 specimens, which were subjected to low levels of bending did not exhibit the same extent of protection from anular degradation (not shown). By comparing Group 5 with the first set of experiments, we can see that no marked changes occurred within those final 3 weeks (Fig. 6). These images also demonstrate the dramatic and consistent preservation of lamellar architecture.
Fig. 5.
Effects of varying the bending duration immediately after cessation of load, and then allowing discs to recover unloaded for the remaining 6 weeks. Safranin-O/Fast-green stained sections of 1 week bending (a, b) and 3 week bending (c, d) specimens revealed organized lamellae in both groups. This was evident under brightfield microscopy (a, c) and accentuated when viewed under polarized light microscopy (b, d). Results show that immediate tension was beneficial for preserving architecture throughout the anulus, with slightly superior results for 3 week specimens
Fig. 6.
Comparison of discs assessed immediately after loading and 3 weeks of bending (a–c) with those assessed after loading, 3 weeks of bending, and 3 weeks of recover (d–f). For all images, the convex side of bending (tissue level tension) is on the right; the concave side of bending (tissue level compression) is on the left. Preservation of anular lamellar structure is clearly evident in all specimens and suggests that no further degenerative changes occurred after the initial 3 weeks of bending during the recovery period
To corroborate our histologic/collagen birefringence findings, discs subjected to (a) sham operated procedures, (b) recovery only, and (c) 3-week bending–recovery were analyzed using PM-SHGI (Table 1). Disorder indices obtained for the anulus regions of these discs were 11.64 for the sham discs and 70.11 for recovery only discs. For discs subjected to 3 weeks of bending then 3 weeks of recovery, DI values were 15.00 on the convex side of bending and 31.91 on the concave side. This indicates that collagen organization on the convex side of bending resembled the annulus in sham discs, while collagen on the concave side of bending resembled the annulus in discs that were allowed recovery only.
Table 1.
Disorder indices obtained from PM-SHGI analyses
| Procedure | Side of anulus analyzed | Disorder index |
|---|---|---|
| Sham | Both | 11.64 |
| Load-recovery only | Both | 70.11 |
| Load 3-week bending–recovery | Concave (compressive) | 31.91 |
| Convex (tensional) | 15.00 |
Discussion
This study provides evidence that maintaining tension in the AF in vivo protects it from degradative processes initiated by supraphysiologic compression. In addition, the observed trends suggest that the ability for MMP-1 to alter biomechanical function is reduced with tension applied to anular rings. These findings are especially relevant given that increased levels of various degradative enzymes, including MMPs and cathepsins, have been shown to be associated with degenerate discs in humans [3, 11, 12, 17, 42, 54]. Several animal models of experimentally induced disc degeneration—either through mechanical loading [13, 14, 23, 24, 34, 38] or anular stab/incision [2, 45]—have likewise observed increases in activation and/or expression of MMPs shortly after the initiating event. While our study does not characterize modulation of MMP activation or secretion of TIMPs and serine proteinase inhibitors, it appears that bending may impart some benefit to the AF on the convex aspect.
Our biomechanical testing results exhibited trends that are consistent with those reported in other collagenous tissues. Specifically, Nabeshima and colleagues found that rabbit tendons exhibited a significant decrease in tensile stiffness when incubated slack in bacterial collagenase for 20 h, whereas contralateral tendons placed under a static 4% strain were unaffected [30]. More recently, Ruberti and Hallab demonstrated that loading corneal tissues uniaxially along the direction of one population of collagen fibers resulted in efficient digestion of collagen fibers oriented perpendicular to the load, but not those in the direction of load [43]. While we found that slack anular rings possessed lower stiffness and UTF than those under tension during MMP-1 treatment, these trends were not statistically significant. This is likely due in part to our low sample numbers and in part to the low concentration of human MMP-1 relative to the aforementioned studies. The 100-ng/ml concentration used in our experiments are much lower than bacterial collagenase concentrations previously used: 20–60 μg/ml [30] and 6.8–12.5 mg/ml [43] (estimated based on their manufacturer’s current specifications). Our previous study on quantification of MMP-2 in murine disc tissues found levels on the order of 0.5 ng/ml per mg of tissue [14].
Although there have been no previous reports on the role of tension in maintaining resistance to enzymatic digestion in the intervertebral disc, a recent study discovered that once residual stress in the anulus is eliminated by tissue excision from vertebral attachments, thermal denaturation occurs much more quickly in vitro [4] due to the increase in Gibbs free energy required to denature collagen [27]. This same increase in collagen thermal stability has a concurrently beneficial effect on enzyme resistance as well [28], suggesting that tension is likely the mechanism whereby anular architecture is preserved after supraphysiologic loading.
Loss of tension in the anulus and a concommitant vulnerability to matrix degradation could be important in the etiology of disc degeneration. Prolonged disc compression has been implicated in reducing anular tension within minutes of creep compression, as predicted by finite element models [20]. Decreased anular tension could also result from removal or degradation of the NP. Loss of proteoglycans from the nucleus by experimental chondroitinase ABC treatment in vivo results directly in decreased intradiscal pressure [35, 44]. This reduces not only the pressurization generated during loading, but also swelling pressure of the disc at rest, both of which would adversely affect anular tension. Partial or complete discectomy also prevents the nucleus from pressurizing, resulting in inward anular bulging and radial separation of lamellae [25, 26]. These experimental models could explain how enhanced proteoglycan degradation that occurs with aging and disc degeneration grade could consequently affect anular health [16, 21, 39, 50, 52]. Simultaneous loss of anular tension with increased MMP activity/expression, both of which are found in supraphysiologic compression and stab/incision animal models point to the possibility that both phenomena are required for producing the degenerative characteristics of the anulus. Our findings that bending after an injurious supraphysiologic load inhibits lamellar disorganization in AF on the convex side supports this notion.
The study design allowed us to investigate the role of timing and duration of anular tension on the degree of protection imparted. Notably, results underscored the importance of an initiating event followed by a progression of events; anulus tissues that were placed in tension soon after the mechanical insult were preserved, but those subjected to delayed tension were not. In addition, the adverse biochemical environment that is generated from long-term supraphysiologic compression appears to have a lengthy but limited duration, as discs subjected to 1 week of immediate bending were somewhat spared of degradation, but those with 3 weeks of immediate bending were fully preserved. These results also demonstrate that histologic and polarized light micrograph appearance were not skewed by the state of the tissue at the time of sectioning. Tissues that recovered for 3 weeks, then placed in bending for 3 weeks did not possess organized lamellae.
One limitation of this study is the small sample numbers that were used, making it difficult to identify conclusively the mechanisms underlying our observations. Because effect sizes were smaller than anticipated due to low MMP concentrations, biomechanical tests exhibited only trends with no statistical significance. It should be noted that because of low sample number, statistical power of the analyses (1-β) fell between 0.30–0.44, so the possibility for type II error (incorrectly concluding no significant difference) is rather high. A sample number calculation indicated that 20 specimens/group were needed to achieve desired statistical power of 0.90. Because we were using a commercial source human MMP-1, the number of trials to achieve this sample number was cost prohibitive. In terms of in vivo studies, most of the assessments were qualitative, but the dramatic visual results observed for the preservation of anular architecture was such that sacrificing more animals did not seem warranted.
Two additional limitations preclude direct relationships from being drawn between our murine tail disc model and human motion segments. The first is the unphysiologic bending durations that were applied to these motion segments. These durations are clearly longer than could be practically applied but, because of the severity in the biochemical environment generated by static supraphysiologic compression, may be necessary in this animal model to provide the necessary protection to the anulus. A second limitation is that the magnitudes of bending used in our study are greater than those typically experienced in human motion segments. Whereas respective low and high bending angles of 15.3° and 23.2° were used in this current study, various in vivo studies in humans have found that the segmental range of motion across the cervical spine to be only approximately 8° for each direction in flexion-extension, and approximately 6° for each side in lateral bending [29, 36, 47]. However, the murine caudal motion segment is also more flexible than that of the human spine. The neutral zone of the former is approximately 26° in one direction [9], while that of human lumbar motion segments has been measured to range from 3.2 to 4.6° [46]. In order to translate our findings to human discs, it is likely more appropriate to utilize some direct measure of AF tissue strain rather than on motion segment bending angle, which relies more on geometric differences [33] of the disc and its supporting soft tissues. Such measures are further complicated by the microstructural and interlamellar non-uniformities [8] in tissue deformation.
Conclusions
Evidence from this study supports the argument that tension on the tissue level—imposed by motion segment bending—can have a beneficial effect against degradative changes in the AF. Although our in vitro results exhibit trends of an impaired ability for MMP to digest anulus tissue under tension, this is likely only one contribution to the observed preservation of the anulus during bending in vivo. Since the disc is not likely to experience as hostile a degenerative stimulus as we have used, the drastic bending configurations examined here are probably not necessary under normal circumstances. How these findings can be translated into a potentially preventative measure against degradative processes in intervertebral discs of humans require further, more extensive, studies.
Acknowledgment
Financial support for this study was received from the National Institutes of Health AR48033 (A. H. Hsieh) and AR46173 (J. C. Lotz).
References
- 1.An HS, Anderson PA, Haughton VM, Iatridis JC, Kang JD, Lotz JC, et al. Introduction: disc degeneration: summary. Spine. 2004;29:2677–2678. doi: 10.1097/01.brs.0000147573.88916.c6. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DG, Izzo MW, Hall DJ, Vaccaro AR, Hilibrand A, Arnold W, et al. Comparative gene expression profiling of normal and degenerative discs: analysis of a rabbit annular laceration model. Spine. 2002;27:1291–1296. doi: 10.1097/00007632-200206150-00009. [DOI] [PubMed] [Google Scholar]
- 3.Ariga K, Yonenobu K, Nakase T, Kaneko M, Okuda S, Uchiyama Y, et al. Localization of cathepsins D, K, and L in degenerated human intervertebral discs. Spine. 2001;26:2666–2672. doi: 10.1097/00007632-200112150-00007. [DOI] [PubMed] [Google Scholar]
- 4.Bass EC, Wistrom EV, Diederich CJ, Nau WH, Pellegrino R, Ruberti J, et al. Heat-induced changes in porcine annulus fibrosus biomechanics. J Biomech. 2004;37:233–240. doi: 10.1016/j.jbiomech.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 6.Budde B, Blumbach K, Ylostalo J, Zaucke F, Ehlen HW, Wagener R, et al. Altered integration of matrilin-3 into cartilage extracellular matrix in the absence of collagen IX. Mol Cell Biol. 2005;25:10465–10478. doi: 10.1128/MCB.25.23.10465-10478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ching CT, Chow DH, Yao FY, Holmes AD. The effect of cyclic compression on the mechanical properties of the inter-vertebral disc: an in vivo study in a rat tail model. Clin Biomech (Bristol, Avon) 2003;18:182–189. doi: 10.1016/S0268-0033(02)00188-2. [DOI] [PubMed] [Google Scholar]
- 8.Costi JJ, Stokes IA, Gardner-Morse M, Laible JP, Scoffone HM, Iatridis JC. Direct measurement of intervertebral disc maximum shear strain in six degrees of freedom: motions that place disc tissue at risk of injury. J Biomech. 2007;40:2457–2466. doi: 10.1016/j.jbiomech.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Court C, Colliou OK, Chin JR, Liebenberg E, Bradford DS, Lotz JC. The effect of static in vivo bending on the murine intervertebral disc. Spine J. 2001;1:239–245. doi: 10.1016/S1529-9430(01)00056-0. [DOI] [PubMed] [Google Scholar]
- 10.Dominguez LJ, Barbagallo M, Moro L. Collagen overglycosylation: a biochemical feature that may contribute to bone quality. Biochem Biophys Res Commun. 2005;330:1–4. doi: 10.1016/j.bbrc.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 11.Fujita K, Nakagawa T, Hirabayashi K, Nagai Y. Neutral proteinases in human intervertebral disc. Role in degeneration and probable origin. Spine. 1993;18:1766–1773. doi: 10.1097/00007632-199310000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Gruber HE, Ingram JA, Hanley EN., Jr Immunolocalization of MMP-19 in the human intervertebral disc: implications for disc aging and degeneration. Biotech Histochem. 2005;80:157–162. doi: 10.1080/10520290500387607. [DOI] [PubMed] [Google Scholar]
- 13.Guehring T, Omlor GW, Lorenz H, Bertram H, Steck E, Richter W, et al. Stimulation of gene expression and loss of anular architecture caused by experimental disc degeneration—an in vivo animal study. Spine. 2005;30:2510–2515. doi: 10.1097/01.brs.0000186591.17114.e9. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh AH, Lotz JC. Prolonged spinal loading induces matrix metalloproteinase-2 activation in intervertebral discs. Spine. 2003;28:1781–1788. doi: 10.1097/01.BRS.0000083282.82244.F3. [DOI] [PubMed] [Google Scholar]
- 15.Iatridis JC, Mente PL, Stokes IA, Aronsson DD, Alini M. Compression-induced changes in intervertebral disc properties in a rat tail model. Spine. 1999;24:996–1002. doi: 10.1097/00007632-199905150-00013. [DOI] [PubMed] [Google Scholar]
- 16.Inkinen RI, Lammi MJ, Lehmonen S, Puustjarvi K, Kaapa E, Tammi MI. Relative increase of biglycan and decorin and altered chondroitin sulfate epitopes in the degenerating human intervertebral disc. J Rheumatol. 1998;25:506–514. [PubMed] [Google Scholar]
- 17.Konttinen YT, Kaapa E, Hukkanen M, Gu XH, Takagi M, Santavirta S, et al. Cathepsin G in degenerating and healthy discal tissue. Clin Exp Rheumatol. 1999;17:197–204. [PubMed] [Google Scholar]
- 18.Kroeber M, Unglaub F, Guehring T, Nerlich A, Hadi T, Lotz J, et al. Effects of controlled dynamic disc distraction on degenerated intervertebral discs: an in vivo study on the rabbit lumbar spine model. Spine. 2005;30:181–187. doi: 10.1097/01.brs.0000150487.17562.b1. [DOI] [PubMed] [Google Scholar]
- 19.Lotz JC, Chin JR. Intervertebral disc cell death is dependent on the magnitude and duration of spinal loading. Spine. 2000;25:1477–1483. doi: 10.1097/00007632-200006150-00005. [DOI] [PubMed] [Google Scholar]
- 20.Lotz JC, Colliou OK, Chin JR, Duncan NA, Liebenberg E. Compression-induced degeneration of the intervertebral disc: an in vivo mouse model and finite-element study. Spine. 1998;23:2493–2506. doi: 10.1097/00007632-199812010-00004. [DOI] [PubMed] [Google Scholar]
- 21.Lyons G, Eisenstein SM, Sweet MB. Biochemical changes in intervertebral disc degeneration. Biochim Biophys Acta. 1981;673:443–453. doi: 10.1016/0304-4165(81)90476-1. [DOI] [PubMed] [Google Scholar]
- 22.MacLean JJ, Lee CR, Grad S, Ito K, Alini M, Iatridis JC. Effects of immobilization and dynamic compression on intervertebral disc cell gene expression in vivo. Spine. 2003;28:973–981. doi: 10.1097/00007632-200305150-00004. [DOI] [PubMed] [Google Scholar]
- 23.MacLean JJ, Lee CR, Alini M, Iatridis JC. Anabolic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res. 2004;22:1193–1200. doi: 10.1016/j.orthres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 24.MacLean JJ, Lee CR, Alini M, Iatridis JC. The effects of short-term load duration on anabolic and catabolic gene expression in the rat tail intervertebral disc. J Orthop Res. 2005;23:1120–1127. doi: 10.1016/j.orthres.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 25.Meakin JR, Hukins DW. Effect of removing the nucleus pulposus on the deformation of the annulus fibrosus during compression of the intervertebral disc. J Biomech. 2000;33:575–580. doi: 10.1016/S0021-9290(99)00215-8. [DOI] [PubMed] [Google Scholar]
- 26.Meakin JR, Redpath TW, Hukins DW. The effect of partial removal of the nucleus pulposus from the intervertebral disc on the response of the human annulus fibrosus to compression. Clin Biomech (Bristol, Avon) 2001;16:121–128. doi: 10.1016/S0268-0033(00)00075-9. [DOI] [PubMed] [Google Scholar]
- 27.Miles CA, Ghelashvili M. Polymer-in-a-box mechanism for the thermal stabilization of collagen molecules in fibers. Biophys J. 1999;76:3243–3252. doi: 10.1016/S0006-3495(99)77476-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minond D, Lauer-Fields JL, Nagase H, Fields GB. Matrix metalloproteinase triple-helical peptidase activities are differentially regulated by substrate stability. Biochemistry. 2004;43:11474–11481. doi: 10.1021/bi048938i. [DOI] [PubMed] [Google Scholar]
- 29.Miura T, Panjabi MM, Cripton PA. A method to simulate in vivo cervical spine kinematics using in vitro compressive preload. Spine. 2002;27:43–48. doi: 10.1097/00007632-200201010-00011. [DOI] [PubMed] [Google Scholar]
- 30.Nabeshima Y, Grood ES, Sakurai A, Herman JH. Uniaxial tension inhibits tendon collagen degradation by collagenase in vitro. J Orthop Res. 1996;14:123–130. doi: 10.1002/jor.1100140120. [DOI] [PubMed] [Google Scholar]
- 31.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 32.Niosi CA, Oxland TR. Degenerative mechanics of the lumbar spine. Spine J. 2004;4:202S–208S. doi: 10.1016/j.spinee.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 33.O’Connell GD, Vresilovic EJ, Elliott DM. Comparison of animals used in disc research to human lumbar disc geometry. Spine. 2007;32:328–333. doi: 10.1097/01.brs.0000253961.40910.c1. [DOI] [PubMed] [Google Scholar]
- 34.Omlor GW, Lorenz H, Engelleiter K, Richter W, Carstens C, Kroeber MW, et al. Changes in gene expression and protein distribution at different stages of mechanically induced disc degeneration—an in vivo study on the New Zealand white rabbit. J Orthop Res. 2006;24:385–392. doi: 10.1002/jor.20055. [DOI] [PubMed] [Google Scholar]
- 35.Ono A, Harata S, Takagaki K, Endo M. Proteoglycans in the nucleus pulposus of canine intervertebral discs after chondroitinase ABC treatment. J Spinal Disord. 1998;11:253–260. doi: 10.1097/00002517-199806000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Ordway NR, Seymour RJ, Donelson RG, Hojnowski LS, Edwards WT. Cervical flexion, extension, protrusion, and retraction. A radiographic segmental analysis. Spine. 1999;24:240–247. doi: 10.1097/00007632-199902010-00008. [DOI] [PubMed] [Google Scholar]
- 37.Palmer EI, Lotz JC. The compressive creep properties of normal and degenerated murine intervertebral discs. J Orthop Res. 2004;22:164–169. doi: 10.1016/S0736-0266(03)00161-X. [DOI] [PubMed] [Google Scholar]
- 38.Palmer EI, Lotz JC (2004) The time-dependent role of cytokines in mechanically induced disc degeneration. In: Transactions of the 50th annual meeting of the Orthopaedic Research Society. San Francisco, p 128
- 39.Pearce RH, Grimmer BJ, Adams ME. Degeneration and the chemical composition of the human lumbar intervertebral disc. J Orthop Res. 1987;5:198–205. doi: 10.1002/jor.1100050206. [DOI] [PubMed] [Google Scholar]
- 40.Reiser KM, Rocha-Mendoza I, Wang M, Yankelevich DR, Bratton C, Knoesen A, et al. Polarization-modulated second harmonic generation imaging: method for quantitative assessment of disorganization in anulus. Conf Proc IEEE Eng Med Biol Soc. 2004;7:4982–4985. doi: 10.1109/IEMBS.2004.1404377. [DOI] [PubMed] [Google Scholar]
- 41.Reiser KM, Bratton C, Yankelevich DR, Knoesen A, Rocha-Mendoza I, Lotz JC. Quantitative analysis of structural disorder in intervertebral disks using second harmonic generation imaging: Comparison with morphometric analysis. J Biomed Opt. 2007;12:064019. doi: 10.1117/1.2812631. [DOI] [PubMed] [Google Scholar]
- 42.Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine. 2000;25:3005–3013. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 43.Ruberti JW, Hallab NJ. Strain-controlled enzymatic cleavage of collagen in loaded matrix. Biochem Biophys Res Commun. 2005;336:483–489. doi: 10.1016/j.bbrc.2005.08.128. [DOI] [PubMed] [Google Scholar]
- 44.Sasaki M, Takahashi T, Miyahara K, Hirose T. Effects of chondroitinase ABC on intradiscal pressure in sheep: an in vivo study. Spine. 2001;26:463–468. doi: 10.1097/00007632-200103010-00008. [DOI] [PubMed] [Google Scholar]
- 45.Sobajima S, Shimer AL, Chadderdon RC, Kompel JF, Kim JS, Gilbertson LG, et al. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J. 2005;5:14–23. doi: 10.1016/j.spinee.2004.05.251. [DOI] [PubMed] [Google Scholar]
- 46.Spenciner D, Greene D, Paiva J, Palumbo M, Crisco J. The multidirectional bending properties of the human lumbar intervertebral disc. Spine J. 2006;6:248–257. doi: 10.1016/j.spinee.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 47.Steffen T, Rubin RK, Baramki HG, Antoniou J, Marchesi D, Aebi M. A new technique for measuring lumbar segmental motion in vivo. Method, accuracy, and preliminary results. Spine. 1997;22:156–166. doi: 10.1097/00007632-199701150-00006. [DOI] [PubMed] [Google Scholar]
- 48.Stoller P, Reiser KM, Celliers PM, Rubenchik AM. Polarization-modulated second harmonic generation in collagen. Biophys J. 2002;82:3330–3342. doi: 10.1016/S0006-3495(02)75673-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stoller P, Celliers PM, Reiser KM, Rubenchik AM. Quantitative second-harmonic generation microscopy in collagen. Appl Opt. 2003;42:5209–5219. doi: 10.1364/AO.42.005209. [DOI] [PubMed] [Google Scholar]
- 50.Sztrolovics R, Alini M, Roughley PJ, Mort JS. Aggrecan degradation in human intervertebral disc and articular cartilage. Biochem J. 1997;326(Pt 1):235–241. doi: 10.1042/bj3260235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka N, An HS, Lim TH, Fujiwara A, Jeon CH, Haughton VM. The relationship between disc degeneration and flexibility of the lumbar spine. Spine J. 2001;1:47–56. doi: 10.1016/S1529-9430(01)00006-7. [DOI] [PubMed] [Google Scholar]
- 52.Urban JP, McMullin JF. Swelling pressure of the lumbar intervertebral discs: influence of age, spinal level, composition, and degeneration. Spine. 1988;13:179–187. doi: 10.1097/00007632-198802000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Walsh AJ, Lotz JC. Biological response of the intervertebral disc to dynamic loading. J Biomech. 2004;37:329–337. doi: 10.1016/S0021-9290(03)00290-2. [DOI] [PubMed] [Google Scholar]
- 54.Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N. 2002 SSE award competition in basic science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002;11:308–320. doi: 10.1007/s00586-002-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao F, Pollintine P, Hole BD, Dolan P, Adams MA. Discogenic origins of spinal instability. Spine. 2005;30:2621–2630. doi: 10.1097/01.brs.0000188203.71182.c0. [DOI] [PubMed] [Google Scholar]