Abstract
The objective of this study was to assess the impact of a landmark annular lesion model on our understanding of the etiopathogenesis of IVD degeneration and to appraise current IVD repairative strategies. A number of studies have utilised the Osti sheep model since its development in 1990. The experimental questions posed at that time are covered in this review, as are significant recent advances in annular repair strategies. The ovine model has provided important spatial and temporal insights into the longitudinal development of annular lesions and how they impact on other discal and paradiscal components such as the NP, cartilaginous end plates, zygapophyseal joints and vertebral bone and blood vessels. Important recent advances have been made in biomatrix design for IVD repair and in the oriented and dynamic culture of annular fibrochondrocytes into planar, spatially relevant, annular type structures. The development of hyaluronan hydrogels capable of rapid in situ gelation offer the possibility of supplementation of matrices with cells and other biomimetics and represent a significant advance in biopolymer design. New generation biological glues and self-curing acrylic formulations which may be augmented with slow delivery biomimetics in microcarriers may also find application in the non-surgical repair of annular defects. Despite major advances, significant technical challenges still have to be overcome before the biological repair of this intractable connective tissue becomes a realistic alternative to conventional surgical intervention for the treatment of chronic degenerate IVDs.
Keywords: Annular injury, Annular remodelling/repair, Intervertebral disc degeneration, Intervertebral disc repair, Rim lesions, Perlecan
Introduction
The intervertebral disc (IVD) is a tough, composite biomaterial which acts as a viscoelastic weight-bearing cushion and is a major contributor to the spinal flexibility and mechanical stability properties of the spine [48]. The outer regions of the IVD, the annulus fibrosus (AF) consist of lamellae containing counter-gradients of types I and II collagen with the former predominating in the outer AF and the latter in the central region of the IVD and the nucleus pulposus (NP) [24]. Elastin fibrils are also present in interlamellar regions and in translamellar cross bridges [10, 106, 158] which interconnect and stabilise the annular lamellae [106, 131]. The type II collagen fibrillar network in the NP is relatively disorganised compared to the fibrillar arrangements of annular type I collagen. The interconnectivity provided by collagen and elastin fibres provides exceptional tensile strength to the AF and allows it to withstand very significant hoop stresses.
Aggrecan is the major space-filling proteoglycan of the NP where it forms massive macromolecular aggregates with hyaluronan which are entrapped within an amorphous type II collagen network [122]. These networks imbibe water into the IVD resulting in the generation of an internal hydrostatic pressure which equips the NP with the ability to act as a viscoelastic cushion to counter compressive axial spinal loading. Versican is another large CS-rich IVD proteoglycan found in the NP and in proteoglycan-rich regions between adjacent annular lamellae [74, 80] and in translamellar annular cross bridges [82] where it may have a role in the lubrication of collagen bundles. Furthermore, the G3 domain of versican is interactive with elastic networks and this may also facilitate its inter-lamellar localisation [56]. Members of the small leucine repeat proteoglycans (SLRPs), decorin, biglycan, fibromodulin, lumican and keratocan are also prominent disc proteoglycans. The SLRPs have important linking, shape determining and matrix organising roles and regulate cellular proliferation, matrix adhesion and response to growth factors and cytokines [27, 154, 155]. Direct evidence for the importance of the SLRPs in musculoskeletal tissues has been demonstrated using knockout mice. While functional overlap between SLRP members is evident, a major phenotype of biglycan, decorin, fibromodulin and lumican single or double knockout mice is age-dependent tendon laxity, ectopic calcification and arthritis [14, 18, 25, 121]. A recent study using the ovine annular lesion model has demonstrated fragmentation of fibromodulin and biglycan in areas of annular remodelling [83]. Isolation and characterisation of these fragments may demonstrate their utility as bio-markers for the assessment of annular remodelling processes.
Changes in the intervertebral disc during ageing and degeneration
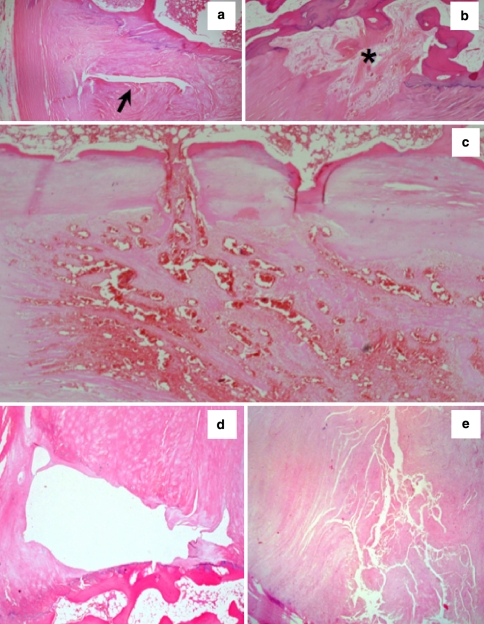
The IVD undergoes profound age-related cellular and matrix changes leading to degeneration and a diminution in its weight-bearing capability. Discs of older spines exhibit a range of pathologies including abnormalities in the AF. These include the vertebral “rim lesion” (Fig. 1) originally described by Schmorl and Junghans [125] arising from discontinuities in the vertebral attachment to the AF and may arise from avulsion of the annular bony attachment in close proximity to the cartilaginous end-plate (CEP) (Fig. 1). At 30 years of age approximately 20% of L4L5 IVDs have rim lesions. This incidence rises to 90% at 80 years and the number of rim lesions per IVD also increases [146]. The anterior AF has 10% more rim lesions than the posterior annulus. Connections between rim lesions and radiating tears are rare before the age of 50 but are present in 50% of older spines [146]. Concentric (circumferential) annular tears are separations of the annular lamellae (de-lamellations), while radial annular defects result from clefts initiating within the NP. Despite vascular ingrowth around annular rim lesions (see Fig. 1b, c) [79], evidence from human post-mortem studies indicates that these show a very limited ability to undergo spontaneous repair.
Fig. 1.
Morphology of rim lesions in H&E stained human IVDs. a Typical cleft type rim lesion (arrow), b repaired rim lesion (asterisk), c vasoproliferative rim lesion, d cystic rim lesion and e fragmented rim lesion
The ovine annular lesion model of experimental IVD degeneration
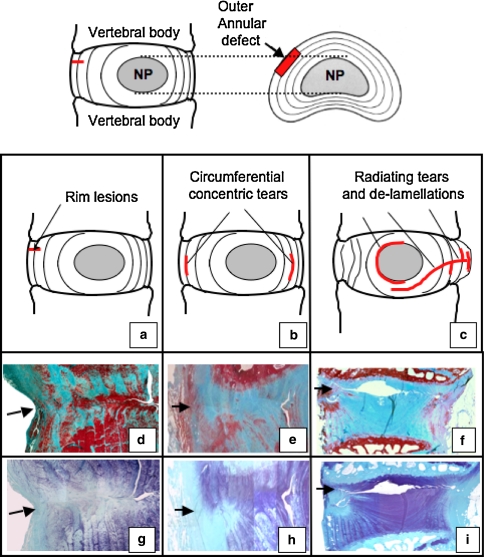
The ovine annular lesion model developed by Osti et al. [103] utilises controlled annular defects 5 mm deep by 5 mm wide [75, 77, 81] (Fig. 2). This model has provided some important insights into the temporal ECM changes that occur following induction of rim lesions. Compositional changes previously noted may contribute to the degenerative process, like loss of large high-buoyant density aggrecan type proteoglycans and an elevation in levels of the small leucine repeat proteoglycans (SLRPs) decorin and biglycan in the injured disc [76, 77]. Fragmentation of fibromodulin and biglycan further illustrate the ECM remodelling events in the AF-defect site. Transforming growth factor-beta (TGF-β) and basic fibroblast growth factor (bFGF) have been immunolocalised with blood vessel and nerve in-growth around experimental annular lesions [79, 81] and with cells expressing α-smooth muscle cell actin [81] (Figs. 3, 4). Six months after the creation of the lesion, penetration of blood vessels and the influx of cells into the inner AF were more advanced. However after 12 months, the blood vessels ceased to extend further into the defect and the number of associated cells had decreased [79, 81]. This increased cellularity in the mid and inner annular lesion may have resulted from increased proliferation of endogenous disc cell populations in response to TGF-β and FGF-2 which are known to stimulate cellular division and matrix synthesis [136].
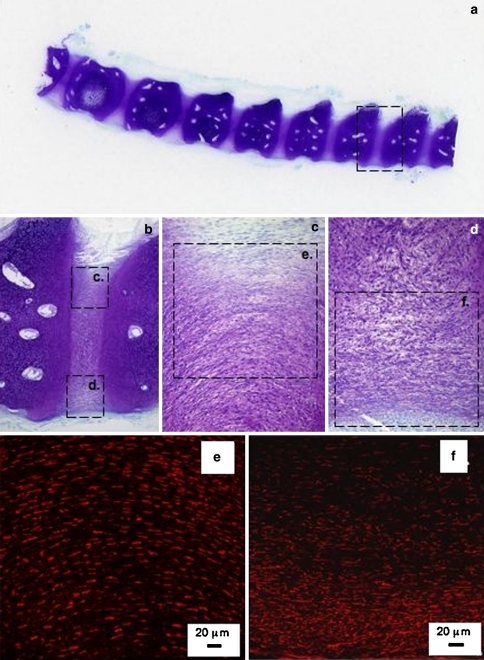
Fig. 2.
Schematic representation of the controlled rim lesions (top, arrow) used in the ovine model of experimental intervertebral disc degeneration and the defects which have been observed using this model (a–c). Histological evaluation of vertical disc sections stained with Masson-Trichrome (d–f) to assess collagenous reorganisation and toluidine blue–fast green to delineate the focal loss of anionic proteoglycan in the vicinity of the initial lesion site (g–i). Remodelling of the outer lesion consistent with a spontaneous repair response is evident in the outer lesion, however, experimental rim lesions are capable of propagating to form de-lamellations after 3 months (d, g) which may form circumferential concentric tears after 6 months (e, h) and radiating tears spanning from the original defect site to the contralateral annulus after 12 months (f, i)
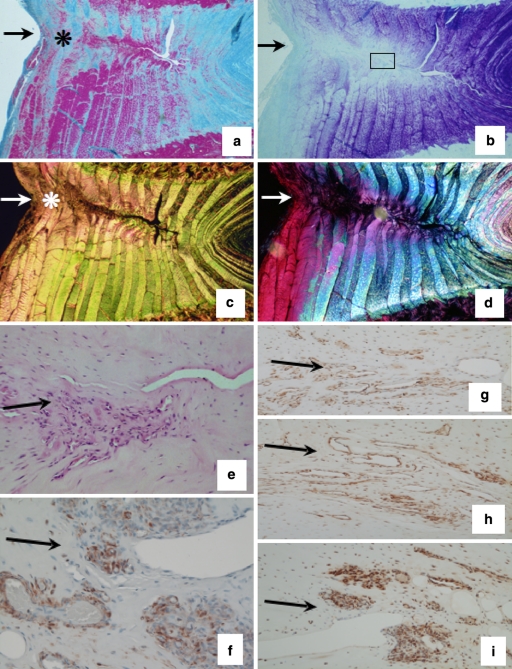
Fig. 3.
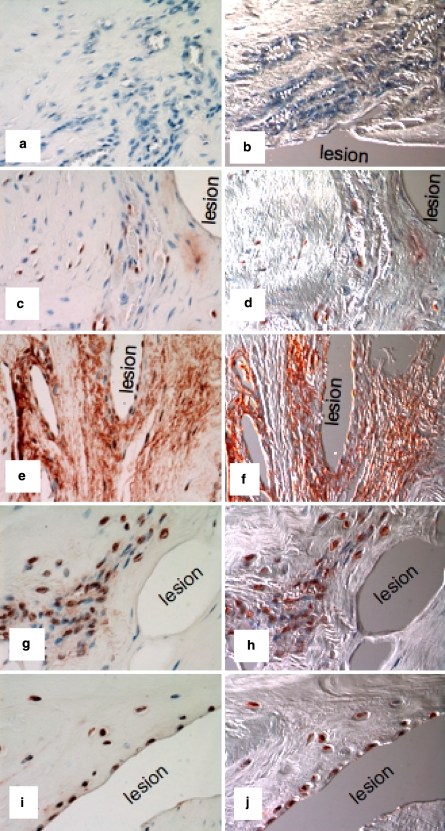
Experimental ovine annular lesions demonstrating a spontaneous repair response in the outer annulus fibrosus (asterisk) after 3 months and lack of repair of the defect internally (a–d). The non-healed defect is visible towards the right hand side of each photosegment, an arrow at the left hand side marks where the defect was initially made. a. Focal collagenous remodelling of outer AF spanning the original defect site (Masson Trichrome); b Focal loss of anionic proteoglycan along the plane of the experimental annular defect, (toluidine blue/fast green stain). c Re-organisation of collagen in the outer AF in response to the experimental annular defect (asterisk), (picrosirius red stain viewed under polarised light). d Polarised light view of segment (b) depicting similar detail to segment (c). e Cellular infiltration into a defect site 6 months post-operatively, (H&E). (f) Cells in the vicinity of a defect site stain positively for α-smooth muscle actin 6 months post-operatively. A large proportion of the cells which migrate into inner annular repair sites express FGF-2 (g–i) and are associated with small blood vessels in regions of the AF undergoing remodelling consistent with an active repair response at least in the outer AF but not so in the inner AF; (g) 3 months; (h) 6 months; (i) 12 months post-operatively. The photosegments in e–i are typical higher power views from an area approximating to the boxed region in segment b
Fig. 4.
Immunolocalisation of TGF-β in vertical sections of lesion affected ovine IVDs with a pan TGF-β Ab which detects all TGF-β isoforms. The areas shown are in the approximate area of the mid AF depicted by the boxed area in Fig. 2b. Negative control using equivalent concentration of mouse IgG in place of primary antibody (a, b); 3-month lesion (c, d); 6-month lesion (e, f); 12-month lesion (g, h); 26-month lesion (i, j). The photosegments depicted in the right hand column (b, d, f, h, j) are viewed with Nomarski DIC optics and are of the corresponding photosegments to their left (a, c, e, g, i), red blood cells are prominent in the DIC imaged blood vessels. The inner AF lesions depicted were incapable of undergoing repair despite the influx of cells and blood vessels to the lesion site and the demonstrated localisation of FGF-2 and TGF-β which are known to promote such processes
Repair of the outer third of the experimentally injured AF is a universal finding but this repair process does not extend into the inner regions of the AF (Fig. 3a–d). Rim lesions also propagate in some cases along the track of the original defect as far as the contralateral AF or between adjacent annular lamellae leading to delamellations which may induce circumferential defects (Fig. 2d, e, g, h). Propagation of outer annular defects into the NP as radial defects are also frequently seen (Fig. 2i). All of these defects have previously been described in pathological human IVDs [104, 137] and severely impact on the biomechanical competence of the IVD [137]. Other changes which have been observed in the ovine annular lesion model of relevance to the etiology of human disc degeneration include changes in endplate vascularity adjacent to annular rim lesions [92], remodelling of vertebral bone [93], and osteoarthritic changes in the facet joints secondary to the development of experimental annular rim lesions [90]. Ovine IVDs containing experimental outer and circumferential annular lesions have compromised biomechanical properties [26]. Fixation failed to stabilise such lesion affected IVDs and did not prevent subsequent degenerative changes in the IVD and other spinal components [91].
Rim lesions and the etiopathogenesis of disc degeneration in man
There has been much debate over the years as to whether rim lesions result from degenerative changes in another component of the axial spinal motion segment or are themselves causal agents leading to degeneration in discal and paradiscal components. There is no doubt with the longitudinal experimental design possible and the numerous well-controlled studies already conducted using the ovine annular lesion model on healthy ovine IVDs, that standardised, experimental, outer, annular lesions can set in motion a series of events which lead to (1) early spontaneous repair of outer annular defects; (2) time-dependant propagation of the non-repaired inner annular defect resulting in the development of delamellations, circumferential or radial tears and/or transverse tears through the NP to the contralateral AF resulting in severe distortion of annular architecture, loss of disc height and disc degeneration. It is self evident that the biomechanical properties of such degenerate IVDs (and spinal motion segment) are severely, possibly irreversibly compromised; (iii) resultant changes also occur in the vascular supply underlying the endplate adjacent to the rim-lesion site and in vertebral subchondral bone density. These vascular changes further impact on the nutrition of the endogenous cell populations in the affected IVDs which rely heavily on diffusive processes exacerbating degenerative processes in the IVD; (4) consequential degenerative changes in the posterior zygapophyseal joints of the spinal motion segment affected by the rim lesion and in which disc degeneration has ensued. As to whether the above sequence of events (1–4) accurately reflect the initiating and perpetuating events underlying the etiopathogenesis of human IVD degeneration has yet to be definitively answered. Rim lesions certainly occur at similar frequencies in young, otherwise structurally intact, healthy human IVDs and in older discs containing early degenerative changes. Rim lesions are therefore present in human IVDs devoid of any other obvious defect, apparently indicating that they could be an initiating event in the etiopathogenesis of human disc degeneration rather than occurring as a secondary feature in consequence to changes in some other spinal component. Similar longitudinal studies to those undertaken in sheep are not possible with human IVDs. Despite advances in high-resolution imaging techniques which can identify rim lesions, such longitudinal approaches have yet to be undertaken to follow systematically the impact of rim lesions on disc degeneration. The question of rim lesions and IVD degeneration causality, therefore, must remain open despite compelling evidence from experimental studies using the ovine annular lesion model implicating these as causative agents in IVD degeneration.
Immunolocalisation of FGF-2 and TGF-β in the remodelling AF: evidence of their potential in intervertebral disc repair processes
As already noted, FGF-2 has been localised to areas of the AF undergoing a reparative response in the ovine model (Fig. 2g–i). FGF-2 sustains the differentiated phenotype of cultured NP cells by maintaining their responsiveness to TGF-β [140]. FGF-2 also increases the numbers of inflammatory cells, including macrophages, lymphocytes, and fibroblasts in herniated disc and facilitates the resorption of material extruded into the epidural space [86]. Basic fibroblast growth factor also promotes the proliferation of chondrocytes in degenerated discs in an autocrine or paracrine manner [97]. IVD cells are also responsive to TGF-β in culture which stimulates MMP-2 and matrix production as well as cell proliferation [16, 105, 118].
TGF-β has been strongly localised to regions of annular lesions containing cells of a more rounded morphology than the typical elongated annular fibrochondrocyte (Fig. 4). The former cells also express α-smooth muscle cell actin suggesting a myofibroblastic phenotype. TGF-β exerts anabolic effects on articular chondrocytes and IVD cells [105], stimulates repair processes in cartilage defects [36] and enhances periosteal chondrogenesis [133]. A number of delivery systems have been developed for TGF-β to articular chondrocytes including hydrogels, periosteal grafts dipped in growth factor, liposomes and alginate beads [1, 43, 84]. Bone morphogenetic protein-7 (osteogenic protein-1, OP-1) is also a member of the TGF-β superfamily of growth factors which has been used in a number of applications to promote repair processes in articular cartilage [17, 57], IVD [7, 134], rotator cuff, ligament [9, 138] and genetically modified cells over expressing OP-1 have been developed to promote repair processes [42].
Perlecan and its roles in annulus development, remodelling and potential in therapeutic repair
An improved understanding of annular development and assembly may be insightful with regard to potential annular repair strategies. Our laboratory has embarked on a series of studies in the last few years to better understand the role of a multifunctional HS-proteoglycan, perlecan in FGF growth factor signalling in cartilaginous tissues and to assess how this regulates chondrogenic events relevant in annular development and remodelling [78]. Perlecan is a, large, modular, multifunctional proteoglycan with a 467-kDa protein core containing five distinct domains with homology to the laminin A chain, low density lipoprotein receptor, neural cell adhesion molecule and epidermal growth factor [62, 94, 102]. In man, domain-I in perlecan is the main region of GAG substitution [19, 62, 94, 102] while domain V may also have an additional two HS attachment sites [54, 65]. When substituted with HS, perlecan domain-1 promotes binding to laminin-1 and collagen IV and can also act as a low affinity co-receptor for FGF-1, 2, 7, 9 and 18 while domains III and V act as a receptor for FGF-7 [63–65]. The low density lipoprotein (LDL) repeats in perlecan domain II bind connective tissue growth factor (CTGF) [2, 101], CTGF modulates bone morphogenetic protein (BMP) and transforming growth factor (TGF)-β signalling to co-ordinate morphogenesis, and chondrogenic and angiogenic events during skeletal development [2, 101]. Perlecan also interacts with a number of matrix stabilising molecules including fibrillin-1, nidogen-1 and 2, fibulin-2, fibronectin, proline/arginine-rich and leucine-rich repeat protein/prolargin (PRELP), von Willebrand factor protein-A related protein (WARP) and types XIII and XVIII collagen; and cell attachment proteins such as laminin, fibronectin and thrombospondin [6, 8, 45, 54, 78, 87, 141] and thus also has important roles to play in matrix organisation and stabilisation.
Immunolocalistion of perlecan in foetal human IVDs demonstrates its prominent pericellular distribution in the arcades of cells in the developing annulus and NP as well as the vertebral rudimentary cartilages (Fig. 5). Perlecan is particularly prominently expressed by the hypertrophic chondrocytes of the vertebral growth plates in the cartilaginous rudiments which is consistent with its known chondrogenic properties. It may be instructive to understand the pathobiology of perlecan in such transient developmental cartilages since it may be possible to harness some of these traits in annular remodelling processes in the mature IVD. Perlecan is also a prominent pericellular proteoglycan in the mature AF, although its levels are severely diminished with the age-dependant decline in cell numbers (Fig. 6e).
Fig. 5.
Perlecan has important roles to play in matrix assembly and the development of the annulus fibrosus and other discal components. Low and medium power longitudinal mid sagittal sections through a 12-week-old human foetal spine stained with toluidine blue–fast green to depict the cartilaginous rudiments and developing intervertebral discs (a–d). The boxed area in (a) is presented at higher magnification in (b), as are the boxed areas in (b) in segments (c and d). Perlecan immunolocalisation in the boxed areas (e, f) is presented in segments (e, f), respectively by indirect fluorescent confocal microscopy using a rat anti perlecan primary mAb A7L6 to domain IV of perlecan and Alexa 594 conjugated donkey anti rat igG secondary Ab (red chromophore)
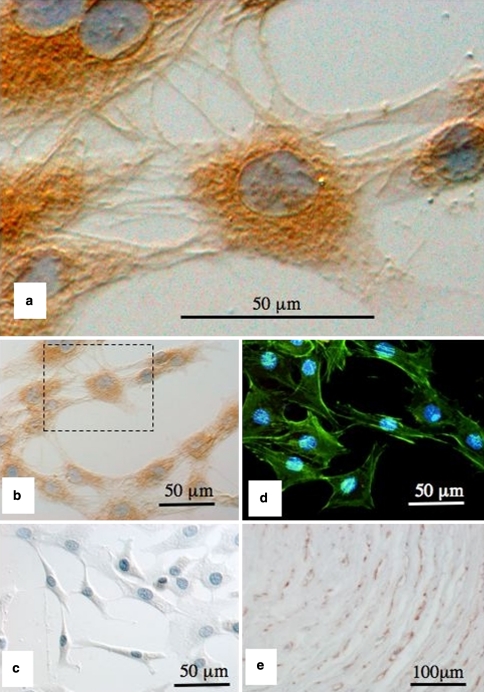
Fig. 6.
Perlecan provides cell-cell and cell-ECM interconnections important for cellular attachment and possibly mechanosensory processes. Immunolocalisation of pericellular perlecan in short-term primary monolayer culture of ovine AF cells using a primary mAb A76 to perlecan domain-I and horse radish conjuagated secondary antibodies and diaminobenzidene substrate for visualisation (brown chromogen), nuclei are counterstained with eosin (a, b). A negative control using equivalent concentration of mouse IgG in place of primary antibody is presented in (c). Localisation of the actin cytoskeleton layed down by the AF cells in monolayer culture using Phalloidin-oregon green, cell nuclei stained with DAPI (d). Immunolocalisation of perlecan in the pericellular matrix of AF cells in the native tissue (brown chromogen) using a mAb to perlecan domain IV (mAb A7L6) (e). Perlecan provides both cell–cell and cell–matrix connections which allow the monolayer cells to attach and spread out and also attaches the AF cells to the pericellular and interterritorial matrix in the native tissue
The pericellular matrix, cell–matrix interactions and annular mechanosensory processes
A number of studies have demonstrated that the pericellular matrix of AF cells display a complex morphology [12, 22, 23, 37, 60]. Extensive sinuous cellular processes interwoven through annular collagenous fibres are evident in cells of the outer AF as well as strings of 10–12 cells interconnected by type VI collagen which is a prominent pericellular AF component particularly in the mid and inner AF [23, 120]. This is consistent with type VI collagen’s documented roles in the assembly of the basket-like chondron functional unit around cells of a chondrocytic background [46, 107]. The cells in the outer AF are extensively interconnected through functional gap junctions forming a network through which the cells can communicate [23]. Loading of the IVD results in shearing forces between collagen fibrils in the annular lamellae and rotation of adjacent lamellae which absorbs the mechanical strains. The strings of mid and inner annular cells embedded in a dense pericellular matrix, located between adjacent lamellae, largely spared these tensile strains. However, the annular cells of the outer AF with their extensive interdigitating cellular processes woven into the collagenous architecture would be expected to be subjected to tensional forces in situ and may be involved in annular mechanotransduction events [38]. The morphologic complexity of the annular cells with their interwoven ECM attachments to the annular collagen fibres indicates that the load transfer from ECM to cell membrane to cytoskeleton in all likelihood occurs by a complex pathway. The physical connections between focal cellular adhesions, the actin cytoskeleton and nuclear scaffold is thought to provide a pathway for mechanical signal transfer from the cell surface to the nucleus [50, 51]. Deformation of the cytoskeleton by intrinsic biomechanical stresses provides a physical basis for transducing mechanical signals into transcriptional gene expression and protein synthesis through cell signalling [49, 52, 70].
Perlecan is a prominent pericellular matrix component of AF cells in vitro and in vivo. Furthermore, FGF-2, a major ligand for perlecan, has been suggested as a mechanotransducer molecule in articular cartilage and perlecan modulates FGF-2 bioactivity in growth plate cartilage [147–150]. Thus, the proposal of perlecan having some accessory role in mechanosensory processes in the IVD is not without merit. We have examined the expression of perlecan in the pericellular matrix of AF cells in the native tissue (Figs. 5, 6e) and in short term monolayer culture (Fig. 6a–d). The AF cells are cultured in primary short-term (4 day) cultures to minimise cellular de-differentiation, in the absence of ascorbic acid, thus the intracellular procollagens are not hydroxylated or assembled into fibrillar collagen in the pericellular matrix. The triple helix of fibrillar collagens is not thermally stable at 37°C without hydroxylation, and it is not secreted. Collagen VI also does not assemble into tetramers in the absence of ascorbate although the collagen VI monomers (α1, α2, α3) are secreted into the medium. Using this monolayer approach greatly simplifies observation of the early stages of the assembly of the annular pericellular matrix. Furthermore, without the surrounding ECM and the instructive properties it normally conveys [110], the AF cells synthesise a number of perlecan positive interconnections to adjacent cells and to the tissue culture plastic, and along with the actin cytoskeleton (visualised using phalloidin-oregon green, Fig. 6d) this facilitates the spreading out of these cells in culture. The fibrillar cellular extensions containing perlecan in the native AF are pericellular due to ECM constraining and intrinsic biomechanical forces. These monolayer experiments serve to demonstrate the propensity of the annular cells to assemble a pericellular matrix containing perlecan as an interactive component. This is consistent with perlecan’s known multifunctional properties in a number of tensional and weight bearing connective tissues. Perlecan also has a pericellular localisation in the native AF and important interactive roles controlling matrix assembly and tissue homeostasis (Fig. 6e). Perlecan’s chondrogenic, matrix organisational and stabilising roles and ability to sequester a range of anabolic growth factors in the pericellular matrix indicates that it has important attributes relevant to annular remodelling and repair processes, reinforcing its potential as an effector molecule worthy of further investigation in the context of repair biology. It will be very interesting in this regard to ascertain whether the therapeutic potential of 3D scaffolds of electrospun fibres of collagen and gelatin functionalised with recombinant domain-I of perlecan are fully realised [13].
Biological strategies for annular and intervertebral disc repair
Tissue engineering strategies for intervertebral disc repair have utilised a number of bioscaffolds [127]. Collagen sponge, collagen gel, agarose, alginate or fibrin gel cell carriers, alginate/chitosan hybrid fibre scaffolds [129], scaffolds assembled from electrospun collagen and gelatin functionalised with perlecan domain-I [13], chitosan salts cross-linked to genipen [95], a chitosan glycerophosphate hydrogel [114], a porous calcium polyphosphate carrier [128], elastic poly (1, 8-octanediol malate) 3D scaffolds[151], a composite demineralised bone matrix gelatin-(polycaprolactone triol malate)- poly(caprolactone triol malate) biphasic scaffold[152] and collagen/hyaluronan hybrid scaffolds [5] have all been examined in IVD and/or annular repair strategies. Exciting possibilities also exist for the use of replacement total IVDs using biocompatible and biomechanically competent PLGA and PLA scaffolds seeded with AF and NP cells [88, 89]. Novel injectable hydrogels which can be seeded with a number of cell types have also been developed for NP replacement strategies [111, 145].
Therapeutic strategies focussed specifically on annular repair
Injectable scaffolds have potential application in the repair of annular defects since they allow easy filling of irregularly shaped defects and implantation of cells through minimally invasive surgical procedures. Due to the intricate structural organisation of the AF which is composed of highly oriented arcade-like, alternating stressed and relaxed lamellae in which fibrochondrocytes are aligned and containing counter-gradients of types II and I collagen in the inner and outer AF, respectively, this tissue has special requirements from an engineering perspective. AF cells have been grown on PDLLA/45S5 Bioglass composite films [156] and composite foam scaffolds [40], an atelocollagen honeycomb-shaped scaffold with a membrane seal (ACHMS-scaffold) [124], and on micro-grooved membranes of polycaprolactone [61]. This provides control over the directional growth of the annular fibrochondrocytes in the constructs and recapitulates some of the spatial organisation of this tissue. Since engineering, a functional replacement for the AF is contingent upon recapitulation of AF structure, composition, and mechanical properties, some of the more recent advances in oriented electrospun scaffolds [58, 99], porous silk fibroin scaffolds [15] and microgrooved membranes [61] hold great promise in replacement strategies for the repair of annular defects. The interconnectivity and shape of tendon cells are important determinants which regulate production of type I collagen in vitro [68], a greater appreciation of the micromechanical control of cell and tissue development by biomatrices is emerging and newer approaches are attempting to guide cellular behaviour to facilitate correct tissue assembly and seamless integration with adjacent living tissues [31]. A novel 3D elastic scaffold which simulates the deformability of the AF and has good biocompatibility has also been developed using a malic acid based polyester poly (1,8-ocatanediol malate) (POM) scaffold [151]. This polymer supported the growth of AF cells, maintained their phenotype in culture and the cells produced proteoglycan and type II collagen in vitro. POM is metabolised through the tricarboxylic acid cycle, its degradative products are well tolerated, and POM consequently does not elicit a strong inflammatory response in situ [151]. Furthermore, POM has predictable degradative decay rates which allow the accurate determination of the culture period required for production of an engineered replacement AF. A biphasic scaffold has been developed based on an outer demineralised bone matrix gelatin (BMG) scaffold which simulates the type I collagen rich outer AF and an inner biomaterial based on poly(polycaprolactone triol malate) (PPCLM) which has biomechanical properties more similar to the inner AF [152]. Incorporation of BMG into PPCLM resulted in a 50-fold increase in the tensile properties of the composite scaffold over PPCLM alone and produced a scaffold which could be prepared as sheets which could be subsequently assembled in concentric layers to mimic the lamellar structure present in the native AF. Both BGM and PPCLM demonstrated excellent biocompatability and supported the growth of AF cells in culture. The phenotype of the cells was also maintained in culture with strong production of proteoglycan and type II collagen by AF cells on BMG-PPCLM and PPCLM matrix. Modification of porous silk fibroin scaffolds by functionalisation with RGD peptides transforms the properties of this scaffold material from that of the unmodified fibroin scaffold [15]. Coupling of RGD peptide influences AF cell phenotype when they are cultured on this matrix for 8 weeks with the cells synthesising higher levels of aggrecan and type II collagen than when grown on non-modified fibroin. The AF cells grown on the RGD functionalised fibroin displayed a phenotype similar to the cells of the inner AF while the AF cells grown on non-modified fibroin displayed a phenotype more similar to that of outer AF cells. This ability to differentially modulate gene expression profiles to simulate inner and outer AF cells in culture may prove useful in repair strategies aimed at recapitulating the spatial organisation of the AF.
An innovative development in scaffold design for annular repair is the use of natural polymer 3D scaffolds of electrospun collagen and gelatin functionalised with recombinant domain-I of the HS-proteoglycan, perlecan [13]. Recombinant perlecan domain-I substituted with HS has also been expressed in HEK-293 kidney cells, its FGF binding capability demonstrated and potential use as a scaffold additive to enhance FGF cell signalling by endogenous cell populations established [153]. Perlecan domain-I in native human perlecan contains up to three HS side chains. The rationale for the development of perlecan domain-I functionalised scaffolds for annular repair resides in their ability to bind FGF-2 although these HS chains can also bind FGF-1, 9, 18.
Integration of engineered annular constructs in repair environments
A major obstacle in IVD repair biology is how to effectively anchor the construct to facilitate its biointegration with the cartilaginous margins of a defect site which may be somewhat devitalised [130]. Biological glues based on an adhesive protein extracted from the marine mussel Mytilus edulis [100] and a protein-based adhesive elastomer secreted by the Australian frog Notaden bennetti [34] have been used to glue porcine small intestinal submucosa [100] and may also be useful in annular repair strategies. “Frog-glue” offers a stronger alternative to fibrin glue, has been used for the reattachment of bucket handle tears in knee joint menisci and offers potential in the non-surgical sealing of annular defects as an integral part of a repair strategy [35]. Injectable, self-curing, acrylic formulations, also display potential in this regard and may be additionally supplemented with drugs and other biomimetics in microcarriers for their controlled release in a therapeutic setting to stimulate cellular responses and promote tissue repair [66]. Biphasic scaffolds have also been developed to mimic the known differences in the spatial organisation of the outer and inner AF and these should aid in the integration of constructs which recapitulate at least some aspects of the structure of the native AF. Multiphasic scaffolds have also been developed to produce biomaterials appropriate for replacement of osteochondral defects [71, 126]. Although designed specifically for the treatment of defects in articular cartilage a similar approach could be adopted for repair of IVD defects to effect direct integration of constructs into vertebral bone.
The search for cellular marker proteins and their use as phenotypic markers for the identification of AF and NP cells
A fundamental requirement to a proper understanding of disc degeneration and regeneration at the cellular level is a full appreciation of the cellular biology of the intervertebral disc. Currently, there are no definitive cellular biomarkers which can be used to define a normal AF or NP cell to differentiate these from one another or from an articular chondrocyte. Micro-array and RT-PCR approaches have been used to determine which disc cell markers are most appropriate as phenotypic markers in rat IVDs [28, 67]. In an initial study, CD-24 was suggested as an NP cell marker [28]. In a follow up study [67] while CD-24 was shown to be more highly expressed in NP cells than articular chondrocytes (AC), the discriminative criteria adopted (NP gene expression ≥5-fold of AC cells) excluded CD-24 as a candidate NP marker. Sixty-three genes were expressed at a 5-fold greater level in NP compared to AF cells; 41 genes had ≥5-fold greater expression in NP than AC cells. Real time RT-PCR confirmed that the expression of annexin A3, glypican-3 (gpc-3), keratin-19 (k-19), and pleiotropin were all significantly higher in NP cells than AF or AC cells, a finding confirmed using immunohistochemistry on rat disc tissues [67]. However, it remains to be established how useful these markers will be for the human intervertebral disc.
Several proteins have been investigated which convey functional adaptations to NP cells allowing them to survive under hostile low oxygen tension and high osmotic pressure environments. Tonicity enhancer binding protein (TonEBP) [139] and hypoxia inducible factor-1 alpha (HIF-1α) [4, 115, 119] are two candidate proteins which allow NP cells to withstand hyperosmotic stress and an avascular hypoxic environment. TonEBP binds to the aggrecan promoter, regulates its transcriptional activity and the hydration status of the IVD. TonEBP is an interesting and functionally relevant marker for NP cells, however, it is also expressed by AF and kidney cells [11] and by costal chondrocytes but at considerably lower levels negating its suitability as an NP cell specific marker. HIF-1 α is produced by NP cells and is a key regulator of mammalian oxygen homeostasis [4, 109, 119]. HIF-1α is tightly regulated by the oxygen tension of tissues, hypoxic conditions prevent ubiquitination and proteasomal degradation of HIF-1α resulting in its accumulation and the trans-activation of downstream genes including those encoding glucose transporters and glycolytic enzymes capable of increasing anaerobic ATP synthesis [4, 109, 119]. Unlike other mammalian cells, HIF-1α expression in NP cells is stabilised under normoxic conditions [119]. However, HIF-1α is also expressed in a number of tumours [20, 21, 29] where it promotes the transcription of glycolytic enzymes which switch cells from aerobic to anaerobic glycolysis. HIF-1α expression in the ovine and human IVD correlates with IVD pathology [39, 115, 119]. The normoxic stabilization of HIF-1α in rat NP cells drives glycolytic processes which regulate aggrecan gene expression [4]. The glucose transporters GLUT-1, 3 and 9 are also targets for HIF-1α in rat NP cells (but not AF cells) [115]. Thus, while HIF-1 α in isolation is not suitable as a specific NP marker, assessment of GLUT-1, 3 and 9 expression in combination with HIF-1α and possibly other markers such as Sox 9, aggrecan and type II collagen in a mini array profile may facilitate the functional identification and differentiation of NP cells from related cell types. While in themselves each proposed marker may not be absolutely specific for NP cells, in combination and utilising threshholding techniques an identifiable NP signature may be identified. For example, the ratio of aggrecan to collagen in the IVD is 27:1 whereas in articular cartilage it is 2:1 [96]. Such assays will need to be validated in the adult human IVD which unlike rat IVDs lack notochordal cells and the regulatory factors they secrete.
Two-dimensional fluorescence difference gel electrophoresis (2D-DIGE) is a powerful new technique whereby the proteome of several cell types can be compared simultaneously in one 2D gel format using in-gel enzymatic digestion, mass spectrometry and bioinformatics software to identify the full cellular proteome [69, 135]. 2D-DIGE has been used to search for NP cell biomarkers to differentiate these from cells of a chondrocytic background [32]. Thirty differentially expressed proteins were identified in a preliminary study using DIGE, thus it may well be possible to identify useful NP and AF cell protein biomarkers using such technology in the not too distant future.
Cell-based therapies for the biologic treatment of disc degeneration
The IVD has a relatively sparse cell population available to effect repair processes and cell viabilities are also known to be significantly diminished with aging. The mean IVD cell density of 6,000 cells/mm3 compares with 14,000–15,000 cells/mm3 in human articular cartilage [47, 72]. The avascular nature of the IVD, poor nutrition and severe biomechanical demands placed on it are all refractory to repair processes and it consequently has an intrinsically poor healing capability. Of the cells to use for cell-based therapies for IVD degeneration, IVD cells themselves are the least likely candidates for use given the difficulty of harvesting sufficient numbers of viable autologous disc cells from symptomatic IVDs [112–114, 116, 117]. However, a number of cell types have provided promising results in therapeutic cell replacement strategies for disc repair, including costal and articular chondrocytes [30, 55, 73, 111], auricular chondrocytes [33] and mesenchymal stem cells (MSCs) [3, 41, 113, 114, 117, 123, 132]. A common pathological features of IVD degeneration is a chondroid metaplasia of the NP with clusters (chondrones) of chondrocyte-like cells within a dense hyaline-like matrix [59] which is different from the fibrocartilage of the normal NP. This is considered a degenerative feature, thus despite similarities in morphology between the chondrocyte and NP cells the rationale for the use of the latter cell type to effect disc “repair” must be questioned.
Nutritional constraints on cell-based therapeutic approaches for IVD repair
A number of factors affect disc cell metabolism apart from nutrient supply and removal of metabolic waste products. These include the presence of growth factors, changes in local biomechanical stresses and in the osmotic environment of the disc cell [44, 47, 72, 98, 143, 144, 157]. Stimulation of endogenous disc cell populations by administered growth factors and other biomimetics in slow delivery carriers within bioscaffolds aim to address some of these questions [53, 85].
The metabolism of disc cells is regulated by oxygen and nutrient levels in the tissue and also by the extracellular pH [44, 47, 143, 144]. The adult human IVD is virtually avascular apart from a sparse penetration of small vessels in the outermost AF. The NP, inner AF and part of the outer AF is supplied by a network of small vessels that arise from the vertebral arteries and penetrates the subchondral plate terminating in capillary buds in the bone–cartilage end plate junction through which diffusive processes effect the nutrition of endogenous disc cell populations [44, 47, 143, 144]. The density of the capillary network feeding the disc diminishes with age and across the disc, with the highest density overlying the NP and lowest in the endplate adjacent to the outer AF. The density of the subchondral plate through which the capillaries pass also varies across the disc and it’s permeability decreases with age as the end plate becomes sclerotic. Such changes significantly diminish the nutritional status of the disc cell and undoubtedly contribute to cell death (apoptosis, senescence) with aging. This has to be taken into account in any prospective use of administered cells to replenish ECM components through cellular replicative and matrix biosynthetic processes. It may simply not be possible for a relatively large number of administered cells to undertake such tasks given that the degenerate IVD would be expected to have severely compromised routes of nutrition which have led to its demise in the first place. The use of stimulatory growth factors and/or slow release biomimetics in scaffolds similarly may be a futile gesture and may fail to stimulate replicative and matrix biosynthetic processes in endogeneous disc cells unless the normal pathways to the disc cells for the supply of nutrients and removal of metabolic waste products have first been re-established.
Remodelling of the vertebral subchondral capillary bed in response to calcium agonists such as Nimodipine (marketed by Bayer as Nimotop®) has been observed in animal models of disc degeneration suggesting that the IVD nutritional status can be improved at least experimentally [142]. Nimodipine is a dihydropyridine calcium channel blocker originally developed for the treatment of high blood pressure, it has been used to increase blood flow to injured brain tissue and in the treatment of migraines. Oral administration of Nimodipine in 40 patients (30 mg QID for 5 days) significantly enhanced signal intensity of the subchondral bone and cartilaginous endplates in serial post contrast MRI studies using gadodiamide [108]. This indicated that the diffusive pathways to the human lumbar IVDs were significantly enhanced by Nimodipine treatment and suggests that this treatment may be beneficial in the nutrition of endogenous cell populations in age onset disc degeneration in man but has yet to be evaluated as part of a discal repair strategy.
Concluding remarks
The ovine annular lesion model [103] has provided useful spatial, temporal and longitudinal information on how rim lesions may impact on other discal components and has provided important insights into annular remodelling and how it can influence the etiopathogenesis of disc degeneration in specific situations. There is a clear need to identify specific phenotypic marker proteins for AF and NP cells. This lack of cellular markers makes it difficult to evaluate the specific contributions of disc cell populations in cell-based IVD repair strategies. AF and NP cell markers would also be useful to assess how effectively an MSC or pluripotent progenitor cell population has been guided along a particular differentiation pathway by specific culture conditions to produce AF or NP cells for therapeutic applications. The nutrition of outer annulus is less critical than the inner annulus, however the nutrition of disc cells remains a centrally important issue in any repair strategy involving large numbers of administered reparative cells. Tissue engineering of the IVD has undergone impressive advances over the last few years particularly in the development of new biomatrices and the oriented and dynamic culture of annular fibrochondrocytes into planar, spatially relevant, annulus type structures. Improvements in our understanding of annular assembly has also provided insightful information applicable to new repair strategies. The multifunctional proteoglycan, perlecan, in particular has many attributes conducive to matrix assembly, remodelling and repair processes and is worthy of further consideration in annular and discal repair biology. Despite exciting advances in biological IVD repair, significant technical obstacles still have to be overcome before such approaches become realistic alternative therapeutic options to conventional surgical intervention procedures.
Acknowledgments
This study was funded by NHMRC Project Grant 211266.
References
- 1.Abe T, Yamada H, Nakajima H, Kikuchi T, Takaishi H, Tadakuma T, et al. Repair of full-thickness cartilage defects using liposomal transforming growth factor-beta1. J Orthop Sci. 2003;8:92–101. doi: 10.1007/s007760300016. [DOI] [PubMed] [Google Scholar]
- 2.Abreu JG, Ketpura NI, Reversade B, Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acosta FL, Jr, Lotz J, Ames CP. The potential role of mesenchymal stem cell therapy for intervertebral disc degeneration: a critical overview. Neurosurg Focus. 2005;19:E4. doi: 10.3171/foc.2005.19.3.5. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal A, Guttapalli A, Narayan S, Albert TJ, Shapiro IM, Risbud MV. Normoxic stabilization of HIF-1alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am J Physiol Cell Physiol. 2007;293:C621–C631. doi: 10.1152/ajpcell.00538.2006. [DOI] [PubMed] [Google Scholar]
- 5.Alini M, Li W, Markovic P, Aebi M, Spiro RC, Roughley PJ. The potential and limitations of a cell-seeded collagen/hyaluronan scaffold to engineer an intervertebral disc-like matrix. Spine. 2003;28:446–454. doi: 10.1097/00007632-200303010-00007. [DOI] [PubMed] [Google Scholar]
- 6.Allen JM, Bateman JF, Hansen U, Wilson R, Bruckner P, Owens RT, et al. WARP is a novel multimeric component of the chondrocyte pericellular matrix that interacts with perlecan. J Biol Chem. 2006;281:7341–7349. doi: 10.1074/jbc.M513746200. [DOI] [PubMed] [Google Scholar]
- 7.An HS, Takegami K, Kamada H, Nguyen CM, Thonar EJ, Singh K, et al. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine. 2005;30:25–31. doi: 10.1097/01.brs.0000148002.68656.4d. [DOI] [PubMed] [Google Scholar]
- 8.Bengtsson E, Morgelin M, Sasaki T, Timpl R, Heinegard D, Aspberg A. The leucine-rich repeat protein PRELP binds perlecan and collagens and may function as a basement membrane anchor. J Biol Chem. 2002;277:15061–15068. doi: 10.1074/jbc.M108285200. [DOI] [PubMed] [Google Scholar]
- 9.Bobacz K, Ullrich R, Amoyo L, Erlacher L, Smolen JS, Graninger WB. Stimulatory effects of distinct members of the bone morphogenetic protein family on ligament fibroblasts. Ann Rheum Dis. 2006;65:169–177. doi: 10.1136/ard.2004.022756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckwalter JA, Cooper RR, Maynard JA. Elastic fibers in human intervertebral discs. J Bone Joint Surg Am. 1976;58:73–76. [PubMed] [Google Scholar]
- 11.Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev. 2007;87:1441–1474. doi: 10.1152/physrev.00056.2006. [DOI] [PubMed] [Google Scholar]
- 12.Cao L, Guilak F, Setton LA. Three-dimensional morphology of the pericellular matrix of intervertebral disc cells in the rat. J Anat. 2007;211:444–452. doi: 10.1111/j.1469-7580.2007.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casper CL, Yang W, Farach-Carson MC, Rabolt JF. Coating electrospun collagen and gelatin fibers with perlecan domain I for increased growth factor binding. Biomacromolecules. 2007;8:1116–1123. doi: 10.1021/bm061003s. [DOI] [PubMed] [Google Scholar]
- 14.Chakravarti S. Functions of lumican and fibromodulin: lessons from knockout mice. Glycoconj J. 2002;19:287–293. doi: 10.1023/A:1025348417078. [DOI] [PubMed] [Google Scholar]
- 15.Chang G, Kim HJ, Kaplan D, Vunjak-Novakovic G, Kandel RA. Porous silk scaffolds can be used for tissue engineering annulus fibrosus. Eur Spine J. 2007;16:1848–1857. doi: 10.1007/s00586-007-0364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen WH, Lo WC, Lee JJ, Su CH, Lin CT, Liu HY, et al. Tissue-engineered intervertebral disc and chondrogenesis using human nucleus pulposus regulated through TGF-beta1 in platelet-rich plasma. J Cell Physiol. 2006;209:744–754. doi: 10.1002/jcp.20765. [DOI] [PubMed] [Google Scholar]
- 17.Cook SD, Patron LP, Salkeld SL, Rueger DC. Repair of articular cartilage defects with osteogenic protein-1 (BMP-7) in dogs. J Bone Joint Surg Am. 2003;85-A(Suppl 3):116–123. doi: 10.2106/00004623-200300003-00018. [DOI] [PubMed] [Google Scholar]
- 18.Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, et al. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers–Danlos-like changes in bone and other connective tissues. J Bone Miner Res. 2002;17:1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- 19.Costell M, Gustafsson E, Aszodi A, Morgelin M, Bloch W, Hunziker E, et al. Perlecan maintains the integrity of cartilage and some basement membranes. J Cell Biol. 1999;147:1109–1122. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Cristofano C, Minervini A, Menicagli M, Salinitri G, Bertacca G, Pefanis G, et al. Nuclear expression of hypoxia-inducible factor-1alpha in clear cell renal cell carcinoma is involved in tumor progression. Am J Surg Pathol. 2007;31:1875–1881. doi: 10.1097/PAS.0b013e318094fed8. [DOI] [PubMed] [Google Scholar]
- 21.Du Z, Fujiyama C, Chen Y, Masaki Z. Expression of hypoxia-inducible factor 1alpha in human normal, benign, and malignant prostate tissue. Chin Med J (Engl) 2003;116:1936–1939. [PubMed] [Google Scholar]
- 22.Duncan NA. Cell deformation and micromechanical environment in the intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl 2):47–51. doi: 10.2106/JBJS.F.00035. [DOI] [PubMed] [Google Scholar]
- 23.Errington RJ, Puustjarvi K, White IR, Roberts S, Urban JP. Characterisation of cytoplasm-filled processes in cells of the intervertebral disc. J Anat. 1998;192(Pt 3):369–378. doi: 10.1046/j.1469-7580.1998.19230369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eyre DR, Muir H. Types I and II collagens in intervertebral disc. Interchanging radial distributions in annulus fibrosus. Biochem J. 1976;157:267–270. doi: 10.1042/bj1570267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ezura Y, Chakravarti S, Oldberg A, Chervoneva I, Birk DE. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J Cell Biol. 2000;151:779–788. doi: 10.1083/jcb.151.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fazzalari NL, Costi JJ, Hearn TC, Fraser RD, Vernon-Roberts B, Hutchinson J, et al. Mechanical and pathologic consequences of induced concentric anular tears in an ovine model. Spine. 2001;26:2575–2581. doi: 10.1097/00007632-200112010-00010. [DOI] [PubMed] [Google Scholar]
- 27.Feng H, Danfelter M, Stromqvist B, Heinegard D. Extracellular matrix in disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):25–29. doi: 10.2106/JBJS.E.01341. [DOI] [PubMed] [Google Scholar]
- 28.Fujita N, Miyamoto T, Imai J, Hosogane N, Suzuki T, Yagi M, et al. CD24 is expressed specifically in the nucleus pulposus of intervertebral discs. Biochem Biophys Res Commun. 2005;338:1890–1896. doi: 10.1016/j.bbrc.2005.10.166. [DOI] [PubMed] [Google Scholar]
- 29.Fujiwara S, Nakagawa K, Harada H, Nagato S, Furukawa K, Teraoka M, et al. Silencing hypoxia-inducible factor-1alpha inhibits cell migration and invasion under hypoxic environment in malignant gliomas. Int J Oncol. 2007;30:793–802. [PubMed] [Google Scholar]
- 30.Ganey T, Libera J, Moos V, Alasevic O, Fritsch KG, Meisel HJ, et al. Disc chondrocyte transplantation in a canine model: a treatment for degenerated or damaged intervertebral disc. Spine. 2003;28:2609–2620. doi: 10.1097/01.BRS.0000097891.63063.78. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh K, Ingber DE. Micromechanical control of cell and tissue development: implications for tissue engineering. Adv Drug Deliv Rev. 2007;59:1306–1318. doi: 10.1016/j.addr.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 32.Gilson A, Dreger M, Urban J (2008) Towards the identification of intervertebral disc biomarkers: their role in tissue engineering for degenerated disc repair. In: Proceedings of World Forum for Spine Research, Kyoto, Japan, 23–26 January 2008
- 33.Gorensek M, Jaksimovic C, Kregar-Velikonja N, Gorensek M, Knezevic M, Jeras M, et al. Nucleus pulposus repair with cultured autologous elastic cartilage derived chondrocytes. Cell Mol Biol Lett. 2004;9:363–373. [PubMed] [Google Scholar]
- 34.Graham LD, Glattauer V, Huson MG, Maxwell JM, Knott RB, White JW, et al. Characterization of a protein-based adhesive elastomer secreted by the Australian frog Notaden bennetti. Biomacromolecules. 2005;6:3300–3312. doi: 10.1021/bm050335e. [DOI] [PubMed] [Google Scholar]
- 35.Graham LD, Glattauer V, Peng YY, Vaughan PR, Werkmeister JA, Tyler MT, et al. An adhesive secreted by Australian frogs of the genus Notaden. In: Smith AM, Callow JA, et al., editors. Biological adhesives. Berlin: Springer; 2006. pp. 207–233. [Google Scholar]
- 36.Grimaud E, Heymann D, Redini F. Recent advances in TGF-beta effects on chondrocyte metabolism. Potential therapeutic roles of TGF-beta in cartilage disorders. Cytokine Growth Factor Rev. 2002;13:241–257. doi: 10.1016/S1359-6101(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 37.Gruber HE, Ingram JA, Hanley EN., Jr Morphologic complexity of the pericellular matrix in the annulus of the human intervertebral disc. Biotech Histochem. 2007;82:217–225. doi: 10.1080/10520290701713999. [DOI] [PubMed] [Google Scholar]
- 38.Guilak F, Alexopoulos LG, Upton ML, Youn I, Choi JB, Cao L, et al. The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage. Ann N Y Acad Sci. 2006;1068:498–512. doi: 10.1196/annals.1346.011. [DOI] [PubMed] [Google Scholar]
- 39.Ha KY, Koh IJ, Kirpalani PA, Kim YY, Cho YK, Khang GS, et al. The expression of hypoxia inducible factor-1alpha and apoptosis in herniated discs. Spine. 2006;31:1309–1313. doi: 10.1097/01.brs.0000219493.76081.d6. [DOI] [PubMed] [Google Scholar]
- 40.Helen W, Merry CL, Blaker JJ, Gough JE. Three-dimensional culture of annulus fibrosus cells within PDLLA/Bioglass composite foam scaffolds: assessment of cell attachment, proliferation and extracellular matrix production. Biomaterials. 2007;28:2010–2020. doi: 10.1016/j.biomaterials.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Helm GA, Gazit Z. Future uses of mesenchymal stem cells in spine surgery. Neurosurg Focus. 2005;19:E13. doi: 10.3171/foc.2005.19.6.14. [DOI] [PubMed] [Google Scholar]
- 42.Hidaka C, Goodrich LR, Chen CT, Warren RF, Crystal RG, Nixon AJ. Acceleration of cartilage repair by genetically modified chondrocytes over expressing bone morphogenetic protein-7. J Orthop Res. 2003;21:573–583. doi: 10.1016/S0736-0266(02)00264-4. [DOI] [PubMed] [Google Scholar]
- 43.Holland TA, Tessmar JK, Tabata Y, Mikos AG. Transforming growth factor-beta 1 release from oligo(poly(ethylene glycol) fumarate) hydrogels in conditions that model the cartilage wound healing environment. J Control Rel. 2004;94:101–114. doi: 10.1016/j.jconrel.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Holm S, Maroudas A, Urban JP, Selstam G, Nachemson A. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8:101–119. doi: 10.3109/03008208109152130. [DOI] [PubMed] [Google Scholar]
- 45.Hopf M, Gohring W, Kohfeldt E, Yamada Y, Timpl R. Recombinant domain IV of perlecan binds to nidogens, laminin-nidogen complex, fibronectin, fibulin-2 and heparin. Eur J Biochem. 1999;259:917–925. doi: 10.1046/j.1432-1327.1999.00127.x. [DOI] [PubMed] [Google Scholar]
- 46.Horikawa O, Nakajima H, Kikuchi T, Ichimura S, Yamada H, Fujikawa K, et al. Distribution of type VI collagen in chondrocyte microenvironment: study of chondrons isolated from human normal and degenerative articular cartilage and cultured chondrocytes. J Orthop Sci. 2004;9:29–36. doi: 10.1007/s00776-003-0737-4. [DOI] [PubMed] [Google Scholar]
- 47.Horner HA, Urban JP. 2001 Volvo Award Winner in basic science studies: effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine. 2001;26:2543–2549. doi: 10.1097/00007632-200112010-00006. [DOI] [PubMed] [Google Scholar]
- 48.Humzah MD, Soames RW. Human intervertebral disc: structure and function. Anat Rec. 1988;220:337–356. doi: 10.1002/ar.1092200402. [DOI] [PubMed] [Google Scholar]
- 49.Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 50.Ingber DE. Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci. 2003;116:1157–1173. doi: 10.1242/jcs.00359. [DOI] [PubMed] [Google Scholar]
- 51.Ingber DE. Tensegrity II. How structural networks influence cellular information processing networks. J Cell Sci. 2003;116:1397–1408. doi: 10.1242/jcs.00360. [DOI] [PubMed] [Google Scholar]
- 52.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 53.Ingber DE, Mow VC, Butler D, Niklason L, Huard J, Mao J, et al. Tissue engineering and developmental biology: going biomimetic. Tissue Eng. 2006;12:3265–3283. doi: 10.1089/ten.2006.12.3265. [DOI] [PubMed] [Google Scholar]
- 54.Iozzo RV. Basement membrane proteoglycans: from cellar to ceiling. Nat Rev Mol Cell Biol. 2005;6:646–656. doi: 10.1038/nrm1702. [DOI] [PubMed] [Google Scholar]
- 55.Isogai N, Kusuhara H, Ikada Y, Ohtani H, Jacquet R, Hillyer J, et al. Comparison of different chondrocytes for use in tissue engineering of cartilage model structures. Tissue Eng. 2006;12:691–703. doi: 10.1089/ten.2006.12.691. [DOI] [PubMed] [Google Scholar]
- 56.Isogai Z, Aspberg A, Keene DR, Ono RN, Reinhardt DP, Sakai LY. Versican interacts with fibrillin-1 and links extracellular microfibrils to other connective tissue networks. J Biol Chem. 2002;277:4565–4572. doi: 10.1074/jbc.M110583200. [DOI] [PubMed] [Google Scholar]
- 57.Jelic M, Pecina M, Haspl M, Kos J, Taylor K, Maticic D, et al. Regeneration of articular cartilage chondral defects by osteogenic protein-1 (bone morphogenetic protein-7) in sheep. Growth Factors. 2001;19:101–113. doi: 10.3109/08977190109001079. [DOI] [PubMed] [Google Scholar]
- 58.Ji Y, Ghosh K, Shu XZ, Li B, Sokolov JC, Prestwich GD, et al. Electrospun three-dimensional hyaluronic acid nanofibrous scaffolds. Biomaterials. 2006;27:3782–3792. doi: 10.1016/j.biomaterials.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 59.Johnson WE, Eisenstein SM, Roberts S. Cell cluster formation in degenerate lumbar intervertebral discs is associated with increased disc cell proliferation. Connect Tissue Res. 2001;42:197–207. doi: 10.3109/03008200109005650. [DOI] [PubMed] [Google Scholar]
- 60.Johnson WE, Roberts S. Human intervertebral disc cell morphology and cytoskeletal composition: a preliminary study of regional variations in health and disease. J Anat. 2003;203:605–612. doi: 10.1046/j.1469-7580.2003.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson WE, Wootton A, El Haj A, Eisenstein SM, Curtis AS, Roberts S. Topographical guidance of intervertebral disc cell growth in vitro: towards the development of tissue repair strategies for the anulus fibrosus. Eur Spine J. 2006;15(Suppl 3):S389–S396. doi: 10.1007/s00586-006-0125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kallunki P, Tryggvason K. Human basement membrane heparan sulfate proteoglycan core protein: a 467-kD protein containing multiple domains resembling elements of the low density lipoprotein receptor, laminin, neural cell adhesion molecules, and epidermal growth factor. J Cell Biol. 1992;116:559–571. doi: 10.1083/jcb.116.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knox S, Melrose J, Whitelock J. Electrophoretic, biosensor, and bioactivity analyses of perlecans of different cellular origins. Proteomics. 2001;1:1534–1541. doi: 10.1002/1615-9861(200111)1:12<1534::AID-PROT1534>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 64.Knox S, Merry C, Stringer S, Melrose J, Whitelock J. Not all perlecans are created equal: interactions with fibroblast growth factor (FGF) 2 and FGF receptors. J Biol Chem. 2002;277:14657–14665. doi: 10.1074/jbc.M111826200. [DOI] [PubMed] [Google Scholar]
- 65.Knox SM, Whitelock JM. Perlecan: how does one molecule do so many things? Cell Mol Life Sci. 2006;63:2435–2445. doi: 10.1007/s00018-006-6162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Larraz E, Elvira C, Fernandez M, Parra J, Collia F, Lopez-Bravo A, et al. Self-curing acrylic formulations with applications in intervertebral disk restoration: drug release and biological behaviour. J Tissue Eng Regen Med. 2007;1:120–127. doi: 10.1002/term.10. [DOI] [PubMed] [Google Scholar]
- 67.Lee CR, Sakai D, Nakai T, Toyama K, Mochida J, Alini M, et al. A phenotypic comparison of intervertebral disc and articular cartilage cells in the rat. Eur Spine J. 2007;16:2174–2185. doi: 10.1007/s00586-007-0475-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li F, Li B, Wang QM, Wang JH. Cell shape regulates collagen type I expression in human tendon fibroblasts. Cell Motil Cytoskeleton. 2008;65(4):332–341. doi: 10.1002/cm.20263. [DOI] [PubMed] [Google Scholar]
- 69.Lilley KS, Friedman DB. All about DIGE: quantification technology for differential-display 2D-gel proteomics. Expert Rev Proteomics. 2004;1:401–409. doi: 10.1586/14789450.1.4.401. [DOI] [PubMed] [Google Scholar]
- 70.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci USA. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mano JF, Reis RL. Osteochondral defects: present situation and tissue engineering approaches. J Tissue Eng Regen Med. 2007;1:261–273. doi: 10.1002/term.37. [DOI] [PubMed] [Google Scholar]
- 72.Maroudas A, Stockwell RA, Nachemson A, Urban J. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat. 1975;120:113–130. [PMC free article] [PubMed] [Google Scholar]
- 73.Meisel HJ, Siodla V, Ganey T, Minkus Y, Hutton WC, Alasevic OJ. Clinical experience in cell-based therapeutics: disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol Eng. 2007;24:5–21. doi: 10.1016/j.bioeng.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 74.Melrose J, Ghosh P, Taylor TK. A comparative analysis of the differential spatial and temporal distributions of the large (aggrecan, versican) and small (decorin, biglycan, fibromodulin) proteoglycans of the intervertebral disc. J Anat. 2001;198:3–15. doi: 10.1046/j.1469-7580.2001.19810003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Melrose J, Ghosh P, Taylor TK, Hall A, Osti OL, Vernon-Roberts B, et al. A longitudinal study of the matrix changes induced in the intervertebral disc by surgical damage to the annulus fibrosus. J Orthop Res. 1992;10:665–676. doi: 10.1002/jor.1100100509. [DOI] [PubMed] [Google Scholar]
- 76.Melrose J, Ghosh P, Taylor TK, Latham J, Moore R. Topographical variation in the catabolism of aggrecan in an ovine annular lesion model of experimental disc degeneration. J Spinal Disord. 1997;10:55–67. doi: 10.1097/00002517-199702000-00008. [DOI] [PubMed] [Google Scholar]
- 77.Melrose J, Ghosh P, Taylor TK, Vernon-Roberts B, Latham J, Moore R. Elevated synthesis of biglycan and decorin in an ovine annular lesion model of experimental disc degeneration. Eur Spine J. 1997;6:376–384. doi: 10.1007/BF01834063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Melrose J, Hayes AJ, Whitelock JM, Little CB. Perlecan, the “jack of all trades” proteoglycan of cartilaginous weight-bearing connective tissues. Bioessays. 2008;30:457–469. doi: 10.1002/bies.20748. [DOI] [PubMed] [Google Scholar]
- 79.Melrose J, Roberts S, Smith S, Menage J, Ghosh P. Increased nerve and blood vessel ingrowth associated with proteoglycan depletion in an ovine anular lesion model of experimental disc degeneration. Spine. 2002;27:1278–1285. doi: 10.1097/00007632-200206150-00007. [DOI] [PubMed] [Google Scholar]
- 80.Melrose J, Smith S, Ghosh P. Differential expression of proteoglycan epitopes by ovine intervertebral disc cells. J Anat. 2000;197(Pt 2):189–198. doi: 10.1046/j.1469-7580.2000.19720189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Melrose J, Smith S, Little CB, Kitson J, Hwa SY, Ghosh P. Spatial and temporal localization of transforming growth factor-beta, fibroblast growth factor-2, and osteonectin, and identification of cells expressing alpha-smooth muscle actin in the injured anulus fibrosus: implications for extracellular matrix repair. Spine. 2002;27:1756–1764. doi: 10.1097/00007632-200208150-00014. [DOI] [PubMed] [Google Scholar]
- 82.Melrose J, Smith SM, Appleyard RC, Little CB. Aggrecan, versican and type VI collagen are components of annular translamellar crossbridges in the intervertebral disc. Eur Spine J. 2007;17(2):314–324. doi: 10.1007/s00586-007-0538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Melrose J, Smith SM, Fuller ES, Young AA, Roughley PJ, Dart A, et al. Biglycan and fibromodulin fragmentation correlates with temporal and spatial annular remodelling in experimentally injured ovine intervertebral discs. Eur Spine J. 2007;16:2193–2205. doi: 10.1007/s00586-007-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mierisch CM, Cohen SB, Jordan LC, Robertson PG, Balian G, Diduch DR. Transforming growth factor-beta in calcium alginate beads for the treatment of articular cartilage defects in the rabbit. Arthroscopy. 2002;18:892–900. doi: 10.1053/jars.2002.36117. [DOI] [PubMed] [Google Scholar]
- 85.Mikos AG, Herring SW, Ochareon P, Elisseeff J, Lu HH, Kandel R, et al. Engineering complex tissues. Tissue Eng. 2006;12:3307–3339. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Minamide A, Hashizume H, Yoshida M, Kawakami M, Hayashi N, Tamaki T. Effects of basic fibroblast growth factor on spontaneous resorption of herniated intervertebral discs. An experimental study in the rabbit. Spine. 1999;24:940–945. doi: 10.1097/00007632-199905150-00003. [DOI] [PubMed] [Google Scholar]
- 87.Miosge N, Simniok T, Sprysch P, Herken R. The collagen type XVIII endostatin domain is co-localized with perlecan in basement membranes in vivo. J Histochem Cytochem. 2003;51:285–296. doi: 10.1177/002215540305100303. [DOI] [PubMed] [Google Scholar]
- 88.Mizuno H, Roy AK, Vacanti CA, Kojima K, Ueda M, Bonassar LJ. Tissue-engineered composites of anulus fibrosus and nucleus pulposus for intervertebral disc replacement. Spine. 2004;29:1290–1297. doi: 10.1097/01.BRS.0000128264.46510.27. [DOI] [PubMed] [Google Scholar]
- 89.Mizuno H, Roy AK, Zaporojan V, Vacanti CA, Ueda M, Bonassar LJ. Biomechanical and biochemical characterization of composite tissue-engineered intervertebral discs. Biomaterials. 2006;27:362–370. doi: 10.1016/j.biomaterials.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 90.Moore RJ, Crotti TN, Osti OL, Fraser RD, Vernon-Roberts B. Osteoarthrosis of the facet joints resulting from anular rim lesions in sheep lumbar discs. Spine. 1999;24:519–525. doi: 10.1097/00007632-199903150-00003. [DOI] [PubMed] [Google Scholar]
- 91.Moore RJ, Latham JM, Vernon-Roberts B, Fraser RD. Does plate fixation prevent disc degeneration after a lateral anulus tear? Spine. 1994;19:2787–2790. doi: 10.1097/00007632-199412150-00010. [DOI] [PubMed] [Google Scholar]
- 92.Moore RJ, Osti OL, Vernon-Roberts B, Fraser RD. Changes in endplate vascularity after an outer anulus tear in the sheep. Spine. 1992;17:874–878. doi: 10.1097/00007632-199208000-00003. [DOI] [PubMed] [Google Scholar]
- 93.Moore RJ, Vernon-Roberts B, Osti OL, Fraser RD. Remodeling of vertebral bone after outer anular injury in sheep. Spine. 1996;21:936–940. doi: 10.1097/00007632-199604150-00006. [DOI] [PubMed] [Google Scholar]
- 94.Murdoch AD, Dodge GR, Cohen I, Tuan RS, Iozzo RV. Primary structure of the human heparan sulfate proteoglycan from basement membrane (HSPG2/perlecan). A chimeric molecule with multiple domains homologous to the low density lipoprotein receptor, laminin, neural cell adhesion molecules, and epidermal growth factor. J Biol Chem. 1992;267:8544–8557. [PubMed] [Google Scholar]
- 95.Mwale F, Iordanova M, Demers CN, Steffen T, Roughley P, Antoniou J. Biological evaluation of chitosan salts cross-linked to genipin as a cell scaffold for disk tissue engineering. Tissue Eng. 2005;11:130–140. doi: 10.1089/ten.2005.11.130. [DOI] [PubMed] [Google Scholar]
- 96.Mwale F, Roughley P, Antoniou J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cell Mater. 2004;8:58–63. doi: 10.22203/ecm.v008a06. [DOI] [PubMed] [Google Scholar]
- 97.Nagano T, Yonenobu K, Miyamoto S, Tohyama M, Ono K. Distribution of the basic fibroblast growth factor and its receptor gene expression in normal and degenerated rat intervertebral discs. Spine. 1995;20:1972–1978. doi: 10.1097/00007632-199509150-00002. [DOI] [PubMed] [Google Scholar]
- 98.Neidlinger-Wilke C, Wurtz K, Urban JP, Borm W, Arand M, Ignatius A, et al. Regulation of gene expression in intervertebral disc cells by low and high hydrostatic pressure. Eur Spine J. 2006;15(Suppl 3):S372–S378. doi: 10.1007/s00586-006-0112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nerurkar NL, Elliott DM, Mauck RL. Mechanics of oriented electrospun nanofibrous scaffolds for annulus fibrosus tissue engineering. J Orthop Res. 2007;25(8):1018–1028. doi: 10.1002/jor.20384. [DOI] [PubMed] [Google Scholar]
- 100.Ninan L, Stroshine RL, Wilker JJ, Shi R. Adhesive strength and curing rate of marine mussel protein extracts on porcine small intestinal submucosa. Acta Biomater. 2007;3(5):687–694. doi: 10.1016/j.actbio.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nishida T, Kubota S, Fukunaga T, Kondo S, Yosimichi G, Nakanishi T, et al. CTGF/Hcs24, hypertrophic chondrocyte-specific gene product, interacts with perlecan in regulating the proliferation and differentiation of chondrocytes. J Cell Physiol. 2003;196:265–275. doi: 10.1002/jcp.10277. [DOI] [PubMed] [Google Scholar]
- 102.Noonan DM, Fulle A, Valente P, Cai S, Horigan E, Sasaki M, et al. The complete sequence of perlecan, a basement membrane heparan sulfate proteoglycan, reveals extensive similarity with laminin A chain, low density lipoprotein-receptor, and the neural cell adhesion molecule. J Biol Chem. 1991;266:22939–22947. [PubMed] [Google Scholar]
- 103.Osti OL, Vernon-Roberts B, Fraser RD. 1990 Volvo Award in experimental studies. Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine. 1990;15:762–767. doi: 10.1097/00007632-199008010-00005. [DOI] [PubMed] [Google Scholar]
- 104.Osti OL, Vernon-Roberts B, Moore R, Fraser RD. Annular tears and disc degeneration in the lumbar spine. A post-mortem study of 135 discs. J Bone Joint Surg Br. 1992;74:678–682. doi: 10.1302/0301-620X.74B5.1388173. [DOI] [PubMed] [Google Scholar]
- 105.Pattison ST, Melrose J, Ghosh P, Taylor TK. Regulation of gelatinase-A (MMP-2) production by ovine intervertebral disc nucleus pulposus cells grown in alginate bead culture by Transforming Growth Factor-beta(1) and insulin like growth factor-I. Cell Biol Int. 2001;25:679–689. doi: 10.1006/cbir.2000.0718. [DOI] [PubMed] [Google Scholar]
- 106.Pezowicz CA, Robertson PA, Broom ND. Intralamellar relationships within the collagenous architecture of the annulus fibrosus imaged in its fully hydrated state. J Anat. 2005;207:299–312. doi: 10.1111/j.1469-7580.2005.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Poole CA, Ayad S, Gilbert RT. Chondrons from articular cartilage. V. Immunohistochemical evaluation of type VI collagen organisation in isolated chondrons by light, confocal and electron microscopy. J Cell Sci. 1992;103(Pt 4):1101–1110. doi: 10.1242/jcs.103.4.1101. [DOI] [PubMed] [Google Scholar]
- 108.Rajasekaran S (2008) Evaluation of disc nutrition by serial post contrast MRI studies. In: Proceedings world forum for spine research, Kyoto, Japan, p 49
- 109.Rajpurohit R, Risbud MV, Ducheyne P, Vresilovic EJ, Shapiro IM. Phenotypic characteristics of the nucleus pulposus: expression of hypoxia inducing factor-1, glucose transporter-1 and MMP-2. Cell Tissue Res. 2002;308:401–407. doi: 10.1007/s00441-002-0563-6. [DOI] [PubMed] [Google Scholar]
- 110.Ramirez F, Rifkin DB. Cell signalling events: a view from the matrix. Matrix Biol. 2003;22:101–107. doi: 10.1016/S0945-053X(03)00002-7. [DOI] [PubMed] [Google Scholar]
- 111.Revell PA, Damien E, Di Silvio L, Gurav N, Longinotti C, Ambrosio L. Tissue engineered intervertebral disc repair in the pig using injectable polymers. J Mater Sci Mater Med. 2007;18:303–308. doi: 10.1007/s10856-006-0693-6. [DOI] [PubMed] [Google Scholar]
- 112.Richardson SM. Tissue engineering today, not tomorrow. Regen Med. 2007;2:91–94. doi: 10.2217/17460751.2.1.91. [DOI] [PubMed] [Google Scholar]
- 113.Richardson SM, Curran JM, Chen R, Vaughan-Thomas A, Hunt JA, Freemont AJ, et al. The differentiation of bone marrow mesenchymal stem cells into chondrocyte-like cells on poly-L-lactic acid (PLLA) scaffolds. Biomaterials. 2006;27:4069–4078. doi: 10.1016/j.biomaterials.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 114.Richardson SM, Hughes N, Hunt JA, Freemont AJ, Hoyland JA. Human mesenchymal stem cell differentiation to NP-like cells in chitosan-glycerophosphate hydrogels. Biomaterials. 2008;29:85–93. doi: 10.1016/j.biomaterials.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 115.Richardson SM, Knowles R, Tyler J, Mobasheri A, Hoyland JA. Expression of glucose transporters GLUT-1, GLUT-3, GLUT-9 and HIF-1alpha in normal and degenerate human intervertebral disc. Histochem Cell Biol. 2008;129(4):503–511. doi: 10.1007/s00418-007-0372-9. [DOI] [PubMed] [Google Scholar]
- 116.Richardson SM, Mobasheri A, Freemont AJ, Hoyland JA. Intervertebral disc biology, degeneration and novel tissue engineering and regenerative medicine therapies. Histol Histopathol. 2007;22:1033–1041. doi: 10.14670/HH-22.1033. [DOI] [PubMed] [Google Scholar]
- 117.Richardson SM, Walker RV, Parker S, Rhodes NP, Hunt JA, Freemont AJ, et al. Intervertebral disc cell-mediated mesenchymal stem cell differentiation. Stem Cells. 2006;24:707–716. doi: 10.1634/stemcells.2005-0205. [DOI] [PubMed] [Google Scholar]
- 118.Risbud MV, Di Martino A, Guttapalli A, Seghatoleslami R, Denaro V, Vaccaro AR, et al. Toward an optimum system for intervertebral disc organ culture: TGF-beta 3 enhances nucleus pulposus and anulus fibrosus survival and function through modulation of TGF-beta-R expression and ERK signaling. Spine. 2006;31:884–890. doi: 10.1097/01.brs.0000209335.57767.b5. [DOI] [PubMed] [Google Scholar]
- 119.Risbud MV, Guttapalli A, Stokes DG, Hawkins D, Danielson KG, Schaer TP, et al. Nucleus pulposus cells express HIF-1alpha under normoxic culture conditions: a metabolic adaptation to the intervertebral disc microenvironment. J Cell Biochem. 2006;98:152–159. doi: 10.1002/jcb.20765. [DOI] [PubMed] [Google Scholar]
- 120.Roberts S, Ayad S, Menage PJ. Immunolocalisation of type VI collagen in the intervertebral disc. Ann Rheum Dis. 1991;50:787–791. doi: 10.1136/ard.50.11.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Robinson PS, Huang TF, Kazam E, Iozzo RV, Birk DE, Soslowsky LJ. Influence of decorin and biglycan on mechanical properties of multiple tendons in knockout mice. J Biomech Eng. 2005;127:181–185. doi: 10.1115/1.1835363. [DOI] [PubMed] [Google Scholar]
- 122.Roughley PJ. The structure and function of cartilage proteoglycans. Eur Cell Mater. 2006;12:92–101. doi: 10.22203/ecm.v012a11. [DOI] [PubMed] [Google Scholar]
- 123.Sakai D, Mochida J, Iwashina T, Hiyama A, Omi H, Imai M, et al. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials. 2006;27:335–345. doi: 10.1016/j.biomaterials.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 124.Sato M, Asazuma T, Ishihara M, Kikuchi T, Masuoka K, Ichimura S, et al. An atelocollagen honeycomb-shaped scaffold with a membrane seal (ACHMS-scaffold) for the culture of annulus fibrosus cells from an intervertebral disc. J Biomed Mater Res A. 2003;64:248–256. doi: 10.1002/jbm.a.10287. [DOI] [PubMed] [Google Scholar]
- 125.Schmorl G, Junghans H (1971) The human spine in health and disease. 2nd American edition translated and edited by EF Besemann. Grune and Stratton, New York and London
- 126.Schumann D, Ekaputra AK, Lam CX, Hutmacher DW. Biomaterials/scaffolds. Design of bioactive, multiphasic PCL/collagen type I and type II-PCL-TCP/collagen composite scaffolds for functional tissue engineering of osteochondral repair tissue by using electrospinning and FDM techniques. Methods Mol Med. 2007;140:101–124. doi: 10.1007/978-1-59745-443-8_6. [DOI] [PubMed] [Google Scholar]
- 127.Sebastine IM, Williams DJ. Current developments in tissue engineering of nucleus pulposus for the treatment of intervertebral disc degeneration. Conf Proc IEEE Eng Med Biol Soc. 2007;1:6400–6405. doi: 10.1109/IEMBS.2007.4353821. [DOI] [PubMed] [Google Scholar]
- 128.Seguin CA, Grynpas MD, Pilliar RM, Waldman SD, Kandel RA. Tissue engineered nucleus pulposus tissue formed on a porous calcium polyphosphate substrate. Spine. 2004;29:1299–1306. doi: 10.1097/01.BRS.0000127183.43765.AF. [DOI] [PubMed] [Google Scholar]
- 129.Shao X, Hunter CJ. Developing an alginate/chitosan hybrid fiber scaffold for annulus fibrosus cells. J Biomed Mater Res A. 2007;82(3):701–710. doi: 10.1002/jbm.a.31030. [DOI] [PubMed] [Google Scholar]
- 130.Silverman RP, Bonasser L, Passaretti D, Randolph MA, Yaremchuk MJ. Adhesion of tissue-engineered cartilage to native cartilage. Plast Reconstr Surg. 2000;105:1393–1398. doi: 10.1097/00006534-200004050-00060. [DOI] [PubMed] [Google Scholar]
- 131.Smith LJ, Fazzalari NL. Regional variations in the density and arrangement of elastic fibres in the anulus fibrosus of the human lumbar disc. J Anat. 2006;209:359–367. doi: 10.1111/j.1469-7580.2006.00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Steck E, Bertram H, Abel R, Chen B, Winter A, Richter W. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells. 2005;23:403–411. doi: 10.1634/stemcells.2004-0107. [DOI] [PubMed] [Google Scholar]
- 133.Stevens MM, Marini RP, Martin I, Langer R, Prasad Shastri V. FGF-2 enhances TGF-beta1-induced periosteal chondrogenesis. J Orthop Res. 2004;22:1114–1119. doi: 10.1016/j.orthres.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 134.Takegami K, Thonar EJ, An HS, Kamada H, Masuda K. Osteogenic protein-1 enhances matrix replenishment by intervertebral disc cells previously exposed to interleukin-1. Spine. 2002;27:1318–1325. doi: 10.1097/00007632-200206150-00014. [DOI] [PubMed] [Google Scholar]
- 135.Tannu NS, Hemby SE. Two-dimensional fluorescence difference gel electrophoresis for comparative proteomics profiling. Nat Protocols. 2006;1:1732–1742. doi: 10.1038/nprot.2006.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thompson JP, Oegema TR, Jr, Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine. 1991;16:253–260. doi: 10.1097/00007632-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 137.Thompson RE, Pearcy MJ, Downing KJ, Manthey BA, Parkinson IH, Fazzalari NL. Disc lesions and the mechanics of the intervertebral joint complex. Spine. 2000;25:3026–3035. doi: 10.1097/00007632-200012010-00010. [DOI] [PubMed] [Google Scholar]
- 138.Tsai AD, Yeh LC, Lee JC. Effects of osteogenic protein-1 (OP-1, BMP-7) on gene expression in cultured medial collateral ligament cells. J Cell Biochem. 2003;90:777–791. doi: 10.1002/jcb.10666. [DOI] [PubMed] [Google Scholar]
- 139.Tsai TT, Danielson KG, Guttapalli A, Oguz E, Albert TJ, Shapiro IM, et al. TonEBP/OREBP is a regulator of nucleus pulposus cell function and survival in the intervertebral disc. J Biol Chem. 2006;281:25416–25424. doi: 10.1074/jbc.M601969200. [DOI] [PubMed] [Google Scholar]
- 140.Tsai TT, Guttapalli A, Oguz E, Chen LH, Vaccaro AR, Albert TJ, et al. Fibroblast growth factor-2 maintains the differentiation potential of nucleus pulposus cells in vitro: implications for cell-based transplantation therapy. Spine. 2007;32:495–502. doi: 10.1097/01.brs.0000257341.88880.f1. [DOI] [PubMed] [Google Scholar]
- 141.Tu H, Sasaki T, Snellman A, Gohring W, Pirila P, Timpl R, et al. The type XIII collagen ectodomain is a 150-nm rod and capable of binding to fibronectin, nidogen-2, perlecan, and heparin. J Biol Chem. 2002;277:23092–23099. doi: 10.1074/jbc.M107583200. [DOI] [PubMed] [Google Scholar]
- 142.Turgut M, Uysal A, Uslu S, Tavus N, Yurtseven ME. The effects of calcium channel antagonist nimodipine on end-plate vascularity of the degenerated intervertebral disc in rats. J Clin Neurosci. 2003;10:219–223. doi: 10.1016/S0967-5868(02)00336-3. [DOI] [PubMed] [Google Scholar]
- 143.Urban JP, Holm S, Maroudas A, Nachemson A. Nutrition of the intervertebral disk. An in vivo study of solute transport. Clin Orthop Relat Res. 1977;129:101–114. [PubMed] [Google Scholar]
- 144.Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine. 2004;29:2700–2709. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 145.Vernengo J, Fussell GW, Smith NG, Lowman AM. Evaluation of novel injectable hydrogels for nucleus pulposus replacement. J Biomed Mater Res B Appl Biomater. 2007;84(1):64–69. doi: 10.1002/jbm.b.30844. [DOI] [PubMed] [Google Scholar]
- 146.Vernon-Roberts B, Moore RJ, Fraser RD (2007) The natural history of age related disc degeneration. The pathology and sequelae of tears. Spine 32(25):2797-2804 [DOI] [PubMed]
- 147.Vincent T, Hermansson M, Bolton M, Wait R, Saklatvala J. Basic FGF mediates an immediate response of articular cartilage to mechanical injury. Proc Natl Acad Sci USA. 2002;99:8259–8264. doi: 10.1073/pnas.122033199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vincent T, Saklatvala J. Basic fibroblast growth factor: an extracellular mechanotransducer in articular cartilage? Biochem Soc Trans. 2006;34:456–457. doi: 10.1042/BST0340456. [DOI] [PubMed] [Google Scholar]
- 149.Vincent TL, Hermansson MA, Hansen UN, Amis AA, Saklatvala J. Basic fibroblast growth factor mediates transduction of mechanical signals when articular cartilage is loaded. Arthritis Rheum. 2004;50:526–533. doi: 10.1002/art.20047. [DOI] [PubMed] [Google Scholar]
- 150.Vincent TL, McLean CJ, Full LE, Peston D, Saklatvala J. FGF-2 is bound to perlecan in the pericellular matrix of articular cartilage, where it acts as a chondrocyte mechanotransducer. Osteoarthr Cartil. 2007;15:752–763. doi: 10.1016/j.joca.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 151.Wan Y, Feng G, Shen FH, Balian G, Laurencin CT, Li X. Novel biodegradable poly(1, 8-octanediol malate) for annulus fibrosus regeneration. Macromol Biosci. 2007;7:1217–1224. doi: 10.1002/mabi.200700053. [DOI] [PubMed] [Google Scholar]
- 152.Wan Y, Feng G, Shen FH, Laurencin CT, Li X. Biphasic scaffold for annulus fibrosus tissue regeneration. Biomaterials. 2008;29:643–652. doi: 10.1016/j.biomaterials.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 153.Whitelock JM, Ma JL, Davies N, Nielsen N, Chuang C, Rees M, Iozzo RV, Knox S, Lord M. Recombinant heparan sulphate for use in tissue engineering applications. J Chem Technol Biotechnol. 2008;83:496–504. doi: 10.1002/jctb.1835. [DOI] [Google Scholar]
- 154.Wiberg C, Heinegard D, Wenglen C, Timpl R, Morgelin M. Biglycan organizes collagen VI into hexagonal-like networks resembling tissue structures. J Biol Chem. 2002;277:49120–49126. doi: 10.1074/jbc.M206891200. [DOI] [PubMed] [Google Scholar]
- 155.Wiberg C, Klatt AR, Wagener R, Paulsson M, Bateman JF, Heinegard D, et al. Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan. J Biol Chem. 2003;278:37698–37704. doi: 10.1074/jbc.M304638200. [DOI] [PubMed] [Google Scholar]
- 156.Wilda H, Gough JE. In vitro studies of annulus fibrosus disc cell attachment, differentiation and matrix production on PDLLA/45S5 Bioglass composite films. Biomaterials. 2006;27:5220–5229. doi: 10.1016/j.biomaterials.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 157.Wuertz K, Urban JP, Klasen J, Ignatius A, Wilke HJ, Claes L, et al. Influence of extracellular osmolarity and mechanical stimulation on gene expression of intervertebral disc cells. J Orthop Res. 2007;25:1513–1522. doi: 10.1002/jor.20436. [DOI] [PubMed] [Google Scholar]
- 158.Yu J. Elastic tissues of the intervertebral disc. Biochem Soc Trans. 2002;30:848–852. doi: 10.1042/BST0300848. [DOI] [PubMed] [Google Scholar]