Abstract
Structural discontinuity in the spinal cord after injury results in a disruption in the impulse conduction resulting in loss of various bodily functions depending upon the level of injury. This article presents a summary of the scientific research employing electrical stimulation as a means for anatomical or functional recovery for patients suffering from spinal cord injury. Electrical stimulation in the form of functional electrical stimulation (FES) can help facilitate and improve upper/lower limb mobility along with other body functions lost due to injury e.g. respiratory, sexual, bladder or bowel functions by applying a controlled electrical stimulus to generate contractions and functional movement in the paralysed muscles. The available rehabilitative techniques based on FES technology and various Food and Drug Administration, USA approved neuroprosthetic devices that are in use are discussed. The second part of the article summarises the experimental work done in the past 2 decades to study the effects of weakly applied direct current fields in promoting regeneration of neurites towards the cathode and the new emerging technique of oscillating field stimulation which has shown to promote bidirectional regeneration in the injured nerve fibres. The present article is not intended to be an exhaustive review but rather a summary aiming to highlight these two applications of electrical stimulation and the degree of anatomical/functional recovery associated with these in the field of spinal cord injury research.
Keywords: Spinal cord injury, Oscillating field stimulation, Functional electrical stimulation, Axonal regeneration, Advances in spinal cord research
Introduction
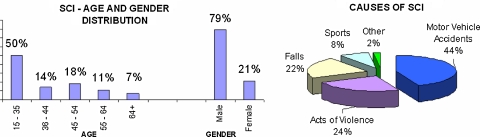
Spinal cord injury (SCI) is one of the most catastrophic injuries of the nervous system resulting in permanent neurological deficits. Typically SCI occurs in young, otherwise healthy adults as a result of trauma. Nearly 50% of SCI occurs in the age bracket of 16–30 years (Fig. 1) [18, 25]. The number of people suffering from SCI is quite significant. There are approximately 11,000 new cases each year in the United States [25]. The number of people living with SCI in June 2006 was approximately 253,000 [25]. The average yearly health care and living expenses are directly proportional to the level of injury and have been estimated to be between USD 1.0 and 2.9 million per person [25]. Each year in Australia, about 300–400 new cases of SCI are added to an estimated prevalent SCI population of about 10,000 cases [18] (Figs. 2, 3).
Fig. 1.
SCI statistics (National Spinal Cord Injury Statistical Centre, University of Alabama at Birmingham)
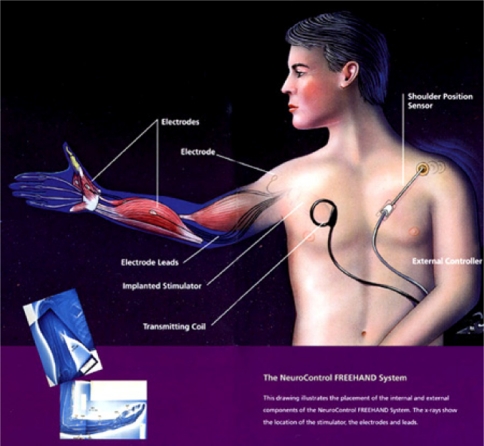
Fig. 2.
Neurocontrol Freehand (Neurocontrol Corporation)
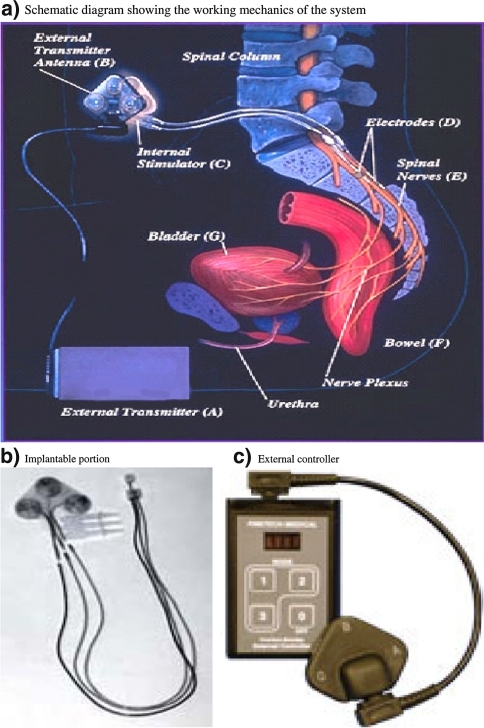
Fig. 3.
Finetech-Brindley Bladdersystem (Finetech Medical) (previously marketed as Vocare bladder system by Neurocontrol Corporation in US). a Schematic diagram showing the working mechanics of the system, b implantable portion, c external controller
A few decades ago, most of the people with SCI usually died in a fairly short period of time due to limited emergency care available at the time of injury. Their life expectancy today is becoming close to that of the normal population [25], yet for the rest of their lives they remain dependent on others for managing their day to day living. Those surviving the initial brunt would suffer from many other associated medical conditions like respiratory infections, urinary tract infections, decubitus ulcers, cardiovascular diseases, etc. Recent advances in emergency and rehabilitative medical care have greatly reduced the premature mortality and morbidity in these patients.
Spinal cord injury is classified as acute or chronic, although there is no clear demarcation as to when an acute injury converts into a chronic one. Generally, the time elapsed between initial hours and a few weeks after the injury is considered as acute phase, whereas months to years after injury is characterised as chronic phase. This clinical classification is necessary as the biology of these two phases is significantly different from each other and different strategies are needed while developing experimental therapies for functional recovery. Hence an in depth understanding of the pathophysiology of SCI is vital for developing new strategies to target the cellular mechanisms involved in the development and perpetuation of acute or chronic injury. SCI can be described as a disconnection syndrome [33] that disrupts the descending motor fibres from the motor cortex to the spinal motoneurons, and the ascending somato-sensory fibres from the spinal cord to the brain. The functional loss seen in SCI is due to interruption of electrical impulse conduction through the lesioned axons. Intrinsic circuits below the level of injury remain intact but disconnected from the descending controls of the cerebral cortex. Anywhere else in the body this physiological conduction blockade is tackled with regrowth, regeneration and sometimes functional re-connectivity of the axons to the designated end-organ resulting in variable levels of functional recovery [26]. In the event of a central nervous system (CNS) injury, regrowth of axons is not possible because CNS has multiple additional factors (at the cellular as well as molecular level) which act as barriers towards regeneration and make the environment hostile to inherent regenerative ability [77, 88].
The initial mechanical injury to the spinal cord results in localised oedema and haemorrhage in the central grey matter along with vasospasm of arteries supplying the spinal cord. This leads to severe ischaemia of the cord triggering a secondary injury from within due to the release of various inflammatory biochemical mediators such as arachidonic acid metabolites, free radicals and many other apoptotic molecules. Further injury occurs due to the disruption of the ionic homeostasis in the cells such as accumulation of Ca++ ions intracellularly and of K+ ions extracellularly [28, 95]. This ionic disruption in concert with ischaemic, inflammatory, haemorrhagic and biochemical reactions induces a secondary axonal dieback and retraction that contributes towards the self-destruction of the spinal cord leading to loss of structural integrity and severe functional loss. At present, there are no known definitive treatment strategies that can alter the pathophysiology and bring a significant change to the condition of patients suffering from this injury.
Although complete recovery of function in an injured spinal cord is still not possible in clinical setting, numerous research efforts are being conducted to promote regeneration and repair the interruption of nerve impulses as seen in SCI. Currently there are three main research avenues under progress (see Table 1). The first approach aims to minimise the extent of initial and secondary injury in the spinal cord and attempts to limit or even reverse the physiological conduction blockade by preserving the surviving viable non-functioning white matter with the help of various pharmacological compounds such as Methylprednisolone [44, 68, 83], Polyethylglycol [14, 22, 80, 81] and 4-aminopyridine [21, 38, 62].
Table 1.
Summary of current research approaches in SCI
| Current research approaches in SCI | |||
|---|---|---|---|
| Neural transplantation | Neural stem cells, foetal neural cells | ||
| Olfactory ensheathing cells | |||
| Peripheral neural bridges, Schwann cells | |||
| Modification of CNS environment | Antibodies to various endogenous inhibitory factors | ||
| Promotion of neurotrophic factors | |||
| Pharmacological interventions | |||
| Electrical stimulation | Functional electrical stimulation (FES) | Therapeutic applications | |
| Functional application | |||
| Axonal regeneration | Direct current stimulation | Uni-directional growth | |
| Oscillating field stimulation | Bi-directional growth | ||
The second approach concentrates on ways to regenerate and reconnect the injured axons within the spinal cord by modifying the various factors responsible for the antagonistic CNS environment and make it more receptive towards regeneration. Various kinds of cellular transplants such as foetal neuronal cells, stem cells, peripheral nerves, Schwann cells and olfactory glial cells have been employed in an effort to bridge the gap in experimental models [52, 54]. Antibodies are currently being designed to neutralise the endogenous inhibitors of nerve regeneration such as Nogo, Oligodendrocyte myelin glycoprotein and other apoptotic factors associated with the glial scar that forms at the site of injury [35, 49, 77, 84]. Other neural repair strategies are aiming to supplement the endogenous neurotrophic factors like brain-derived neurotrophic factor (BDNF) or Neurotrophin-3 (NT-3) etc., to achieve greater sprouting and elongation of damaged axons [51–53, 77]. Another rather new regenerative strategy is application of weak electrical fields around the lesion to induce a regeneration permissive environment at the injury site in the CNS [5]. This will be discussed in detail in the second part of this review. All of these neuro-regenerative strategies are looking quite promising at this stage and some have even entered clinical trials but we do not know to what extent the results will be mirrored in human patients.
The third approach is directed towards regaining the functional recovery, regardless of the anatomical connections within the spinal cord. This involves the use of electrical stimulation through neural prosthetic devices for partially restoring the lost functions. Several Food and Drug Administration (FDA) approved devices are commercially available at present. These are also discussed in detail in this review.
The basic goal of these experimental therapeutic interventions is to re-create a regenerative environment for structural re-connectivity and to bring functional recovery in an injured spinal cord. This paper summarises the applications of electrical stimulation in spinal cord injury for rehabilitation purposes, and for the regeneration of the severed axons and the degree of functional/ anatomical recovery associated with these.
Restoration of function through electrical stimulation
Nearly half a century has been dedicated to the research involving use of electric currents for stimulating the paralysed muscles with intact peripheral motor nerves. Electrical stimulation can overcome the deficit produced by the lesion in the spinal cord and maintain the integrity of various bodily functions through direct neuromuscular stimulation.
Functional electrical stimulation
Functional electrical stimulation (FES) is the technique of applying safe levels of electric current to activate the damaged or disabled neuromuscular system in a coordinated manner in order to achieve the lost function. Neuro-prosthesis is a device that uses electrical stimulation to activate the nervous system. These initiate a physiological-like stimulation in the intact peripheral nerves, providing functional restoration of various body organs in the neurologically impaired individuals.
External neuromuscular excitation has been attempted since the eighteenth century, when Luigi Galvani discovered that external electric current can cause the severed nerves to generate an action potential leading to muscular contractions [30]. However, achieving functionally useful movements remained a challenge till the development of the first functional electrical stimulator (FES) to prevent foot-drop in hemiplegic patients by Liberson et al. in 1961 [50]. Functional electrical stimulator was first defined as the technique used to artificially stimulate the muscles deprived of nervous control by appropriate sequencing bursts of electrical pulses with a view to generate muscular contractions and produce a functionally useful movement. The efforts to develop a suitable human functional stimulator which can achieve synergistic activity of various muscles accelerated in the late 1980s and early 1990s. In 1987, Davis proposed the development of a FES system based on multi-cochlear implant technology to restore function in paraplegic patients [19]. Kralj, Bajd and Turk in 1988 applied FES to SCI patients with lesions between T4 and T12 in an effort to restore standing and walking [46]. Other parallel studies at that time also concluded that FES assisted walking is feasible in patients with incomplete SCI even with severe motor loss [37, 48].
The initial goal of FES technology was to provide greater mobility to the patients after SCI. However, with the advances in biomedical engineering within the last 2 decades, FES is no more limited to locomotion alone. Therefore, the definition of FES has changed considerably and is now considered to be the technique of applying safe levels of electric current to stimulate various organs of the body rendered disabled due to SCI. Examples include assistance with respiration, bowel/bladder activity or some return of upper or lower limb function.
Mechanism of FES operation
Both nerves and muscle fibres respond to electric current. However, for practical purposes FES is used to directly stimulate nerve fibres only, as a much lower amount of current is required to generate an action potential in a nerve than the one required for muscular depolarisation.
The main component of a FES system is the microprocessor-based electronic stimulator which determines when and how the stimulation is provided, with channels for delivery of individual pulses through a set of electrodes connected to the neuromuscular system. The microprocessor contains programs for sitting, standing, walking, hand grasp etc. It serves to generate a train of impulses that grossly imitate the neural triggers that would have normally passed through the spinal cord to the appropriate peripheral nerves below spinal cord lesion for these different programs. These stimuli thus trigger action potentials in the peripheral nerves which inturn activate muscle contractions in the associated muscles fibres [72]. The pulse amplitude (magnitude of current), duration, frequency, waveform (a display of a signal on an oscilloscope that shows the magnitude of current or voltage with respect to time) and duty cycle (the total time to complete one on/off cycle) regulate the stimulation parameters. The numbers of channels, which can range from one to several, govern the sophistication required for complex outputs like FES assisted standing. The programmable microprocessor activates the various channels sequentially or in unison to synchronize the complex output of the stimulator. Electrodes provide the interface between the electrical stimulator and the nervous system. Various types of electrodes have been developed and are available ranging from non-invasive surface electrodes to invasive implantable ones. Implantable electrodes provide more specific and selective stimulation to the desired muscle group than the surface electrodes. The feedback control of the FES system can be either open-looped or closed-looped. Open-looped control is used for simple tasks such as for muscle strengthening alone, and requires a constant electrical output from the stimulator. In a closed-looped system, the parameters for electrical stimulation are constantly modified by a computer via feedback information on muscle force and joint position thus stimulating various muscle groups simultaneously leading to a combination of muscular contraction needed for a complex sophisticated functional activity such as walking [34]. Paraplegic patients using FES for ambulation still require the use of walker or other orthotic devices for stabilising the ankle, knees and hips.
Current scope, applications and limitations of FES
There are clinically two applications of FES devices which have been designed to help SCI patients according to their need. Therapeutic applications include cardiovascular conditioning and the prevention of muscular atrophy through exercise. Functional applications assist with vital body functions lost due to SCI. Examples include ambulation and locomotive support in cases of paraplegia, assistance with respiration or hand grasp in case of quadriplegia, in addition to electro ejaculation, bowel or bladder voiding. Several commercial as well as research based FES devices have been developed in different centres around the world for various therapeutic and functional applications. For commercially available system (see Table 2).
Table 2.
Various available FES systems
| Name of system | Name of manufacturer | FES category | Manufacturing status | Type of system | Implantable /external | FDA approval status |
|---|---|---|---|---|---|---|
| PARASTEP-I | Sigmedics Inc. USA | Functional | Commercial | Ambulation | External | Approved 1994 |
| ERGYS 2 CLINICAL REHABILITATION SYSTEM | Therapeutic Technologies Inc. USA | Therapeutic | Commercial | Exercise/ cardiac conditioning | External | Not approved |
| HANDMASTER NMS I | NESS Co. Israel | Functional | Commercial | Hand grasping Palmer and lateral | External | Approved 2001 |
| NEC FESS MATE | NEC Inc. Japan | Functional | Commercial | Hand grasping Palmer and lateral | Implantable | Not approved |
| BIONIC GLOVE | Neuromotion Inc. Canada | Functional | Experimental | Hand grasping Tenodesis | External | Not approved |
| FINETECH-BRINDLEY SYSTEM (previously VOCARE) | Finetech medical Ltd. UK | Functional | Commercial | Bladder & bowel function | Implantable | Approved 1998 |
| MARKIV BREATHING PACEMAKER SYSTEM | Avery Biomedical devices Inc. USA | Functional | Commercial | Breathing assistance | Implantable | Approved 1998 |
| IDE: ATROSTIM PHRENIC NERVE STIMULATOR V1.0 & V2.0 | Atrotech Ltd. Finland | Functional | Commercial | Breathing assistance | Implantable | IDE not approved |
| MEDIMPLANT | MedImplant Biotechnisches Labor. Austria | Functional | Commercial | Breathing assistance | Implantable | Not approved |
Functional applications
The FES devices were initially designed in an attempt to provide assistance with standing or walking, provided the paraplegic patient had adequate upper body motor control and strength. There are more than 24 centres in the world that are actively assessing the role of FES induced walking or standing and many systems are under development [34]. However, the only FDA approved FES system for short distance ambulation is Parastep I that uses a walker support for balance. [36]. It is a transcutaneous, micro-computerised, electrical stimulation system built into a small unit powered by batteries and is controlled by a finger touch button located on a walker’s handbars for manual selection of stimulation menus. The system provides stimulation output to 12 surface electrodes that are attached to the skin at appropriate placements. These stimulation pulses trigger action potentials in the intact peripheral nerves to generate muscle contraction. It is the only system which is widely available and has been evaluated for its ambulation performance and medical/psychological effects [16, 31, 34, 36]. To be a candidate for FES induced ambulation patients need to be selected after a thorough evaluation conducted by a physician and physical therapist. Factors considered include the presence of neurologically stable and complete SCI, level of injury (preferably between T4 and T12), patient motivation, degree of spasticity, muscle contractile response to electrical stimulation, cardio-respiratory capacity, and musculoskeletal integrity [36]. The use of these FES devices designed to permit or improve ambulation is not simple or without risks. Paraplegic patients require extensive training to build muscle strength in the upper body in order to achieve FES assisted ambulation. The amount of energy spent with FES walking is almost twice than that for normal walking, although the achievable speed is slower than that of normal walking [16, 45]. The risk of injury with FES assisted ambulation is more likely to be higher due to fatigue of the stimulated muscle causing an increase incidence of fall and fractures. These factors limit the true functional utilisation of this system. Another major practical problem associated with the current FES locomotive models is mainly related to feedback control. There is a need for more sensors to measure the muscular force, muscular fatigue, joint position, angular velocity and trunk position, which are to be constantly analysed by the microprocessor. In response to all these sensory inputs, the FES system should be able to redefine the stimulation parameters according to the feedback it receives thus delivering more natural responses, smoother transitions and making ambulation on rough surfaces a possibility as well. In spite of these associated limitations for everyday mobility in daily life, there are potential functional, medical and psychological benefits of FES assisted standing and walking. These devices can help increase their level of independence by providing some assistance with standing while transferring from the wheelchair to a car, climbing a few steps or reaching for a higher object. The medical advantages of short distance ambulation include increased blood flow to lower limbs, increase in lower limb muscle mass, reduced spasticity, lower heart rate at sub peak work intensities and beneficial effects on digestion, bowel and bladder function [34, 36]. Psychological benefits achieved through FES assisted walking such as the associated increase in self esteem and reduction in depression are all well documented [16, 31, 34, 36].
Most of the studies conducted which have evaluated the role of FES assisted walking have a very small sample size and a short follow up time. Thus, the limited amount of data is currently insufficient to demonstrate a viable role for this technology in the management of SCI. FES assisted walking is likely to be accepted clinically as a form of locomotion for SCI patients only if it can be proven to be a more reliable, convenient, safer and faster system than an average wheelchair.
FES devices for quadriplegic patients mainly focuses on restoring the grasp and pinch function. Presently there are quite a few FES devices available for restoration of hand function out of which only two have been approved by FDA: Freehand system and HandMaster [67]. These devices enable the restoration of palmer grasp for holding bigger/heavier objects and lateral grasp for smaller and thinner ones. These can also stimulate the triceps brachii muscle to generate elbow extension for reaching as well.
The Freehand system was the first to be granted the FDA approval. It is a surgically implantable, 8 channel stimulator, with an external control unit that can be programmed to synthesize the movement of muscles through subcutaneously implanted electrodes to provide smooth grasp. Patients control the device through a joystick placed on the shoulder or wrist. Auditory or sensory feedback provide the user with information regarding the system state. Patients can choose between lateral and palmer grasp in order to assist with handling large or small objects. The main disadvantage of the Freehand system is that in case of failed hardware components, additional surgery is required to fix the problem. This is the only system that has been extensively evaluated and several multicentre studies support the Freehand system’s safety and efficacy along with user’s satisfaction [41, 59, 89]. Unfortunately the company previously marketing the Freehand system in United States has recently decided to stop manufacturing new devices but still provides maintenance to previously implanted systems [63].
Another device for restoration of hand function that received FDA approval in 2001 is called HandMaster. At present this is the only FDA approved system that is commercially available. It comprises a hinged shell with three built in surface electrodes to stimulate the finger flexors and extensors and the thenar musculature and has a spiral splint to stabilise the wrist. A separate control unit is used by the user to start the stimulation pattern and choose between different programs for muscle activation. Traditionally it has been used as an exercise tool for stroke patients. A literature search for its role in the SCI population showed that it has only been evaluated in a handful of these patients [3, 86]. The advantages of HandMaster are that it is non-invasive and has ease of application. However; it does not allow the user to fully supinate the arm due to the splint that fixes the wrist joint angle and is less customisable than the Freehand system. Both these devices are designed for patients with C5-C6 injury with adequate motor innervation of the forearm and hand muscles, good passive range of motion, good upper trunk support, intact vision and controlled spasticity. Both these devices––by restoring grasp, hold and release function of the hand––increase the level of independence in the tetraplegic patients. There is a time delay of 1–2 s between the command generation and execution of grasp function that interferes with the speed with which the patient can grasp and release objects. Future designs should focus on increasing the stimulation channels and sensors technology in order to provide increased flexibility in controlling the muscle movement and providing a feedback about the wrist joint movement.
Electrical stimulation of the phrenic nerve in order to achieve effective diaphragmatic contraction is used in high cervical tetraplegics (usually C3 or above) as an alternative to long term mechanical ventilation. Three companies are commercially manufacturing these stimulators, although only Avery’s Mark IV breathing pacemaker system has been successful in achieving FDA approval [34]. This is a surgically implanted phrenic nerve stimulator which delivers electrical impulses to the diaphragm in order to restore breathing function. Small electrodes are sutured to the phrenic nerves either in the neck or by a trans-thoracic approach through the second intercostal space, connected by leads to receivers implanted subcutaneously. Radio signals from an external transmitter and antenna activate the receivers, and the stimulating pulses delivered to the phrenic nerve cause the diaphragm to contract, producing inhalation [34]. Post operative care requires 4–6 weeks of mostly in patient training. Candidates for phrenic nerve pacing must have the phrenic nerve intact, as well as normal lung function and normal chest wall compliance. It is more suitable for C1/C2 injuries than those at C3-C5 injuries where anterior horn cells might have been damaged. Therefore pre-implantation screening includes verification of phrenic nerve function by either a nerve conduction study or visualising diaphragmatic movements under fluoroscopy [34, 74]. To avoid the risk of nerve damage implantation is done at one year post SCI. It has a number of advantages over the traditional ventilator assisted respiration. It improves the patient’s ability to speak, reduces the amount of respiratory secretions, improves the level of comfort in the patient and reduces the level of required nursing care as needed with ventilator dependent respiration [34, 74]. The possible complications include infection, intraoperative mechanical damage to the phrenic nerve, reduction in ventilation and technical malfunctions leading to failure, with a need to retain a tracheostomy stoma. The future work investigates the diaphragmatic stimulation through laparoscopic implantation of intramuscular electrodes in order to avoid the chances of per operative damage to phrenic nerves [23].
Other functional applications of FES which help to restore useful functions and thus improve the quality of life include bladder and bowel voiding and electro-ejaculation. Voluntary control of bowel and bladder function is either lost or considerably impaired depending upon the level and severity of SCI and can lead to multiple complications. The Vocare bladder system (currently available as Finetech-Brindley bladder system) is a surgically implantable sacral anterior root stimulator that allows individuals with complete spinal cord injury to urinate on demand [74]. Secondary use of the device is to aid in bowel evacuation. It was approved by FDA in 1998. It consists of an external controller and transmitter and an implantable receiver-stimulator and electrodes. This system is operated by radio frequency signals transmitted to electrodes placed on the sacral spinal nerves (S2-S4) and leads to bladder/large bowel and urethral/ anal sphincter contraction [34]. At the time of implantation, a posterior rhizotomy through laminectomy at sacral level is performed to abolish the uninhibited reflex bladder contractions. This eliminates the reflex incontinence caused by the activation of the sensory reflex pathway [24]. However it also causes a loss of perineal sensations and reflex erection and ejaculation if present. Patient selection criteria for Vocare implantation include neurologically stable and clinically complete supra-sacral SCI and intact parasympathetic innervation to detrusor musculature. The major disadvantage of this system is the need for major surgery for implantation and posterior rhizotomy. However, this device offers an improved quality of life, social ease, as well as a reduction and prevention of urinary tract infections and their associated complications [15, 17, 34]. Another added benefit of this system is enhanced bowel evacuation with most patients reporting a reduction in the time required for bowel evacuation along with a reduction in constipation and faecal impaction [17]. A slower stimulation time sequence is required for defeacation than for micturation. Approximately 60% of men can also produce penile erection using this device [15]. Electroejaculation is one of the several techniques now available to harvest viable sperm for the purposes of artificial insemination or in vitro fertilization [34]. An electric probe is inserted into the rectum near the prostate to stimulate the nerves and contract the pelvis muscles, causing ejaculation [39]. The ejaculate is collected from the urethra and prepared for use in artificial insemination. Caution need to be taken in men with SCI who have a history of autonomic dysreflexia as electroejaculation can cause a significant increase in blood pressure and heart rate. This system was also previously manufactured and supplied by a US based company which is no longer functioning [63] however it is still available in Europe through a new manufacturer (see Table 2).
Therapeutic applications
In SCI the problem is not confined to spine alone rather the whole body is affected. Depending upon the level of injury, unused paralysed muscles undergo disuse atrophy with reduced peripheral circulation and demineralisation in the bones leading to osteoporosis. The paralysis forces the patients towards a more sedentary life style. The autonomic nervous system impairment coupled with the lack of exercise and mobility leads to various cardiovascular problems thus increasing the morbidity in SCI sufferers. A significant rehabilitative application of FES is in improving muscle bulk and strength and prevention of denervation muscular atrophy. It also facilitates cardiovascular conditioning and improvement in peripheral circulation through the use of stationary exercise devices. The most common system for lower extremity FES exercise is the bicycle ergometer. Two commercial systems which are approved under Class II devices are currently available: StimMaster and ERGYS 2 clinical rehabilitation system, which is a FDA approved, commercially available ergometer. These are computer controlled closed-looped systems that bilaterally activate the gluteals, quadriceps and hamstrings through surface electrodes in a sequential and alternating pattern. Both these systems are fully portable thus allowing the user to use these at home as well. Patients with partially preserved sensations in their lower limbs are usually unable to tolerate stimulation since the intensity of electric current applied is quite high [74].
The neurological benefits of exercise after SCI have been studied in animal models. Gazula et al. [32] demonstrated the effects of lower limb exercise on spinal motor neurons in animal models. They compared the effect of lower limb exercise in three groups of animals; a group with intact spinal cords, animals with complete spinal cord transection (SCT) and a third group of animals with complete spinal cord transaction who underwent a daily exercise program. They were able to show that the group with daily exercise had none of the atrophic/ regressive changes in the motor neurons that the group with SCT exhibited.
The clinical benefits that can be achieved through the exercises conducted via the FES systems include cardiac conditioning when training for at least 30 min a day for 3–5 days/week [69, 74]. However in individuals with injury above T5, the increase in heart rate, stroke volume and cardiac output is limited due to the loss of supra-spinal sympathetic control [70]. Other benefits include improvement of venous return from lower limbs thus reducing the incidence of deep venous thrombosis; increase in muscle bulk, strength and endurance and an improvement in bowel functions [34, 74]. The ability to regulate the body functions through FES also leads to additional psychological benefits [34, 74]. However, there is no conclusive data on whether these FES induced exercises can retard osteoporosis, increase insulin resistance and improve carbohydrate metabolism as well or not.
All of the above mentioned FES strategies for various SCI related complications have had a varied uptake into clinical trials. It is largely to a variety of factors such as availability and affordability of these devices, regional ethical requirements, and technical difficulties in transforming a theoretical model into a viable clinical tool. The other issues that need to be addressed are the inadequate system reliability, inappropriate cosmetics and portability of the system. These issues can be resolved by changes in the design specifications to miniaturise the system, a better manufacturing processes and a stricter safety and reliability analysis so that the freedom of mobility and other bodily functions is not restricted to the laboratory environment.
Future scope
Although the current development and advances in the design of FES system have made it possible to provide some mobility and functions to the SCI patients, at present, FES alone has multiple inherent limitations. To restore the lost function safely, completely and efficaciously, further research is needed. There are multiple challenges that need to be met before it can be utilised by the SCI population on a regular basis.
Many centres around the world are engaged in further development of FES prototypes with lesser limitations and more control. FES promises a new era in rehabilitation and offers great hope for patients who are wheelchair bound or suffering from ambulatory difficulties as a result of a CNS dysfunction. However, ultimate freedom of motion and function is not likely to be seen in the near future. Experimental models of FES systems with implantable electrical stimulators and portable microprocessors are under development [20, 85]. Another emerging paradigm is intraspinal micro-stimulation (ISMS), where the spinal-cord-locomotor-circuits called Central Pattern Generators (CPG) are directly tapped for stimulation and restoration of limb movements [75]. Future work on these neural prosthetic devices is also focusing on decoding the intended motion trajectories from the cerebral motor cortex and using this signal to control the FES devices. Hybrid Neuro-prosthetics are being investigated and may lead to the development of a cognitive link through cerebral motor cortex to these neural prosthetic devices [61, 65]. Recently, Muller-Putz [60] reported a case study where an implanted FES device Freehand system was connected with an electroencephalogram (EEG) based-Brain-Computer Interface (BCI). The patient was given a short training course in order to be able to generate distinct EEG patterns by imagining the movement of his paralysed hand. The patterns were further classified by the BCI and output signals generated by BCI emulated those of the shoulder joystick for different grasp phases that the Freehand system provides. With consecutive imaginations, the patient was able to move a simple object from one place to another. These and other further improvements in the FES technology would help augment its role as a valuable therapeutic and rehabilitative technique.
Axonal regeneration through electrical stimulation
Contrary to the long held clinical belief that adult CNS, once injured, does not posses any regenerative ability, advances in neuroscience have shown that a mature CNS has a limited ability to regenerate after injury. If the damaged spinal cord has got the potential to grow in an effort to bridge the gap, then why is it unable to do so? Spanish neurologist Ramón Y Cajal concluded some 100 years ago that the failure of CNS regeneration was in fact due to the absence of certain growth promoting factors in the CNS environment, rather than an intrinsic neuronal deficit for regeneration [71]. Therefore, it is necessary to define ways to facilitate regeneration that spontaneously begins after injury to the CNS, but stops prematurely due to its non-permissive environment. It is now well understood that neurotrophic (growth stimulating) and neurotropic (growth guiding) factors are essential for any degree of neuronal regrowth. The majority of work on finding a cure for SCI is focussed on developing ways to induce and support axonal sprouting and elongation in the desired direction.
Axonal regeneration is the ability to extend the tip of the proximal portion of an amputated/ severed axon. This tip of the growing axon, called the growth cone, has got the ability to sense cues from the environment and steer the axon to grow in one direction or another. For successful regeneration to happen, a series of biological events is required. Most importantly, the neurons with lesioned axons must survive the initial injury. Second, the lesioned axons must be able to sprout and grow towards the target area. Finally, the axons must form functional synaptic connections with their target areas in order to conduct the axoplasmic flow. Several research studies conducted on comparative SCI regeneration in other species, have established that a variable degree of functional recovery is possible even without the accurate point to point connectivity of the axonal synapses [73, 93, 94]. Anatomically inappropriate synaptic connections can still serve to provide some behavioural recovery after spinal cord trauma.
Today, many researchers in different countries are striving to regenerate and repair the damage produced by the spinal cord after injury and the pace of their research continues to accelerate. Recent progress in cellular and molecular biology and transplantation techniques has made it possible to induce axonal growth. This has involved manipulating the intrinsic processes that limit the CNS regeneration via neutralisation of growth inhibitory factors and promotion of endogenous neurotrophic factors. Another concept in neural regeneration is of axonal guidance or neurotropic factors which help the regenerating axons to grow in the desired direction. Regenerative studies done in the peripheral nervous system (PNS) have demonstrated that for successful regeneration the growing axons need a guidance channel to follow so that growth can happen in the appropriate direction [90, 94]. In case of the PNS, this guidance is provided by the Schwann cells which are missing in the CNS. In the absence of such guidance, the axonal growth in the CNS results in a haphazard sprouting. Various researchers studying the effects of weakly applied electric fields on the innately regenerating axons have suggested that exogenously applied weak electric fields around the lesioned axons have a role to play in facilitating axonal regeneration, possibly by providing neurotropic guidance to the growing axons [6, 56, 57, 64].
Applied electrical fields for axonal regeneration
Steady, polarized extracellular voltage gradients are a normal environmental component in the early developing nervous system and are required for cranial-to-caudal development [4, 42, 82]. Interfering with these endogenous electrical fields interferes with the morphogenic fields in the embryo, thus certifying their role in normal development [42]. These currents have also been found during normal wound healing when tissue regeneration is required [8], suggesting that endogenous currents play some causal role in morphogenesis.
Invitro experiments conducted by Lionel Jaffe and Moo-ming Poo established the relationship between extracellular applied electric fields and the neurite regeneration [46]. It was demonstrated that the single nerve processes react within minutes of exposure to an applied DC electric field. The growing nerve fibres respond immediately to the voltage gradient and tend to orient themselves parallel with the long axis of this gradient. Another important phenomenon observed was the directional response of the growing nerve fibres within the imposed electrical field. The position of the electrodes determines the direction of neurite growth. Neurites tend to grow three times faster towards the cathode electrode in DC field between 70 and 140 mV/mm. However, after a lag period of 30 min following the exposure to voltage gradient, the neurites facing the anode start to regress as if being repelled by the anodal electrode. Further clarification on the response of applied Direct Current (DC) voltages on single nerve fibres was shown by Hinkle et al. [40] and Patel & Poo [64]. They observed that the neurites that were parallel to the field grow towards the cathode, while the ones that were perpendicular to the electric field turned in order to grow towards the cathode. The cathode-directed reorientation was dependent on the strength of the electric field, and the threshold level for this directional growth was 7 mV/mm.
Borgens et al. [7] studied axonal regeneration with applied DC fields in living animals. He applied a weak voltage gradient to the severed spinal cord of sea lamprey larvae. The applied weak DC field was able to enhance the rate of regeneration in the severed axons towards the implanted cathode. These regenerated axons were also able to make functional synaptic connections with the caudal end of the injured spinal cord. The placement of cathode rostral or caudal to the site of an SCI determined the direction of regeneration. Proliferation and faster growth rate was seen towards the cathode (negative pole). In experiments where the cathode electrode was placed rostral to the injury functionally only sensory recovery (ascending fibres) was established [9, 11]. In other experiments, Borgens et al. created specific lesions in the spinal cords of guinea pigs, which permanently eliminated a specific spinal reflex in the dorsal column. Application of steady voltage gradients induced both anatomical and functional recovery of the reflex in a significant proportion of animals [10–12]. Wallace, Tator and Piper studied the effects of DC field polarity in axons after severe compressive injuries in rats. This type of injury closely resembles the injury sustained by humans after spinal cord trauma. Stimulating electrodes were positioned with anodes proximal and cathodes distal to the injury site and the response of axons to the weak DC electric fields was studied for a period of 15 weeks [92]. These electrodes were attached to a subcutaneously implanted stimulator. Results of these experimental studies also indicated that electrical stimulation can lead to significant functional recovery as compared to the control animals. However, they were unable to elicit any histological difference in the Schwann cell or ependymal cells proliferation and in the new myelin formation in the spinal cords of the two groups. The functional recovery seen can be explained by the development of alternate synaptic pathways which could be picked up by the anterograde and retrograde tracer techniques in the studied histological region of spinal cord. Fehlings et al. [27, 29] conducted similar in vivo experiments in animal models of SCI to study the effect of applied DC fields on regeneration of axons and associated functional recovery. In all of these experiments significant functional recovery was observed in the cathode caudal groups. They were also able to demonstrate that motor evoked potentials, counts of neurons retrogradely labelled by horse radish peroxidise (HRP) and the axons count at the injury site were greater in the group treated with DC field with cathode caudal to injury as compared to the sham and cathode rostral groups.
In another set of experiments Wallace studied the effect of alternating current (AC) with square pulse wave. After 15 weeks of continuous spinal cord stimulation, no significant difference in functional recovery was observed among the experimental group and the control group [91].
In most of these studies conducted to evaluate the effects of electrical stimulation on nerve growth, electrical fields were applied immediately after SCI. Politis and Zanakis [66] investigated whether similar recovery could be established after a delay in the initiation of treatment. They contused spinal cords of rats and allowed untreated recovery for 10 days. DC fields were then applied to the dorsal region of the cord. The results indicated that applied electric fields facilitate behavioural recovery and regeneration even after a delay in the treatment.
Recently, Shen et al. [79] showed that if the concurrently occurring spinal oedema is reduced via the use of large dose methylprednisolone, the efficacy of DC field improves and results in earlier recovery of nerve function.
In summary, various experiments conducted in the last 2 decades revealed that a weakly imposed electrical field affects orientation and regeneration of axons invitro and invivo, and also leads to functional recovery after spinal cord injury. Applied DC electrical fields give directional cues to the regenerating axons. The position of the cathode electrode either rostral or caudal to the injury site facilitates regeneration in one direction only. After a lag period regression is seen in nerve fibres facing the anodal electrode. Symmetric AC fields do not provide directional cues unless it is converted into an asymmetric AC field. The rate of growth of axons in an applied electric field is dependent on the magnitude of the imposed electric field [55].
Mechanism for electrical stimulation
The exact mechanisms responsible for enhancing axonal regeneration in response to applied voltage gradients are not yet fully known. The axonal growth seen with the application of electrical field has been proposed to be mediated via membrane bound receptors and some secondary messengers like adenyl cyclase and interaction with other physiological neurotrophins present in the CNS [56, 57]. The cathode is believed to lead to a reduction of the cyto-destructive effects of the endogenous calcium current of injury into damaged axons, which in turn brings marked reduction in the degeneration of the axonal growth tip facing the cathode [55, 56, 87]. Other possible mechanisms include reduction in the number of astrocytes within the injury site [55, 58] and changes to post-traumatic spinal cord blood flow [92]. Electrical stimulation has also shown to enhance the expression of regeneration-associated genes (RAGs) and accelerate regeneration in peripheral nervous system [1, 2]. There are no studies to comment whether such an effect exists in CNS regeneration as well.
Recent advances and future scope in electrical stimulation
The observation that neurites grow faster towards the cathode and are repelled by the anode after the imposition of a DC electric field has led to the development of a special stimulatory technique referred to as oscillating field stimulation (OFS). This implantable device reverses the polarity of the applied field being exposed to the injured axons every 15 min. Thus facilitates the cathodal growth before the anodal regression sets in, and leads to a bidirectional axonal growth. Two randomised double-blinded clinical trials were conducted to evaluate the efficacy and biocompatibility of the implantable OFS in canines with naturally occurring SCI [12, 13]. The OFS was implanted for a period of 14 weeks. The dogs were evaluated for behavioural recovery at 1 week, 6 week and 6 month after injury. Significantly greater neurological improvement was observed in the OFS treated group. The dogs regained the ability to walk although not perfectly. The ascending and descending axons projected to the plane of transection, whereas degeneration of ascending and descending axons was seen in sham-treated control animals. The device also proved to be safe. The FDA approval for the ‘human use OFS device’ was attained in late 2000 and the first Phase I human clinical trial began in 2001. In this trial, OFS was surgically implanted in 10 patients with acute neurologically complete SCI between C5 and T-10 [78]. Patients also underwent intravenous methylprednisolone therapy according to the NASCIS III protocol prior to the entry into the trial. OFS was implanted within 18 days of sustaining the SCI and left in place for 15 weeks. At the end of this period it was explanted and studied for bio-compatibility. Patients were observed for functional recovery at 6 weeks, 6 and 12 months. The net gain in neurological improvement was assessed. Significant neurological improvement from baseline in motor and sensory function was observed in all the patients except for one patient who was lost to follow-up. The outcome measures included behavioural assessment of recovery and electrophysiological studies. All patients reported improvement in proprioceptive and exteroceptive sensations. The application of the device was safe and well tolerated by all the patients. Based on the positive data from this phase I trial, FDA has given approval for ten additional patients to be enrolled in the study [78].
Research on the application of electrical stimulation in an effort to enhance axonal regeneration has existed for more than 2 decades, but significantly increased therapeutic ratios have not been demonstrated. Borgens’ work represents a new success in electrical stimulation technology. Oscillating field stimulation seems to be safe and effective in enhancing neurological outcome after SCI. However, OFS is an invasive intervention and multiple risks (surgical technique, wound infection, breakage of wires, battery life, and control device) are associated with this kind of experimental therapy. Second, in the first phase I trial, all ten patients also underwent the methylprednisolone therapy and decompression procedure before the implantation of the OFS device. Thus the true nature and extent of neurological recovery directly associated with the OFS alone cannot be assessed. The full therapeutic potential of OFS technology can only be discussed after the OFS technique goes to test by a multicentre randomised double-blind controlled clinical trial.
Future studies need to explore the effects of various neuro-regenerative approaches when combined with electrical stimulation (for example NT3, Nogo, BDNF or OEC transplantation etc.), in conjunction with a DC field application. The applied DC field would in these cases provide the essentially needed directional cue to the already sprouting axons.
Conclusion
The long standing belief that the central nervous system––once injured––could never be repaired, still lingers on to some extent in the clinical world. However, recent advances in experimental regenerative research have created optimism that the spinal cord is not a rigid and unrepairable structure. Researchers in this field believe that it is merely a matter of time before a therapy is developed to change the quality of life of individuals suffering from this devastating condition. However there are a certain standard criteria to be satisfied before any success for real axonal regeneration can be claimed (see Table 3).
Table 3.
Criteria for evaluating spinal cord regeneration experiments (National Institute of Neurological and Communicative Disorders and Stroke Guidelines)
| Key criteria to assess axonal regeneration after experimental SCI | |
|---|---|
| 1. | The experimental lesion must cause disconnection of nerve processes |
| 2. | Processes of CNS neurons must bridge the level of injury |
| 3. | The regenerated fibres must make Synaptic contacts |
| 4. | The regenerated fibres must generate post-synaptic responses |
| 5. | Functional responses must derive from regenerated connections |
Studies on the pathophysiology of SCI have identified multiple factors believed to be involved in the generation of the final outcome of the injury and the associated neurological deficit. All the factors that lead to secondary injury e.g. blood flow changes at the injury site, excito-toxicity, inflammation and the free radical injury, need to be dealt with before any potential for recovery or curative treatment is seen. Neural regeneration after SCI is likely to become a clinical reality and a major contributor towards recovery and re-connectivity of the damaged site. The question remains however, as to the level of functional recovery that can be attained with the neural re-connectivity. Assistive neuroprosthetic devices will therefore continue to play an important role in rehabilitation. FES is more likely to provide functional recovery for people with longstanding SCI, whereas the research on axonal regeneration with applied electric field is expected to benefit those with acute SCI.
A combination of interventions would be significantly more effective than either alone, suggesting a synergistic action between biological, pharmacological and electrical stimulation. SCI research represents an exciting new evolving field, with more promising results demonstrated in the last few years than in the past 2 decades. Overall, insights arising from the result of all these experimental strategies will eventually lead to better therapeutic interventions that can lessen the functional disability and enhance the quality of life in patients with SCI.
References
- 1.Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci. 2000;12(12):4381–4390. doi: 10.1046/j.1460-9568.2000.01341.x. [DOI] [PubMed] [Google Scholar]
- 2.Al-Majed AA, Tam SL, Gordon T. Electrical stimulation accelerates and enhances expression of regeneration-associated genes in regenerating rat femoral motoneurons. Cell Mol Neurobiol. 2004;24(3):379–402. doi: 10.1023/B:CEMN.0000022770.66463.f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alon G, McBride K. Persons with C5 or C6 tetraplegia achieve selected functional gains using a neuroprosthesis. Arch Phys Med Rehabil. 2003;84(1):119–124. doi: 10.1053/apmr.2003.50073. [DOI] [PubMed] [Google Scholar]
- 4.Borgens RB. What is the role of naturally produced electric current in vertebrate regeneration and healing? Int Rev Cytol. 1982;76:245–298. doi: 10.1016/S0074-7696(08)61793-3. [DOI] [PubMed] [Google Scholar]
- 5.Borgens RB (2003) Restoring function to the injured human spinal cord. Adv Anat Embryol Cell Biol 171:III-IV, 1–155 [PubMed]
- 6.Borgens RB, Bohnert DM. The responses of mammalian spinal axons to an applied DC voltage gradient. Exp Neurol. 1997;145:376–389. doi: 10.1006/exnr.1997.6499. [DOI] [PubMed] [Google Scholar]
- 7.Borgens RB, Roederer E, Cohen MJ. Enhanced spinal cord regeneration in lamprey by applied electric fields. Science. 1981;213:611–617. doi: 10.1126/science.7256258. [DOI] [PubMed] [Google Scholar]
- 8.Borgens RB, McGinnis Vanable JW, et al. Stump currents in regenerating salamanders and newts. J Exp Zool. 1984;231(2):249–256. doi: 10.1002/jez.1402310209. [DOI] [PubMed] [Google Scholar]
- 9.Borgens RB, Blight AR, Murphy DJ. Transected dorsal column axons within the guinea pig spinal cord regenerate in the presence of an applied electric field. J Comp Neurol. 1986;250(2):168–180. doi: 10.1002/cne.902500204. [DOI] [PubMed] [Google Scholar]
- 10.Borgens RB, Blight AR, McGinnis ME. Behavioural recovery induced by applied electric fields after spinal cord hemisection in guinea pig. Science. 1987;238(4825):366–369. doi: 10.1126/science.3659920. [DOI] [PubMed] [Google Scholar]
- 11.Borgens RB, Blight AR, McGinnis ME. Functional recovery after spinal cord hemisection in guinea pigs: the effects of applied electric fields. J Comp Neurol. 1990;296(4):634–653. doi: 10.1002/cne.902960409. [DOI] [PubMed] [Google Scholar]
- 12.Borgens RB, Toombs JP, Blight AR, et al. Effects of applied electric fields on clinical cases of complete paraplegia in dogs. J Restor Neurol Neurosci. 1993;5:305–322. doi: 10.3233/RNN-1993-55601. [DOI] [PubMed] [Google Scholar]
- 13.Borgens RB, Toombs JP, Bauer G, et al. An imposed oscillating electrical field improves the recovery of function in neurologically complete paraplegic dogs. J Neurotrauma. 1999;16:639–657. doi: 10.1089/neu.1999.16.639. [DOI] [PubMed] [Google Scholar]
- 14.Borgens RB, Shi R, Bohnert D. Behavioural recovery from spinal cord injury following delayed application of polyethylene glycol. J Exp Biol. 2002;205:1–12. doi: 10.1242/jeb.205.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Brindley G. The first 500 patients with sacral anterior root stimulator implants:General description. Paraplegia. 1994;32:795–805. doi: 10.1038/sc.1994.126. [DOI] [PubMed] [Google Scholar]
- 16.Brissot R, Gallien P, Le Bot MP, et al. Clinical experience wit functional electrical stimulation-assisted gait with parastep in spinal cord injured patients. Spine. 2000;25(4):501–508. doi: 10.1097/00007632-200002150-00018. [DOI] [PubMed] [Google Scholar]
- 17.Creasey GH, Grill JH, Hoi SU, et al. An Implantable neuroprosthesis for restoring bladder and bowel control to patients with spinal cord injuries: a multicenter trial. Arch Phys Med Rehabil. 2001;82:1512–1519. doi: 10.1053/apmr.2001.25911. [DOI] [PubMed] [Google Scholar]
- 18.Cripps RA (2004) Spinal cord injury, Australia, 2002–03. Injury research and statistics series no 22. AIHW (AIHW Cat no INJCAT64), Adelaide
- 19.Davis R, Eckhouse J, Patrick J, et al. Computerised 22 channel stimulator for limb movement. Appl Neurophysiol. 1987;50:444–448. doi: 10.1159/000100760. [DOI] [PubMed] [Google Scholar]
- 20.Davis R, Patrick J, Barriskill A. Development of functional electrical stimulators utilizing cochlear implant technology. Med Eng Phys. 2001;23:61–68. doi: 10.1016/S1350-4533(01)00023-6. [DOI] [PubMed] [Google Scholar]
- 21.DeForge D, Nymark J, Lemaire E, et al. Effects of 4-aminopyridine on gait in ambulatory spinal cord injuries: a double-blind, placebo-controlled, crossover trial. Spinal Cord. 2004;42(12):674–685. doi: 10.1038/sj.sc.3101653. [DOI] [PubMed] [Google Scholar]
- 22.Donaldson J, Shi R, Borgens R. Polyethylene glycol rapidly restores physiological functions in damaged sciatic nerves of guinea pigs. Neurosurgery. 2002;50:147–157. doi: 10.1097/00006123-200201000-00023. [DOI] [PubMed] [Google Scholar]
- 23.DiMarco AF, Onders RP, Ignagni A, et al. Phrenic nerve pacing via intramuscular diaphragm electrodes in tetraplegic subjects. Chest. 2005;127(2):671–678. doi: 10.1378/chest.127.2.671. [DOI] [PubMed] [Google Scholar]
- 24.Egon G, Barat M, Colombel P, et al. Implantation of anterior sacral root stimulators combined with posterior sacral rhizotomy in spinal injury patients. World J Urol. 1998;16:342–349. doi: 10.1007/s003450050078. [DOI] [PubMed] [Google Scholar]
- 25.Facts and figures at a glance (2006) National Spinal Cord Injury Statistical Centre, University of Alabama at Birmingham web site. Available at http://images.main.uab.edu/spinalcord/pdffiles/Facts06.pdf. Accessed 18 Oct 2006
- 26.Fawcett JW, Keynes RJ. Peripheral nerve regeneration. Annu Rev Neurosci. 1990;13:43–60. doi: 10.1146/annurev.neuro.13.1.43. [DOI] [PubMed] [Google Scholar]
- 27.Fehlings MG, Tator CH. The effect of direct current field polarity on recovery after acute experimental spinal cord injury. Brain Res. 1992;579:32–42. doi: 10.1016/0006-8993(92)90738-U. [DOI] [PubMed] [Google Scholar]
- 28.Fehlings MG, Sekhon LHS. Cellular, ionic and biomolecular mechanisms of the injury process. In: Tator CH, Benzel EC, editors. Contemporary management of spinal cord injury: from impact to rehabilitation. New York: American Association of Neurological Surgeons; 2000. pp. 33–50. [Google Scholar]
- 29.Fehlings MG, Tator CH, Linden RD. The effect of an applied direct current field on recovery from acute experimental spinal cord injury. J Neurosurg. 1988;68:781–792. doi: 10.3171/jns.1988.68.5.0781. [DOI] [PubMed] [Google Scholar]
- 30.Galvani L. Commentary on the effect of electricity on muscular motion translated by Green RM. Cambridge: Elizabeth Litcht Publishing co 1953; 1791. [Google Scholar]
- 31.Gallien P, Brissot R, Eyssette M, et al. Restoration of gait by functional electrical stimulation for spinal cord injured patients. Paraplegia. 1995;33(11):660–664. doi: 10.1038/sc.1995.138. [DOI] [PubMed] [Google Scholar]
- 32.Gazula VR, Roberts M, Luzzio C, et al. Effects of limb exercise after spinal cord injury on motor neuron dendrite structure. J Comp Neurol. 2004;476(2):130–145. doi: 10.1002/cne.20204. [DOI] [PubMed] [Google Scholar]
- 33.Geschwind N. Disconnexion syndrome in animals and man. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- 34.Gorman PH. An update on functional electrical stimulation after spinal cord injury. Neurorehabil Neural Repair. 2000;14:251–263. doi: 10.1177/154596830001400402. [DOI] [PubMed] [Google Scholar]
- 35.Grados- Munro EM, Fournier AE. Myelin-associated inhibitors of axon regeneration. J Neurosci Res. 2003;74:479–485. doi: 10.1002/jnr.10803. [DOI] [PubMed] [Google Scholar]
- 36.Graupe D. An overview of the state of the art of noninvasive FES for independent ambulation by thoracic level paraplegics. Neurol Res. 2002;24(5):431–442. doi: 10.1179/016164102101200302. [DOI] [PubMed] [Google Scholar]
- 37.Graupe D, Kohn KH. Functional neuromuscular stimulator for short distance ambulation by certain thoracic level spinal cord injured paraplegics. Surg Neurol. 1998;50(3):202–207. doi: 10.1016/S0090-3019(98)00074-3. [DOI] [PubMed] [Google Scholar]
- 38.Grijalva I, Guizar-Sahagun G, Castaneda-Hernandez G, et al. Efficacy and safety of 4-aminopyridine in patients with long term spinal cord injury: a randomized, double-blind, placebo-controlled trial. Pharmacotherapy. 2003;23(7):823–834. doi: 10.1592/phco.23.7.823.32731. [DOI] [PubMed] [Google Scholar]
- 39.Heruti RJ, Katz H, Menashe Y, et al. Treatment of male infertility due to spinal cord injury using rectal probe electroejaculation: the Israeli experience. Spinal Cord. 2001;39(3):168–175. doi: 10.1038/sj.sc.3101120. [DOI] [PubMed] [Google Scholar]
- 40.Hinkle L, McCaig CD, Robinson KR. The direction of growth of differentiating neurons and myeloblasts from frog embryos in an applied electric field. J Physiol. 1981;314:121–135. doi: 10.1113/jphysiol.1981.sp013695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hobby J, Taylor PN, Esnouf J. restoration of tetraplegics hand function by the use of the neurocontrol freehand system. J Hand Surg Br. 2001;26(5):459–464. doi: 10.1054/jhsb.2001.0587. [DOI] [PubMed] [Google Scholar]
- 42.Hotary KB, Robinson KR. Endogenous electric currents and the resultant voltage gradients in the chick embryo. Dev Biol. 1990;140(1):149–160. doi: 10.1016/0012-1606(90)90062-N. [DOI] [PubMed] [Google Scholar]
- 43.Hotary KB, Robinson KR. Endogenous electric currents and voltage gradients in xenopus embryos and the consequences of their disruption. Dev Biol. 1994;166(2):789–800. doi: 10.1006/dbio.1994.1357. [DOI] [PubMed] [Google Scholar]
- 44.Hulbert RJ. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J Neurosurg (Spine 1) 2000;93:1–7. doi: 10.3171/spi.2000.93.1.0001. [DOI] [PubMed] [Google Scholar]
- 45.Jacobs PL, Johnson B, Mahoney ET. Physiologic responses to electrically assisted and frame-supported standing in persons with paraplegia. J Spinal Cord Med. 2003;26(4):384–389. doi: 10.1080/10790268.2003.11753710. [DOI] [PubMed] [Google Scholar]
- 46.Jaffe LF, Poo M-M. Neurites grow faster towards the cathode than the anode in a steady field. J Exp Zool. 1979;209:115–127. doi: 10.1002/jez.1402090114. [DOI] [PubMed] [Google Scholar]
- 47.Kralj AR, Bajd T. Functional electrical stimulation: standing and walking after spinal cord injury. Florida: CRC Press; 1989. [Google Scholar]
- 48.Kralj A, Bajd T, Turk R. Use of functional electrical stimulation in the rehabilitation of patients with incomplete spinal injury. J Biomed Eng. 1989;11(2):96–102. doi: 10.1016/0141-5425(89)90115-5. [DOI] [PubMed] [Google Scholar]
- 49.Kwon BK, Borisoff JF, Tetzlaff W. Molecular targets for therapeutic intervention after spinal cord injury. Mol Interv. 2002;2:244–258. doi: 10.1124/mi.2.4.244. [DOI] [PubMed] [Google Scholar]
- 50.Liberson WT, Holmquest HJ, Scott D, et al. Functional electrotherapy: stimulation of the peroneal nerve synchronized with the swing phase of the gait of hemiplegic patients. Arch Phys Med Rehabil. 1961;42:101. [PubMed] [Google Scholar]
- 51.Liu Y, Himes BT, Murray M, et al. Grafts of BDNF-producing fibroblasts rescue axotomised rubrospinal neurons and prevent their atrophy. Exp Neurol. 2002;178:150–164. doi: 10.1006/exnr.2002.7977. [DOI] [PubMed] [Google Scholar]
- 52.Lu J, Waite P. Advances in spinal cord regeneration. Spine. 1999;24(9):926–930. doi: 10.1097/00007632-199905010-00019. [DOI] [PubMed] [Google Scholar]
- 53.Lu P, Yang H, Jones LL, et al. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J Neurosci. 2004;24:6402–6409. doi: 10.1523/JNEUROSCI.1492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mackay-Sim A. Olfactory ensheathing cells and spinal cord repair. Keio J Med. 2005;54(1):8–14. doi: 10.2302/kjm.54.8. [DOI] [PubMed] [Google Scholar]
- 55.McCaig CD. Dynamic aspects of amphibian neurite growth and the effects of an applied electric field. J Physiol. 1986;375:55–69. doi: 10.1113/jphysiol.1986.sp016105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCaig CD, Erskine L. Nerve growth and nerve guidance in a physiological electrical field. In: McCaig CD, editor. Nerve growth and guidance. London: Portland Press Ltd; 1996. pp. 151–170. [Google Scholar]
- 57.McCaig CD, Sangster L, Stewart R. Neurotrophins enhance electric field-directed growth cone guidance and directed nerve branching. Dev Dyn. 2000;217:299–308. doi: 10.1002/(SICI)1097-0177(200003)217:3<299::AID-DVDY8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 58.Moriarty LJ, Borgens RB. An oscillating extracellular voltage gradient reduces the density and influences the orientation of astrocytes in injured mammalian spinal cord. J Neurocytol. 2001;30(1):45–57. doi: 10.1023/A:1011917424450. [DOI] [PubMed] [Google Scholar]
- 59.Mulcahey MJ, Betz RR, Kozin SH, et al. Implantation of the Freehand system during initial rehabilitation using minimally invasive technique. Spinal Cord. 2004;42(3):146–155. doi: 10.1038/sj.sc.3101573. [DOI] [PubMed] [Google Scholar]
- 60.Muller-Putz GR, SchererR Pfurtscheller G, et al. EEG based neuroprosthesis control: a step towards clinical practice. Neurosci Lett. 2005;381:169–174. doi: 10.1016/j.neulet.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 61.Musallam S, Corneil BD, Scherberger H, et al. Cognitive control signals for neural prosthetics. Science. 2004;305:258–262. doi: 10.1126/science.1097938. [DOI] [PubMed] [Google Scholar]
- 62.Nashmi R, Fehlings MG. Mechanism of axonal dysfunction after spinal cord injury: with an emphasis on the role of voltage gated potassium channels. Brain Res. 2001;38(1–2):165–191. doi: 10.1016/s0165-0173(01)00134-5. [DOI] [PubMed] [Google Scholar]
- 63.NeuroControl exit leaves holes in spinal injury market. Neurotech Business Report. Available at http://www.neurotechreports.com/pages/SCImarket.html. Accessed 15 Feb 2008
- 64.Patel M, Poo M-M. Orientation of neurite growth by extracellular electric fields. J Neuro Sci. 1982;2:483–496. doi: 10.1523/JNEUROSCI.02-04-00483.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pfurtscheller G, Muller GR, Pfurtscheller J, et al. ‘Thought’ control of functional electrical stimulation to restore hand grasp in a patient with tetraplegia. Neurosci Lett. 2003;351(1):33–36. doi: 10.1016/S0304-3940(03)00947-9. [DOI] [PubMed] [Google Scholar]
- 66.Politis MJ, Zanakis MF. The short term effects of delayed application of electric fields in the damaged rodent spinal cord. Neurosurgery. 1989;25(1):71–75. doi: 10.1097/00006123-198907000-00012. [DOI] [PubMed] [Google Scholar]
- 67.Popovic MR, Popovic DB, Keller T. Neuroprostheses for grasping. Neurol Res. 2002;24:443–452. doi: 10.1179/016164102101200311. [DOI] [PubMed] [Google Scholar]
- 68.Qian T, Guo X, Levi AD, et al. High dose methylprednisolone may cause myopathy in acute spinal cord injury patients. Spinal Cord. 2005;43:199–203. doi: 10.1038/sj.sc.3101681. [DOI] [PubMed] [Google Scholar]
- 69.Ragnarsson KT, Pollack S, WJr O’Daniel. Clinical evaluation of computerized functional electrical stimulation after spinal cord injury:a multicenter pilot study. Arch Phys Med Rehabil. 1988;69(9):672–677. [PubMed] [Google Scholar]
- 70.Ragnarsson KT, Pollack SF, Twist D. Lower limb endurance exercise after spinal cord injury: implications for health and functional ambulation. J Neurol Rehabil. 1991;5:37–48. [Google Scholar]
- 71.Ramon Y, Cajal S (1928) Degeneration and regeneration of the nervous system. In: May RM (ed) Oxford University Press, London
- 72.Rattay F, Resatz S, Dimitrijevic MR, et al. Mechanisms of electrical stimulations with neural prosthesis. Neuromodulation. 2003;6(1):42–56. doi: 10.1046/j.1525-1403.2003.03006.x. [DOI] [PubMed] [Google Scholar]
- 73.Rovainen CM. Regeneration of Muller and Mauthner axons after spinal transaction in larval lampreys. J Comp Neurol. 1976;168(4):545–554. doi: 10.1002/cne.901680407. [DOI] [PubMed] [Google Scholar]
- 74.Sadowsky CL. Electrical stimulation in spinal cord injury. Neurorehabilitation. 2001;16:165–169. [PubMed] [Google Scholar]
- 75.Saigal R, Renzi C, Mushahwar VK. Intraspinal microstimulation generates functional movements after spinal-cord injury. IEEE Trans Neural Syst Rehabil Eng. 2004;12(4):430–440. doi: 10.1109/TNSRE.2004.837754. [DOI] [PubMed] [Google Scholar]
- 76.Schnell L, Schneider R, Kolbeck R, et al. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after spinal cord lesion. Nature. 1994;367:170–173. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- 77.Schwab ME, Kapfhammer JP, Bandtlow CE. Inhibitors of neurite growth. Annu Rev Neurosci. 1993;16:565–595. doi: 10.1146/annurev.ne.16.030193.003025. [DOI] [PubMed] [Google Scholar]
- 78.Shapiro S, Borgens RB, Pascuzzi R, et al. Oscillating field stimulation for complete spinal cord injury in humans: a phase 1 trial. J Neurosurg Spine. 2005;2:3–10. doi: 10.3171/spi.2005.2.1.0003. [DOI] [PubMed] [Google Scholar]
- 79.Shen NJ, Wang YT, Lin QB, et al. Using methylprednisolone to supplement direct current electrical field in promoting spinal cord regeneration. J Reconstr Microsurg. 2005;21:251–255. doi: 10.1055/s-2005-871752. [DOI] [PubMed] [Google Scholar]
- 80.Shi R, Borgens RB. Anatomical repair of nerve membranes in crushed mammalian spinal cord with polyethylene glycol. J Neurocytol. 2000;29:633–643. doi: 10.1023/A:1010879219775. [DOI] [PubMed] [Google Scholar]
- 81.Shi R, Asano T, Wining NC, et al. Control of membrane sealing in injured mammalian spinal cord axons. J Neurophysiol. 2000;84:1762–1769. doi: 10.1152/jn.2000.84.4.1763. [DOI] [PubMed] [Google Scholar]
- 82.Shi R, Borgens RB. Three-dimensional gradients of voltage during development of the nervous system as invisible coordinates for the establishment of embryonic patterns. Dev Dyn. 1995;202:101–114. doi: 10.1002/aja.1002020202. [DOI] [PubMed] [Google Scholar]
- 83.Short DJ, Masry El WS, Jones PW. High dose methylprednisolone in the management of acute spinal cord injury––a systematic review from a clinical perspective. Spinal Cord. 2000;38:273–286. doi: 10.1038/sj.sc.3100986. [DOI] [PubMed] [Google Scholar]
- 84.Miller JH, Silver J. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 85.Simcox S, Davis G, Barriskill A, et al. A portable, 8-channel transcutaneous stimulator for paraplegic muscle training and mobility–a technical note. JRRD. 2004;41(1):41–52. doi: 10.1682/JRRD.2004.01.0041. [DOI] [PubMed] [Google Scholar]
- 86.Snoek GJ, IJzerman MJ, in’t Groen FA , et al. Uses of the NESS Handmaster to restore handfunction in tetraplegia: clinical experience in ten patients. Spinal Cord. 2000;38(4):244–249. doi: 10.1038/sj.sc.3100980. [DOI] [PubMed] [Google Scholar]
- 87.Strautman AF, Cook RJ, Robinson KR. The distribution of free calcium in transected spinal axons and its modulation by applied electric fields. J Neurol Sci. 1990;10:3564–3575. doi: 10.1523/JNEUROSCI.10-11-03564.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tatagiba M, Brosamle C, Schwab ME. Regeneration of injured axons in the adult mammalian central nervous system. Neurosurgery. 1997;40(3):541–547. doi: 10.1097/00006123-199703000-00023. [DOI] [PubMed] [Google Scholar]
- 89.Taylor PN, Esnouf J, Hobby J. The functional impact of the freehand system on tetraplegics hand function. Spinal Cord. 2002;40(11):560–566. doi: 10.1038/sj.sc.3101373. [DOI] [PubMed] [Google Scholar]
- 90.Uzman BG, Snyder DS, Villegas GM. Status of peripheral nerve regeneration. In: Seil F, editor. Neural regeneration and transplantation. New York: Alan R LissInc; 1989. pp. 15–28. [Google Scholar]
- 91.Wallace MC, Tator CH, Gentles WM. Effect of alternating current stimulation of the spinal cord on recovery from acute spinal cord injury in rats. Surg Neurol. 1987;28(4):269–276. doi: 10.1016/0090-3019(87)90305-3. [DOI] [PubMed] [Google Scholar]
- 92.Wallace MC, Tator CH, Piper I. Recovery of spinal cord function induced by direct current stimulation of the injured rat spinal cord. Neurosurg. 1987;20(6):878–884. doi: 10.1097/00006123-198706000-00010. [DOI] [PubMed] [Google Scholar]
- 93.Wood MR, Cohen MJ. Synaptic regeneration in identified neurons of the lamprey spinal cords. Science. 1979;206(4416):344–347. doi: 10.1126/science.482943. [DOI] [PubMed] [Google Scholar]
- 94.Wood MR, Cohen MJ. Synaptic regeneration and glial reactions in the transected spinal cord of the lamprey. J Neurocytol. 1981;10(1):57–79. doi: 10.1007/BF01181745. [DOI] [PubMed] [Google Scholar]
- 95.Young W. Secondary injury mechanisms in acute spinal cord injury. J Emerg Med. 1993;11:13–22. doi: 10.1016/0736-4679(93)90002-O. [DOI] [PubMed] [Google Scholar]