Abstract
To examine racial differences in mammography use and its determinants in the City of St. Louis, MO, USA, we recruited women age 40 or older using random-digit dialing to (1) examine the difference in mammography use between white women and African American women and (2) identify individual- and census-tract-level risk factors of nonadherence to mammography. During telephone interviews, we inquired about mammography use and several demographic, psychosocial, and health behavior variables. We determined the residential census tracts of study subjects using a geographic information system. The rate of mammography use was 68.0% among white women and 74.7% among African American women (P = 0.022). African American women were more likely to have mammograms than white woman (adjusted odds ratio [OR] = 1.71; 95% confidence interval [CI] = 1.09–2.69). System-level barriers to mammography and heavy smoking were associated with lower mammography use among both white and African American women. Personal-experience barriers to mammography and no physician recommendation also were independently associated with mammography use among white women. White women residing within a historic geographic cluster area of late-stage breast cancer were less likely to have mammograms (adjusted OR = 0.42, 95% CI = 0.22–0.80), while African American women residing within a historic geographic cluster area of late-stage breast cancer were equally likely to have mammograms (adjusted OR = 0.79, 95% CI = 0.28–2.24). Neither individual- nor census-tract-level socioeconomic status was associated with mammography screening. These findings suggest that there may be a greater need for increasing mammography use among white women, especially in the historic cluster area of late-stage breast cancer in St. Louis.
Keywords: Breast cancer, Cluster, Geocoding, Mammography, Random digit dialing
Introduction
Breast cancer is the leading cancer and the second leading cause of cancer death among women in the USA.1 Mammography use reduces the likelihood of late-stage breast cancer and subsequent mortality.2
Increased use of screening mammography during the 1990s was reported by several national studies, including the National Health Interview Survey (NHIS)3 and the Behavioral Risk Factor Surveillance System (BRFSS).4 By 2000, 70% of women 40 years of age and older reported being screened by mammography within the previous two years.5 Recently, it has been shown that racial disparities in screening mammography have largely disappeared.6–8 While trends in disparities over time in the USA or specific states are important to monitor, they may mask disparities in screening practices for much smaller areas. Evidence of declining mammography rates from 2000 to 2005 also may be cause for concern.9,10 If mammography rates continue to decline, the issue of where to target interventions locally (geographic targeting) to increase mammography use, such as by offering flexible clinic hours, health education, or mobile mammography vans, will become crucial to reduce the incidence of late-stage breast cancer diagnoses.
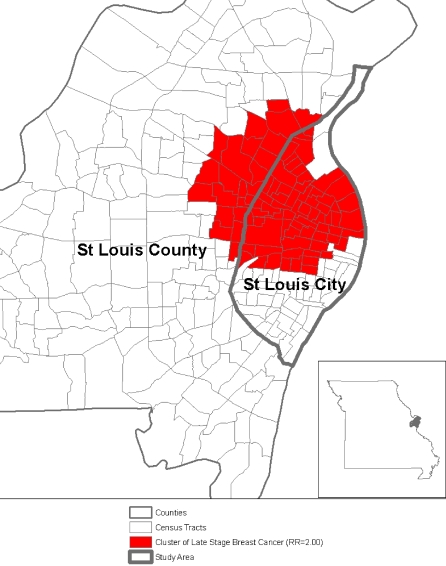
The diagnosis of late-stage breast cancer, which is associated with greater mortality from breast cancer, has been considered a marker for lower breast cancer screening.11 In the analysis of the 1996–1998 statewide breast cancer data at the census-tract level from the Missouri Cancer Registry using a Geographic Information System, we detected a geographic cluster of elevated first primary diagnosis of late-stage (distant) breast cancer, which was located in the St. Louis, MO, metropolitan area. Women age 50 or older in this area (Figure 1) were two times more likely to be diagnosed with late-stage disease than elsewhere in Missouri.12 While other studies have identified geographic clusters of higher-than-expected incidence of late-stage breast cancer,13,14 these studies did not report potential reasons for the higher-than-expected late-stage disease they observed in these areas.
FIGURE 1.
Geographic clustering of late-stage breast cancer among women age 50 or older in St. Louis County and St. Louis City, 1996–1998. This paper reports on comparisons between women living within and outside the geographic cluster within the limits of St. Louis City only. Women living in St. Louis County were not surveyed.
To examine local racial disparities in mammography screening use, the current study examined racial differences in nonadherence to mammography screening guidelines in the City of St. Louis both within and outside the cluster of late-stage breast cancer. We also examined the extent to which several individual-level factors accounted for any observed associations between census-tract-level factors and mammography screening, using the conceptual framework of Anderson’s behavioral model,15,16 which is frequently applied in healthcare utilization studies.
Materials and Methods
Data Collection
From March 2004 through June 2006, we used the equal probability of selection method of randomly selecting residential telephone numbers in order to contact women age 40 or older residing within the St. Louis City limits. This age group was selected because routine breast cancer screening is recommended for women age 40 and older.17,18 Each telephone number was called until response but no more than eight times. Women who reported having a prior history of breast cancer with double mastectomy, who had prophylactic double mastectomy, or who did not speak English were not eligible to participate. Women whose geocoded address placed them outside the St. Louis City limits were excluded from the study, and those women whose self-reported address was not able to be geocoded were set as a category “ungeocoded” in our analysis. All data were collected by trained female interviewers using a computer-assisted telephone interview system. This study was approved by the Washington University Institutional Review Board.
As a result, a total of 40,162 telephone numbers were dialed, 29,159 of which were a fax or answering machine or nonworking numbers. Of the 11,003 household numbers reached, 7,438 (67.6%) were not eligible and were excluded because no woman lived in the household or met the inclusion criteria. A total of 1,033 women completed the telephone interview, 35 of whom were subsequently excluded because their residential address was located outside the St. Louis City limits based on geocoding. Of the remaining 998 women (556 white, 429 African American, 8 other minority, and 5 race unknown), 215 could not be geocoded because of unreported or inaccurate street addresses. The Council of American Survey Research Organizations (CASRO) response rate was 50.9%, which was similar to the overall response rates for the Missouri 2001–2006 BRFSS, which ranged from 49.3% to 54.2%.19 The CASRO rate reflects telephone sampling efficiency as well as the degree of cooperation from eligible people contacted, with higher percentages indicating lower potential for bias in the data. For this reason, the CASRO rate is a more appropriate measure of participation rates in surveys using random-digit dialing methods than the simple response rate.
Mammography Screening
During the interview, we inquired about ever having had a mammogram and about the time since the last mammogram. All women who had a mammogram for health-related reasons were excluded, and only those women who had a mammogram as part of a routine checkup for screening were included in the analysis. We considered women to be adherent to mammography guidelines if they reported having a mammogram in the past 2 years for women age 40–49 and in the past year for women age 50 or older.17,18,20 Women answering “don’t know” or “refused” were set to missing and excluded from the analysis.
Geocoding
Self-reported residential addresses were geocoded to create longitude and latitude using the US Census Tiger Line 2000 street database, both to determine eligibility for participation in the study (i.e., living within the St. Louis City limits) and to determine if participants resided within or outside the geographic cluster of elevated late-stage breast cancer incidence. Subjects whose addresses could not be geocoded were categorized as such.
Census-tract-level Factors
To compare mammography use among women living within and outside the geographic cluster of late-stage breast cancer, we created a census-tract-level variable “cluster”. A census tract was categorized with the value one if it lay in the cluster area shown in Figure 1, otherwise as zero. In addition, we also examined the role of census-tract socioeconomic position in mammography use. Census-tract-level percentage of the population living below the US federal poverty line from 2000 census was used to estimate the census-tract socioeconomic position. Census-tract-level poverty rate is a robust measure for determining the census-tract socioeconomic contextual effects on health and is comparable across areas.21
Potential Individual-level Mediating Pathways
We used the conceptual framework of Anderson’s behavioral model15,16 to create groups of factors that have been previously associated with breast cancer screening use and that might serve as potential mediating pathways to account for an observed association between lower screening use and living within the geographic cluster of elevated incidence of late-stage breast cancer. We grouped variables as follows based on the Anderson model: predisposing characteristics, enabling resources, health-related behavior, and self-rated health status.
Predisposing factors previously found to be associated with screening included age,22 race/ethnicity,23 education level,24 marital status, and family history of breast cancer. Enabling resources associated with screening included family income,24,25 employment status, health insurance coverage, barriers to seeing a doctor, having access to medical care when needed,15,23,26 knowledge about breast cancer and mammography,27–29 perceived barriers and benefits of mammography,27–32 and having a physician recommend a mammogram during the past 2 years.23,24,33,34 These questions were obtained from the BRFSS, Medical Expenditure Panel Survey, or based on items used in previous studies.27,29–31,35,36 Participants’ knowledge about breast cancer and mammography was measured using nine questions; we computed a total score based on the number of correct answers reported. A 12-item measure of perceived barriers of mammography was developed based on previous research,27–32 using five-point Likert scale responses ranging from 1 = “strongly disagree” to 5 = “strongly agree.” Higher scores reflected greater perceived barriers to mammography.
Principal components factor analysis with varimax rotation was used for data reduction to evaluate the component structure of the 12 barriers-to-mammography items. Rotation technique in the factor analysis can produce more useful patterns of common factors, and the varimax orthogonal rotation is one of the most popular rotation techniques.37 Cronbach alpha coefficients measured the internal consistency of items on the resulting factors. Factor analysis of the 12 barriers to mammography items resulted in a two-factor solution based on the following criteria: eigenvalues greater than 1.0 and number of components confirmed using a parallel analysis method described by Lautenschlager,38 which used constructed tables of Monte Carlo-simulated random data to accurately estimate the number of components to retain (Table 1). A five-item factor described system-level barriers (e.g., cost, facility location, lack of physician recommendation) and negative beliefs about early-detection value of mammography. Cronbach alpha for this factor was 0.72. A three-item factor described barriers relating to personal experience (e.g., mammograms are painful, embarrassing, and too much trouble); Cronbach alpha for this factor was 0.61. Mean scores for these two new variables were used as candidate covariates in the analysis. Four of the 12 items loaded on more than one factor and were excluded from further analysis.
Table 1.
Factor loadings of items measuring barriers to mammography
| Item | Factor loading | |
|---|---|---|
| System-level barriers | Personal-experience-related barriers | |
| 1. Compared with your other health problems, having a mammogram is not important | 0.48 | 0.45 |
| 2. You are afraid to have a mammogram because it might show a problem | 0.41 | 0.42 |
| 3. Having a mammogram is a lot of trouble for you | 0.28 | 0.72 |
| 4. Having a mammogram is painful for you | −0.19 | 0.72 |
| 5. Having a mammogram is embarrassing for you | 0.23 | 0.67 |
| 6. You don’t have the time to get a mammogram | 0.49 | 0.42 |
| 7. Cost would keep you from having a mammogram | 0.54 | 0.20 |
| 8. You have trouble remembering to get a mammogram | 0.34 | 0.35 |
| 9. Your doctor did not tell you that you should have a mammogram | 0.71 | −0.04 |
| 10. Breast cancer that is found when it is just getting started has a good chance of being cured (score was reversed) | 0.53 | 0.10 |
| 11. You do not know where to get a mammogram | 0.73 | 0.16 |
| 12. You do not get a mammogram because the nearest mammography facility is too far from your home | 0.72 | 0.26 |
Factor loadings from principal components analysis with varimax rotation. Coefficients in italics indicate items that were included in the computation of the mean score for that factor.
In addition to the predisposing factors and enabling resources described above, we included the Short Form 12 (SF-12) measures of physical and mental health functioning,39 since functioning also has been associated with breast cancer screening.26,40 We also asked participants about their current smoking status using questions from the BRFSS, since engagement in health-related behaviors, such as smoking,41 has been associated with breast cancer screening.
Statistical Analysis
Using generalized linear mixed models, multilevel logistic regression was fitted to determine the individual- and census-tract-level factors that may be associated with mammography screening. To compare racial differences in the determinants of nonadherence to mammography screening guidelines, we fitted separate models for white women and African American women. We added the following groups of variables in a hierarchical fashion: (1) census-tract-level factors, (2) predisposing factors, (3) enabling resources, (4) smoking, and (5) physical and mental health functioning to the regression model to examine the associations between census-tract-level factors and mammography use and to determine whether and to what extent individual-level variables could explain census-tract effects. Changes in the odds ratios (ORs) of census-tract-level variables were considered to be evidence of mediation when adding the groups of variables.
All models were weighted based on the number of women age 40 or older in the household, the number of residential telephones available in each household, and a poststratification weight, which was calculated based on the number of women living in the City of St. Louis by age and race. The fit of the multilevel models was evaluated using scaled deviance. The scaled deviance is a log-likelihood ratio statistic used to evaluate the goodness-of-fit of a generalized linear model.42 Smaller values of scaled deviance reflect better model fitting. All statistical procedures were executed using the SAS System (Version 9.1, SAS Institute, Cary, NC, USA).
Sensitivity Analysis
We conducted two types of sensitivity analyses to examine the robustness of our findings. First, a sensitivity analysis was performed to determine the effect on our results of excluding the women who were unable to be geocoded because they declined to report their address or because of inaccurate reporting of the street address but stated that they resided in the City of St. Louis. Second, we examined the mediational effects of only those variables that were associated with screening use. A mediator should be associated both with adverse census-tract conditions and with mammography screening. This also reduces the potential for collinear effects between variables as part of a pathway.
Results
Characteristics of the 985 study participants are described in Table 2. About 21.6% of white women and 62.2% of African American women lived within the cluster area. In addition, compared with white women, African American women were more likely to live within high-poverty census tracts, have lower education level, be unmarried, have low family income, be unemployed, have lower health insurance coverage, report more barriers to see a doctor, less knowledge about breast cancer, and more personal-experience-related barriers to mammography but have better self-rated health status.
Table 2.
Characteristics of the study population by race in St. Louis City, 2004–2006
| Variable | White (n = 556) | African American (n = 429) | P value | ||
|---|---|---|---|---|---|
| Number | Percent | Number | Percent | ||
| Mammography | |||||
| No | 177 | 32.0 | 108 | 25.3 | 0.022 |
| Yes | 376 | 68.0 | 319 | 74.7 | |
| Census-tract factors | |||||
| Cluster | <0.001 | ||||
| Outside | 326 | 58.6 | 62 | 15.5 | |
| Within | 120 | 21.6 | 267 | 62.2 | |
| Ungeocoded | 110 | 19.8 | 100 | 23.3 | |
| Poverty | <0.001 | ||||
| <10% | 167 | 30.0 | 2 | 0.5 | |
| 10–20% | 140 | 25.2 | 65 | 15.2 | |
| 20–30% | 107 | 19.2 | 126 | 29.4 | |
| ≥30% | 32 | 5.8 | 136 | 31.7 | |
| Unknown | 110 | 19.8 | 100 | 23.3 | |
| Predisposing factors | |||||
| Age | 0.135 | ||||
| 40–49 | 177 | 31.8 | 148 | 34.5 | |
| 50–64 | 222 | 39.9 | 184 | 42.9 | |
| 65+ | 157 | 28.6 | 97 | 22.6 | |
| Education | <0.001 | ||||
| >High school | 358 | 64.4 | 214 | 50.0 | |
| ≤High school | 198 | 35.6 | 214 | 50.0 | |
| Marital status | <0.001 | ||||
| Married | 286 | 51.6 | 117 | 27.3 | |
| Not | 268 | 48.4 | 311 | 72.7 | |
| Family history | 0.340 | ||||
| No | 460 | 82.7 | 369 | 86.0 | |
| Yes | 87 | 15.7 | 53 | 12.4 | |
| Uncompleted | 9 | 1.6 | 7 | 1.6 | |
| Enabling resources | |||||
| Income | <0.001 | ||||
| >$75K | 116 | 20.9 | 16 | 3.7 | |
| $25K–75K | 239 | 43.0 | 149 | 34.7 | |
| <$25K | 137 | 24.6 | 214 | 49.9 | |
| Unknown/refused | 64 | 11.5 | 50 | 11.7 | |
| Employment | <0.001 | ||||
| Yes | 327 | 58.8 | 193 | 45.0 | |
| No | 229 | 41.2 | 236 | 55.0 | |
| HI coverage | <0.001 | ||||
| Yes | 490 | 88.1 | 313 | 73.0 | |
| No | 22 | 4.0 | 47 | 11.0 | |
| Unknown/re fused | 44 | 7.9 | 69 | 16.1 | |
| Reasons for not seeing a doctor | 0.002 | ||||
| No | 408 | 73.4 | 276 | 64.3 | |
| Yes | 148 | 26.6 | 153 | 35.7 | |
| Access to medical care | 0.440 | ||||
| Yes | 539 | 96.9 | 412 | 96.0 | |
| No | 17 | 3.1 | 17 | 4.0 | |
| Knowledge score | <0.001 | ||||
| 5–9 | 47 | 8.5 | 79 | 18.4 | |
| 2–4 | 292 | 52.5 | 268 | 62.5 | |
| 0/1 | 217 | 39.0 | 82 | 19.1 | |
| System-level barriers to mammography | 1.78 ± 0.54 | 1.93 ± 0.50 | <0.001 | ||
| Personal-experience barriers to mammography | 2.28 ± 0.75 | 2.14 ± 0.62 | 0.001 | ||
| Physician recommendation | 0.099 | ||||
| Yes | 431 | 77.5 | 313 | 73.0 | |
| No | 125 | 22.5 | 116 | 27.0 | |
| Personal health practices | |||||
| Smoking | 0.004 | ||||
| No | 278 | 50.2 | 192 | 45.0 | |
| <10 per day | 197 | 35.6 | 150 | 35.1 | |
| 10–19 per day | 28 | 5.1 | 53 | 12.4 | |
| ≥20 per day | 51 | 9.2 | 32 | 7.5 | |
| Self-rated health | |||||
| Physical health functioning | <0.001 | ||||
| 1st quartile (poorest) | 168 | 30.2 | 80 | 18.7 | |
| 2nd quartile | 149 | 26.8 | 95 | 22.1 | |
| 3rd quartile | 133 | 23.9 | 113 | 26.3 | |
| 4th quartile | 106 | 19.1 | 141 | 32.9 | |
| Mental health functioning | <0.001 | ||||
| 1st quartile (poorest) | 149 | 26.8 | 105 | 24.5 | |
| 2nd quartile | 162 | 29.1 | 77 | 18.0 | |
| 3rd quartile | 134 | 24.1 | 114 | 26.6 | |
| 4th quartile | 111 | 20.0 | 133 | 31.0 | |
Test of significance were chi-square tests for categorical variables and t tests for continuous variables
Overall, mammography use was 70.9%, and the rate was lower among white than among African American women (68.0% vs. 74.7%, P = 0.022, Table 3). Within the cluster area, the mammography-use rate was 69.5% (60.2% among white women vs. 73.7% among African American women, P = 0.008); outside the cluster area, the rate was 70.6% (69.6% among white women vs. 75.8% among African American women, P = 0.328). After adjustment for census-tract-level factors and all individual-level factors, African American women were more likely to have mammograms than white women (OR = 1.71, 95% confidence interval [CI] = 1.09–2.69, Table 4).
Table 3.
Unadjusted mammography rates among white women and African American women in St. Louis City, 2004–2006
| Populationa | Race | Mammography rate (%) | P valueb |
|---|---|---|---|
| Total (n = 985) | White + African American | 70.9 | 0.022 |
| White | 68.0 | ||
| African American | 74.7 | ||
| Within the cluster (n = 384) | White + African American | 69.5 | 0.008 |
| White | 60.2 | ||
| African American | 73.7 | ||
| Outside the cluster (n = 388) | White + African American | 70.6 | 0.328 |
| White | 69.6 | ||
| African American | 75.8 |
aUngeocoded subjects were not listed individually
bChi-square tests comparing mammography screening use between white women and African American women
Table 4.
Associations between individual-level factors and mammogram receiving among white and African American women age 40 or older using multilevel logistic regression in St. Louis City, 2004–2006
| Variable | Odds ratio (95% confidence Interval) | ||
|---|---|---|---|
| White + African American (n = 985) | White (n = 556) | African American (n = 429) | |
| Predisposing factors | |||
| Age (vs. 40 ∼ 49) | |||
| 50 ∼ 64 | 0.36 (0.24–0.54) | 0.32 (0.18–0.58) | 0.32 (0.18–0.59) |
| 65+ | 0.38 (0.20–0.71) | 0.30 (0.13–0.73) | 0.49 (0.19–1.27) |
| Race (vs. white) | |||
| Black | 1.71 (1.09–2.69) | – | – |
| Education (vs. high school+) | |||
| ≤high school | 1.61 (1.07–2.41) | 1.69 (0.96–2.98) | 1.82 (0.97–3.41) |
| Marriage (vs. married) | |||
| Not married | 1.38 (0.92–2.06) | 1.34 (0.79–2.27) | 1.35 (0.71–2.59) |
| Family history (vs. no) | |||
| Yes | 1.08 (0.64–1.82) | 1.47 (0.72–3.03) | 0.79 (0.36–1.74) |
| Uncompleted | 0.95 (0.25–3.66) | 0.51 (0.08–3.13) | 1.86 (0.19–18.0) |
| Enabling resources | |||
| Income (vs. >$75K) | |||
| $25K–75K | 0.61 (0.32–1.13) | 0.67 (0.33–1.35) | 0.32 (0.06–1.77) |
| <$25K | 0.69 (0.32–1.46) | 0.72 (0.28–1.84) | 0.35 (0.06–2.11) |
| Unknown/refused | 0.72 (0.32–1.64) | 0.69 (0.26–1.80) | 0.54 (0.08–3.66) |
| Employment (vs. yes) | |||
| No | 1.29 (0.85–1.97) | 1.81 (0.98–3.35) | 0.95 (0.51–1.74) |
| HI coverage (vs. yes) | |||
| No | 0.69 (0.37–1.29) | 0.35 (0.12–1.00) | 1.25 (0.53–2.95) |
| Unknown/refused | 0.52 (0.30–0.88) | 0.36 (0.15–0.83) | 0.73 (0.36–1.50) |
| Reasons for not seeing a doctor (vs. no) | |||
| Yes | 0.77 (0.51–1.16) | 1.05 (0.59–1.85) | 0.63 (0.34–1.15) |
| Access to medical care (vs. yes) | |||
| No | 0.56 (0.24–1.27) | 0.34 (0.08–1.35) | 0.68 (0.22–2.16) |
| Knowledge score (vs. 5–9) | |||
| 2–4 | 1.53 (0.89–2.62) | 0.83 (0.32–2.15) | 2.60 (1.29–5.27) |
| 0/1 | 1.18 (0.64–2.17) | 1.28 (0.47–3.44) | 1.08 (0.45–2.58) |
| System-level barriers to mammography | 0.39 (0.26–0.59) | 0.45 (0.27–0.76) | 0.22 (0.11–0.45) |
| Personal-experience barriers to mammography | 0.59 (0.45–0.76) | 0.46 (0.33–0.63) | 0.93 (0.57–1.51) |
| Physician recommendation (vs. yes) | |||
| No | 0.40 (0.27–0.60) | 0.24 (0.14–0.43) | 0.60 (0.33–1.08) |
| Personal health practices | |||
| Smoking (vs. no) | |||
| <10 per day | 0.77 (0.52–1.14) | 0.55 (0.33–0.92) | 0.87 (0.46–1.64) |
| 10–19 per day | 0.56 (0.30–1.04) | 0.59 (0.19–1.78) | 0.34 (0.15–0.77) |
| >= 20 per day | 0.39 (0.21–0.73) | 0.42 (0.18–0.98) | 0.17 (0.06–0.46) |
| Need for medical care | |||
| Physical health functioning (vs. 1st quartile, poorest) | |||
| 2nd quartile | 0.89 (0.53–1.48) | 1.26 (0.65–2.47) | 0.40 (0.17–0.97) |
| 3rd quartile | 0.88 (0.53–1.45) | 0.83 (0.44–1.58) | 0.87 (0.38–1.97) |
| 4th quartile | 0.89 (0.53–1.50) | 0.54 (0.26–1.11) | 1.44 (0.65–3.17) |
| Mental health functioning (vs. 1st quartile, poorest) | |||
| 2nd quartile | 0.90 (0.53–1.50) | 1.57 (0.81–3.03) | 0.24 (0.10–0.60) |
| 3rd quartile | 0.89 (0.53–1.50) | 1.37 (0.69–2.72) | 0.35 (0.15–0.82) |
| 4th quartile | 0.82 (0.47–1.43) | 1.04 (0.49–2.23) | 0.44 (0.18–1.06) |
Three models were adjusted for all individual factors and census-tract-level covariates, including census-tract-level poverty rate based on US 2002 Census and whether a census tract lies in the cluster area of late-stage breast cancer or not.
Table 4 shows that older age, system-level barriers to mammography, and heavy smoking were associated with lower mammography screening among both white women and African American women. Personal-experience barriers to mammography and physician recommendation were independent risk factors for nonadherence to mammography-screening guidelines among white women.
Model I in Table 5 shows that white women living within the cluster area were as likely to report having a mammogram as white women living outside this area (OR = 0.70). When adding predisposing factors in model II, the OR was similar (OR = 0.71) to model I. The OR was reduced to 0.49 after adding enabling resources variables in model III. The OR and model significance were not altered much when adding smoking to model IV (OR = 0.46) and subsequently adding the SF-12 physical and mental health functioning measures in model V (OR = 0.42). African American women living within the cluster area were as likely to have received a mammogram within the past year as African American women living outside the cluster area in unadjusted analysis (model I) and in each of the adjusted models (models II–V). Neither individual- nor census-tract-level socioeconomic status was associated with mammography screening among white women and African American women (Tables 4 and 5).
Table 5.
Associations between census-tract-level factors and mammogram receiving among white and African American women age 40 or older using multilevel logistic regression in St. Louis City, 2004–2006
| Race | Variable | Model I | Model II | Model III | Model IV | Model V |
|---|---|---|---|---|---|---|
| White + African American (n = 985) | Cluster (vs. outside)a | |||||
| Within | 0.87 (0.60–1.24) | 0.75 (0.51–1.11) | 0.62 (0.39–1.00) | 0.62 (0.38–1.00) | 0.62 (0.38–1.02) | |
| Poverty (vs. <10%) | ||||||
| 10–20% | 0.90 (0.55–1.47) | 0.83 (0.50–1.37) | 1.11 (0.61–2.03) | 1.07 (0.58–1.99) | 1.09 (0.58–2.03) | |
| 20–30% | 0.97 (0.59–1.59) | 0.84 (0.50–1.41) | 1.21 (0.65–2.25) | 1.26 (0.67–2.38) | 1.27 (0.67–2.42) | |
| ≥30% | 1.01 (0.58–1.75) | 0.79 (0.44–1.42) | 1.13 (0.56–2.28) | 1.27 (0.62–2.62) | 1.27 (0.61–2.64) | |
| Scaled deviance | 1,174.0 | 1,131.4 | 838.0 | 836.6 | 827.0 | |
| White (n = 556) | Cluster (vs. outside)a | |||||
| Within | 0.70 (0.44–1.13) | 0.71 (0.43–1.16) | 0.49 (0.27–0.89) | 0.46 (0.25–0.84) | 0.42 (0.22–0.80) | |
| Poverty (vs. <10%) | ||||||
| 10–20% | 0.82(0.49–1.38) | 0.83 (0.48–1.43) | 1.20 (0.63–2.31) | 1.21 (0.62–2.33) | 1.30 (0.66–2.59) | |
| 20–30% | 0.86 (0.50–1.50) | 0.87 (0.49–1.54) | 1.26 (0.64–2.48) | 1.37 (0.69–2.74) | 1.41 (0.69–2.88) | |
| ≥30% | 0.60 (0.26–1.38) | 0.64 (0.27–1.52) | 1.12 (0.41–3.05) | 1.38 (0.49–3.90) | 1.24 (0.43–3.60) | |
| Scaled deviance | 675.1 | 643.8 | 507.4 | 492.2 | 471.3 | |
| African American (n = 429) | Cluster (vs. Outside)a | |||||
| Within | 0.76 (0.37–1.56) | 0.78 (0.37–1.65) | 0.78 (0.32–1.91) | 0.81 (0.30–2.17) | 0.79 (0.28–2.24) | |
| Poverty (vs. <10%) | ||||||
| 10–20% | 1.79 (0.11–28.2) | 1.34 (0.08–22.4) | 2.14 (0.10–44.0) | 2.73 (0.12–60.8) | 2.07 (0.08–54.0) | |
| 20–30% | 1.70 (0.11–26.2) | 1.35 (0.08–21.9) | 2.87 (0.14–57.8) | 3.97 (0.18–85.7) | 3.11 (0.12–78.6) | |
| ≥30% | 1.82 (0.12–27.8) | 1.40 (0.09–22.7) | 2.75 (0.14–55.1) | 4.09 (0.19–87.9) | 2.83 (0.11–71.3) | |
| Scaled deviance | 485.3 | 465.9 | 388.2 | 385.6 | 365.8 | |
Model I was not adjusted for any individual-level factors; model II was adjusted for predisposing factors (age, education, marital status, family history of breast cancer); model III was adjusted for predisposing factors and enabling resources (annual family income, employment status, health insurance coverage, reasons for not seeing a doctor, access to medical care, knowledge about breast cancer, barriers to mammography, and physician recommendation); model IV was adjusted for predisposing factors, enabling resources, and smoking; model V was adjusted for predisposing factors, enabling resources, smoking, and self-rated health status (physical and mental health functioning).
aThe category “ungeocoded” was not listed in the table because it was not statistically different with the category “outside.”
The sensitivity analyses performed did not significantly alter the OR estimates. This suggests that women whose addresses could not be geocoded were similar to the women who remained in the analysis. Results were not altered when including only variables associated with mammography use as part of the mediating pathways suggesting that collinearity among the independent variables was not an issue.
Discussion
Identification and measurement of disparities in screening are critical to substantially improve rates of mammography screening in local community settings. Previous studies have reported individual-level risk factors and census-tract socioeconomic effect on mammography use.7,8,22–27,29,30,32–34,43 In our study, system-level barriers to mammography and heavy smoking were associated with mammography use among white and African American women. Personal-experience barriers to mammography and no physician recommendation also were independently associated with mammography screening among white women. White women but not African American women residing in a historic geographic cluster area of late-stage breast cancer were less likely to have mammograms. Increased risk of late-stage breast cancer in women age 40 or older can result from nonadherence to recommended breast cancer screening guidelines or from failure to have timely and adequate follow-up of positive screening results or of an actual breast cancer diagnosis. In community settings, nonadherence to breast cancer screening guidelines contributes to the increased risk of late-stage breast cancer among women more than 40 years of age.2 Thus, it is reasonable to target efforts to improve adherence to mammography screening guidelines in a geographic area previously found to have higher-than-expected incidence of late-stage breast cancer.
The rates of mammography screening use in the City of St. Louis were 69.5% within and 70.6% outside the geographic cluster of elevated incidence of late-stage breast cancer; these rates are slightly lower than the 74.6% nationally reported on the BRFSS.10 Our results show that before and after adjusting for four groups of individual-level variables, significantly higher mammography use was found for African American compared with white women. Although unexpected, this reverse racial difference is similar to a previous study6 and suggests that mammography screening rates may have been successfully improved, to some degree, among African American women living in the City of St. Louis. Recent studies also indicated women residing in areas with higher percentage of non-Hispanic African American women were more likely to have mammograms.7,8 Additionally, our study also indicated that white women residing within the geographic cluster of late-stage breast cancer diagnosis had lower mammography screening relative to the rest of the city. These findings suggest a need to increase routine-screening mammography use among white women, especially for those living within the cluster area. Although we have examined 16 individual-level factors previously reported to be associated with mammography use, these factors did not fully explain why white women living within the St. Louis City limits overall and white women who lived in the cluster area were less likely to receive mammograms. Possible explanations for these findings could include individual-level factors that we did not measure, such as incentives for primary care physicians,44 a woman’s perceived susceptibility to breast cancer,45 or travel distance to mammography facilities.46 Contextual factors, such as spatial availability and accessibility of low-fee or no-fee screening mammography, also could play a role.47 Future studies focusing on these factors will provide support for the effectiveness of targeted interventions, such as flexible clinic hours, health education, or provision of services using mobile mammography vans. Although previous studies have found socioeconomic position to be an important indicator of breast cancer screening, neither individual-level nor census-tract-level socioeconomic status was found to be associated with mammography screening in our study.
In this study, we only surveyed women in the City of St. Louis, which was previously identified in the analysis of Missouri statewide data as having a cluster of increased incidence of late-stage breast cancer. Therefore, our results may not be directly generalized to other geographic areas in Missouri or elsewhere. For example, it has been reported that rural residents were less likely to receive preventive healthcare services than urban residents.7,8,48 Our findings, however, suggest that a historic geographic cluster of late-stage breast cancer may serve as a geographic marker for nonadherence to recommended breast cancer screening, so we can better target interventions to improve mammography screening in areas of greater need. Since the proportion of cases of late-stage (distant) breast cancers changed little over time in the City of St. Louis according to the Missouri Cancer Registry (from 40.2% in 1996–1998 to 43.3% in 2002–2004),49 it is unlikely that the cluster of late-stage breast cancer has disappeared. Although it still remains unclear why late-stage breast cancer aggregated in this area, we can speculate this cluster may result partly from lower rates of mammography use. Although our study did not indicate that African American women also had a lower mammography screening in this area, other research has reported that African American women were less likely to receive adequate follow-up of abnormal mammographic results than white women.50,51 This observation might partially explain the clustering of late-stage breast cancer cases in this area.
Other limitations include our reliance on telephone interviews. Since low-income households are less likely to have telephones or may be more likely to have intermittent telephone service and since they are more likely to be located in the geographic cluster of elevated late-stage breast cancer incidence, this limitation could have biased our findings. However, such bias would have underestimated our findings.
Conclusions
Mammography utilization was lower among white women than African American women residing in the St. Louis City limits and among white women residing within the geographic cluster of elevated late-stage breast cancer incidence in northern St. Louis compared with white women living outside the geographic cluster area. Factors investigated in our study did not explain fully the racial and geographic disparities, and the reasons still remain unclear. But, these findings suggest that screening mammography should be improved among white women living in the St. Louis City limits and especially among white women living in the geographic cluster of elevated late-stage breast cancer. Geographic clustering of late-stage breast cancer is a potential geographic marker that can be used to target areas for improvement in mammography use. Future studies should examine other risk factors accounted for racial and geographic disparities for improving mammography screening in the St. Louis City limits.
Acknowledgments
This research was supported in part by grants from the National Cancer Institute (CA91842, CA91734, and CA98594) and the Agency for Healthcare Research and Quality (HS 14095-01). We thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, MO, for the use of the services of the Health Behavior and Outreach Core, especially for database development and management and geocoding services provided by Ms. Jennifer Tappenden and Mr. Jim Struthers. We also thank the interviewers and the women who participated in the study.
Footnotes
Lian, Jeffe, and Schootman are with the Department of Medicine, Washington University School of Medicine, St. Louis, MO, USA; Jeffe and Schootman are with the Alvin J. Siteman Cancer Center, Barnes-Jewish Hospital, Washington University School of Medicine, St. Louis, MO, USA.
Contributor Information
Min Lian, Phone: +1-314-2861988, FAX: +1-314-2861919, Email: mlian@dom.wustl.edu.
Donna B. Jeffe, Phone: +1-314-2861914, FAX: +1-314-2861919, Email: djeffe@dom.wustl.edu
Mario Schootman, Phone: +1-314-2861956, FAX: +1-314-2861919, Email: mschootm@dom.wustl.edu.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. [DOI] [PubMed]
- 2.Elmore JG, Armstrong K, Lehman CD, et al. Screening for breast cancer. JAMA. 2005;293:1245–1256. [DOI] [PMC free article] [PubMed]
- 3.Breen N, Wagener DK, Brown ML, et al. Progress in cancer screening over a decade: results of cancer screening from the 1987, 1992, and 1998 National Health Interview Surveys. J Natl Cancer Inst. 2001;93:1704–1713. [DOI] [PubMed]
- 4.Blackman DK, Bennett EM, Miller DS. Trends in self-reported use of mammograms (1989–1997) and Papanicolaou tests (1991–1997)—Behavioral Risk Factor Surveillance System. Morb Mortal Wkly Rep CDC Surveill Summ. 1999;48:1–22. [PubMed]
- 5.Swan J, Breen N, Coates RJ, et al. Progress in cancer screening practices in the United States: results from the 2000 National Health Interview Survey. Cancer. 2003;97:1528–1540. [DOI] [PubMed]
- 6.Jones AR, Caplan LS, Davis MK. Racial/ethnic differences in the self-reported use of screening mammography. J Commun Health. 2003;28:303–316. [DOI] [PubMed]
- 7.Benjamins MR, Kirby JB, Bond Huie SA. County characteristics and racial and ethnic disparities in the use of preventive services. Prev Med. 2004;39:704–712. [DOI] [PubMed]
- 8.Coughlin SS, Leadbetter S, Richards T, et al. Contextual analysis of breast and cervical cancer screening and factors associated with health care access among United States women, 2002. Soc Sci Med. 2008;66:260–275. [DOI] [PubMed]
- 9.Breen N, Cronin KA, Meissner HI, et al. Reported drop in mammography: is this cause for concern? Cancer. 2007;109:2405–2409. [DOI] [PubMed]
- 10.Ryerson AB, Miller J, Eheman CR, et al. Use of mammograms among women aged >/= 40 years—United States, 2000–2005. Morb Mortal Wkly Rep. 2007;56:49–51. [PubMed]
- 11.Roche LM, Skinner R, Weinstein RB. Use of a geographic information system to identify and characterize areas with high proportions of distant stage breast cancer. J Public Health Manag Pract. 2002;8:26–32. [DOI] [PubMed]
- 12.Schootman M, Jeffe DB, Gillanders WE, et al. Geographic clustering of adequate diagnostic follow-up after abnormal screening results for breast cancer among low-income women in Missouri. Ann Epidemiol. 2007;17:704–712. [DOI] [PubMed]
- 13.Sheehan TJ, DeChello LM. A space-time analysis of the proportion of late stage breast cancer in Massachusetts, 1988 to 1997. Int J Health Geogr. 2005;4:15. [DOI] [PMC free article] [PubMed]
- 14.MacKinnon JA, Duncan RC, Huang Y, et al. Detecting an association between socioeconomic status and late stage breast cancer using spatial analysis and area-based measures. Cancer Epidemiol Biomarkers Prev. 2007;16:756–762. [DOI] [PubMed]
- 15.Lane DS, Zapka J, Breen N, et al. A systems model of clinical preventive care: the case of breast cancer screening among older women. For the NCI Breast Cancer Screening Consortium. Prev Med. 2000;31:481–493. [DOI] [PubMed]
- 16.Mandelblatt JS, Yabroff KR, Kerner JF. Equitable access to cancer services: a review of barriers to quality care. Cancer. 1999;86:2378–2390. [DOI] [PubMed]
- 17.US Preventive Services Task Force. Summaries for patients. Screening for breast cancer: recommendations from the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:I–47. [DOI] [PubMed]
- 18.Leitch AM, Dodd GD, Costanza M, et al. American Cancer Society guidelines for the early detection of breast cancer: update 1997. CA Cancer J Clin. 1997;47:150–153. [DOI] [PubMed]
- 19.Behavioral Risk Factor Surveillance System. Technical Information and Data: Summary Data Quality Reports. http://www.cdc.gov/brfss/technical_infodata/quality.htm. Accessed on May 26, 2007.
- 20.Fletcher SW, Elmore JG. Clinical practice. Mammographic screening for breast cancer. N Engl J Med. 2003;348:1672–1680. [DOI] [PMC free article] [PubMed]
- 21.Krieger N, Chen JT, Waterman PD, et al. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter? the Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156:471–482. [DOI] [PubMed]
- 22.Harris RP, Fletcher SW, Gonzalez JJ, et al. Mammography and age: are we targeting the wrong women? A community survey of women and physicians. Cancer. 1991;67:2010–2014. [DOI] [PubMed]
- 23.Caplan LS, Wells BL, Haynes S. Breast cancer screening among older racial/ethnic minorities and whites: barriers to early detection. J Gerontol. 1992;47:101–110. [PubMed]
- 24.Breen N, Kessler L. Changes in the use of screening mammography: evidence from the 1987 and 1990 National Health Interview Surveys. Am J Public Health. 1994;84:62–67. [DOI] [PMC free article] [PubMed]
- 25.Hardy RE, Ahmed NU, Hargreaves MK, et al. Difficulty in reaching low-income women for screening mammography. J Health Care Poor Underserved. 2000;11:45–57. [DOI] [PubMed]
- 26.Iezzoni LI, McCarthy EP, Davis RB, et al. Mobility impairments and use of screening and preventive services. Am J Public Health. 2000;90:955–961. [DOI] [PMC free article] [PubMed]
- 27.Bryant H, Mah Z. Breast cancer screening attitudes and behaviors of rural and urban women. Prev Med. 1992;21:405–418. [DOI] [PubMed]
- 28.Champion VL. Instrument refinement for breast cancer screening behaviors. Nurs Res. 1993;42:139–143. [DOI] [PubMed]
- 29.Skinner CS, Arfken CL, Sykes RK. Knowledge, perceptions, and mammography stage of adoption among older urban women. Am J Prev Med. 1998;14:54–63. [DOI] [PubMed]
- 30.Champion VL. Beliefs about breast cancer and mammography by behavioral stage. Oncol Nurs Forum. 1994;21:1009–1014. [PubMed]
- 31.Fulton JP, Buechner JS, Scott HD, et al. A study guided by the Health Belief Model of the predictors of breast cancer screening of women ages 40 and older. Public Health Rep. 1991;106:410–420. [PMC free article] [PubMed]
- 32.Aiken LS, West SG, Woodward CK, et al. Health beliefs and compliance with mammography-screening recommendations in asymptomatic women. Health Psychol. 1994;13:122–129. [DOI] [PubMed]
- 33.Smith RA, Haynes S. Barriers to screening for breast cancer. Cancer. 1992;69:1968–1978. [DOI] [PubMed]
- 34.Conry CM, Main DS, Miller RS, et al. Factors influencing mammogram ordering at the time of the office visit. J Fam Pract. 1993;37:356–360. [PubMed]
- 35.Ware JE Jr. The status of health assessment 1994. Annu Rev Public Health. 1995;16:327–354. [DOI] [PubMed]
- 36.Skinner CS, Sykes RK, Monsees BS, et al. Learn, share, and live: breast cancer education for older, urban minority women. Health Educ Behav. 1998;25:60–78. [DOI] [PubMed]
- 37.Crawford CB, Ferguson GA. A general rotation criterion and its use in orthogonal rotation. Psychometrika. 1970;35:321–332. [DOI]
- 38.Lautenschlager GJ. A comparison of alternatives to conducting Monte Carlo analyses for determining parallel analysis criteria. Multivariate Behavioral Research. 1989;24:365–395. [DOI] [PubMed]
- 39.Ware J, Kosinski M, Keller S. SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales. 2nd ed. Boston, MA: The Health Institute, New England Medical Center; 1995.
- 40.Schootman M, Jeffe DB. Identifying factors associated with disability-related differences in breast cancer screening (United States). Cancer Causes Control. 2003;14:97–107. [DOI] [PubMed]
- 41.Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav. 1995;36:1–10. [DOI] [PubMed]
- 42.McCullagh P, Nelder JA. Generalized Linear Models. 2nd ed. London: Chapman and Hall; 1989.
- 43.Dailey AB, Kasl SV, Holford TR, et al. Neighborhood-level socioeconomic predictors of nonadherence to mammography screening guidelines. Cancer Epidemiol Biomarkers Prev. 2007;16:2293–2303. [DOI] [PubMed]
- 44.O’Malley AS, Renteria-Weitzman R, Huerta EE, et al. Patient and provider priorities for cancer prevention and control: a qualitative study in Mid-Atlantic Latinos. Ethn Dis. 2002;12:383–391. [PubMed]
- 45.Calvocoressi L, Kasl SV, Lee CH, et al. A prospective study of perceived susceptibility to breast cancer and nonadherence to mammography screening guidelines in African American and White women ages 40 to 79 years. Cancer Epidemiol Biomarkers Prev. 2004;13:2096–2105. [PubMed]
- 46.Hyndman JC, Holman CD, Dawes VP. Effect of distance and social disadvantage on the response to invitations to attend mammography screening. J Med Screen. 2000;7:141–145. [DOI] [PubMed]
- 47.Zenk SN, Tarlov E, Sun J. Spatial equity in facilities providing low- or no-fee screening mammography in Chicago neighborhoods. J Urban Health. 2006;83:195–210. [DOI] [PMC free article] [PubMed]
- 48.Casey MM, Thiede Call K, Klingner JM. Are rural residents less likely to obtain recommended preventive healthcare services? Am J Prev Med. 2001;21:182–188. [DOI] [PubMed]
- 49.Missouri Cancer Registry. Cancer MICA. http://www.dhss.mo.gov/CancerMICA/index.html. Accessed on September 26, 2007.
- 50.Chang SW, Kerlikowske K, Napoles-Springer A, et al. Racial differences in timeliness of follow-up after abnormal screening mammography. Cancer. 1996;78:1395–1402. [DOI] [PubMed]
- 51.Jones BA, Reams K, Calvocoressi L, et al. Adequacy of communicating results from screening mammograms to African American and White women. Am J Public Health. 2007;97:531–538. [DOI] [PMC free article] [PubMed]