Summary
Increased airway responsiveness occurs in normal young individuals compared to adults. A maturation of airway smooth muscle (ASM) contractility is likely a mechanism of this juvenile airway hyperresponsiveness. Indeed, we showed in guinea pig tracheal smooth muscle (TSM) that maximum shortening velocity decreases dramatically after the first 3 weeks of life. Because the phosphorylation of the 20-kDa myosin light chain (MLC20) was shown to be a key event in ASM contractility, in the present work we sought to investigate it during ontogenesis. In three age groups (1-week-old, 3-week-old, and adult guinea pigs), we assessed the amount of MLC20 phosphorylation achieved either in TSM crude protein homogenates exposed to Mg2+·ATP·CaCl2 or in tracheal strips during electrical field stimulation (EFS). Phosphorylated and unphosphorylated MLC20 were separated on nondenaturing 10% polyacrylamide gels, and the ratio of phosphorylation was obtained by densitometric analysis of chemiluminescent Western immunoblots. Maximum MLC20 phosphorylation (% of total MLC20) in TSM tissue homogenate was, respectively, 32.6 ± 5.7, 32.2 ± 5.7, and 46.8 ± 5.8 in 1-week, 3-week, and adult guinea pigs. Interestingly, in nonstimulated intact tracheal strips, we found a substantial degree of MLC20 phosphorylation: respectively, 42.2 ± 5.8, 36.5 ± 7.8, and 46.4 ± 4.7 in 1-week, 3-week, and adult guinea pigs. Maximal EFS-induced MLC20 phosphorylation (% increase over baseline) in the 3-week age group was attained after 3 sec of EFS, and was 161.2 ± 17.6, while in 1-week and adult guinea pigs, it was attained at 1.5 sec of EFS and was, respectively, 133.3 ± 9.3 and 110.2 ± 3.9 (P<0.05). We conclude that MLC20 phosphorylation in guinea pig intact tracheal strips correlates with ontogenetic changes in shortening velocity and changes in myosin light chain kinase content. These results further suggest that the maturation of ASM contractile properties plays a role in the greater airway responsiveness reported in children and young animals.
Keywords: airway reactivity, airway smooth muscle, asthma, contraction, maturation
INTRODUCTION
Airway responsiveness, i.e., the extent to which a given contractile stimulus constricts the airway lumen, is elevated in young healthy individuals and declines toward adulthood.1–3 This change in normal airway physiology may contribute to the age differences reported for diseases characterized by airway hyperresponsiveness, such as asthma. Indeed, the incidence of this disease has a peak during infancy and early childhood, and its prevalence decreases in adulthood.4–7 Mechanisms potentially involved in the ontogenetic modification of airway responsiveness include neural, pharmacological, and structural factors.8–10 A crucial role might be played by a maturation of the response of airway smooth muscle (ASM),11–13 as shown by the change with age of several ASM factors involved in contractility.14–16 Whereas ample evidence suggests an important role of ASM in pathological airway hyperresponsiveness in adults,17–20 little is known on the role of ASM function in the ontogenesis of airway responsiveness.21 To elucidate the mechanisms behind the maturation of ASM function is essential for revealing the possible role of ASM in the onset of diseases characterized by airway hyperresponsiveness during childhood.
While the ability of ASM to generate force has not shown a consistent correlation with in vivo airway responsiveness, accumulating data suggest that shortening velocity may closely resemble the dynamics of airway responsiveness.22–24 It is worth mentioning that airway myocytes from mild asthmatic subjects reveal a substantial increase of shortening velocity and shortening capacity compared with normal control volunteers.19 We used the guinea pig trachealis as a maturational model for ASM, and showed that maximum shortening velocity decreases after age 3 weeks, without a parallel change in force generation. This decrease is associated with an increase of internal resistance to shortening.25 On the one hand, this reduction in shortening velocity may be explained by a downregulating effect of mechanical resistive components, e.g., higher tissue stiffness, in adult guinea pigs. On the other hand, it might arise from differences in the biochemical pathway that lead to activation of the actomyosin ATPase.26
The phosphorylation of the 20-kDa myosin light chain (MLC20) activates the actomyosin ATPase, with subsequent recruitment of fast-cycling cross-bridges and the generation of muscle contraction. The regulation of MLC20 phosphorylation occurs by the counteracting action of myosin light chain kinase (MLCK) and myosin light chain phosphatase. We recently reported that the content of MLCK in guinea pig tracheal smooth muscle significantly increases during the first 3 weeks of life, and later declines to the levels observed in 1-week-old tissue.27 We suggested that this transient increase in MLCK content is one of the mechanisms responsible for the increased shortening velocity we previously reported at 3 weeks in the same animal model.
In the current study, we sought to further investigate the mechanisms responsible for smooth muscle hyperresponsiveness at 3 weeks of age. We therefore studied MLC20 phosphorylation in tracheal smooth muscle from 1-week-old, 3-week-old, and adult guinea pigs. To evaluate the inducible activity of MLCK in tracheal smooth muscle of different ages, MLC20 phosphorylation was studied, using an in vitro assay in tracheal smooth muscle tissue homogenates. To evaluate the involvement of MLC20 phosphorylation in maturational changes in contractility, we measured it in intact tracheal strips either at rest or during electrical field stimulation.
MATERIALS AND METHODS
Animals and Tissue Preparation
We employed Hartley guinea pigs (Charles River Laboratories, Inc., Wilmington, MA) of three different ages: 1 week old (1 wk, n = 42, 140.1 ± 21.3 (SD) g, 7.0 ± 1.2 days old), 3 weeks old (3 wk, n = 22,280.8 ± 24.8 g, 23.2 ± 3.0 days old), and 3 months old (adult, n = 16, 699.7 ± 121.2 g, 86.3 ± 26.6 days old). Only male animals were used for the 3-week and adult groups. The animal protocol was approved by the Duke University Institutional Animal Care and Use Committee.
Anesthesia was achieved with an intraperitoneal injection of 200 mg/kg Na-pentobarbital (Abbot Laboratories, Chicago, IL). When all reflexes observed in response to a toe-clamping were completely abolished, the trachea and lungs were exposed, excised, and immediately put into ice-cold Krebs-Henseleit buffer solution (K-H), aerated with 95% O2 and 5% CO2. The composition of K–H was the following (mM): 115 NaCl, 25 NaHCO3, 1.38 NaH2PO4, 2.5 KCl, 2.46 MgSO4, 1.9 CaCl2, and 5.55 dextrose. After cleaning away loose connective tissue, either tracheal strips or tracheal smooth muscle (TSM) tissues were obtained under a dissecting stereomicroscope (model SZH10, Olympus, Lake Success, NY). All procedures were performed in K-H solution buffered to pH 7.35–7.45 by continuous aeration with 95% O2/5% CO2.
MLC20 Phosphorylation Assay in Homogenated TSM
We previously showed that smooth muscle is only about 18% of the tissue forming the guinea pig paries membranaceus trachea.25 Therefore, after cleaning away loose connective tissue, a more careful dissection was carried out in order to obtain a preparation predominantly composed of smooth muscle. Cartilage rings were cut ventrally along the longitudinal axis of the trachea. The inner surface was exposed by mounting the trachea in a dissection Petri dish with stainless-steel insect pins (size 00, Ward’s, Rochester, NY), and the epithelium was gently removed with the smooth edge of curved forceps. The trachea was then flipped in the dissection dish, and the layer of connective tissue on the external surface of the trachea was removed. Finally, the muscle was detached from the edges of the cartilage, weighed (after removing the excess liquid with absorptive paper), frozen in liquid nitrogen, and stored at −80°C until used for the phosphorylation assay. The average quantity of tissue isolated from a single trachea was, respectively, 2.3, 4.0, and 7.9 mg for 1-week, 3-week, and adult animals. Histological analysis confirmed that the tissue isolated according to this procedure was primarily smooth muscle.
TSM tissue from different animals was pooled to prepare crude protein homogenates. We obtained, respectively, 4, 3, and 4 homogenate samples (average weight = 22.5 mg) from 1-week (n = 37), 3-week (n = 17), and adult (n = 12) guinea pigs. Each 1-week, 3-week, and adult sample was composed of TSM tissue from 6–11, 4–7, and 2–4 animals, respectively. The homogenate was dissolved in a buffer containing 60 mM KCl, 20 mM imidazole, 10 mM sodium azide, 1 mM L-cysteine, 1 mM MgCl2, 1 mM ouabain, 1 mM dithiothreitol, 0.25 mM phenylmethylsulfonyl fluoride, and 0.001% leupeptin. The suspension of each sample was then split into five aliquots of equal amounts, in which MLC20 phosphorylation was induced with 0.1 M Mg++-ATP/5 mM CaCl2 and stopped after 0, 10, 20, 40, and 60 sec with 50% trichloroacetic acid. After spinning at 13,000g, at 4°C, for 30 min, a solution of 10% TCA and 10 mM DTT in acetone was added to the pellet and stored overnight at −80°C. To extract proteins, the solution was replaced with 10 mM DTT in acetone for 1 hr at room temperature, and then the pellet was resuspended in urea sample buffer and shaken at room temperature on a tube rotator for 90 min. The composition of urea sample buffer was: 6.4 M urea, 29 mM glycine, 27 mM tris, 10 mM DTT, 10 mM EGTA, 1 mM Na2EDTA, 5 mM NaF, and 1 mM phenylmethylsulfonyl fluoride. Then, phosphorylated and unphosphorylated MLC20 were separated by electrophoresis, and the ratio of phosphorylation was obtained by densitometric analysis of chemiluminescent immunoblots.
Electrical Field Stimulation-Induced Phosphorylation in Intact Tracheal Strips
For this set of experiments, we used, respectively, 5, 5, and 4 animals of the 1-week, 3-week, and adult groups. From each animal, five parallel–fibered tracheal strips (2–3 mm in width) were obtained from transverse sections of the trachea and were dissected with ~2-mm cartilaginous attachments at both ends. The two cartilaginous ends were used to mount the strip, with 4-0 braided silk surgical thread, in a 1-ml organ bath filled with K-H solution, prepared as above (PO2 600 mmHg, PCO2 40 mmHg, pH 7.4, 37°C) and to connect it to a force transducer. The organ bath was specifically designed for the quick-freeze procedure needed to obtain phosphorylation measurements at any given time during a contraction. This apparatus allows a solution of acetone at dry-ice temperature to replace the K-H in the bath, so that the strip freezes and the phosphorylation reaction is arrested within 300 msec from the trigger time.28
In the presence of 10−5 M indomethacin, after equilibrating for 60 min, supramaximal electrical field stimulation (EFS: 18 V, 60 Hz, 400 mA/cm2) was elicited by plate platinum electrodes positioned on both sides of the strip. A partial length-tension curve was obtained by stretching the strips to increasing length and recording the isometric response to EFS until the optimal length was attained. Then the strip was frozen at a given time after onset of electrical stimulation. The five different strips obtained from each trachea were frozen, respectively, at 0, 0.7, 1.5, 3, and 9 sec during a stimulus. Frozen strips were quickly removed from the organ bath, transferred to acetone containing 10% TCA and 10 mM DTT at dry-ice temperature, and stored at −80°C. To perform protein extraction, the solution was replaced first with 10 mM DTT in acetone for 1 hr at room temperature, then with urea sample buffer (described above), and put on a tube rotator for 90 min. To quantify MLC20 phosphorylation, electrophoresis and Western immunoblotting were then performed.
Electrophoresis and Immunoblotting
Phosphorylated and unphosphorylated MLC20 were separated by nondenaturing 10% polyacrylamide gel electrophoresis, according to a modification of the method of Hathaway and Haeberle.29 Gels were subjected to 1 hr of pre-electrophoresis at 400 V, before loading equal amounts of each sample and performing electrophoresis at 400 V, at 10°C, for 14 hr. Lower chamber buffer contained (in mM) 22 glycine and 20 tris, while upper chamber buffer contained 22 glycine, 20 tris, 3 dithiothreitol, and 1 L-cysteine. Proteins were then transferred to nitrocellulose membrane at 1.5 A for 1.5 hr, using a 25-mM Na2HPO4 transfer buffer. Nonspecific binding sites were blocked by incubating the membrane for 2 hr at room temperature in tris-base saline (TBS) containing 5% nonfat dry milk. Western immunoblots were developed using, in sequence, a monoclonal mouse antibody to smooth muscle MLC20 (1:1,000, Clone MY-21), biotinylated anti-mouse-specific IgG (1:1,000), and streptavidin-horseradish peroxidase conjugate (1:5,000). Before and after incubation with anti-MLC20 antibody, membranes were rinsed three times in TBS containing 1% nonfat dry milk and 0.1% Tween-20. After incubation with anti-IgG and streptavidin-horseradish peroxidase, membranes were rinsed three times in TBS containing 0.1% Tween-20. Enhanced chemiluminescence (ECL+) was employed to detect MLC20 bands on X-OMAT AR film (Kodak, Rochester, NY). Densitometric quantification of Western immunoblots was then obtained with NIH Image 1.62 analytical software.
Drugs and Chemicals
Monoclonal antibodies anti-myosin light chain 20K (clone MY-21), leupeptin, imidazole, ouabain, phenylmethylsulfonyl fluoride, EGTA, Na2EDTA, NaF, L-cysteine, and indomethacin were purchased from Sigma Chemical Co. (St. Louis, MO); biotinylated anti-mouse-specific IgG, streptavidin-horseradish peroxidase, nitrocellulose membrane, and ECL+ were obtained from Amersham Pharmacia Biotech (Piscataway, NJ); dithiothreitol was from ICN Biomedicals (Aurora, OH); sodium azide was from Fisher Scientific (Fair Lawn, NJ); glycine, tris, and tricloroacetic acid were from EM Science (Gibbstown, NJ); and urea was from Mallinckrodt Chemicals (Paris, KY).
Data Analysis
Data are expressed as mean ± standard error of the mean, except where otherwise indicated. Statistical analyses performed were analysis of variance (ANOVA), and the post hoc least significant difference (PLSD) Fisher’s test to find out which groups were responsible for differences shown by ANOVA. The software employed was Statistix (Analytical Software, Tallahassee, FL). P <0.05 was considered significant.
RESULTS
MLC20 Phosphorylation Assay in Homogenated TSM
MLC20 phosphorylation induced in TSM tissue homogenate was significantly greater in samples from adults than in samples from both 1-week and 3-week animals. A typical chemilumigram showing phosphorylated and unphosphorylated MLC20 bands is shown in Figure 1A. The results of densitometric analysis of the bands expressed as phosphorylation ratio, i.e., phosphorylated MLC20 as fraction of total MLC20, attained at different time points of the in vitro assay, are shown in Figure 1B. The phosphorylation ratio was higher in homogenates from adult tissue than in homogenates from both 1-week and 3-week tissue (P <0.05 by ANOVA). As revealed by the absence of the related band, no MLC20 phosphorylation was observed in tissue homogenate prior to the addition of Mg++-ATP-CaCl2 (time 0).
Fig. 1.
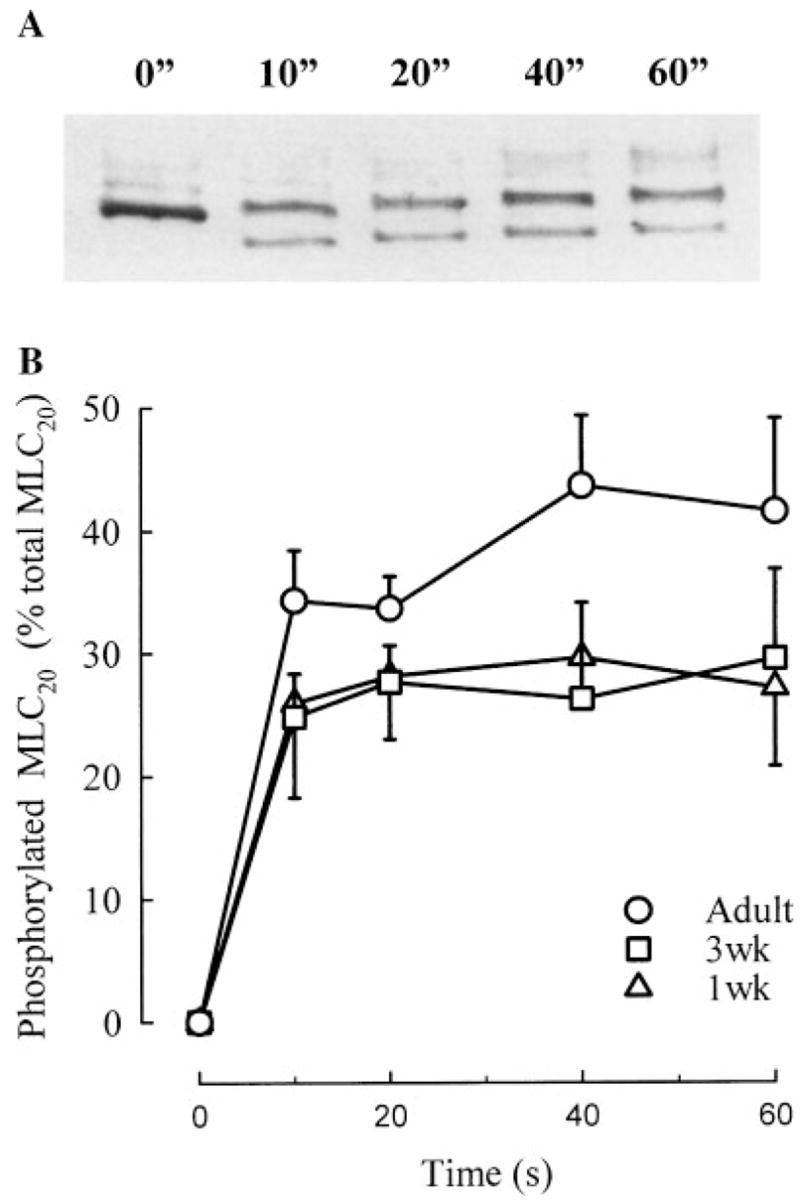
A: Typical chemilumigram with unphosphorylated and phosphorylated MLC20 bands at different times after activation with Mg++-ATP-CaCl2. Lowest band is phosphorylated MLC20, and is not present at time 0. B: Average values of MLC20 phosphorylation, expressed as % of total MLC20, in tracheal smooth muscle from 1-week-old (1 wk, n = 4), 3 week-old (3 wk, n = 3), and adult guinea pigs (n = 4). Values are means ± SE. MLC20 phosphorylation was significantly higher in adult compared to 1-week and 3-week samples (P<0.05 by ANOVA;”, seconds).
Electrical Field Stimulation-Induced Phosphorylation in Intact Tracheal Strips
When we measured it in intact strips, we found an elevated ratio of MLC20 phosphorylation in the absence of any stimulation (Fig. 2). A similar degree of phosphorylation was present in strips from all age groups. Strips were incubated with 10−5 M indomethacin, a concentration that inhibits the guinea pig ASM characteristic intrinsic tone, suggesting that baseline phosphorylation may not be related to spontaneous smooth muscle tone. As a control, we also measured baseline phosphorylation in TSM from adult swine, a species in which no intrinsic tone is present. In this preparation, we found a similar extent of MLC20 phosphorylation, i.e., 37 ± 10.3%.
Fig. 2.
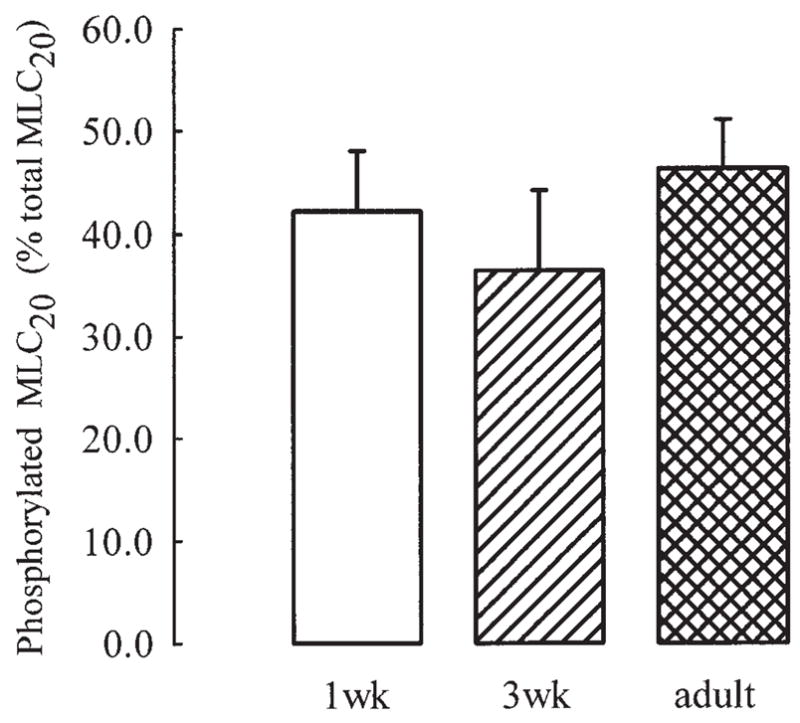
Phosporylated MLC20, expressed as % of total MLC20, in unstimulated tracheal strips from guinea pigs of different ages. Values are means ± SE.
In strips frozen during EFS, MLC20 phosphorylation rapidly increased and reached its maximum at 1.5 sec in 1-week and adult animals, while it increased further and reached a maximum at 3 sec in 3-week animals. Maximal phosphorylation in 1-week, 3-week, and adult strips was, respectively, 56.6 ± 4.0%, 60.1 ± 4.5%, and 51.9 ± 4.8% of total MLC20. We used level of phosphorylation at rest as a baseline to express MLC20 phosphorylation induced by EFS, i.e., for each age group, phosphorylation at rest was set as the baseline (100%), and phosphorylation levels reached during EFS were expressed as percentage of baseline in that group. The increase in phosphorylation produced by EFS was significantly higher in 3-week-old guinea pigs than in strips from younger and older animals (Fig. 3, P <0.01 by ANOVA). Similar results were obtained by expressing EFS-induced phosphorylation as a percentage of total MLC20 in each sample. Using this form of normalization, a maximal phosphorylation increment of 14.4%, 23.6%, and 5.5% was found in 1-week, 3-week, and adult samples, respectively. At 9 sec of EFS, MLC20 phosphorylation was noticeably reduced in all three age groups. No significant difference from baseline was found at this time point.
Fig. 3.
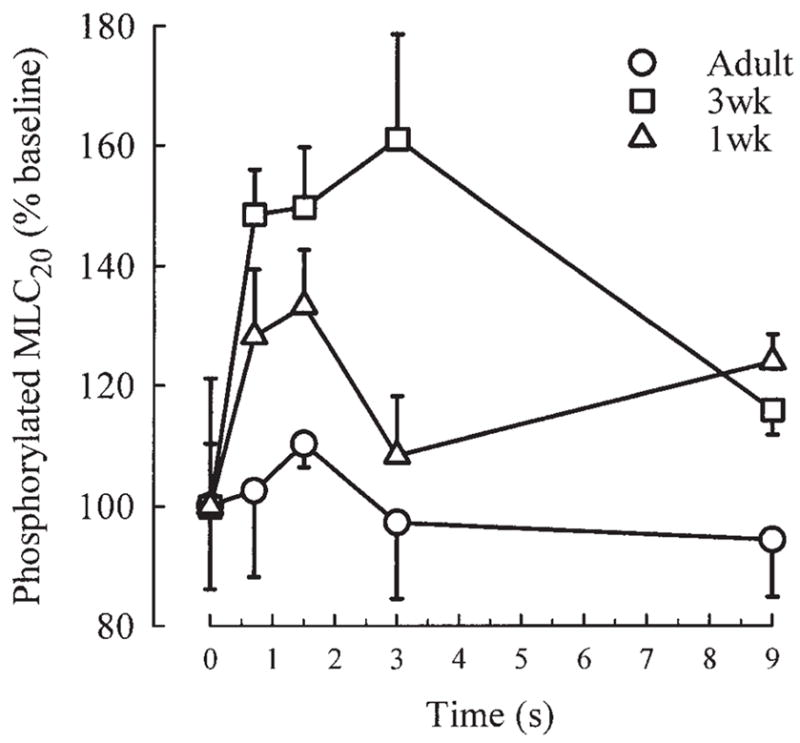
Phosporylated MLC20, expressed as % of level of MLC20 phosporylated at rest (% baseline), in tracheal strips from guinea pigs of different ages quick-frozen at different time points after onset of electrical field stimulation. Values are means ± SE. Increment of MLC20 phosphorylation over baseline was significantly higher in 3 wk (n = 5) compared to 1 wk (n = 5) and adult (n= 4) strips (P<0.01 by ANOVA).
In each strip used for these experiments, we performed a partial length-tension curve in order to freeze them at their optimal length. Therefore, we could compare the level of MLC20 phosphorylation found in each strip with the tension generated in the same strip during EFS at the time when the strip was frozen. This time course of EFS-induced tension is given in Figure 4 and shows no correlation between MLC20 phosphorylation and tension development.
Fig. 4.
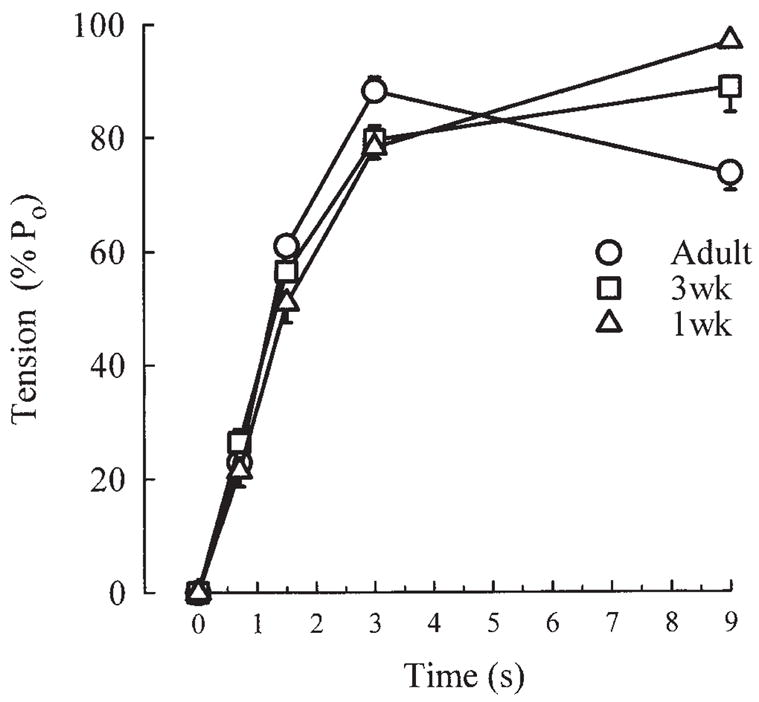
Tension produced in response to EFS by same tracheal strips as in Figure 3 at time points when strips were frozen. Values are means ± SE, and are expressed as % of maximal tension produced in each strip at optimal length (%P0).
DISCUSSION
In the present study, we investigated the phosphorylation of the 20-kDa MLC20 in 1-week-old, 3-week-old, and 2–3-month-old guinea pigs. We assessed the level of MLC20 phosphorylation induced either in tracheal strips by EFS or in TSM crude protein homogenates by Mg2+·ATP·CaCl2. We found that a substantial MLC20 phosphorylation occurs in basal conditions in intact strips of all ages, and that EFS induces a greater increase of MLC20 phosphorylation in 3-week-old animals. These results show that the amount of EFS-induced phosphorylation of the regulatory MLC20 at a given age reflects the measure of shortening velocity and the content of MLCK, as we previously reported in TSM for the same age.25,27 By contrast, the phosphorylation assay showed a greater ratio of phosphorylation in homogenates from adults compared to younger animals, suggesting that maturational changes of other factors may contribute to determine MLC20 phosphorylation in intact tissue during ontogenesis.
We previously showed that ASM contractility varies during ontogenesis,25 with a trend that parallels airway responsiveness.1–3 We used guinea pigs at different stages of maturation, and showed that maximal shortening velocity decreases significantly after the third week of life, but maximal force production per unit cross-sectional area of tracheal smooth muscle does not change significantly. In order to integrate the results of the present study with our previous findings and suggest a role for MLC20 phosphorylation in the maturation of ASM contractility, we must consider three distinct issues that may affect our results and/or their interpretation. A first point to consider is how different factors may participate in regulating the level of MLC20 phosphorylation in different experimental conditions and tissues. Second, one has to ponder to what extent the level of MLC20 phosphorylation influences ASM shortening velocity. Finally, factors not related to the level of MLC20 phosphorylation may also contribute to ASM shortening velocity. These three issues may all be essential in understanding the relevance of MLC20 phosphorylation in the ontogenesis of ASM contractility.
The phosphorylation of MLC20 is mainly regulated by the activity of MLCK and myosin light chain phosphatase. Both these enzymes are in turn regulated by protein kinases, with consequent further modulation of MLC20 phosphorylation. Among those potentially responsible for increased MLC20 phosphorylation, the content of MLCK is the only factor that was shown to be increased in sensitized dogs.30 An increased content of MLCK was also reported in sensitized human airways.17 We recently showed in guinea pig tracheal smooth muscle that MLCK content increases significantly during the first 3 weeks of life and later declines to the levels observed in tissue from 1-week-old animals.27 Therefore, MLCK protein content is conceivably an important factor in determining the level of MLC20 phosphorylation in hyperresponsive ASM. In the present investigation, we used an in vitro MLC20 phosphorylation assay to evaluate MLCK activity in TSM of different ages. The assay is based on the measurable ratio of induced phosphorylation in tissue homogenate, and was successfully used in canine ASM to show that the MLCK content and MLC20 phosphorylation in the in vitro phosphorylation assay have a similar increment in sensitized compared to control animals.30 This observation indicates that, although the amount of MLCK was altered by allergen-sensitization, its specific activity was not. In our study, we found that the homogenate of adult tissue generated a higher ratio of phosphorylation upon activation than tissue from younger animals. It is therefore possible that MLCK-specific activity is lower in 3-week animals, in which MLCK protein is more abundant. We also found that the level of MLC20 phosphorylation was higher in intact tracheal strips than in tissue homogenates. This suggests that factors affecting MLCK and/or phosphatase activity may have a different degree of effect in homogenate compared to intact strips. The lower level of phosphorylation in homogenized tissue could be due to competition among different kinases for the same substrate, and consequent insufficient ATP available for MLCK. However, this is unlikely because the concentration of Mg++ ATP used in the present study was chosen to provide a sufficient substrate for maximum phosphorylation of MLC20. An alternative explanation of our result derives from the notion that MLCK can be phosphorylated by different kinases, resulting in a reduction of its activity.31 The activation of these kinases cannot be ruled out in the activity assay we employed. If these inhibitory kinases are activated in the homogenates, our result would suggest that the inhibitory effect in reducing MLC20 phosphorylation varies with age and is more pronounced in younger animals. More conceivably, the negative regulation of phosphatase activity normally occurring in intact tissue26 is reduced in tissue homogenate, therefore reducing the level of phosphorylation. Indeed, we performed the assay in the absence of phosphatase inhibitors for consistency with the experiments in intact strips. In intact cells, myosin light chain phosphatase is regulated by the action of small GTPase Rho32 on Rho kinase, which in turn increases MLC20 phosphorylation by phosphorylating, and thus inhibiting, myosin light chain phosphatase.33 If this inhibitory regulation is reduced or absent in tissue homogenates, our results could be explained by a greater myosin light chain phosphatase inhibition in young animals compared to adults. The removal of this inhibition in the homogenate would have a stronger effect in young tissue, allowing a more pronounced MLC20 dephosphorylation and therefore a lower maximal ratio of phosphorylated MLC20. Whether a change in phosphatase activity, produced by a variation of either its content or its negative regulation, occurs in our maturational model is not known, and calls for further investigation.
MLC20 phosphorylation in intact strips is of course also affected by neurohumoral components involved in ASM activation. A nonadrenergic noncholinergic, as well as an adrenergic, innervation is present in guinea pigs.34,35 Both components indirectly affect MLCK activity by inducing cyclic nucleotide generation.36 Indeed, the main nonadrenergic noncholinergic mediator in guinea pigs is nitric oxide, which induces cGMP elevation and in turn lowers the intracellular concentration of Ca++, thus reducing MLCK activity. The adrenergic response is instead mediated by cAMP, which reduces MLCK activity through protein kinase A-dependent phosphorylation of MLCK. Although it would be important to reveal to what extent these two components participate in the regulation of MLC20 phosphorylation during ontogenesis, we designed our experiments to maintain both of them active. Our aim was to evaluate the level of MLC20 phosphorylation in minimally altered strips, i.e., with intact epithelium and without pharmacological inhibitions. The use of indomethacin was justified by the unstable resting tone characteristic of guinea pig, which would not have allowed us to obtain reproducible shortening velocity data in our previous investigation.25 As discussed below, shortening velocity correlates better than force with MLC20 phosphorylation, and we needed to maintain the same experimental conditions in order to compare these two parameters. Moreover, although indomethacin may affect MLC20 phosphorylation, we showed that this cyclooxygenase inhibitor increases relaxation in younger animals, hence reducing age differences in relaxation.37 This suggests that indomethacin might also reduce MLC20 phosphorylation in younger animals, and that the actual age differences could be stronger than those shown in the present paper. Therefore, we are confident that our results reflect physiologically relevant differences that can be related to ASM function and airway responsiveness.
A key relationship upon which we base the interpretation of our results is the notion that MLC20 phosphorylation is a major contributor to ASM shortening velocity. Several studies suggested a linear correlation between MLC20 phosphorylation and shortening velocity, but not force generation, in ASM.38,39 However, a few studies showed that this is not always the case,40–43 suggesting that other factors, e.g., the inhibitory action of thin filament proteins on actomyosin ATPase activity,40,41 may reduce the velocity of shortening. Dissociation between the two parameters was recently shown in canine TSM during the first 2–3 sec of stimulation.42 Similar to our study, a quick freeze at different times during EFS was used. Nonetheless, while in dog strips maximal phosphorylation was attained at 7 sec after the onset of stimulation, we found that it is reached at 1.5–3 sec of EFS in guinea pig strips. Therefore, one would expect that the dissociation of shortening velocity and MLC20 phosphorylation observed in canine TSM to be at least less pronounced in guinea pigs. The difference between the two studies may be dependent on species or on the presence of epithelium in our strips. Indeed, in our study, the preservation of an intact tracheal strip was preferred because removal of the epithelium was shown to increase phosphorylation,44 and our aim was to compare phosphorylation with our previous mechanical data obtained in intact strips. In the present study, we show that the increase in MLC20 phosphorylation produced by EFS in juvenile strips is significantly higher than in infant and adult strips. We previously showed that maximal EFS-induced tension in guinea pig tracheal strips does not change with age, and that the maximal rate of tension development slightly and progressively increases with age.25 We now show that the time course of force development during EFS is similar at all ages, despite the strong age differences in EFS-induced MLC20 phosphorylation. By contrast, we showed that shortening velocity at 2.5 sec during EFS increases, although not significantly, from 1-week to 3-week and later decreases significantly. We find now that EFS-induced MLC20 phosphorylation follows the same age trend. More specifically, one can infer by interpolation of the MLC20 phosphorylation curve at 2.5 sec that shortening velocity and MLC20 phosphorylation closely correlate.
Although our study showed that the ontogenesis of MLC20 phosphorylation induced by EFS parallels the maturation of shortening velocity, other factors may play a role in the ontogenesis of shortening velocity. Indeed, it was observed that, in response to different agonists or in smooth muscle from different origins, the same level of phosphorylation may be associated with a different velocity of shortening.43 The regulation of shortening velocity in different tissues may derive from changes in both contractile and cytoskeletal proteins, such as those reported during development and remodeling.45 The insertion of a seven-amino-acid domain in the myosin heavy chain seems responsible for the differences in shortening velocity between vascular and intestinal smooth muscle.46 Moreover, the alteration of either the external load or the internal resistance to shortening may modify shortening velocity. Indeed, we showed a conspicuous increase of the internal resistance to shortening in adult tracheal strips,25 and we suggested that this is a mechanism to reduce the shortening velocity toward adulthood. In light of our present results, we suggest that the reduction of shortening velocity with maturation originates from the concomitant reduced level of MLC20 phosphorylation and increased resistance to shortening.
The high baseline phosphorylation in unstimulated tracheal strips was partially unexpected, since a phosphorylation not exceeding 30% of the total MLC20 was reported in unstimulated intact tissue. However, data reported in the literature are extremely variable both among and within species. In species that do not show smooth muscle intrinsic tone, MLC20 phosphorylation at rest varies from 4.0–14.4% in healthy dog trachealis,30,42 to 29% in bovine,47 and 31.5% in allergic dog tracheal smooth muscle.30 Assuming that the characteristically elevated intrinsic tone of guinea pig ASM would have affected the basal level of MLC20 phosphorylation, we performed stimulation and quick freeze of tracheal strips in a solution containing the cyclooxygenase inhibitor indomethacin, which abolishes intrinsic tone in this species. Nonetheless, we found about 40% phosphorylation in the absence of EFS, although no age difference was observed. As a control, we measured basal phosphorylation in TSM from adult swine, a species in which no intrinsic tone is present, and found a similar degree of MLC20 phosphorylation. It is therefore possible that either intrinsic tone is not the result of basal level of MLC20 phosphorylation, or an uncoupling of MLC20 phosphorylation and tone occurs in given species or conditions. In the first case, a function of MLC20 phosphorylation other than the activation of actomyosin ATPase should be hypothesized. In the second case, the administration of a stimulus and the consequent signaling cascade would reestablish the required coupling of MLC20 phosphorylation and tension, thus allowing smooth muscle to contract. Further studies will be required to test these hypotheses. An important implication of elevated baseline MLC20 phosphorylation is that the smooth muscle contractile response could be determined more by the portion of phosphorylation attained after stimulation than by its total level. In our results, only a 10.2% increase in MLC20 phosphorylation was observed in adult strips in response to EFS. This is a particularly low value, which fits the observation that shortening velocity at that age is also dramatically reduced compared to the other two age groups. Similarly, in the canine allergic model, maximum phosphorylation produced by EFS in control tracheal smooth muscle (32.6%) was similar to the MLC20 phosphorylation in sensitized strips at rest (31.5%), suggesting once again that the total level of phosphorylation does not reflect the level of activation.
In conclusion, we showed that the MLC20 phosphorylation induced by EFS in intact tracheal strips is greater in 3-week-old than in younger and older guinea pigs. The ontogenetic variation in the amount of EFS-induced phosphorylation correlates with changes we previously reported in both the MLCK content of TSM and the maximal shortening velocity of guinea pig trachealis. The present results give further evidence that ASM undergoes maturational changes, and strengthen our suggestion that the ontogenesis of ASM responsiveness contributes to maturational changes in airway responsiveness and potentially to determine the higher susceptibility of young individuals to develop obstructive airway diseases.
Acknowledgments
The authors thank Tao Fan, Kelley Hutcheson, and Dr. Shyamala Dakshinamurti for help in setting up the experimental conditions of different sets of experiments, Viviana Cantillana for technical assistance, and Dr. Andrew J. Halayko for valuable advice. The authors thank Dr. Jonathan A. Hata (Department of Surgery, Duke University Medical Center) for kindly providing the swine tracheas used to obtain control tracheal smooth muscle, as described in Results.
Grant sponsor: ALA; Grant sponsor: NIH; Grant numbers: HL48376, HL61899; Grant sponsor: Duke Children’s Miracle Network.
Footnotes
This work was partially presented in preliminary form at the 2000 meeting of the European Respiratory Society and at the 2003 meeting of the American Thoracic Society.
References
- 1.Hopp RJ, Bewtra A, Nair NM, Townley RG. The effect of age on methacholine response. J Allergy Clin Immunol. 1985;76:609–613. doi: 10.1016/0091-6749(85)90783-3. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery GL, Tepper RS. Changes in airway reactivity with age in normal infants and young children. Am Rev Respir Dis. 1990;142:1372–1376. doi: 10.1164/ajrccm/142.6_Pt_1.1372. [DOI] [PubMed] [Google Scholar]
- 3.Shen X, Bhargava V, Wodicka GR, Doerschuk CM, Gunst SJ, Tepper RS. Greater airway narrowing in immature than in mature rabbits during methacholine challenge. J Appl Physiol. 1996;81:2637–2643. doi: 10.1152/jappl.1996.81.6.2637. [DOI] [PubMed] [Google Scholar]
- 4.Larsen GL. Differences between adult and childhood asthma. J Allergy Clin Immunol. 2000;106:S153–S157. doi: 10.1067/mai.2000.109421. [DOI] [PubMed] [Google Scholar]
- 5.Lemanske RF. Issues in understanding pediatric asthma: epidemiology and genetics. J Allergy Clin Immunol. 2002;109:S521–S524. doi: 10.1067/mai.2002.124564. [DOI] [PubMed] [Google Scholar]
- 6.Martinez FD. Links between pediatric and adult asthma. J Allergy Clin Immunol. 2001;107:S449–S455. doi: 10.1067/mai.2001.114993. [DOI] [PubMed] [Google Scholar]
- 7.Von Mutius E. The burden of childhood asthma. Arch Dis Child. 2000;82:ii2–ii5. doi: 10.1136/adc.82.suppl_2.ii2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busse WW, Banks-Schlegel SP, Larsen GL. Effects of growth and development on lung function. Models for study of childhood asthma. Am J Respir Crit Care Med. 1997;156:314–319. doi: 10.1164/ajrccm.156.1.9612121. [DOI] [PubMed] [Google Scholar]
- 9.Ishida K, Fukuchi Y. The effect of aging on airway responsiveness. Nippon Kyobu Shikkan Gakkai Zasshi. 1992;30:182–186. [PubMed] [Google Scholar]
- 10.Saunder RA, McNikol KJ, Stecenko AA. Effect of age on lung mechanics and airway reactivity in lambs. J Appl Physiol. 1986;61:2074–2080. doi: 10.1152/jappl.1986.61.6.2074. [DOI] [PubMed] [Google Scholar]
- 11.Panitch HB, Allen JL, Ryan JP, Wolfson MR, Shaffer TH. A comparison of preterm and adult airway smooth muscle mechanics. J Appl Physiol. 1989;66:1760–1765. doi: 10.1152/jappl.1989.66.4.1760. [DOI] [PubMed] [Google Scholar]
- 12.Sparrow MP, Mitchell HW. Contraction of smooth muscle of pig airway tissues from before birth to maturity. J Appl Physiol. 1990;68:468–477. doi: 10.1152/jappl.1990.68.2.468. [DOI] [PubMed] [Google Scholar]
- 13.Tepper RS, Du T, Styhler A, Ludwig M, Martin JG. Increased maximal pulmonary response to methacholine and airway smooth muscle in immature compared with mature rabbits. Am J Respir Crit Care Med. 1995;151:836–840. doi: 10.1164/ajrccm.151.3.7881679. [DOI] [PubMed] [Google Scholar]
- 14.Fisher JT. Airway smooth muscle contraction at birth: in vivo versus in vitro comparisons to the adult. Can J Physiol Pharmacol. 1992;70:590–596. doi: 10.1139/y92-075. [DOI] [PubMed] [Google Scholar]
- 15.Murphy TM, Mitchell RW, Phillips JP, Leff AR. Ontogenic expression of acetylcholinesterase activity in trachealis of young swine. Am J Physiol. 1991;261:L322–L326. doi: 10.1152/ajplung.1991.261.4.L322. [DOI] [PubMed] [Google Scholar]
- 16.Wills-Karp M. Effects of ageing upon airway smooth muscle contractility. In: Raeburn D, Giembycz MA, editors. Airway smooth muscle: development and regulation of contractility. Basel: Birkhäuser Verlag; 1994. pp. 185–218. [Google Scholar]
- 17.Ammit AJ, Armour CL, Black JL. Smooth-muscle myosin light-chain kinase content is increased in human sensitized airways. Am J Respir Crit Care Med. 2000;161:257–263. doi: 10.1164/ajrccm.161.1.9901005. [DOI] [PubMed] [Google Scholar]
- 18.Bramley AM, Thomson RJ, Roberts CR, Schellenberg RR. Hypothesis: excessive bronchoconstriction in asthma is due to decreased airway elastance. Eur Respir J. 1994;7:337–341. doi: 10.1183/09031936.94.07020337. [DOI] [PubMed] [Google Scholar]
- 19.Ma X, Cheng Z, Kong H, Wang Y, Unruh H, Stephens NL, Laviolette M. Changes in biophysical and biochemical properties of bronchial smooth muscle cells from asthmatic subjects. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1181–L1189. doi: 10.1152/ajplung.00389.2001. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell RW, Ruhlmann E, Magnussen H, Leff AR, Rabe KF. Passive sensitization of human bronchi augments smooth muscle shortening velocity and capacity. Am J Physiol. 1994;267:L218–L222. doi: 10.1152/ajplung.1994.267.2.L218. [DOI] [PubMed] [Google Scholar]
- 21.Chitano P, Murphy TM. Maturational changes in airway smooth muscle shortening and relaxation. Implications for asthma. Respir Physiol Neurobiol. 2003;137:347–359. doi: 10.1016/s1569-9048(03)00158-7. [DOI] [PubMed] [Google Scholar]
- 22.Antonissen LA, Mitchell RW, Kroeger EA, Kepron W, Tse KS, Stephens NL. Mechanical alterations of airway smooth muscle in a canine asthmatic model. J Appl Physiol. 1979;46:681–687. doi: 10.1152/jappl.1979.46.4.681. [DOI] [PubMed] [Google Scholar]
- 23.Duguet A, Wang CG, Gomes R, Ghezzo H, Eidelman DH, Tepper RS. Greater velocity and magnitude of airway narrowing in immature than in mature rabbit lung explants. Am J Respir Crit Care Med. 2001;164:1728–1733. doi: 10.1164/ajrccm.164.9.2011045. [DOI] [PubMed] [Google Scholar]
- 24.Fan T, Yang M, Halayko A, Mhapatra SS, Stephens NL. Airway responsiveness in two inbred strains of mouse disparate in IgE and IL-4 production. Am J Respir Cell Mol Biol. 1997;17:156–163. doi: 10.1165/ajrcmb.17.2.2628. [DOI] [PubMed] [Google Scholar]
- 25.Chitano P, Wang J, Cox CM, Stephens NL, Murphy TM. Different ontogeny of rate of force generation and shortening velocity in guinea pig tracheals. J Appl Physiol. 2000;88:1338–1345. doi: 10.1152/jappl.2000.88.4.1338. [DOI] [PubMed] [Google Scholar]
- 26.Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- 27.Chitano P, Voynow JA, Pozzato V, Cantillana V, Burch LH, Wang L, Murphy TM. Ontogenesis of myosin light chain kinase mRNA and protein content in guinea pig tracheal smooth muscle. Pediatr Pulmonol. 2004;38:456–464. doi: 10.1002/ppul.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maass-Moreno R, Burdyga T, Mitchell RW, Seow CY, Ragozzino J, Ford LE. Simple freezing apparatus for resolving rapid metabolic events associated with smooth muscle activation. J Appl Physiol. 2001;90:2453–2459. doi: 10.1152/jappl.2001.90.6.2453. [DOI] [PubMed] [Google Scholar]
- 29.Hathaway D, Haeberle JR. A radioimmunoblotting method for measuring myosin light chain phosphorylation levels in smooth muscle. Am J Physiol. 1985;249:C345–C351. doi: 10.1152/ajpcell.1985.249.3.C345. [DOI] [PubMed] [Google Scholar]
- 30.Jiang H, Rao K, Halayko AJ, Liu X, Stephens NL. Ragweed sensitization-induced increase of myosin light chain kinase content in canine airway smooth muscle. Am J Respir Cell Mol Biol. 1992;7:567–573. doi: 10.1165/ajrcmb/7.6.567. [DOI] [PubMed] [Google Scholar]
- 31.Word RA, Tang DC, Kamm KA. Activation properties of myosin light chain kinase during contraction/relaxation cycles of tonic and phasic smooth muscles. J Biol Chem. 1994;269:21596–21602. [PubMed] [Google Scholar]
- 32.Somlyo AP, Somlyo AV. From pharmacomechanical coupling to G-proteins and myosin phosphatase. Acta Physiol Scand. 1998;164:437–448. doi: 10.1046/j.1365-201X.1998.00454.x. [DOI] [PubMed] [Google Scholar]
- 33.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatzu A, Kaibuchi K. Regulation of myosin phosphorylation by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 34.Coburn RF, Tomita T. Evidence for nonadrenergic inhibitory nerves in the guinea pig trachealis muscle. Am J Physiol. 1973;224:1072–1080. doi: 10.1152/ajplegacy.1973.224.5.1072. [DOI] [PubMed] [Google Scholar]
- 35.Yip P, Palombini B, Coburn RF. Inhibitory innervation to the guinea pig trachealis muscle. J Appl Physiol. 1981;50:374–382. doi: 10.1152/jappl.1981.50.2.374. [DOI] [PubMed] [Google Scholar]
- 36.De Lanerolle P, Paul RJ. Myosin phosphorylation/dephosphorylation and regulation of airway smooth muscle contractility. Am J Physiol. 1991;261:L1–L14. doi: 10.1152/ajplung.1991.261.2.L1. [DOI] [PubMed] [Google Scholar]
- 37.Chitano P, Cox CM, Murphy TM. Relaxation of guinea pig trachealis during electrical field stimulation increases with age. J Appl Physiol. 2002;92:1835–1842. doi: 10.1152/japplphysiol.00688.2001. [DOI] [PubMed] [Google Scholar]
- 38.Hai CM, Murphy RA. Regulation of shortening velocity by cross-bridge phosphorylation in smooth muscle. Am J Physiol. 1988;255:C86–C94. doi: 10.1152/ajpcell.1988.255.1.C86. [DOI] [PubMed] [Google Scholar]
- 39.Dillon PF, Aksoy MO, Driska SP, Murphy RA. Myosin phosphorylation and the crossbridge cycle in arterial smooth muscle. Science. 1981;211:495–497. doi: 10.1126/science.6893872. [DOI] [PubMed] [Google Scholar]
- 40.Ngai PK, Walsh MP. Inhibition of smooth muscle actin-activated myosin Mg2+-ATPase activity by caldesmon. J Biol Chem. 1984;259:13656–13659. [PubMed] [Google Scholar]
- 41.Malmqvist U, Trybus KM, Yagi S, Carmichael J, Fay FS. Slow cycling of unphosphorylated myosin is inhibited by calponin, thus keeping smooth muscle relaxed. Proc Natl Acad Sci USA. 1997;94:7655–7660. doi: 10.1073/pnas.94.14.7655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell RW, Seow CY, Burdyga T, Maass-Moreno R, Pratusevich VR, Ragozzino J, Ford LE. Relationship between myosin phosphorylation and contractile capability of canine airway smooth muscle. J Appl Physiol. 2001;90:2460–2465. doi: 10.1152/jappl.2001.90.6.2460. [DOI] [PubMed] [Google Scholar]
- 43.Miller-Hance WC, Kamm KE. Force-velocity relation and myosin light chain phosphorylation in bovine coronary arterial smooth muscle. Circ Res. 1991;69:1207–1214. doi: 10.1161/01.res.69.5.1207. [DOI] [PubMed] [Google Scholar]
- 44.Wong CT, Hai CM. Mucosal modulation of agonist-induced myosin phosphorylation and contraction in airway smooth muscle. Respir Physiol. 1999;115:103–111. doi: 10.1016/s0034-5687(98)00106-6. [DOI] [PubMed] [Google Scholar]
- 45.Low RB, White SL. Lung smooth muscle differentiation. Int J Biochem Cell Biol. 1998;30:869–883. doi: 10.1016/s1357-2725(98)00049-1. [DOI] [PubMed] [Google Scholar]
- 46.Kelley CA, Takahashi M, Yu JH, Adelstein RS. An insert of seven amino acids confers functional differences between smooth muscle myosins from the intestines and vasculature. J Biol Chem. 1993;268:12848–12854. [PubMed] [Google Scholar]
- 47.Tseng S, Kim R, Kim T, Morgan KG, Hai CM. F-actin disruption attenuates agonist-induced [Ca2+], myosin phosphorylation, and force in smooth muscle. Am J Physiol. 1997;272:C1960–C1967. doi: 10.1152/ajpcell.1997.272.6.C1960. [DOI] [PubMed] [Google Scholar]


