Abstract
Evidence for contributions of airway smooth muscle (ASM) to the hyperresponsiveness of newborn and juvenile airways continues to accumulate. In our laboratory three novel paradigms of hyperresponsiveness of newborn and young ASM have recently emerged using a guinea pig model of maturation in three age groups-- 1 week (newborn); 3 week (juvenile) and 2−3 months (adult). These include 1) evidence for a natural decline after newborn and juvenile life of the shortening velocity of ASM shortening associated with a decrease in regulatory myosin light chain (MLC) phosphorylation and a parallel decline in the content of MLC kinase. Associated with the decrease in ASM shortening with age is an increase in the internal resistance to shortening. This relationship can be approximated as dP/dtmax ≈ dP/dLpassive × dL/dtmax (the maximal rate of increase of active stress generation ≈ the passive stiffness × the maximal shortening velocity V0). 2) The second paradigm demonstrates that newborn ASM, unlike that in adults, does not relax with prolonged electrical field stimulation. The impaired relaxation is related to changes in prostaglandin synthesis and acetylcholinesterase function; 3) the third paradigm demonstrates that while oscillatory strain serves to relax adult ASM, the response in newborns is the potentiation of active stress. This is related to developmental changes in the cytoskeleton. Oscillatory stiffness is shown to relate inversely to the expression of myosin light chain kinase. This suggests that developmental changes in shortening relate inversely to the stiffness of the ASM early in shortening, suggesting a dynamic role for the cytoskeleton in facilitating and opposing ASM shortening. Together these paradigms demonstrate that ASM contributes by multiple mechanisms to the natural hyperresponsiveness of newborn and juvenile airways. Future studies will elaborate the mechanisms and extend these paradigms relate to ASM hyperresponsiveness that is increased following sensitization in early life.
Keywords: ontogeny, airway hyperresponsiveness, airway smooth muscle, myosin light chain kinase, myosin light chain phosphorylation, stiffness, prostaglandins, contraction, relaxation
Introduction
Increased airway responsiveness in normal juveniles of many species including humans has been well documented (Hopp et al. 1985; Montgomery and Tepper 1990; Shen et al. 1996; Tepper et al. 1995). While common and normal during ontogenesis the expression of increased responsiveness may predispose to persistent airway hyperresponsiveness (AHR) following adverse events including inflammation or injury (Chitano et al., 2005). Much of the earlier work that described contributions of airway smooth muscle (ASM) to this phenomenon (Rothberg et al. 1987, Sparrow and Mitchell 1990, Murphy et al. 1991, Rosenberg et al. 1991) focused on mechanisms that contributed to altered active stress generation during contraction. Since then it has become apparent that there are several models of smooth muscle contractility other than altered active stress generation that might contribute to increased airway responsiveness. In this manuscript we will review three novel paradigms of increased responsiveness in newborn and/or juvenile ASM developed in our laboratory. These include transiently increased shortening velocity in juvenile ASM, impaired relaxation in newborns and juveniles during sustained electrical field stimulation, and force potentiation (rather than relaxation) following stretch in newborn ASM.
Increased shortening velocity in juvenile airway smooth muscle
Over the span of the three ages of guinea pigs maximal active stress does not change significantly with age. In addition the maximal rate of development of active stress does not differ in juveniles from that of adults or newborns (Chitano et al., 2000a). However, shortening velocities over a wide range of afterloads and maximal shortening velocity V0 are maximal in juvenile guinea pig trachealis and ∼3 times greater than in adults (Figures 1a and 1b). These results are similar to those from washed bronchial smooth muscle from asthmatic humans, whose active stress generation in strips is not increased in comparison with non-asthmatic adults (Whicker et al., 1988), but the unloaded shortening is ∼50% greater in isolated myocytes (Ma, 2002). Maximal power, expressed as the maximal product of active stress multiplied by the corresponding shortening velocity, is considered a robust measure of ASM thick filament activation. During development this parameter doubles from the newborn to the juvenile age group and then declines to newborn levels in adults (Figure 1c). The internal resistance to shortening Rsi, calculated from the force-velocity curves (Figure 1b), is a measure of the mechanical opposition to shortening. This varied inversely with the age-related maximal shortening velocities (Chitano et al., 2000a). Since this parameter is mathematically derived from the force-velocity relationship and not a direct physical measurement, there is a need for a more direct assessment of mechanical opposition to shortening.
Fig.1.
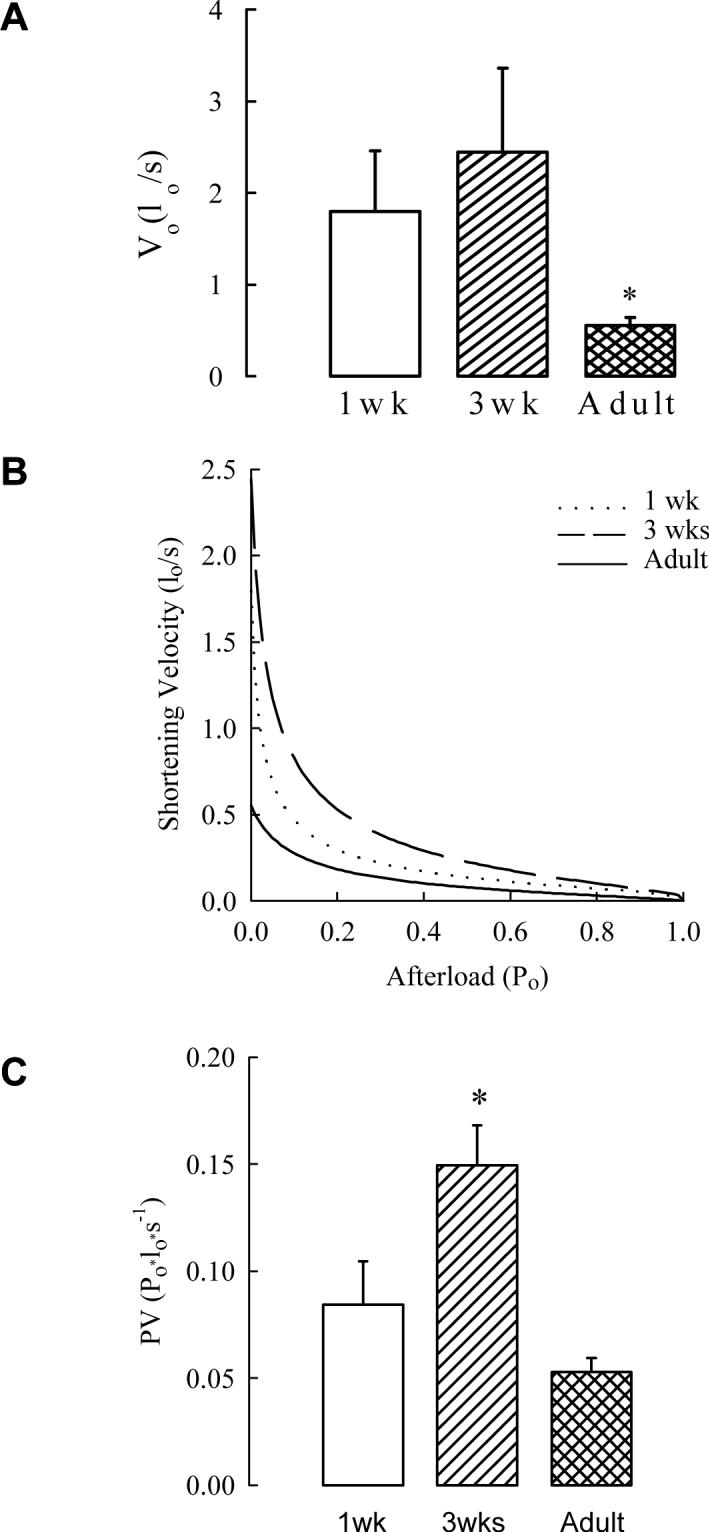
A. Maximum shortening velocity at zero load (Vo) in tracheal strips from different age guinea pigs. In strips from 1wk and 3wks guinea pig Vo was 3 to 5-fold greater compared to adult animals (*P<0.05 by ANOVA and PLSD Fisher's test). B. Force-Velocity curves calculated as average of single curves obtained by fitting experimental data with a modification of the Hill's equation for ASM. C. Maximum power in tracheal strips from different age guinea pigs. Po is the maximum stress generated by EFS, lo is length. Maximum power in 3wks strips was 2 to 3-fold greater than in strips from 1wk and adult guinea pigs (P<0.01 by ANOVA, *P<0.05 by PLSD Fisher's test). Means and standard errors are shown, n=7, n=5, and n=15 for 1wk, 3wks, and adult animals, respectively. The following are the 95% confidence intervals for 1wk, 3wks, and adult animals, respectively. Vo (panel A): 0.159−3.429, −0.104−4.994, 0.363−0.743; PV (panel C): 0.035−0.134, 0.201−0.341, 0.039−0.067 (from Chitano et al. 2000a, with permission).
Measurements of shortening differ from those of active stress generation in their timing. In the standard quick release method, maximal shortening velocity is achieved in the first few hundred milliseconds. It also turns out that measurements of the maximal rate of increase of active stress, whether performed isometrically or isotonically, also occur in the first few hundred milliseconds. We have already determined in the three ages of guinea pigs there is no statistical difference in comparing the 3 week animals to the newborns and adults between either maximal active stress or the rate of increase of the maximal active stress. This combination of results allows for semi-quantitative predictions between shortening velocity and stiffness.
The rate of increase in active stress is related to the stiffness of the ASM strip and the shortening velocity by the following equation--
where dP/dt is the rate of increase in active stress, dP/dL is the stiffness, and dL/dt is the shortening velocity. Because shortening velocity and rate of increase of active stress are maximal almost immediately after the quick release, the stiffness at this time can be estimated as the passive stiffness. This force-velocity relationship then is reduced to the following estimate--
where (dP/dL)passive is the passive stiffness and (dL/dt)max is the maximal shortening velocity or V0. Since the maximal rate of increase of the active stress (dP/dt)max is constant across the three age groups (or nearly so), this predicts that the passive stiffness will vary inversely with V0 as V0 changes with age. Since V0 is maximal in development at 3 weeks of age, we would expect that the passive stiffness would be minimal at this age and be relatively increased in the newborn and adult age groups. This is, in fact, the case. Figure two demonstrates that the passive stiffness of guinea pig trachealis decreases by ∼50% in juvenile guinea pig trachealis and returns to newborn levels, whether or not the strips were set at a preset length to maximize active stress or a preset load of 5 mN. Since active stress following contractile stimulation is equivalent in these three age groups and because this added stress will add substantially to the stiffness, one would predict that there would not be significant age-related differences in active stiffness following cholinergic stimulation. This also turns out to be true (Wang et al., 2005). The implications of these findings are that differences in the passive stiffness of airway smooth muscle, which likely involve the cytoskeleton or the extracellular matrix, play a “hand-in-glove” role in facilitating differences in shortening. These links are currently under investigation.
The motor driving age-related differences in shortening that are not associated with differences in active stress generation is likely related to contractile elements with increased ATPase activity. In addition, most contractile elements and proteins are likely not playing a major role, because these have been shown to alter active stress (which does not change in the three ages of guinea pigs) when altered themselves. This immediately brings focus to myosin light chain kinase (MLCK) and the phosphorylation of myosin light chain (MLC), both of which have been shown to be increased in another model of increased shortening without alteration of active stress, the ragweed sensitized dog (Jiang et al., 1994). If MLCK plays an important role in the increased shortening seen in 3 week old juvenile guinea pigs, then MLC phosphorylation and the content of MLCK would be predicted to increase significantly at this age. This is, in fact, the case. Figure 3 demonstrates that the content of MLCK (normalized to the total quantity of protein in the muscle strip) approximately doubles at age three weeks and then returns to a newborn level in adulthood (Chitano et al., 2004). The functional correlate of MLCK activity is the degree of phosphorylation of MLC. A technique of flash-freezing of strips either early (1, 2 or 3 seconds; times that relate to shortening) or late (9 seconds; a time that relates to maximal isometric stress generation) was developed (Chitano et al., 2005) to analyze early MLC phosphorylation. Figure 4 demonstrates that MLC phosphorylation was greatest early in contraction and expressed, as expected, to a significantly greater degree in the 3 week old animals. At nine seconds there was no significant difference among the three age groups. Together, there is enzymatic and functional phosphorylation evidence to support increased activation and cross-bridge cycling of myosin heavy chain related to the increased shortening velocity documented in the three week old guinea pig trachealis. Future studies will investigate a potential role for the 7-amino acid inserted myosin heavy chain, which is known to be endowed with greater ATPase activity, in contributing to the increased shortening velocity of newborn and juvenile trachealis.
Fig. 3.
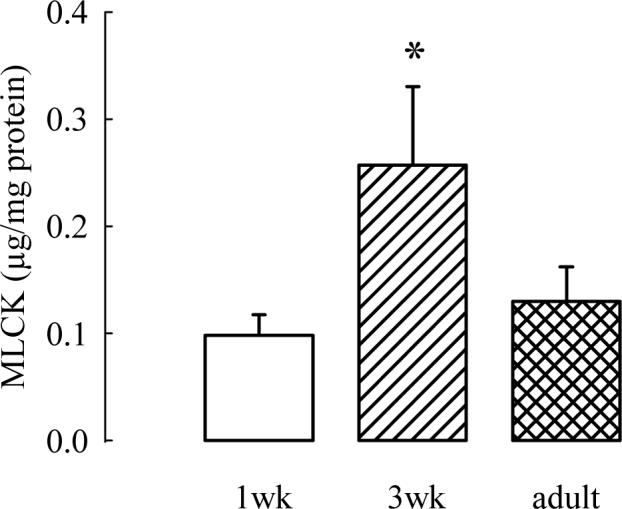
Densitometric measurements from immunoblots of MLCK, which show a transient increase in the content of MLCK at 3 weeks of age (P<0.05 by ANOVA) in extracts of tracheal smooth muscle from 1wk, 3wk, and adult guinea pigs. Values are mean ± SE of twelve 1wk, eleven 3wk, and twelve adult samples (from Chitano et al. 2004, with permission).
Fig. 4.
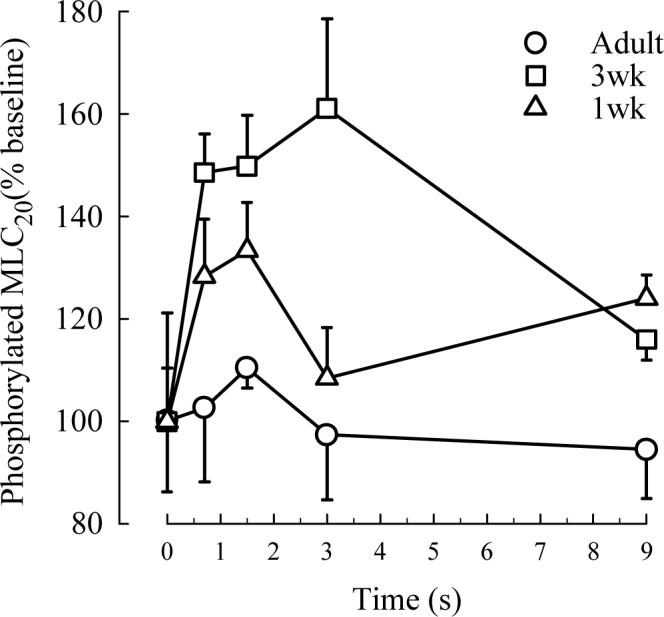
Phosphorylated MLC20, expressed as % of the level of MLC20 phosphorylated at rest (% baseline), in tracheal strips from guinea pigs of different ages quick frozen at different time points after the onset of electrical field stimulation. Values are means ± SE. The increment of MLC20 phosphorylation over baseline was significantly higher in 3wk (n=5) compared to 1wk (n=5) and adult (n=4) strips (p<0.01 by ANOVA) (from Chitano et al. 2005×, with permission).
Spontaneous relaxation of ASM and ontogenesis of airway responsiveness
The second paradigm focuses on the contribution of the spontaneous relaxation process to the overall ASM response to stimulation during the course of ontogenesis. In studies on maturational changes of ASM function as potential contributors to the elevated airway responsiveness in healthy young individuals, the role of impaired relaxing properties of ASM could not be disregarded a priori. The rationale for investigating ASM spontaneous relaxation is based upon the effect that the spontaneous reversal of ASM contraction might exert on bronchospasm and consequently on airway responsiveness. To elucidate this rationale we need to stress that, by definition, spontaneous relaxation is the intrinsic physiological ability of ASM to reverse the status of contraction. In contrast to pharmacologically induced relaxation, the notion of spontaneous relaxation implies that ASM activation, in addition to initiating the contractile response, also activates the signaling leading to relaxation. While induced ASM relaxation has obvious relevance to the therapeutic approach to removal of bronchospasm, the occurrence of spontaneous relaxation has implications for the maximal extent and duration of ASM contraction. Relaxation can be achieved both by antagonizing the contractile response and accelerating its removal. As a consequence, spontaneous relaxation would affect both the extent and time course of a bronchoconstriction and would therefore influence the degree of airway responsiveness.
To show that spontaneous relaxation is a general phenomenon during ASM contraction, representative recordings are display to illustrate clearly the occurrence of spontaneous relaxation in response to different contractile agonists. Recordings shown in the following figures of this section were obtained from parallel-fibered strips cleaned of loose connective tissue and dissected from transverse sections of the trachea. To excise the trachea, animals were anaesthetized with intra-peritoneal injection of Na-pentobarbital, following procedures approved by the Duke University Institutional Animal Care and Use Committee. Tracheal strips contained ∼2 mm cartilaginous attachments at both ends, which were used to connect the smooth muscle between a force-displacement transducer and a holder without damaging the natural structural organization of the muscle. Preparation of the strips and experiments were performed in Krebs-Henseleit solution buffered to pH 7.35−7.45 by continuous aeration with 95% O2/5% CO2. Fig. 1A shows the response to 10−7M ACh in a strip from an adult Hartley guinea pig. Contraction reached the maximum in less than 1 minute and was followed by a substantial relaxation that in about five minutes returned force to un-stimulated level, while ACh concentration was kept unchanged in the tissue bath. Even more remarkable is the level of tension reached later during the response, which was lower than the tension at rest. This suggests that the relaxing phase is an active process and does not involve factors such as uneven diffusion, degradation of the agonist, or mechanical effects due to readjustment of the cellular and tissue tensors that transfer the force to the measurement device. Although spontaneous relaxation was not as dramatic to reach levels of force lower than resting, similar responses are shown in Fig. 5B and 5C, reporting respectively the recording of force generation induced by 10−8M CCh and 10−6M histamine in a tracheal strip from adult guinea pigs. To confirm that force relaxation may indeed translate into re-elongation of the strips and consequently into re-opening of the airway lumen, we show in Fig. 6A and 6B the recordings of both spontaneous force and length relaxation. The force generating ability of ASM is measured by keeping the length of the muscle constant (isometric mode), while the shortening ability is measured by keeping constant the load (isotonic mode). We recorded in the same tracheal strip from an adult guinea pig, both the isometric and the isotonic response to 20-second electric field stimulation (18V, 60Hz, 400mA/mm2). The isotonic response was elicited while the tracheal strip shortened against a load equal to the tension measured in the strip at rest. A substantial relaxation can be observed during the course of contraction in both conditions. In response to electrical stimulation, force generation reached a maximum in less than 10 seconds and then spontaneously declined so that the generated force was completely abrogated by the end of the stimulation. Similarly, the muscle shortened to a maximum in about 10 seconds and then started to re-elongate spontaneously so that by the 20th second of stimulation it had reversed about 40% of the shortening. These recordings not only suggest that spontaneous relaxation is a general phenomenon during ASM contraction but also that ASM spontaneous relaxation may directly translate into relief of airway narrowing and play a role in the reversal of bronchospasm.
Whether the reversal of bronchospasm influences the degree of airway responsiveness has not been conclusively demonstrated. However, a link between airway responsiveness and the time course of bronchospasm removal in asthmatics has been suggested. Cartier et al. (1983) studied the time course of bronchoconstriction in the absence of bronchodilators in 11 asthmatic subjects by measuring the specific lung conductance for as long as the bronchospasm lasted. They analyzed both the sustained and the recovery phase of the bronchoconstriction induced by either histamine or methacholine and demonstrated an enormous variability in the duration of both phases. Although starting at a comparable degree of induced bronchoconstriction, the fastest recovery was completed in less than 10 min for histamine and about 30 min for methacholine while the slowest recovery for the two agonists took 1.5 and 4 hours respectively. This wide range of bronchospasm duration can not be explained by agonist degradation or metabolism and raises the question whether bronchospasm reversal in non-asthmatics would have a much faster time course. This study could not discriminate among subjects with different airway responsiveness because, in order to obtain the same starting level of bronchoconstriction for all subjects, very different agonist doses were used in different subjects. However, in a subsequent study on spontaneous resolution of bronchospasm in 32 asthmatics, Mannino et al. (1997) showed that the onset of bronchospasm reversal occurred earlier and its initial intensity was stronger in subjects with mild compared to severe hyperresponsiveness. These reports suggest that the time course of spontaneous bronchodilatation may be associated with the degree of airway responsiveness. It is therefore possible to hypothesize that impaired ASM spontaneous relaxation may contribute to airway hyperresponsiveness by reducing the intensity and prolonging the time course of bronchospasm removal. Within the context of the present paper, our interest was to study whether ASM from immature healthy guinea pigs, which display airway hyperresponsiveness, was characterized by reduced capacity to spontaneously relax. We performed the study using electric field stimulation in tracheal strips from the same three age groups of Hartley guinea pigs as in the studies described in the previous section: 1-week-old, 3-week-old, and 3-month-old (Chitano et al. 2002). In this study, in order to describe and quantify the spontaneous relaxation occurring during the stimulation with electric field, we developed new indexes of relaxation that were not affected by the elevated and unstable intrinsic tone present at rest in guinea pig ASM. We based our indexes on the maximum total contraction tension (TCTmax), which we defined as the sum of the maximum active tension generated in response to the electrical stimulation and intrinsic tone at rest. The TCTmax is the maximum value of possible relaxation during stimulation and is therefore the best parameter to normalize relaxation indexes in ASM strips of different size-age. Two of the indexes we measured were tension relaxation (TR), which is the drop in tension at any given time during spontaneous relaxation, and the maximum rate of TR, which represents the maximum slope of the recording during relaxation and has the advantage of being time-independent, although not reflecting the total amount of spontaneous relaxation. By using these new indexes, we found that the spontaneous relaxation occurring during electric field stimulation in guinea pig tracheal strips increases with ontogenesis, thus suggesting that a reduced ability to spontaneously relax may contribute to the higher airway responsiveness in young animals. An impairment of ASM spontaneous relaxation has been shown in another animal model that displays airway hyperresponsiveness. In strips of isolated canine tracheal smooth muscle stimulated with electric field for 75-second, a substantial relaxation was observed during the stimulation. By contrast, in smooth muscle strips from littermate ragweed sensitized dogs, the relaxation phase was almost completely absent (Mitchell et al. 1987). To evaluate ASM spontaneous relaxation in a third animal model, we have used electrical stimulation in tracheal strips from mice of different strains. Fig. 7A shows the force generated by a strip from a C57BL/6J mouse, a strain which is characterized by low airway responsiveness. A substantial spontaneous relaxation is evident in this recording during the electrical stimulation. By contrast, no relaxation is observed during the course of electrical stimulation in a tracheal strip from an A/J mouse, a strain characterized by airway hyperresponsiveness (Fig. 7B). It seems evident that ASM from hyperresponsive animals is consistently characterized by a reduced ability to spontaneously relax during the course of a contractile stimulation, thus supporting the notion that impaired ASM spontaneous relaxation may play a role in airway hyperresponsiveness.
Although the literature on spontaneous ASM relaxation is still quite fragmentary, partially due to differences in species and experimental conditions, several factors that may be of physiological relevance have been shown to relax ASM through more than one mechanism. The neurotransmitter vasoactive intestinal peptide (VIP) is present in neurons innervating the airways with other VIP-like neuropeptides and has been shown to participate in reducing ASM tone following non-adrenergic-non-cholinergic inhibitory nerve stimulation (Uddam et al. 1994; Berisha et al. 2002). Nitric oxide (NO) has also been shown to be a factor in ASM non-adrenergic-non-cholinergic relaxation (Belvisi and Bai 1994; Bai and Bramley 1993). An impairment of NO synthesis has been reported in asthmatic subjects (Ricciardolo et al. 2001) and in sensitized animals (Samb et al. 2001). Recent evidence suggests that the action of NO may be mainly achieved through formation of S-nitrosothiols, such as S-nitrosogluthatione (Que et al. 2005). Adrenergic innervation has been reported to contribute to ASM relaxation (Yip et al. 1981). The breakdown of acetylcholine by the enzyme acetylcholinesterase, the activity of which has been shown to decline in hyperresponsive ASM (Murphy et al. 1989; Mitchell et al. 1991), has been reported to be involved in relaxation during electric field stimulation. Most of the arachidonic acid metabolites affect ASM tone and some of them have a strong relaxing activity that could be involved in spontaneous relaxation (Janssen et al. 2000; Yamane and Kobayashi 1990; Schmidt and Rabe 2000). Most probably, an important role is played by the airway epithelium, which is a source of NO, arachidonic acid metabolites, and cholinesterases (Spina 1998; Taisne et al. 1997). What remains to be determined is which of these factors is physiologically rlevant while ASM spontaneously relaxes during the course of a contractile response. In our study on maturation of spontaneous relaxation in guinea pigs, we found that inhibition of cyclooxygenase increases ASM spontaneous relaxation during electric field stimulation in tracheal strips from immature animals to a level similar to the relaxation observed in strips from adults (Chitano et al. 2002). We have also shown preliminary data suggesting that the content of prostanoids in guinea pig tracheal tissue, measured by radio-immunoassay as release in the incubation media, declines significantly with age and was not significantly affected by epithelium removal (Chitano et al. 2000a). Thus, the reduced spontaneous relaxation in strips from immature animals would result from an elevated level of prostanoids in the airway tissue. Removal of the epithelium partially reduced spontaneous relaxation in adult strips (Chitano et al. 2002), suggesting that in our animal model the epithelium may release part of the agents that produce spontaneous relaxation, but is likely not the main factor responsible for the prostanoid profile in guinea pig airway tissue. Moreover, we have found in a preliminary study that inhibition of acetylcholinesterase drastically reduces spontaneous relaxation, an effect that is counteracted by cyclooxygenase inhibition (Chitano and Murphy 2004). These results suggest that prostanoids reduce spontaneous relaxation in immature guinea pig ASM and have some important implications on the mechanistic understanding of spontaneous relaxation: a) because the distribution of prostanoids greatly varies among species and different prostanoids have different effects on ASM, the relative abundance of the different types of prostanoids may determine a specific regulation of spontaneous relaxation; b) the action of prostanoids may be due to either a direct modulation or a functional antagonism of the factor/s that actively produces spontaneous relaxation; c) the level of spontaneous relaxation in a given tissue may be the integrated outcome of the effects exerted by more than one agent.
More recently we have studied the role of NAD(P)H oxidase in ASM response, since this important source of reactive oxygen species may affect either directly or indirectly ASM function (Chitano et al. 2007). In one set of experiments we evaluated whether NAD(P)H oxidase has a role in ASM spontaneous relaxation by studying the response to 20s electric field stimulation in mice tracheal strips. We used strips from wild type (C57BL/6J) and from mice lacking one of the NAD(P)H oxidase components (p47phox). The absence of the p47phox subunit resulted in impaired ASM spontaneous relaxation, suggesting that endogenous reactive oxygen species generated by NAD(P)H oxidase may be involved in the signaling pathways regulating ASM spontaneous relaxation.
To conclude this section on spontaneous relaxation, we would like to suggest that ASM spontaneous relaxation is a common behavior in ASM, no matter the species or the type of contractile stimulation. This functional aspect of the ASM response is a physiological process to avoid excessive and long lasting narrowing of the airway lumen and most likely contributes to the degree of airway responsiveness. In particular, with reference to the ontogenesis of airway responsiveness, the combination of impaired spontaneous relaxation and increased contractile function in ASM may confer to immature airways the hyperresponsive phenotype that is typical of early stages of development. In the next section we will discuss the evidence that altered ASM plastic adaptation may further contribute to the development of this phenotype, suggesting that ASM is a central player in the ontogenesis of airway responsiveness.
Maturation of Airway Smooth Muscle Adaptation and Airway Hyperresponsiveness
Breathing imposes passive lengthening and retraction of the ASM. Certain breathing maneuver such as deep inspiration can be beneficial in normal subjects to counteract bronchospasm but can be detrimental to hyperresponsive airways by triggering further bronchoconstriction. Although the exact mechanisms for this contrary response by normal and hyperresponsive airways are unclear, we postulate that ASM from hyperresponsive airways exhibits an altered time profile of adapting/regaining its contractile function after mechanical stretches. It has been demonstrated that healthy ASM has an amazing capacity to adapt quickly to conformational changes by resuming its normal contractility. Remarkably, as we showed recently, a potentiated force generating ability is exhibited after mechanical oscillations by ASM from airways of healthy infant animals. This maturation-specific expression of ASM adaptability may be closely related to the increased airway responsiveness exhibited by healthy infant subjects.
Airway hyperresponsiveness is a hallmark feature in asthma pathology. One of the prominent clinical observations of impaired airway function in asthma is the inability of deep inspiration (DI) to improve airway function (Wheatley et al. 1989). In normal subjects, a DI prior to the administration of a contractile agonist attenuates the subsequent bronchoconstriction, a behavior defined as bronchoprotective effect of DI (Skloot et al. 1995; Moore et al. 1997). Recent studies suggest that asthmatics are mainly lacking the bronchoprotective component of DI (Scichilone et al. 2001). The bronchoprotective effect of DI can be explained by the adaptive behavior that ASM displays in vitro in response to mechanical oscillation (Wang et al. 2000; Wang and Paré 2003). Similar to asthmatics, DI is ineffective in reducing airway reactivity in infant airways (Weist et al. 2002). Recently we showed augmented contractile response of ASM following mechanical perturbation in infant guinea pigs (Wang et al. 2005). This result supports the concept that altered ASM adaptation contributes to the absence of bronchoprotective effect of DI in hyperresponsive airways.
Smooth muscle adaptation refers to the process during which isometric force generating ability recovers after the reduction in response to perturbations to muscle length. These perturbations usually take the form of length changes (Pratusevich et al. 1995) or mechanical length oscillations which returns the muscle preparation to the same length prior to the maneuver (Wang et al. 2000). The completion of an adaptation process often requires about 30 to 40min depending on stimulation frequency. We recently reported the first evidence of different adaptation profile of ASM from hyperresponsive airways (Wang et al. 2005). As shown in Fig. 4, the recovery of active force in adult guinea pig ASM is gradual, complete, and follows a time course similar to adult canine trachealis. On the other hand, active force produced by 1wk trachealis immediately increases to about 110% Fmax (Fmax: the stable maximal active force generated before mechanical oscillation) and is maintained throughout the adaptation process. We termed this increase of force after the initial reduction as force potentiation. We also reported that the initial force reduction is the same in all age groups and that force potentiation is not related to the extent of this reduction. These data suggest a specific expression of ASM adaptation to mechanical perturbations in immature ASM. This is consistent with the clinical observation that DI worsens bronchoconstriction in hyperresponsive airways of asthmatics and children.
When we evaluated the role of actin polymerization in the process of ASM adaptation we found that it is required for the recovery of active force during repeated stimulations but may not be responsible for producing the characteristic force potentiation (Wang et al. 2005). Other cytoskeletal components have not been evaluated with respect to their participation in ASM adaptation process. One the other hand, we showed that the force potentiation observed in infant ASM can be abolished by indomethacin, a cyclooxygenase inhibitor, in a dose dependent manner (Fig. 5), thus suggesting that the force potentiation is associated with prostanoid signaling.
Prostanoids are membrane lipids involved in a wide range of physiological processes such as inflammatory response, cell proliferation, and smooth muscle contraction. Active prostanoids are prostaglandin D2, E2, F2α, I2, and thromboxane A2. The rate limiting step for prostanoid synthesis is the release of arachidonic acid (AA) from the cell membrane by phospholipase A2 (PLA2) (Bos et al. 2004). AA is converted to prostanoids by the enzymes cyclooxygenase and specific prostanoid synthases. It has been known that prostanoids have disparate effects on particular organs, tissue or cell types. In smooth muscle, some of the prostanoids are contracting whereas others are relaxing depending on the receptors that are activated. Prostanoid receptors constitute a subfamily which belongs to the seven transmembrane G-protein coupled receptors superfamily. Currently 8 types of prostanoid receptors have been identified. Although each prostanoid binds with the highest affinity to its cognate receptor, considerable cross-reactivity in ligand binding exists between a given prostanoid and other receptors in the family. Whereas activation of EP2 and EP4 receptors mainly leads to relaxation of smooth muscle (Nakao et al. 1989; Nishigaki et al. 1995; Boie et al. 1995), activation of EP1, EP3, FP, and TP receptors constricts smooth muscle (Funk et al. 1993, Breyer et al. 2001; Dorn and Becker 1993). IP and DP receptors, however, appear to both contract and relax smooth muscle (Vassaux et al. 1993; Hoeper et al. 2000; Giles et al. 1989). Of particular interest to our findings are the EP3 and FP receptors. EP3 receptor (one of the receptors for prostaglandin E2) is a known constrictor of smooth muscle (Coleman et al. 1994) signaling through the small G-protein Rho (Aoki et al. 1999; Katoh et al. 1998). FP (the receptor for prostaglandin F2α) has been shown to be coupled to two independent signaling pathways; one involves phosphatidylinositol hydrolysis and intracellular calcium release (Griffin et al. 1998) and the other involves activation of small G-protein Rho (Pierce et al. 1999). The role of prostanoids in the maturation-specific force potentiation during the adaptation process may be the result of several events that have been shown to occur in the airway tissue. Facilitated by stretches (Gao and Vanhoutte 1993; Copland et al. 2006), synthesized prostanoids are released to the surface of the cell membrane where they bind to their specific receptors. More prostanoid receptors are likely to be activated in infant ASM since prostanoids are most abundant at this maturational stage (Chitano et al. 2000a). One of the signaling pathways that follow the binding and activation of some of the prostanoid receptors, i.e. EP3 and FP receptor, is the activation of the small G-protein Rho by switching it from guanosine diphosphate (GDP)-bound to guanosine triphosphate (GTP)-bound. The small G-protein Rho, through its effector Rho-kinase (ROCK), plays an important role in smooth muscle contraction by regulating cytoskeletal reorganization (Van Aelst and D'Souza-Schorey 1997) and myosin phosphorylation (Somlyo and Somlyo 2003). One of the substrates that are phosphorylated by ROCK is the intermediate filament protein vimentin, which has also been identified as functional adaptor of the enzymes PLA2, cyclooxygenase, and prostaglandin synthases (Murakami et al. 2000). It is possible that prostanoids determine the force potentiation we have shown in immature ASM by affecting cell cytoskeleton through RhoA-ROCK signaling. The different adaptation profile between immature and adult ASM might also be due to more abundant cyclooxygenase in immature lung (Brannon et al. 1998) and more abundant prostanoids PGE2 and PGF2α in infant ASM (Chitano et al. 2000a), leading to more pronounced EP3 and FP receptor activation. We suggest that activation of ROCK may be one of the important mechanisms governing the enhanced ASM contractility in response to mechanical perturbations in hyperresponsive airways.
In chronic airway diseases such as asthma, passive and active shortening of ASM can occur due to chronic inflammation, loss of lung elastic recoil and airway wall remodeling. Chronic shortening could alter both active and passive length-tension properties of the muscle such that the optimal length range for shortening and force generation is shifted to shorter lengths and that the passive structures surrounding ASM become less compliant to re-lengthening (Wang et al. 2001). Therefore a new paradigm has been proposed to suggest that if ASM adapts to shorter lengths under pathological conditions, the capacity of the ASM to narrow the airways could be markedly enhanced (McParland et al. 2003; Wang and Paré 2003). ASM adaptation can explain the increased narrowing in hyperresponsive airways and many of the phenomena related to bronchoprotection of DI. However, it remains unclear whether and how ASM adaptation can account for the difference in response to DI between normal and asthmatic subjects. A step towards the ultimate goal of understanding the response of asthmatic ASM to DI is to understand the response of hyperresponsive ASM. It has been shown that airway hyperresponsiveness occurs in healthy juvenile both in animal and human subjects. For example, immature rabbits have greater airway responsiveness and greater airway narrowing in vivo than adult animals (Tepper et al. 1995; Ramchandani et al. 2003). Evidence suggests that juvenile airway hyperresponsiveness is closely related to ASM function. As discussed above, we have shown an increase in trachealis shortening velocity in the first three weeks of life in guinea pigs, followed by a decline toward adult life (Chitano et al. 2000a). Besides occurring naturally in the early stages of maturation, it is known that allergic sensitization induces both airway and ASM hyperresponsiveness. It has been shown that both the capacity and velocity of shortening are elevated in ASM from asthmatic airways (Ma et al. 2002). Animal models of chronic allergic sensitization have been shown to trigger airway remodeling and alterations in airway function (Jain et al. 2002; Ramos-Barbon et al. 2004). In addition, sensitization combined with repeated exposure to allergen increases ASM force generation (Gosens et al. 2005), further increases airway hyperresponsiveness (Jain et al. 2003), and increases prostanoid synthesis through upregulated cyclooxygenase-2 expression (Oguma et al. 2002). A maturational sensitization model of guinea pigs developed in our laboratory showed that early sensitization in the first week of life could reverse the normal maturation of ASM shortening functions (Chitano et al. 1999), i.e. the reduction in shortening velocity in adulthood did not take place in this sensitization model (Chitano et al. 1999). These data suggests that the changes in airway responsiveness due to allergic sensitization can be partly due to intrinsic changes in ASM contractile function induced by allergen. Whether the altered function of ASM following sensitization determines an adaptive response to mechanical perturbation similar to what we have found in immature ASM remains to be determined. However, it seems evident that the combined effect of the chronic shortening that can occur in asthmatic airways and of a potentiation effect of mechanical perturbation in hyperresponsive ASM could entirely explain the paradoxical effect of DI in asthmatics.
Conclusions
In our studies on maturation of ASM in guinea pigs, we have shown an astonishing range of functions that undergo major changes during the course of ontogenesis. We have shown that shortening velocity declines toward adulthoods after reaching a maximum at 3 weeks, parallel to a decline in the content of myosin light chain kinase. We have also found that spontaneous relaxation is almost absent in 1 week animals and gradually develops with maturation. Finally, we showed that mechanical perturbation of the muscle length simulating deep breathing induces a unique response of potentiated force in 1 week animals only. Prostanoids seem to be involved in this force potentiation as well as in the impaired spontaneous relaxation. The remarkable common feature of all our findings is that, although in different ways and through different mechanisms, all features of ASM function at early stages of maturation favor a more contractile phenotype, thus making the ASM a central candidate at the origin of the airway hyperresponsiveness that characterizes healthy immature individuals, both in animal species and in human. Besides the physiological relevance to the normal maturation of airway responsiveness, this may have strong implications to the development of diseases presenting with airway hyperresponsiveness that initiate at a young age and persist into adulthood. We suggest that the specific regulation of cellular functions during early life that determine the hyperresponsive phenotypes we have shown in immature ASM may be responsible for a particular vulnerability to environmental insults, thus facilitating an irreversibility of the induced alterations and a persistence of the effects. In this respect, those factors that determine ASM hyperresponsiveness in children may play a role in the incidence of childhood asthma and its persistence into adulthood.
Fig.2.
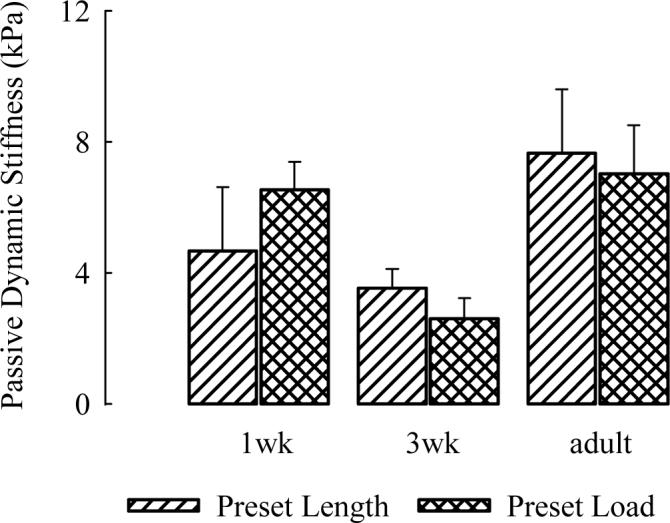
Stiffness measured using dynamic oscillation test in tracheal strips from 1wk, 3wk, and adult guinea pigs. Muscle strips were at rest (no stimulation) either at a preset length (adapted to generate maximal force) or at a preset load of 5mN. Means and standard errors are shown. N=5 in each age group. The stiffness of tracheal strips from 3wk was found to be the least of the 3 age groups (* p<0.05, ANOVA, LSD) (from Wang et al. 2005×, with permission).
References
- Aoki J, Katoh H, Yasui H, Yamaguchi Y, Nakamura K, Hasegawa H, Ichikawa A, Negishi M. Signal transduction pathway regulating prostaglandin EP3 receptor-induced neurite retraction: requirement for two different tyrosine kinases. Biochem. J. 1999;340:365–369. [PMC free article] [PubMed] [Google Scholar]
- Bai TR, Bramley AM. Effect of an inhibitor of nitric oxide synthase on neural relaxation of human bronchi. Am. J. Physiol. 1993;264:L425–L430. doi: 10.1152/ajplung.1993.264.5.L425. [DOI] [PubMed] [Google Scholar]
- Belvisi MG, Bai TR. Inhibitory nonadrenergic, noncholinergic innervation of airway smooth muscle: role of nitric oxide. In: Raeburn D, Giembycz MA, editors. In Airways smooth muscle: structure, innervation and neurotransmission. Birkhauser Verlag Basel; Switzerland: 1994. pp. 157–187. [Google Scholar]
- Berisha HI, Bratut M, Bangale Y, Colasurdo G, Paul S, Said SI. New evidence for transmitter role of VIP in the airways: impaired relaxation by a catalytic antibody. Pulm. Pharmacol. Ther. 2002;15:121–127. doi: 10.1006/pupt.2001.0337. [DOI] [PubMed] [Google Scholar]
- Boie Y, Sawyer N, Slipetz DM, Metters KM, Abramovitz M. Molecular cloning and characterization of the human prostanoid DP receptor. J. Biol. Chem. 1995;270:18910–18916. doi: 10.1074/jbc.270.32.18910. [DOI] [PubMed] [Google Scholar]
- Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Prostanoids and prostanoid receptors in signal transduction. Int. J. Biochem. Cell Biol. 2004;36:1187–1205. doi: 10.1016/j.biocel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Brannon TS, MacRitchie AN, Jaramillo MA, Sherman TS, Yuhanna IS, Margraf LR, Shaul PW. Ontogeny of cyclooxygenase-1 and cyclooxygenase-2 gene expression in ovine lung. Am. J. Physiol. 1998;274:L66–L71. doi: 10.1152/ajplung.1998.274.1.L66. [DOI] [PubMed] [Google Scholar]
- Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu. Rev. Pharmacol. Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- Cartier A, Malo JL, Begin P, Sestier M, Martin RR. Time course of the bronchoconstriction induced by inhaled histamine and methacholine. J. Appl. Physiol. 1983;54:821–826. doi: 10.1152/jappl.1983.54.3.821. [DOI] [PubMed] [Google Scholar]
- Chitano P, Cox CM, Worthington CL, Jenkin JA, Murphy TM. Ovalbuminsensitization reverses the ontogenic changes in internal resistance to shortening and in shortening velocity in guinea pig tracheal smooth muscle. Am. J. Respir. Crit. Care Med. 1999;159(suppl):A471. [Google Scholar]
- Chitano P, Wang J, Cox CM, Stephens NL, Murphy TM. Different ontogeny of rate of force generation and shortening velocity in guinea pig trachealis. J. Appl. Physiol. 2000a;88:1338–1345. doi: 10.1152/jappl.2000.88.4.1338. [DOI] [PubMed] [Google Scholar]
- Chitano P, Turato G, Murphy TM. The release of prostaglandin E2 by sustained electrical field stimulation increases with age in guinea pig trachea. Am. J. Respir. Crit. Care Med. 2000b;161(suppl):A693. [Google Scholar]
- Chitano P, Cox CM, Murphy TM. Relaxation of guinea pig trachealis during electrical field stimulation increases with age. J. Appl. Physiol. 2002;92:1835–1842. doi: 10.1152/japplphysiol.00688.2001. [DOI] [PubMed] [Google Scholar]
- Chitano P, Murphy TM. Role of prostanoids and acetylcholinesterase in the relaxation of airway smooth muscle during electrical stimulation. Am. J. Respir. Crit. Care Med. 2004;169(suppl):A446. [Google Scholar]
- Chitano P, Wang L, Mason SN, Auten RL, Sturrock A, Kennedy TP, Hoidal JR, Murphy TM. Impaired airway smooth muscle relaxation in mice deficient of the NAD(P)H oxidase subunit p47phox. Am. J. Respir. Crit. Care Med. 2007;175(suppl):A303. [Google Scholar]
- Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol. Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- Copland IB, Reynaud D, Pace-Asciak C, Post M. Mechanotransduction of stretch-induced prostanoid release by fetal lung epithelial cells. Am. J. Physiol. 2006;291:L487–L495. doi: 10.1152/ajplung.00510.2005. [DOI] [PubMed] [Google Scholar]
- Dorn GW, 2nd, Becker MW. Thromboxane A2 stimulated signal transduction in vascular smooth muscle. J. Pharmacol. Exp. Ther. 1993;265:447–456. [PubMed] [Google Scholar]
- Funk CD, Furci L, FitzGerald GA, Grygorczyk R, Rochette C, Bayne MA, Abramovitz M, Adam M, Metters KM. Cloning and expression of a cDNA for the human prostaglandin E receptor EP1 subtype. J. Biol. Chem. 1993;268:26767–26772. [PubMed] [Google Scholar]
- Gao Y, Vanhoutte PM. Responsiveness of the guinea pig trachea to stretch: role of the epithelium and cyclooxygenase products. J. Appl. Physiol. 1993;75:2112–2116. doi: 10.1152/jappl.1993.75.5.2112. [DOI] [PubMed] [Google Scholar]
- Giles H, Leff P, Bolofo ML, Kelly MG, Robertson AD. The classification of prostaglandin DP-receptors in platelets and vasculature using BW A868C, a novel, selective and potent competitive antagonist. Br. J. Pharmacol. 1989;96:291–300. doi: 10.1111/j.1476-5381.1989.tb11816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosens R, Bos IS, Zaagsma J, Meurs H. Protective effects of tiotropium bromide in the progression of airway smooth muscle remodeling. Am. J. Respir. Crit, Care Med. 2005;171:1096–1102. doi: 10.1164/rccm.200409-1249OC. [DOI] [PubMed] [Google Scholar]
- Griffin BW, Magnino PE, Pang IH, Sharif NA. Pharmacological characterization of an FP prostaglandin receptor on rat vascular smooth muscle cells (A7r5) coupled to phosphoinositide turnover and intracellular calcium mobilization. J. Pharmacol. Exp. Ther. 1998;286:411–418. [PubMed] [Google Scholar]
- Hoeper MM, Schwarze M, Ehlerding S, Adler-Schuermeyer A, Spiekerkoetter E, Niedermeyer J, Hamm M, Fabel H. Long-term treatment of primary pulmonary hypertension with aerosolized iloprost, a prostacyclin analogue. New Engl. J. Med. 2000;342:1866–1870. doi: 10.1056/NEJM200006223422503. [DOI] [PubMed] [Google Scholar]
- Jain VV, Kitagaki K, Businga T, Hussain I, George C, O'shaughnessy P, Kline JN. CpG-oligodeoxynucleotides inhibit airway remodeling in a murine model of chronic asthma. J. Allergy Clin. Immunol. 2002;110:867–872. doi: 10.1067/mai.2002.129371. [DOI] [PubMed] [Google Scholar]
- Jain VV, Businga TR, Kitagaki K, George CL, O'Shaughnessy PT, Kline JN. Mucosal immunotherapy with CpG oligodeoxynucleotides reverses a murine model of chronic asthma induced by repeated antigen exposure. Am. J. Physiol. 2003;285:L1137–L1146. doi: 10.1152/ajplung.00073.2003. [DOI] [PubMed] [Google Scholar]
- Janssen LJ, Premji M, Netherton S, Catalli A, Cox G, Keshavjee S, Crankshaw DJ. Excitatory and inhibitory actions of isoprostanes in human and canine airway smooth muscle. J. Pharmacol. Exper. Ther. 2000;295:506–511. [PubMed] [Google Scholar]
- Katoh H, Aoki J, Ichikawa A, Negishi M. p160 RhoA-binding kinase ROKalpha induces neurite retraction. J. Biol. Chem. 1998;273:2489–2492. doi: 10.1074/jbc.273.5.2489. [DOI] [PubMed] [Google Scholar]
- Ma X, Cheng Z, Kong H, Wang Y, Unruh H, Stephens NL, Laviolette M. Changes in biophysical and biochemical properties of single bronchial smooth muscle cells from asthmatic subjects. Am. J. Physiol. 2002;283:L1181–L1189. doi: 10.1152/ajplung.00389.2001. [DOI] [PubMed] [Google Scholar]
- Mannino F, Anticoli S, Graziani E, Terzano C. Study of spontaneous resolution of bronchial spasm after methacholine challenge. Comparison of patients with different degree of hyperreactivity. Rec. Prog. Med. 1997;88:115–119. [PubMed] [Google Scholar]
- McParland BE, Macklem PT, Pare PD. Airway wall remodeling: friend or foe? J. Appl. Physiol. 2003;95:426–434. doi: 10.1152/japplphysiol.00159.2003. [DOI] [PubMed] [Google Scholar]
- Mitchell RW, Kroeger EA, Keprom W, Stephens NL. Local parasympathetic mechanisms for ragweed-sensitized canine trachealis hyperresponsiveness. J. Pharmacol. Exper. Ther. 1987;243:907–914. [PubMed] [Google Scholar]
- Mitchell RW, Kelly E, Leff AR. Reduced activity of acetylcholinesterase in canine tracheal smooth muscle homogenates after active immune-sensitization. Am. J. Respir. Cell. Mol. Biol. 1991;5:56–62. doi: 10.1165/ajrcmb/5.1.56. [DOI] [PubMed] [Google Scholar]
- Moore BJ, Verburgt LM, King GG, Paré PD. The effect of deep inspiration on methacholine dose response curves in normal subjects. Am. J. Respir. Crit. Care Med. 1997;156:1278–1281. doi: 10.1164/ajrccm.156.4.96-11082. [DOI] [PubMed] [Google Scholar]
- Murakami M, Nakatani Y, Kuwata H, Kudo I. Cellular components that functionally interact with signaling phospholipase A(2)s. Biochim. Biophys. Acta. 2000;1488:159–166. doi: 10.1016/s1388-1981(00)00118-9. [DOI] [PubMed] [Google Scholar]
- Murphy TM, Mitchell RW, Blake JS, Mack MN, Kelly EA, Munoz NM, Leff AR. Expression of airway contractile properties and acetylcholinesterase activity in swine. J. Appl. Physiol. 1989;67:174–180. doi: 10.1152/jappl.1989.67.1.174. [DOI] [PubMed] [Google Scholar]
- Nakao A, Allen ML, Sonnenburg WK, Smith WL. Regulation of cAMP metabolism by PGE2 in cortical and medullary thick ascending limb of Henle's loop. Am. J. Physiol. 1989;256:C652–C657. doi: 10.1152/ajpcell.1989.256.3.C652. [DOI] [PubMed] [Google Scholar]
- Nishigaki N, Negishi M, Honda A, Sugimoto Y, Namba T, Narumiya S, Ichikawa A. Identification of prostaglandin E receptor 'EP2' cloned from mastocytoma cells EP4 subtype. FEBS Lett. 1995;364:339–341. doi: 10.1016/0014-5793(95)00421-5. [DOI] [PubMed] [Google Scholar]
- Oguma T, Asano K, Shiomi T, Fukunaga K, Suzuki Y, Nakamura M, Matsubara H, Sheldon HK, Haley KJ, Lilly CM, Drazen JM, Yamaguchi K. Cyclooxygenase-2 expression during allergic inflammation in guinea-pig lungs. Am. J. Respir. Crit. Care Med. 2002;165:382–386. doi: 10.1164/ajrccm.165.3.2103093. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Fujino H, Srinivasan D, Regan JW. Activation of FP prostanoid receptor isoforms leads to Rho-mediated changes in cell morphology and in the cell cytoskeleton. J. Biol. Chem. 1999;274:35944–35949. doi: 10.1074/jbc.274.50.35944. [DOI] [PubMed] [Google Scholar]
- Pratusevich VR, Seow CY, Ford LE. Plasticity in airway smooth muscle. J. Gen. Physiol. 1995;105:73–94. doi: 10.1085/jgp.105.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que LG, Liu L, Yan Y, Whitehead GS, Gavett SH, Schwartz DA, Stamler JS. Protection from experimental asthma by an endogenous bronchodilator. Science. 2005;308:1618–1621. doi: 10.1126/science.1108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani R, Shen X, Gunst SJ, Tepper RS. Comparison of elastic properties and contractile responses of isolated airway segments from mature and immature rabbits. J. Appl. Physiol. 2003;95:265–71. doi: 10.1152/japplphysiol.00362.2002. [DOI] [PubMed] [Google Scholar]
- Ramos-Barbon D, Ludwig MS, Martin JG. Airway remodeling: lessons from animal models. Clin. Rev. Allergy Immunol. 2004;27:3–21. doi: 10.1385/CRIAI:27:1:003. [DOI] [PubMed] [Google Scholar]
- Ricciardolo FLM, Timmers MC, Geppetti P, vanSchadewijk A, Brahim JJ, Sont JK, de Gouw HWFM, Hiemstra PS, vanKrieken JHJM, Sterk PJ. Allergen-induced impairment of bronchoprotective nitric oxide synthesis in asthma. J. Allergy Clin. Immunol. 2001;108:198–204. doi: 10.1067/mai.2001.116572. [DOI] [PubMed] [Google Scholar]
- Samb A, Pretolani M, Dinh-Xuan A, Ouksel H, Callebert J, Lisdero C, Aubier M, Boczkowski J. Decreased pulmonary and tracheal smooth muscle expression and activity of type 1 nitric oxide synthase (nNOS) after ovalbumin immunization and multiple aerosol challenge in guinea pigs. Am. J. Respir. Crit. Care Med. 2001;164:149–154. doi: 10.1164/ajrccm.164.1.2004030. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Rabe KF. The role of leukotrienes in the regulation of tone and responsiveness in isolated human airways. Am. J. Respir. Crit. Care Med. 2000;161:S62–S67. doi: 10.1164/ajrccm.161.supplement_1.ltta-13. [DOI] [PubMed] [Google Scholar]
- Scichilone N, Permutt S, Togias A. The lack of the bronchoprotective and not the bronchodilatory ability of deep inspiration is associated with airway hyperresponsiveness. Am. J. Respir. Crit. Care Med. 2001;163:413–419. doi: 10.1164/ajrccm.163.2.2003119. [DOI] [PubMed] [Google Scholar]
- Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J. Clin. Invest. 1995;96:2393–2403. doi: 10.1172/JCI118296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Spina D. Epithelium smooth muscle regulation and interactions. Am. J. Respir. Crit. Care Med. 1998;158:S141–S145. doi: 10.1164/ajrccm.158.supplement_2.13tac100a. [DOI] [PubMed] [Google Scholar]
- Taisne C, Norel X, Walch L, Labat C, Verriest C, Mazmanian GM, Brink C. Cholinesterase activity in pig airways and epithelial cells. Fundam. Clin. Pharmacol. 1997;11:201–205. doi: 10.1111/j.1472-8206.1997.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Tepper RS, Shen X, Bakan E, Gunst SJ. Maximal airway response in mature and immature rabbits during tidal ventilation. J. Appl. Physiol. 1995;79:1190–1198. doi: 10.1152/jappl.1995.79.4.1190. [DOI] [PubMed] [Google Scholar]
- Uddam R, Cardell LO, Luts A, Sundler F. Inhibitory nonadrenergic, noncholinergic innervation of airway smooth muscle: role of vasoactive intestinal peptide and structurally related molecules. In: Raeburn D, Giembycz MA, editors. In Airways smooth muscle: structure, innervation and neurotransmission. Birkhauser Verlag Basel; Switzerland: 1994. pp. 143–156. [Google Scholar]
- Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Vassaux G, Far DF, Gaillard D, Ailhaud G, Negrel R. Inhibition of prostacyclin-induced Ca2+ mobilization by phorbol esters in Ob1771 preadipocytes. Prostaglandins. 1993;46:441–451. doi: 10.1016/0090-6980(93)90080-q. [DOI] [PubMed] [Google Scholar]
- Wang L, Paré PD, Seow CY. Effects of length oscillation on the subsequent force development in swine tracheal smooth muscle. J. Appl. Physiol. 2000;88:2246–2250. doi: 10.1152/jappl.2000.88.6.2246. [DOI] [PubMed] [Google Scholar]
- Wang L, Paré PD, Seow CY. Plasticity in skeletal, cardiac, and smooth muscle, selected contribution: Effect of Chronic passive length change on airway smooth muscle length-tension relationship. J. Appl. Physiol. 2001;90:734–740. doi: 10.1152/jappl.2001.90.2.734. [DOI] [PubMed] [Google Scholar]
- Wang L, Paré PD. Deep inspiration and airway smooth muscle adaptation to length change. Respir. Physiol. Neurobiol. 2003;137:169–178. doi: 10.1016/s1569-9048(03)00145-9. [DOI] [PubMed] [Google Scholar]
- Wang L, Chitano P, Murphy TM. Length oscillation induces force potentiation in infant guinea pig airway smooth muscle. Am. J. Physiol. 2005a;289:L909–L915. doi: 10.1152/ajplung.00128.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chitano P, Murphy TM. Maturation of guinea pig tracheal strip stiffness. Am. J. Physiol. 2005b;289:L902–L908. doi: 10.1152/ajplung.00005.2005. [DOI] [PubMed] [Google Scholar]
- Weist A, Williams T, Kisling J, Clem C, Tepper RS. Volume history and effect on airway reactivity in infants and adults. J. Appl. Physiol. 2002;93:1069–1074. doi: 10.1152/japplphysiol.00986.2001. [DOI] [PubMed] [Google Scholar]
- Wheatley JR, Paré PD, Engel LA. Reversibility of induced bronchoconstriction by deep inspiration in asthmatic and normal subjects. Eur. Respir. J. 1989;2:331–339. [PubMed] [Google Scholar]
- Whicker SD, Armour CL, Black JL. Responsiveness of bronchial smooth muscle from asthmatic patients to relaxant and contractile agonists. Pulmonary Pharm. 1988;1:25–31. doi: 10.1016/0952-0600(88)90007-5. [DOI] [PubMed] [Google Scholar]
- Yamane K, Kobayashi T. Endogenous AA metabolites and their possible role in tracheal smooth muscle tone in guinea pigs. J. Appl. Physiol. 1990;69:26–32. doi: 10.1152/jappl.1990.69.1.26. [DOI] [PubMed] [Google Scholar]
- Yip P, Palombini B, Coburn RF. Inhibitory innervation to the guinea pig trachealis muscle. J. Appl. Physiol. 1981;50:374–382. doi: 10.1152/jappl.1981.50.2.374. [DOI] [PubMed] [Google Scholar]


