Summary
We previously reported in guinea pig tracheal smooth muscle that maximal shortening velocity decreases from 3 weeks of age to adulthood. It is not known whether myosin light chain kinase (MLCK), a key enzyme determining the velocity of smooth muscle contraction, undergoes maturational changes. In the present work, we investigated MLCK protein content and mRNA expression in 1-week-old, 3-week-old, and adult guinea pigs. We extracted either proteins or RNA from isolated tracheal smooth muscle. The content of MLCK was assessed by Western immunoblots. MLCK mRNA was evaluated by Northern analysis and by quantitative real time reverse transcriptase-polymerase chain reaction (RT-PCR). The content of MLCK increased 3-fold at 3 weeks of age and then decreased in adults, being 0.116 30.042, 0.330 30.125 (P<0.05), and 0.153 30.054 mg/mg of total protein, respectively, in 1-week, 3-week, and adult animals. Quantitative RT-PCR revealed that MLCK mRNA increased with age to 135 335% and 177 323% (P<0.01) in 3-week and adult animals, respectively, compared to 1-week animals. The transient increase of MLCK content in juvenile guinea pig tracheal smooth muscle may contribute to the increased shortening velocity at this age. We suggest that this increased content of MLCK is one of the mechanisms leading to maturation of airway smooth muscle contractility, which in turn contributes to the airway hyperresponsiveness reported in children and young animals.
Keywords: airway reactivity, airway smooth muscle, asthma, contractility, maturation
INTRODUCTION
Both in animals and humans, airway responsiveness, defined as degree of airway lumen reduction in response to a given contractile stimulus, undergoes maturational changes. Greater airway responsiveness is commonly observed in juvenile healthy individuals.1–3 Several mechanisms were suggested to be involved in this ontogenesis, including neural, pharmacological, and structural factors.4–6 Among these mechanisms, a central role might be played by the response of airway smooth muscle (ASM),7 in which ontogenetic changes were shown at the prejunctional level, at the receptor level, in the intracellular signal transduction mechanisms, and at the level of contractile proteins.8–10
We recently showed in guinea pig tracheal smooth muscle that, while the ability to generate force does not change substantially with maturation, maximum shortening velocity decreases significantly from 3 weeks of age to adulthood, and is inversely related to the internal resistance to shortening.11 These results suggest a maturation of either mechanical resistive components or biochemical regulatory factors that downregulate shortening velocity in adult animals.
In tonic smooth muscle (as is the case for ASM), a fast shortening, associated with a high ATP consumption rate, characterizes the initial phase of a contractile response.12 It is generally accepted that this initial phase is dependent on phosphorylation of the 20-kDa myosin light chain (MLC20) and the consequent activation of the acto-myosin ATPase, with recruitment of fast-cycling cross-bridges. The regulation of MLC20 phosphorylation is therefore a key limiting event of shortening velocity. Net MLC20 phosphorylation reflects the opposing enzyme activity of myosin light chain kinase (MLCK) and myosin light chain phosphatase, which phosphorylate and dephosphorylate MLC20, respectively. We recently studied the ratio of phosphorylation of MLC20 in tracheal strips from different-aged guinea pigs frozen at different times during electrical field stimulation.13 After 3 sec of stimulation, the ratio of MLC20 phosphorylation obtained by densitometric analysis of Western immunoblots was higher in strips from 3-week-old (60.7 ± 8.2%) than 1-week-old and adult animals (respectively, 45.9 ± 4.1% and 47.9 ± 6.0%). Normalization to basal level of phosphorylation accentuated the age differences and showed a higher level of phosphorylation in strips from 3-week animals at earlier time points of the stimulation. These preliminary data suggest that MLC20 phosphorylation may be a relevant factor in determining the increased shortening velocity in juvenile ASM.
The regulatory mechanisms of MLC20 phosphorylation were well-characterized in sensitized adult mongrel dogs. In that model of allergic hyperresponsiveness, ragweed antigen sensitization results in a substantial increase in ASM shortening velocity and shortening capacity, but not in force generation.14 These changes are determined by an increase in MLCK content,15 but are not related to changes in the activity of myosin light chain phosphatase.16 Similarly, an increase in the content of MLCK was found in excised sensitized human airways,17 suggesting an important role for MLCK in hyperresponsive human airway tissue. Therefore, MLCK likely plays a major regulatory role in the juvenile ASM hyperresponsiveness we previously described. In the current study, we investigated MLCK protein content in tracheal smooth muscle from guinea pigs of different ages: 1-week-old, 3-week-old, and adult. We also studied MLCK mRNA expression to determine whether changes in protein levels were related to ontogenetic changes in gene regulation.
MATERIALS AND METHODS
Animals and Tissue Preparation
Hartley guinea pigs (Charles River Laboratories, Inc., Wilmington, MA) were employed for the present investigation according to a protocol approved by the Duke University Institutional Animal Care and Use Committee. Three age groups were used: 1-week-old guinea pigs (1-week, n = 112, 142.4 ± 24.4 (SD) g, 6.2 ± 0.6 days old), 3-week-old guinea pigs (3-week, n = 74, 250.6 ± 28.4 g, 21.7 ± 1.5 days old), and 3-month-old guinea pigs (adult, n = 43, 707.8 ± 148.1 g, 87.4 ± 37.0 days old). Only male animals were used for the 3-week and adult groups.
Animals were anesthetized with an intraperitoneal injection of 200 mg/kg Na-pentobarbital (Abbott Laboratories, Chicago, IL). When anesthesia was completely achieved (no reflex observed in response to toe clamping), the trachea and lungs were exposed, excised, and immediately put into ice-cold Krebs-Henseleit buffer solution (K-H), aerated with 95% O2 and 5% CO2. The composition of K-H was the following (in mM): 115 NaCl, 25 NaHCO3, 1.38 NaH2PO4, 2.5 KCl, 2.46 MgSO4, 1.9 CaCl2, and 5.55 dextrose. All preparative procedures were performed in K-H solution buffered to pH 7.35–7.45 by continuous aeration with 95% O2/5% CO2.
Because we previously showed that the amount of smooth muscle is only about 18% of the paries membranaceus trachea,11 after cleaning away loose connective tissue, tracheal smooth muscle (TSM) tissue was isolated under a dissecting microscope (SZH10 Olympus stereo-microscope), using the following procedure. Cartilage rings were cut ventrally along the longitudinal axis of the trachea. The lumenal surface was exposed by mounting the trachea in a dissection Petri dish with stainless steel insect pins (size 00, Ward’s, Rochester, NY), and the epithelium was removed by gently scraping the surface with the smooth edge of curved forceps. The trachea was turned upside down and pinned again in the dissection dish, and the layer of connective tissue covering the smooth muscle on the outer surface of the trachea was removed. Finally the muscle was isolated from the edges attached to the cartilage, weighed (after removing the excess liquid with absorptive paper), frozen in liquid nitrogen, and stored at −80°C until used for biochemical analysis. The average quantity of tissue isolated from a single trachea was 2.5, 4.4, and 8.5 mg for 1-week, 3-week, and adult animals, respectively. Histological analysis was performed in samples of the three age groups to confirm that the tissue isolated according to this procedure was mainly smooth muscle. Respectively, 14, 12, and 13 sections were studied in 1-week, 3-week, and adult samples. A section of one adult sample stained with hematoxylineosin is shown in Figure 1. Slides were viewed through an Olympus stereo zoom microscope (model SZhH, Olympus Corp., Lake Success, NY). The smooth muscle and total area were measured using an image measurement software (Sigmascan, Jandel Scientific), and the ratio of smooth muscle in the isolated tissue was calculated. The ratio of smooth muscle in the total area of isolated tissue was 47 ± 12 (SD), 50 ± 6, and 50 ± 11% in 1-week, 3-week, and adult animals, respectively. In freshly isolated tissues of small animals, studies similar to ours normally use full organs. Our dissected preparation gave us more confidence that results reflected changes of MLCK protein and mRNA content occurring in TSM. Since we previously reported that the amount of smooth muscle in the paries membranaceus trachea does not vary with age in guinea pigs,11 no normalization by age was performed on our data.
Fig. 1.
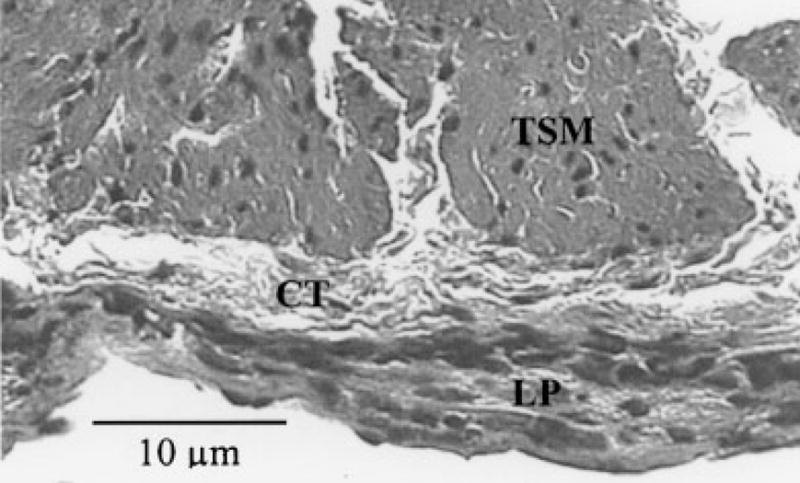
Histological slide showing smooth muscle obtained from adult guinea pig trachea. TSM, tracheal smooth muscle; CT, connective tissue of submucosa; LP, lamina propria.
Myosin Light Chain Kinase Content
Protein Extraction
TSM tissue from different animals of the same age group was in most cases pooled to prepare crude protein homogenate, so that 12, 11, and 12 samples were obtained from 1-week (n = 47), 3-week (n = 27), and adult (n = 17) animals, respectively. The average amount of tissue per sample of the three age groups was 8.1, 8.3, and 11.5 mg, respectively. The homogenate was then suspended in sodium dodecyl sulfate (SDS)-extraction buffer of the following composition: 0.3% SDS, 0.6 M β-mercaptoethanol, 28 mM Tris-HCl, 22 mM Tris, 0.25 mM phenylmethylsulfonyl fluoride, 0.002% leupeptin, and 0.005% soybean trypsin inhibitor. Extracts were heated at 95°C for 10 min and centrifuged at 5,000g or 5 min, the supernatant was collected, and protein concentration was assessed by spectrophotometric analysis at 595 nm. A protein assay was performed with a 96-well plate reader (Model EL311sx, Bio-Tek Instruments, Inc., Winooski, VT), using bovine serum albumin as standard and a protein colorimetric assay (Bio Rad, Hercules, CA). The amount of protein extracted was 43.3 ± 18.3 μ/mg of tissue, and was not statistically different among age groups. Therefore, we inferred that a given amount of protein reflected the same amount of TSM and of tracheal tissue at all ages. Samples were prepared for subsequent electrophoresis by mixing the supernatant with 2 × emmli loading buffer (Sigma, St. Louis, MO).
Electrophoresis and Immunoblotting
MLCK was identified by performing 7.5% SDS-PAGE. Equal amounts of protein, confirmed by silver staining of a preliminary set of gels, from the three age group samples as well as four lanes with known different amounts of MLCK standard from chicken gizzard, were loaded in each gel. A high molecular weight biotinylated marker was also loaded in each gel to visualize in the Western blot the position of the MLCK bands, based on their molecular weight. The composition of the lower chamber buffer was 22 mM glycine and 20 mM tris, while that of the upper chamber buffer was 22 mM glycine, 20 mM tris, 3 mM dithiothreitol, and 1 mM cysteine. Proteins were then transferred to nitrocellulose membrane at 1.5 amp for 1.5 hr. The composition of the transfer buffer was 25 mM Na2HPO4. After blocking nonspecific binding sites by incubating the membrane for 2 hr at room temperature in Tris-base saline (TBS) containing 5% nonfat dry milk, Western immunoblots were developed as follows: overnight at 4°C in a 1:10,000 solution of mouse anti-MLCK monoclonal antibody (Clone K36, Sigma) in TBS containing 0.1% Tween-20 (TBST)-1% milk; 1 hr at room temperature in 1:1,000 solution of biotinylated anti-mouse IgG antibody (Amersham Pharmacia Biotech, Piscat-away, NJ) in TBST-1% milk; and 1 hr at room temperature in a 1:5,000 solution of streptavidin horseradish peroxidase (Amersham Pharmacia Biotech, Piscataway, NJ) in TBST. The enhanced chemiluminescence reagents ECL+ mersham Pharmacia Biotech) were used to detect MLCK bands. Quantification of MLCK content was obtained by densitometric analysis of chemiluminescent immunoblots, using NIH Image 1.62 analytical software, and by interpolation of sample values with the curve obtained from the densitometric values of MLCK standards in the same immunoblot.
Myosin Light Chain Kinase mRNA
Total RNA Isolation
TSM tissue from different animals of the same age group was pooled, and total RNA was isolated. Respectively, 9, 7, and 6 samples were obtained from 1-week (n = 65), 3-week (n = 47), and adult (n = 26) animals. The average amount of tissue per sample of the three age groups was 22.6, 24.8, and 25.5 mg, respectively. TSM was homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA), and total RNA was isolated according to the manufacturer’s instructions. Samples were stored at −80°C.
Northern Analysis
A 218-bp cDNA plasmid encoding a portion of the 3′ terminal domain of guinea pig smooth muscle MLCK was used for Northern analyses to assess the level of MLCK mRNA expression in trachealis from different-aged animals. The cDNA insert was prepared by reverse transcriptase-polymerase chain reaction (RT-PCR). Primers to rabbit smooth muscle MLCK,18 rMLCK1 (sense: 5′ GACTGCAAGATTGAAGGATAC 3′), and rMLCK2 (antisense: 5′ GTTTCCACAATGAGCTCTGC 3′) were used to amplify the 218-bp fragment, using adult guinea pig trachealis smooth muscle cDNA as template. The amplified cDNA was then cloned into TOPOpCR2.1 vector (Invitrogen) according to the manufacturer’s instructions, and the clone was sequenced in both directions. The following MLCK partial cDNA clone (Genebank accession number AY354284.1) was obtained: [GACTG-CAAGATTGA AGGATACCCA GACCCTGAGG TCG-TCTGGTT CAAAGATGAC CAGTCAATCA GAG-AGTCGCG CCACTTCCAA ATAGACTACG ATGAG-GACGG GAACTGCTCT CTGATTATTA GTGATG-TCTG TGGGGATGAC GATGCCAAGT ACACCTGC-AA GGCTGCCAAC AGTCTTGGGG AATCCACCTG CACAGCAGAG CTCATTGTGG AAAC]. The sequence was then analyzed for homology to human19 (accession number HSU48959), rabbit18 (accession number M76233), and chicken gizzard smooth muscle MLCK cDNA20 (accession number M31048), and revealed 94%, 90%, and 81% homology, respectively.
Total RNA (5 μg) was separated by gel electrophoresis on a 1.2% agarose-formaldehyde gel and transferred by capillary blot to nylon membranes (Nytran plus) in 1 M ammonium acetate, and the RNA was cross-linked to the membranes by ultraviolet irradiation. The Northern filter was then hybridized at 62°C in the presence of a 32P-labeled cDNA probe for MLCK. The probe was prepared from the cDNA insert described above using the random priming method (Prime-It II, Stratagene), according to the manufacturer’s instructions. After hybridization, blots were washed twice with saline sodium citrate (SSC) and 0.1% SDS at room temperature for 30 min, and then with 0.1 × SSC and 0.1% SDS at 62°C for 15 min. Autoradiographs were obtained by exposing films to the membrane at −80°C. Autoradiographic bands obtained from three Northern gels were quantitated by densitometry, using NIH Image 1.62 analytical software.
The bands of 18 s and 28 s rRNA obtained by staining the agarose-formaldehyde gel with ethidium bromide revealed that the average relative loading of total RNA for 3-week and adult samples was 1.2 and 1.9 times the loading for 1-week samples, respectively. The densitometric value of the rRNA band was used to normalize mRNA bands of the same sample, and data were expressed as percent of expression in TSM from 1-week animals.
Quantitative Real-Time RT-PCR
MLCK primers were designed for real-time RT-PCR, using ‘‘Primer Express’’ software (Applied Biosystems, Foster City, CA), and included an intron/exon boundary that precluded amplification of contaminating genomic DNA in the RNA samples. Primers amplify a region of guinea pig smooth muscle MLCK 5′ to the fibronectin III domain, and were designed for a sequence21 (Genbank accession number AB070227) with no overlap with telokin or nonmuscle MLCK. Primers had the following sequences: 5′ CCATCTCCAAGACATCTCCGAA 3′ (sense), and 5′ ATGTGCTCGCTGTCCTGGAT 3′ (anti-sense). Primer sequences for 18s rRNA were: 5′ CGGC-TACCACATCCAAGGAA 3′ (sense), and 5′ GCTGGAA-TTACCGCGGCT 3′ (antisense).
Quantitative real-time RT-PCR was performed by the SYBR green method for fluorescence detection during amplification on an ABI Prism 7900 Sequence Detection System (Applied Biosystems). Triplicates of each RNA sample were loaded in 96-well plates (250 ng/well) with a solution of SYBR green master mix, RNase inhibitor, and multiscribe RT enzyme according to the manufacturer’s instructions (Applied Biosystems), and with either MLCK or 18s primers. Prior to RT-PCR, RNA samples were treated with RQ1 RNase-free DNase (Promega, Madison, WI) to remove possible contaminant DNA. PCR conditions were as follows: RT at 50°C for 30 min, initial denaturation at 95°C for 10 min, 40 thermal cycles (95°C for 15 sec, and 62°C for 1 min), and dissociation cycle (95°C for 15 sec, 60°C for 1 min, and ramping to 95°C for 15 sec). This real-time PCR was also performed without the RT step as a control. After determination of the threshold cycle (Ct) for each sample, the relative amount of MLCK mRNA was evaluated by the comparative Ct method (ΔΔCt): the amplification of MLCK was first normalized to the 18s rRNA amplification in the same sample (ΔCt), and then each ΔCt value was compared to the lowest sample ΔCt (average of a triplicate). These ΔΔCt were then transformed to absolute values and expressed as percentage of expression in samples from 1-week animals. The levels of 18s rRNA showed no difference among samples of different ages, with Ct values similar for all samples.
Drugs and Chemicals
Bovine serum albumin, Laemmli loading buffer, leupeptin, soybean trypsin inhibitor, and cystein were purchased from Sigma Chemical Co.; dithiothreitol from ICN Biomedicals (Aurora, OH); Bis, TEMED, ammonium persolphate, and nitrocellulose membrane from BioRad (Richmond, CA); acrylamide, tris, SDS, and glycin from EM Science (Gibbstown, NJ); and glycerol from Mallinckrodt Chemical (Paris, KY). Purified chicken gizzard MLCK to be used as standard in MLCK immunoblots was kindly provided by Dr. Michael P. Walsh (University of Calgary).
Data Analysis
Data are expressed as means ± standard error of the mean, except when differently indicated. The number of samples employed in each set of experiments was used as the n value for all statistics. Statistical analyses performed were ANOVA and the post hoc least-significant-difference Fisher’s test to find out which groups were responsible for differences revealed by ANOVA. ANOVAwith repeated measures was employed when applicable. The software employed was Statistix 7.0 (Analytical Software, Tallahassee, FL). P <0.05 was considered significant.
RESULTS
Myosin Light Chain Kinase Content
An example of a Western immunoblot showing the purified chicken gizzard MLCK standards and the MLCK bands of different age samples is shown in Figure 2. The MLCK bands from guinea pig tracheal smooth muscle migrated more slowly than the chicken gizzard MLCK because of the different molecular weight, which is approximately 150 kDa in most mammalian smooth muscle, and 130 kDa in avian smooth muscle.22 The specificity of all bands was confirmed by their absence from the Western immunoblot when the blot was not exposed to the anti-MLCK monoclonal antibody (clone K36). The 208-kDa embryonic form of MLCK was not present in any of our samples.
Fig. 2.
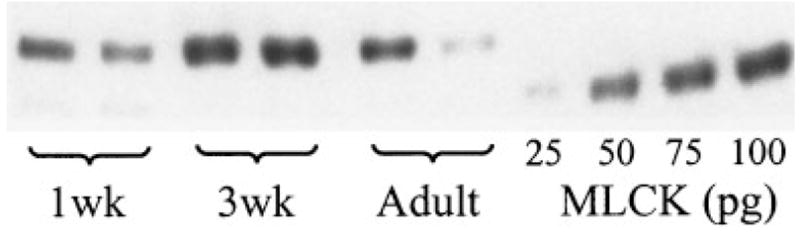
Western immunoblot showing MLCK bands of different-aged (1wk, 1-week-old; 3wk, 3-week-old) guinea pig tracheal smooth muscle samples (150 kDa) and purified chicken gizzard MLCK standards (130 kDa).
The content of MLCK, expressed as microgram per milligram of extracted proteins, in the three age groups is shown in Figure 3. MLCK first increased significantly (about 3-fold) from 1-week to 3-week (P <0.05 by ANOVA) animals, and then returned to 1-week levels in adults.
Fig. 3.
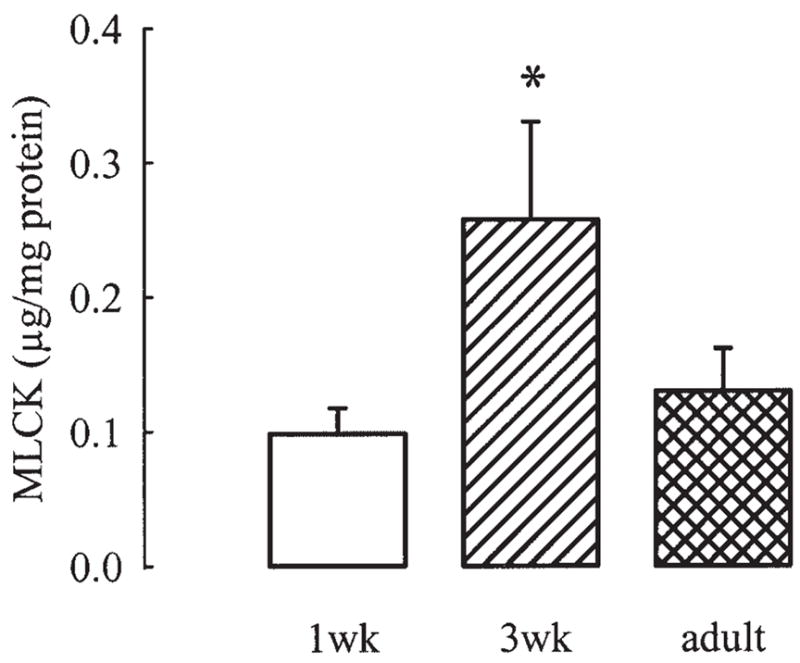
Densitometric measurements from immunoblots of MLCK, which show transient increase in content of MLCK at 3 weeks of age (P<0.05 by ANOVA). Values are mean ± SE of 12 1-week (1wk), 11 3-week (3wk), and 12 adult samples.
Myosin Light Chain Kinase mRNA
The Northern autoradiograph revealed a band for MLCK mRNA at the expected size of 5.8 kb (Fig. 4A). The MLCK cDNA probe also detected a band at approximately 2.6 kb, corresponding to the mRNA encoding telokin, an abundant protein in airway smooth muscle, which has an identical cDNA sequence to the myosin-binding portion of MLCK. The 18s rRNA bands revealed by ethidium staining are shown in Figure 4B. MLCK mRNA:18s rRNA levels, as revealed by densitometric analysis of Northern blots, progressively increased with age (Fig. 4C). Quanti tative real-time RT-PCR was performed to confirm and quantify differences in MLCK expression shown by Northern analysis.
Fig. 4.
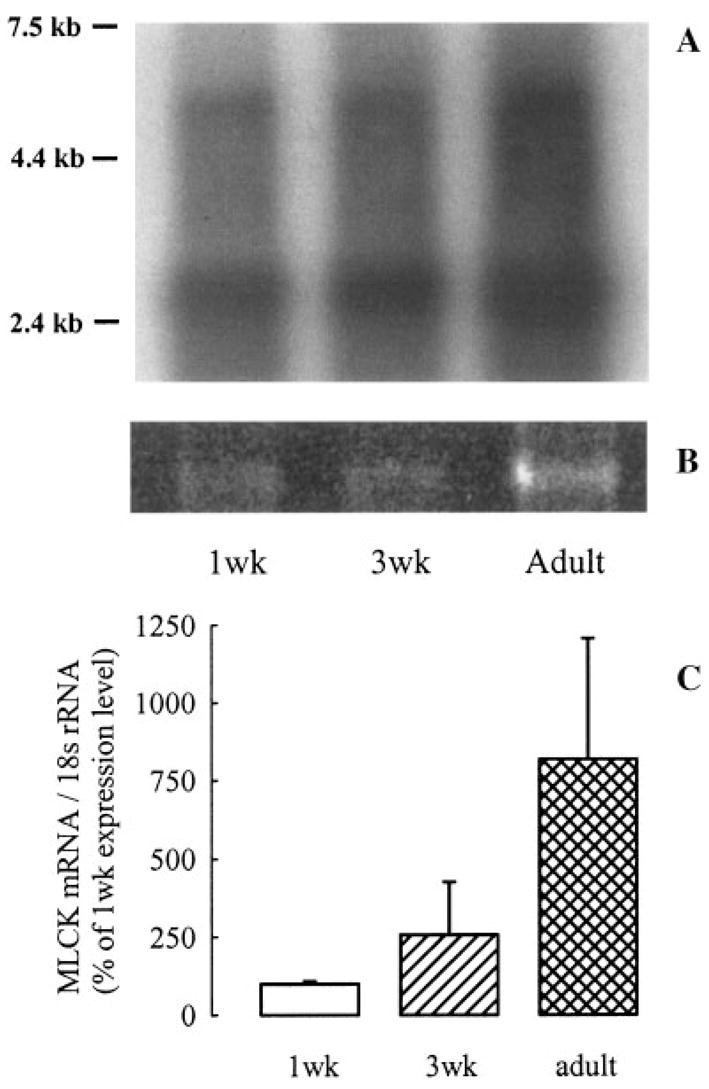
A: Northern autoradiograph showing MLCK (~5.8 kb) and telokin mRNA (~2.6 kb) bands of different-aged (1wk, 1-week-old; 3wk, 3-week-old) guinea pig tracheal smooth muscle samples. Molecular weight markers are shown (kb). B: Ethidium stain of agarose gel revealing 18s rRNA. C: Densitometric values of MLCK bands, normalized to 18s rRNA ethidium bands. Values are mean ± SE, n = 3.
Quantitative real-time RT-PCR, consistent with Northern analysis results, revealed a progressive increase of MLCK mRNA with age (Fig. 5). The mRNA levels in adult TSM samples were significantly higher than in 1-week tissue (P <0.01). PCR amplification in the absence of reverse transcriptase generated Ct values equivalent to absence of DNA in the reaction. Thus these results reflect only mRNA quantitation.
Fig. 5.
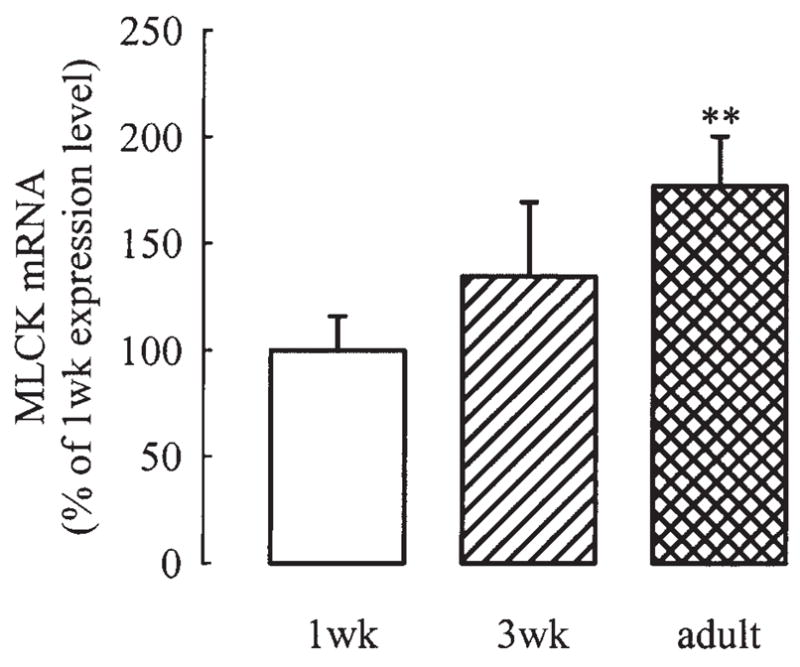
Results of quantitative real-time RT-PCR showing sequential increase in MLCK mRNA expression with age (P<0.01). Values are mean ± SE of 6 1-week (1wk), 4 3-week (3wk), and 3 adult samples.
DISCUSSION
In the present study, we investigated MLCK mRNA and protein content in 1-week, 3-week, and adult guinea pigs. We found that the content of MLCK was higher in 3-week-old compared to younger and older animals, and that the MLCK mRNA increased sequentially with age. This is the first report of a variation in the content and expression of airway smooth muscle MLCK during ontogenesis. The change in MLCK content parallels both the change in shortening velocity and in MLC20 phosphorylation that we reported in maturing TSM. Therefore, these results suggest that a different cellular content of MLCK may be a relevant factor in juvenile airway hyperresponsiveness.
The velocity and extent of a contractile response to most agonists depend on the phosphorylation of MLC20. This is modulated by the phosphorylating action of MLCK and by the opposite dephosphorylating action of myosin light chain phosphatase. Therefore, changes in the cellular content of these two key enzymes may generate substantial modifications of ASM responsiveness. Both enzymes were studied in a canine adult model of allergic airway hyperresponsiveness. In this model, the sensitization of mongrel dogs with ragweed antigen generated an increase in ASM shortening velocity and shortening capacity but not force generation.14 The mechanism of these changes appears to derive entirely from an increase in the content of MLCK15 and its total, but not specific, activity in tissue from sensitized animals. Interestingly, no change of MLCK mRNA was found between sensitized and control smooth muscle,23 suggesting a sensitization-induced regulation at either the translational or posttranslational level. In that same model, no change in activity of myosin light chain phosphatase was produced by ragweed allergen sensitization.16 Investigations from the same laboratory also showed no increase in calcium release, content/activity of calcium-calmodulin,24 or a shift in distribution of myosin heavy chain isoforms14 in sensitized ASM. Altogether those studies suggest that a posttranscriptional regulation of MLCK content may be the major mechanism regulating the increased ASM responsiveness induced by allergic sensitization.
Similar to the allergic canine model discussed above, passive sensitization of human airways induces an increase of the ASM shortening capacity and velocity, without changes in maximal force generation.25 A significant increase in the content of MLCK was also shown to occur in sensitized human airways,17 suggesting that more abundant MLCK may also cause the allergen-induced increase in ASM responsiveness in humans. The same study showed no difference in myosin heavy chain content between control and sensitized tissue. Our results show that an increase in MLCK content is associated with the higher ASM responsiveness of healthy juvenile guinea pigs. As we previously reported, at that stage of ontogenesis, ASM shows an increased shortening velocity but not force generation, resembling the behavior of sensitized canine and human ASM. Taken together, these data suggest that a regulation of the amount of MLCK in ASM cells is a general mechanism in the regulation of ASM responsiveness, as revealed by its increased shortening velocity and capacity.
We focused in the present study on MLCK, because its content was shown in the canine allergic model to be the only factor of the excitation-contraction coupling associated with increased ASM responsiveness.14–16,24 Nonetheless, our study does not exclude the possibility that other factors contribute to the ASM responsiveness in our maturational model. Little is known regarding the alteration of intracellular signal transduction mechanisms and excitation-contraction coupling during ontogenesis. Inositol 1,4,5-trisphosphate accumulation following muscarinic stimulation was reported to be increased in newborn rabbit trachealis,26 suggesting that this second messenger may contribute to the increased juvenile responsiveness. However, no change in inositol phosphate formation was found in tracheal tissue from 1-month-old and adult guinea pigs,27 thus reducing the likelihood of the relevance of this factor in our model. Several regulatory and contractile proteins, such as calmodulin, calponin, caldesmon, myosin light chain phosphatase, myosin, and actin, may play a role in ASM responsiveness. Studies in swine showed that the cellular content of myosin heavy chain (MHC) increased from 0 to 2 weeks, and then decreased,28 while steady-state levels of mRNA for MHC increased in the first 2 weeks.29 A likely candidate regulatory enzyme in the increased juvenile ASM responsiveness is myosin light chain phosphatase. Either a change in its cellular content or an altered modulation of its activity by Rho-kinase and/or by arachidonic acid would affect MLC20 phosphorylation.30 Whether myosin light chain phosphatase and other components involved in the contractile response undergo ontogenetic variations and contribute to juvenile ASM hyperresponsiveness requires further studies that we are currently pursuing.
MLC20 phosphorylation alone was shown to be sufficient to initiate contraction,31 and there is no doubt that MLC20 phosphorylation by MLCK plays a central role in smooth muscle contractility. More controversial is the relationship between MLC20 phosphorylation and shortening velocity. Most early studies suggested a linear relationship between these two parameters. From those studies, a model was proposed that explains the regulation of shortening velocity in smooth muscle as a function of only MLC20 phosphorylation levels. This model hypothesizes the occurrence of slowly cycling “latch-bridges” that form late in contraction, when levels of phosphorylation are low and reduce shortening velocity.12,32 However, data were reported that showed dissociation between MLC20 phosphorylation and shortening velocity in the early phase of a contractile response.33 That study showed that shortening velocity starts to decline when levels of phosphorylation are still rising, and the authors concluded that dephosphorylated “latch-bridges” are not at the origin of early velocity slowing. Therefore, the regulation of shortening velocity in smooth muscle could vary according to the activation state. The issue was addressed in skinned smooth muscle by comparing different levels of activation induced by either okadaic acid or Ca++34 At least in the experimental conditions used in that study, the level of phosphorylation was shown to be the major factor determining shortening velocity. However, the effect of phosphorylation levels on velocity and force was similar. Thus mechanisms other than dephosphorylated “latch-bridges” may also regulate shortening velocity in skinned smooth muscle fibers. One component of smooth muscle cells that was suggested as a candidate for reducing shortening velocity is the action of thin filament system,35,36 i.e., thin filament proteins would reduce actomyosin ATPase activity without altering MLC20 phosphorylation. However, their involvement in the ontogenesis of ASM function is unknown.
A different velocity of shortening was also shown when the same level of phosphorylation was induced by different agonists or in smooth muscle of a different origin.37 This further suggests that the level of MLC20 phosphorylation is not the only factor determining shortening velocity in smooth muscle. The issue of the regulation of shortening velocity in different tissues is certainly of great relevance to our study, because of the changes observed in both contractile and cytoskeletal proteins during development and remodeling.38 A seven-amino-acid insert of MHC was suggested to produce the differences in shortening velocity between vascular and intestinal smooth muscle,39 but it remains to be determined whether this contributes to the ontogenesis of airway smooth muscle function. Moreover, noncontractile components may affect shortening velocity by altering either the external load on smooth muscle or the internal resistance to shortening. This is conceivably the case in our model, since we previously reported a noticeable increase of internal resistance to shortening in adult tracheal strips.11 Because the increased resistance with maturation was not paralleled by a change in force, we suggested that it provides a mechanism to reduce the shortening velocity toward adulthood. Combining those results with the data of the present work, we may conclude that the decline in shortening velocity with maturation results from the combination of reduced MLCK content, which reduces the level of MLC20 phosphorylation, and increases resistance to shortening. Still, the other factors we discussed might also turn out to contribute to reduce shortening velocity during development.
Two different isoforms of MLCK may be present in smooth muscle.40 Only a short form, varying in size between 130–150 kDa, is normally found in differentiated adult tissue, while a long form of 208 kDa is found in embryonic tissues and cultured cells.22 In this study, we used a monoclonal antibody that recognizes both forms of MLCK, and we did not find the long isoform in any of our samples, similar to what was shown for adult tissues.17,22 This suggests that the long isoform of MLCK does not contribute to the maturational changes of ASM contractility. The short isoform of MLCK is known to be nearly ubiquitous,41 and the inclusion of tissue other than ASM could have affected the outcome of our study. Because we previously showed that the amount of smooth muscle is only about 18% of the paries membranaceus trachea,11 we performed a careful dissection of the TSM and confirmed histologically that smooth muscle was the main component of our samples.
As discussed above, an increase of MLCK protein content is produced by allergic sensitization in dogs without change in mRNA levels. This suggests that a regulatory mechanism exists in ASM, which controls either the translation or the posttranslational processing of the MLCK protein. Data from the canine model imply that allergic sensitization alters this regulatory mechanism, thus increasing the MLCK content and consequently ASM responsiveness. By contrast, increased levels of MLCK mRNAwere shown in asthmatic bronchial smooth muscle cells compared to controls,42 although a recent study failed to confirm that observation.43 This suggests that transcriptional mechanisms may also regulate MLCK protein content in asthmatic airway smooth muscle. In our maturational model, we showed that steady-state MLCK mRNA increases sequentially with age, in discord with the decline in MLCK protein content after the initial rise during the first 3 weeks of life. The disparity between MLCK mRNA and protein content during ontogenesis and in canine allergen sensitization suggests that mRNA levels cannot be used as a surrogate for determination of MLCK protein content. However, these studies are a useful tool to determine the mechanisms of this regulation. The reduced MLCK protein content in adult tissue may be the result of either decreased translation or increased protein turnover, e.g., degradation through the proteosome, but further studies will be required to discriminate between these possibilities. With respect to our study, we suggest that the downregulation of MLCK content reduces ASM responsiveness with maturation to adulthood.
In conclusion, we showed that the level of MLCK increases early with maturation and decreases later toward adulthood in guinea pig trachealis. This is in agreement with our previous study in the same model showing that the maximal value of ASM shortening velocity occurs at age 3 weeks and significantly declines in adulthood. In that report, we suggested that an increase of mechanical internal resistance to shortening toward adulthood was a regulatory mechanism to reduce ASM shortening, and thus explained in part the higher airway responsiveness of young animals. In the present paper, we show evidence of a similar regulatory mechanism acting during maturation to decrease the amount of MLCK in adult tissue, thus reducing the ASM contractile capacity. We identified two factors regulated during ontogenesis, whose combination leads to a unique vulnerability for airway hyperresponsiveness in juvenile animals and whose progression toward adulthood provides a protective mechanism to reduce airway responsiveness. Although they need to be further investigated, these two factors are likely involved in the ontogenesis of human ASM and in the airway hyperresponsiveness reported in children.
Acknowledgments
The authors thank Dr. Michael P. Walsh (University of Calgary) for kindly providing purified chicken gizzard MLCK to be used as standard in MLCK immunoblots. The authors thank also Tatiana Vinogradova for performing the preliminary set of experiments with real-time RT-PCR and for the preparation of part of the RNA samples used with this technique.
Grant sponsor: ALA; Grant sponsor: NIH; Grant number: HL61899; Grant sponsor: Duke Children’s Miracle Network; Grant sponsor: Duke Neonatal-Perinatal Research Institute.
Footnotes
This work was partially presented in preliminary form at the 2001 and 2003 meetings of the American Thoracic Society.
References
- 1.Hopp RJ, Bewtra A, Nair NM, Townley RG. The effect of age on methacholine response. J Allergy Clin Immunol. 1985;76:609–613. doi: 10.1016/0091-6749(85)90783-3. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery GL, Tepper RS. Changes in airway reactivity with age in normal infants and young children. Am Rev Respir Dis. 1990;142:1372–1376. doi: 10.1164/ajrccm/142.6_Pt_1.1372. [DOI] [PubMed] [Google Scholar]
- 3.Shen X, Bhargava V, Wodicka GR, Doerschuk CM, Gunst SJ, Tepper RS. Greater airway narrowing in immature than in mature rabbits during methacholine challenge. J Appl Physiol. 1996;81:2637–2643. doi: 10.1152/jappl.1996.81.6.2637. [DOI] [PubMed] [Google Scholar]
- 4.Busse WW, Banks-Schlegel SP, Larsen GL. Effects of growth and development on lung function. Models for study of childhood asthma. Am J Respir Crit Care Med. 1997;156:314–319. doi: 10.1164/ajrccm.156.1.9612121. [DOI] [PubMed] [Google Scholar]
- 5.Ishida K, Fukuchi Y. The effect of aging on airway responsiveness. Nippon Kyobu Shikkan Gakkai Zasshi. 1992;30:182–186. [PubMed] [Google Scholar]
- 6.Saunder RA, McNikol KJ, Stecenko AA. Effect of age on lung mechanics and airway reactivity in lambs. J Appl Physiol. 1986;61:2074–2080. doi: 10.1152/jappl.1986.61.6.2074. [DOI] [PubMed] [Google Scholar]
- 7.Chitano P, Murphy TM. Maturational changes in airway smooth muscle shortening and relaxation. Implications for asthma. Respir Physiol Neurobiol. 2003;137:347–359. doi: 10.1016/s1569-9048(03)00158-7. [DOI] [PubMed] [Google Scholar]
- 8.Fisher JT. Airway smooth muscle contraction at birth: in vivo versus in vitro comparisons to the adult. Can J Physiol Pharmacol. 1992;70:590–596. doi: 10.1139/y92-075. [DOI] [PubMed] [Google Scholar]
- 9.Murphy TM, Mitchell RW, Phillips JP, Leff AR. Ontogenic expression of acetylcholinesterase activity in trachealis of young swine. Am J Physiol Lung Cell Mol Physiol. 1991;261:322–326. doi: 10.1152/ajplung.1991.261.4.L322. [DOI] [PubMed] [Google Scholar]
- 10.Wills-Karp M. Effects of ageing upon airway smooth muscle contractility. In: Raeburn D, Giembycz MA, editors. Airway smooth muscle: development and regulation of contractility. Basel: Birkháuser Verlag; 1994. pp. 185–218. [Google Scholar]
- 11.Chitano P, Wang J, Cox CM, Stephens NL, Murphy TM. Different ontogeny of rate of force generation and shortening velocity in guinea pig trachealis. J Appl Physiol. 2000;88:1338–1345. doi: 10.1152/jappl.2000.88.4.1338. [DOI] [PubMed] [Google Scholar]
- 12.Dillon PF, Aksoy MO, Driska SP, Murphy RA. Myosin phos-phorylation and the crossbridge cycle in arterial smooth muscle. Science. 1981;211:495–497. doi: 10.1126/science.6893872. [DOI] [PubMed] [Google Scholar]
- 13.Chitano P, Dakshinamurti S, Stephens NL, Murphy TM. Myosin light chain phosphorylation at 3s during electrical field stimulation in guinea pig tracheal strips transiently increases at 3 weeks. Am J Respir Crit Care Med. 2003;167 [Abstract]879. [Google Scholar]
- 14.Jiang H, Liu X, Halayko AJ, Liu G, Stephens NL. Early changes in airway smooth muscle hyperresponsiveness. Can J Physiol Pharmacol. 1994;72:1440–1447. doi: 10.1139/y94-208. [DOI] [PubMed] [Google Scholar]
- 15.Jiang H, Rao K, Halayko AJ, Liu X, Stephens NL. Ragweed sensitization-induced increase of myosin light chain kinase content in canine airway smooth muscle. Am J Respir Cell Mol Biol. 1992;7:567–573. doi: 10.1165/ajrcmb/7.6.567. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Halayko AJ, Liu G, Rao K, Jiang H, Stephens NL. Myosin light chain phosphatase activity in ragweed pollen-sensitized canine tracheal smooth muscle. Am J Respir Cell Mol Biol. 1994;11:676–681. doi: 10.1165/ajrcmb.11.6.7946396. [DOI] [PubMed] [Google Scholar]
- 17.Ammit AJ, Armour CL, Black JL. Smooth-muscle myosin light-chain kinase content is increased in human sensitized airways. Am J Respir Crit Care Med. 2000;161:257–263. doi: 10.1164/ajrccm.161.1.9901005. [DOI] [PubMed] [Google Scholar]
- 18.Gallagher PJ, Herring BP, Griffin SA, Stull JT. Molecular characterization of a mammalian smooth muscle myosin light chain kinase. J Biol Chem. 1991;266:23936–23944. [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia JG, Lazar V, Gilbert-McClain LI, Gallagher PJ, Verin AD. Myosin light chain kinase in endothelium: molecular cloning and regulation. Am J Respir Cell Mol Biol. 1997;16:489–494. doi: 10.1165/ajrcmb.16.5.9160829. [DOI] [PubMed] [Google Scholar]
- 20.Olson NJ, Pearson RB, Needleman DS, Hurwitz MY, Kemp BE, Means AR. Regulatory and structural motifs of chicken gizzard myosin light chain kinase. Proc Natl Acad Sci USA. 1990;87:2284–2288. doi: 10.1073/pnas.87.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao J, Oishi K, Yamada T, Liu L, Nakamura A, Uchida MK, Kohama K. Role of the short isoform of myosin light chain kinase in the contraction of cultured smooth muscle cells as examined by its down-regulation. Proc Natl Acad Sci USA. 2002;99:9556–9561. doi: 10.1073/pnas.142298599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallagher PJ, Garcia JGN, Herring BP. Expression of a novel myosin light chain kinase in embryonic tissues and cultured cells. J Biol Chem. 1995;270:29090–29095. doi: 10.1074/jbc.270.49.29090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephens NL, Halayko AJ. Airway smooth muscle contractile, regulatory and cytoskeletal protein expression in health and disease. Comp Biochem Physiol [B] 1998;119:415–424. doi: 10.1016/s0305-0491(98)00004-2. [DOI] [PubMed] [Google Scholar]
- 24.Jiang H, Rao K, Liu X, Liu G, Stephens NL. Increased myosin phosphorylation, but not calmodulin activity in sensitized airway smooth muscles. Am J Physiol Lung Cell Mol Physiol. 1995;268:739–746. doi: 10.1152/ajplung.1995.268.5.L739. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell RW, Ruhlmann E, Magnussen H, Leff AR, Rabe KF. Passive sensitization of human bronchi augments smooth muscle shortening velocity and capacity. Am J Physiol Lung Cell Mol Physiol. 1994;267:218–222. doi: 10.1152/ajplung.1994.267.2.L218. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg SM, Berry GT, Yandrasitz JR, Grunstein MM. Maturational regulation of inositol 1,4,5-trisphosphate metabolism in rabbit airway smooth muscle. J Clin Invest. 1991;88:2032–2038. doi: 10.1172/JCI115531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wills-Karp M. Effects of age on muscarinic agonist-induced contraction and IP accumulation in airway smooth muscle. Life Sci. 1991;49:1039–1045. doi: 10.1016/0024-3205(91)90305-u. [DOI] [PubMed] [Google Scholar]
- 28.Murphy TM, Mitchell RW, Halayko A, Roach J, Roy L, Kelly EA, Munoz NM, Stephens NL, Leff AR. Effect of maturational changes in myosin content and morphometry on airway smooth muscle contraction. Am J Physiol Lung Cell Mol Physiol. 1991;260:471–480. doi: 10.1152/ajplung.1991.260.6.L471. [DOI] [PubMed] [Google Scholar]
- 29.Munoz NM, Zak R, Panettieri RA, Wiesner R, Leff AR. Changes in levels of mRNA encoding myosin heavy chain in porcine trachealis during ontogenesis. Am J Respir Cell Mol Biol. 1993;8:252–257. doi: 10.1165/ajrcmb/8.3.252. [DOI] [PubMed] [Google Scholar]
- 30.Pfitzer G. Regulation of myosin phosphorylation in smooth muscle. J Appl Physiol. 2001;91:497–503. doi: 10.1152/jappl.2001.91.1.497. [DOI] [PubMed] [Google Scholar]
- 31.Walsh MP, Bridenbaugh R, Hartshorne DJ, Kerrick WG. Phosphorylation-dependent activated tension in skinned gizzard muscle fibers in the absence of Ca2+ J Biol Chem. 1982;257:5987–5990. [PubMed] [Google Scholar]
- 32.Hai CM, Murphy RA. Regulation of shortening velocity by cross-bridge phosphorylation in smooth muscle. Am J Physiol Cell Physiol. 1988;255:86–94. doi: 10.1152/ajpcell.1988.255.1.C86. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell RW, Seow CY, Burdyga T, Maass-Moreno R, Pratuse-vich VR, Ragozzino J, Ford LE. Relationship between myosin phosphorylation and contractile capability of canine airway smooth muscle. J Appl Physiol. 2001;90:2460–2465. doi: 10.1152/jappl.2001.90.6.2460. [DOI] [PubMed] [Google Scholar]
- 34.Malmqvist U, Arner A. Regulation of force and shortening velocity by calcium and myosin phosphorylation in chemically skinned smooth muscle. Pflugers Arch. 1996;433:42–48. doi: 10.1007/s004240050246. [DOI] [PubMed] [Google Scholar]
- 35.Ngai PK, Walsh MP. Inhibition of smooth muscle actin-activated myosin Mg2+-ATPase activity by caldesmon. J Biol Chem. 1984;259:13656–13659. [PubMed] [Google Scholar]
- 36.Malmqvist U, Trybus KM, Yagi S, Carmichael J, Fay FS. Slow cycling of unphosphorylated myosin is inhibited by calponin, thus keeping smooth muscle relaxed. Proc Natl Acad Sci USA. 1997;94:7655–7660. doi: 10.1073/pnas.94.14.7655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller-Hance WC, Kamm KE. Force-velocity relation and myosin light chain phosphorylation in bovine coronary arterial smooth muscle. Circ Res. 1991;69:1207–1214. doi: 10.1161/01.res.69.5.1207. [DOI] [PubMed] [Google Scholar]
- 38.Low RB, White SL. Lung smooth muscle differentiation. Int J Biochem Cell Biol. 1998;30:869–883. doi: 10.1016/s1357-2725(98)00049-1. [DOI] [PubMed] [Google Scholar]
- 39.Kelley CA, Takahashi M, Yu JH, Adelstein RS. An insert of seven amino acids confers functional differences between smooth muscle myosins from the intestines and vasculature. J Biol Chem. 1993;268:12848–12854. [PubMed] [Google Scholar]
- 40.Gallagher PJ, Herring BP, Stull JT. Myosin light chain kinase. J Muscle Res Cell Motil. 1997;18:1–16. doi: 10.1023/a:1018616814417. [DOI] [PubMed] [Google Scholar]
- 41.Herring BP, Dixon S, Gallagher PJ. Smooth muscle myosin light chain kinase expression in cardiac and skeletal muscle. Am J Physiol Cell Physiol. 2000;279:1656–1664. doi: 10.1152/ajpcell.2000.279.5.C1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma X, Cheng Z, Kong H, Wang Y, Unruh H, Stephens NL, Laviolette M. Changes in biophysical and biochemical properties of bronchial smooth muscle cells from asthmatic subjects. Am J Physiol Lung Cell Mol Physiol. 2002;283:1181–1189. doi: 10.1152/ajplung.00389.2001. [DOI] [PubMed] [Google Scholar]
- 43.Bellam SK, Patel NM, Laviolette M, Chakir J, Tretiakova M, Solway J. Analysis of gene expression in asthmatic smooth muscle [abstract] Am J Respir Crit Care Med. 2003;167:330. [Google Scholar]


