Abstract
High intake of red meat or processed meat is associated with increased risk of colon cancer. In contrast, consumption of white meat (chicken) is not associated with risk and might even reduce the occurrence of colorectal cancer. We speculated that a diet containing beef or bacon would increase and a diet containing chicken would decrease colon carcinogenesis in rats. One hundred female Fischer 344 rats were given a single injection of azoxymethane (20 mg/kg i.p.), then randomized to 10 different AIN-76-based diets. Five diets were adjusted to 14% fat and 23% protein and five other diets to 28% fat and 40% protein. Fat and protein were supplied by 1) lard and casein, 2) olive oil and casein, 3) beef, 4) chicken with skin, and 5) bacon. Meat diets contained 30% or 60% freeze-dried fried meat. The diets were given ad libitum for 100 days, then colon tumor promotion was assessed by the multiplicity of aberrant crypt foci [number of crypts per aberrant crypt focus (ACF)]. The ACF multiplicity was nearly the same in all groups, except bacon-fed rats, with no effect of fat and protein level or source (p = 0.7 between 8 groups by analysis of variance). In contrast, compared with lard- and casein-fed controls, the ACF multiplicity was reduced by 12% in rats fed a diet with 30% bacon and by 20% in rats fed a diet with 60% bacon (p < 0.001). The water intake was higher in bacon-fed rats than in controls (p < 0.0001). The concentrations of iron and bile acids in fecal water and total fatty acids in feces changed with diet, but there was no correlation between these concentrations and the ACF multiplicity. Thus the hypothesis that colonic iron, bile acids, or total fatty acids can promote colon tumors is not supported by this study. The results suggest that, in rats, beef does not promote the growth of ACF and chicken does not protect against colon carcinogenesis. A bacon-based diet appears to protect against carcinogenesis, perhaps because bacon contains 5% NaCl and increased the rats’ water intake.
Keywords: Adipose Tissue, anatomy & histology, Animals, Azoxymethane, Bile Acids and Salts, analysis, Carcinogens, Cattle, Chickens, Colon, anatomy & histology, Colonic Neoplasms, chemically induced, etiology, prevention & control, Diet, Drinking, Eating, Fatty Acids, analysis, Feces, chemistry, Female, Iron, analysis, Meat, adverse effects, Random Allocation, Rats, Rats, Inbred F344, Risk Factors, Swine, Viscera, Weight Gain
Keywords: colorectal cancer, prevention, promotion, meat, diet, chicken, porc, processed meat, bacon, beef, rat, ACF, aberrant crypt foci, carcinogenesis
Introduction
Colorectal cancer is the second most common cause of death from cancer in the Western countries, exhibiting more than a tenfold excess when compared with rural populations in less affluent countries (1). Diet is supposed to influence the colorectal cancer etiology, but the precise causative factors are yet unknown. International ecological studies show a strong correlation between meat consumption and the colorectal cancer incidence (2). Most case-control studies (22 out of 29) show an increased risk to develop a colorectal cancer for those eating high amounts of meat (reviewed in ref. 3). However, cohort studies of meat intake and colon cancer have been less consistent. For example, Willett and colleagues found an increased risk of colon cancer with high meat intake in both women and men cohorts (4, 5). In contrast, using a similar questionnaire, Bostick and colleagues did not find a significant association between meat intake and colon cancer in Iowa women (6). European prospective studies also show no significant association between meat intake and colon cancer (7–9). Otherwise, the high intake of processed meat is associated with colorectal cancer risk in two cohort studies (4, 8). Two prospective studies also show that consumption of white meat or of fish is not associated with risk, and might even reduce the occurrence of colorectal cancer (4, 5). Animal studies on meat have received little attention compared with epidemiological studies (3). Only seven experimental studies have been published on the effect of meat, or meat fractions, on the colon tumor incidence in rodents initiated with chemical carcinogens (10–16). Data from these studies do not support the belief that red meat has a specific effect on intestinal carcinogenesis, except when it contains high levels of heterocyclic aromatic amines (16,17). The effect of white and of processed meat on experimental colon cancer in rodents has not been studied yet.
The present study was designed to investigate the promoting effect of a diet high in red meat, or high in processed meat, and the protecting effect of white meat, in the context of a high-fat diet. We used the rat/azoxymethane (AOM) model of experimental colon carcinogenesis. The study endpoint was the multiplicity of aberrant crypt foci (ACF), as a measure of ACF growth. We stopped the study after a feeding period of 100 d, adequate to quantify the promotion of ACF growth (18, 19). Some ACF are dysplastic lesions of colonic mucosa thought to represent the earliest precursors of colon cancer (19). We also quantified, in feces, the levels of specific components such as iron, fatty acids and bile acids because they might be meat-borne risk factors for the development of colon cancer. The results suggest that beef or chicken meat does not modulate the growth of ACF in rats. A bacon-based diet might protect rats against colorectal carcinogenesis, maybe because bacon contains 5% NaCl, and increased the water intake of rats.
Materials and Methods
Animals
Female Fischer 344 (F344) rats were obtained from Iffa-Credo (Lyon, France) at 4 weeks of age. One hundred animals were housed two rats per stainless steel wire drop-bottom cage, at 22°C on a 12-hour light: 12-hour dark cycle and were allowed free access to standard laboratory diet (UAR, Villemoisson, France) and to water. After one week of acclimatization, the rats were initiated, between 9 and 11 AM, with a single i.p. injection of azoxymethane (Sigma, St. Quentin, France) at a dose of 20 mg/kg in NaCl 9g/l. Seven days later, they were randomly allocated to ten groups (n = 10 in each group) and given the experimental diets. Body weights were monitored weekly throughout the study, and food and water intakes were measured at periodic intervals. The animals were sacrificed 105–107 days after the carcinogen injection, 98–100 days after the start of the experimental diets, by cervical dislocation between 8 and 11 AM. The abdominal fat was excised and weighed. The colons were removed and fixed in formalin for ACF scoring.
Diets
The hundred F344 rats were randomized to ten groups after initiation, and fed on dry powdered diets, based on a modified AIN 76 formula (UAR, Villemoisson, France). Five groups received a relatively “low fat” diet containing 14% fat and 23.5% protein. Five other groups received a very high fat diet containing 28% fat and 40% protein. Fat represented 32% of calories in “low fat” diets, and 51.5% in high fat diet. These values are below and above the average human intake in affluent countries (40%). Fat and protein were provided either by dry powdered cooked meat, making 30 or 60% of the diet, or with olive oil (Carrefour, France) and vitamin-free casein, or with lard and vitamin-free casein (UAR, Villemoisson, France) (Table 1). Two sources of fat were used to make two control diets: olive oil and lard. Olive oil was chosen because it is “neutral” in colon carcinogenesis studies, since it does not enhance or reduce tumor incidence. Lard was chosen because its fatty acids composition is the same as bacon, and is halfway between beef and chicken. Beef (hamburger Carrefour), chicken (with skin, Gastronome) and bacon (Herta) were obtained from a local supermarket (Carrefour Purpan, France). The 3 types of meat were cooked in an oven for 15 min at 180–185°C. Each dish contained 500 g of meat on a thickness of 1 cm. These cooking conditions may generate 1–15 ng/g of heterocyclic amines in beef, 15–65 ng/g in bacon, and 40 ng/g in chicken (17). After cooling, there were minced, frozen for 24h at −20°C, then freeze dried. After the analysis of fat and protein contents in each type of meat (see values in Table 1, notesb–d), meat diets were supplemented with casein to reach the 23.5 or 40% protein targets. The percentage of fat was adjusted with lard for beef diets and with chicken fat for chicken diets (Table 1).
Table 1.
Composition of experimental diets (g of ingredient per 100 g of diet)
| Low-Meat Diet
|
High-Meat Diet
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Groupa ingredient | Lard | Olive | Beef | Chick | Bacon | Lard | Olive | Beef | Chick | Bacon |
| beefb | - | - | 30 | - | - | - | - | 60 | - | - |
| chickenc | - | - | - | 30 | - | - | - | - | 60 | - |
| bacond | - | - | - | - | 30 | - | - | - | - | 60 |
| casein | 23.5 | 23.5 | 5 | 7.3 | 10.2 | 40 | 40 | 3 | 7.6 | 13.9 |
| lard | 14 | - | 3.8 | - | - | 28 | - | 7.6 | - | - |
| olive oil | - | 14 | - | - | - | - | 28 | - | - | - |
| chicken fat | - | - | - | 0.6 | - | - | - | - | 1.2 | - |
| sucrose | 31.5 | 31.5 | 31.5 | 31.5 | 31.5 | 6 | 6 | 6 | 6 | 6 |
| corn starch | 17 | 17 | 17 | 17 | 17 | 10.2 | 10.2 | 10.2 | 10.2 | 10.2 |
| cellulose | 5.7 | 5.7 | 5.7 | 5.7 | 5.7 | 6.7 | 6.7 | 6.7 | 6.7 | 6.7 |
| choline bitartrate | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 | 0.26 | 0.26 | 0.26 | 0.26 | 0.26 |
| methionine | 0.34 | 0.34 | .34 | 0.34 | 0.34 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| corn oil AIN 76 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| mineral mix | 4 | 4 | 4 | 4 | 4 | 4.68 | 4.68 | 4.68 | 4.68 | 4.68 |
| vitamin mix | 1.13 | 1.13 | 1.13 | 1.13 | 1.13 | 1.33 | 1.33 | 1.33 | 1.33 | 1.33 |
Values are g/100 g diet. The five “low meat” diets contained 16.5% fat and 23.8% protein, the five “high meat” diets contained 30.5% fat and 40.4% protein (including corn oil and methionine). Mineral mix and Vitamin mix according to AIN 76 composition
Freeze-dried cooked beef contained 34% fat and 61.7% protein
Freeze-dried cooked chicken (with skin) contained 44.6% fat and 51.1% protein
Freeze-dried cooked bacon contained 47.1% fat and 44.4% protein
Assay of Bile Acids and Iron in Fecal Water
Fecal Water Preparation
Fecal water was prepared by reconstituting freeze-dried feces by adding to 1 g of freeze-dried feces 0.35 ml of distilled water (20). After homogenizing, the samples were incubated for 1h at 37 °C followed by centrifugation for 10 min at 40,000 g. The supernatant was removed and stored at −20°C until use.
Assay of Iron
Total iron in fecal water was measured using a colorimetric method based on the procedure of Persijn (21) (Kit 565-C, Sigma Chemical France).
Assay of Bile Acids
Bile acids concentration was determined using a fluorometric enzymatic assay based on the technique of Lapre (20). All reagents for bile acid assay were obtained from Sigma Chemical (St. Quentin, France). The reaction mixture was made of 10μl of appropriate dilution of fecal water (substrate source), 1240μl of 0.1 M Tris buffer (pH 9) and 250μl of the “3-alpha-Flu” solution, itself made of 67 mM KH2PO4, 55 mM NaOH, 4.4 mM sucrose, 1.8 mM NAD, 10mM Na4P2O7-10H2O, 0.13% Bovine Serum Albumin, 714 U/l diaphorase and 0.05mM resazurin. To this reaction mixture, 10 μl of 3alpha HydroxySteroid-deshydrogenase (enzyme) 2.5U/ml was added. After 15 min, the fluorescence was measured at 580nm under a 565nm excitation. The intensity of fluorescence was proportional to the bile acid concentration.
Fatty Acids Analysis of Fat in Feces and Food
Total fatty acids concentrations and profiles in feces and in cooked meat were determined according to the one-step extraction-transesterification procedure of Pritam (22). Fatty acids were analyzed on a DANI 1000 gas-liquid chromatograph (DANI, Italy) fitted with a peak simple chromatography Data System (SRI Instruments, California), a split-splitless injector and a flame ionization detector. Conditions: SP2340 fused silica capillary column (Supelco- Aldrich, France), temperature programmed from 74 to 80°C at 1°C/min, from 80 to 160°C at 8°C/min, and for 8 min at 160°C, then from 160 to 180°C at 4°C/min and 1 min at 180°C. Fatty acids were identified by their retention time and quantified in comparison with an internal C19 standard. The profiles of fatty acids in food are shown in Table 2. Lard and bacon showed similar fatty acid profiles with a high content of oleic acid. Compared with proportions found in food, rat feces contained more saturated fatty acids, and less unsaturated fatty acids. For instance, lard contained 16% stearic and 38% oleic acid, while feces from lard-fed rats contained 51% stearic and 15% oleic acid (other data not shown).
Table 2.
Composition (%) of fatty acids in fat, and in meat fat, used in the experimental dietsa
| Fatty Acids | Lard | Olive oil | Beef | Chicken | Bacon | |
|---|---|---|---|---|---|---|
| Myristic acid | C14 :0 | 2.2 | 1.2 | 3.5 | 1,1 | 1.7 |
| Palmitic acid | C16 :0 | 26.3 | 13.6 | 28.1 | 21.0 | 26.3 |
| Stearic acid | C18 :0 | 16.4 | 4.7 | 17.3 | 6.4 | 16.7 |
| Oleic acid | C18 :1 | 38.2 | 68.3 | 40.3 | 45.5 | 42.4 |
| Linoleic acid | C18 :2 | 10.5 | 7.0 | 2.3 | 18.7 | 10.8 |
| Linolenic acid | C18 :3 | 2.2 | 1.0 | 1.1 | 4.4 | 0.6 |
| Other fatty acids | 4.2 | 4.2 | 7.4 | 2.9 | 1.5 |
Values are percentages. Duplicate assays led to quasi-identical values.
Aberrant Crypt Foci Assay
Promotion was assessed by the multiplicity of aberrant crypt foci (number of crypts per ACF). ACF were scored using the procedure of Bird (23). Immediately after sacrifice, the colons were removed and flushed with Kreb’s Ringer solution (Sigma), then opened longitudinally and fixed flat between coded filter papers in 10% buffered formalin (Sigma). The colons were stained with methylene blue (0.1%) for 10 min, then the mucosal side was observed at 32 × magnification. ACF were distinguished by their slit-like opening, increased staining, size and pericryptal zone. The multiplicity was recorded for each ACF in each colon. All colons were scored blindly by a single observer.
Statistical Analysis
Results were analyzed using Systat 5 software for Windows. Data are given as mean ± standard deviations and two-sided p values. Data were analyzed by two-way factorial ANOVA (factor 1, type of meat ; factor 2, level of meat and fat content) using a general linear model. When factorial ANOVA analysis showed a significant difference between groups (F test p<0.05), multiple comparisons were done by the Dunnett’s test, comparing each group with the lard control group. Two separated one-way ANOVA were used when the interaction was significant. Pearson correlation matrix was computed both at the level of groups means (n = 10) and of the individual rats (n = 99), and p values were computed with Bonferroni correction.
Results
Body Weights, Food and Water Intakes
Body weight gains were higher in rats eating high-meat and high-fat diets than in rats eating low-meat and “low-fat” diets (p=0.001; Figure 1 and Table 3). Food and water intakes were measured per cage, during three days, twice (d 26–28 and d 63–65). Consumption of low-fat diets was higher than consumption of high-fat diets (8.6 ± 0.7 and 7.4 ± 0.6 g/d/rat, respectively, p<0.0001). The energy intake was calculated with use of values of 4 kcal/g for carbohydrate and protein, and 9 kcal/g for fat. Rats on low-fat and high-fat diets consumed similar energy levels (37.75 and 37.34 kcal/d respectively), suggesting that they self-regulated their energy intake. During the whole experimental period, rats on the olive oil diets gained less weight per day, and ate less food, than rats on the animal fat diets (Figure 1 and Table 3). Rats fed on the diets containing beef or chicken were the heaviest, but did not eat more food than rats in the other groups (Table 3). As seen in table 3, rats given low-meat and low-fat diets drank less water than rats given high-fat diets (12.3 ± 1.7 and 14.6 ± 2.6 ml/d, p<0.001). Rats fed on bacon-based diets consumed more water than the other rats. The water intake was 32% higher in rats fed 30% bacon than in controls, and 42% higher in rats fed 60% bacon (p<0.0001). This is likely due to the salt in the bacon diets (1.7% and 3.3% NaCl in the diets containing 30 and 60% bacon, respectively).
Figure 1.
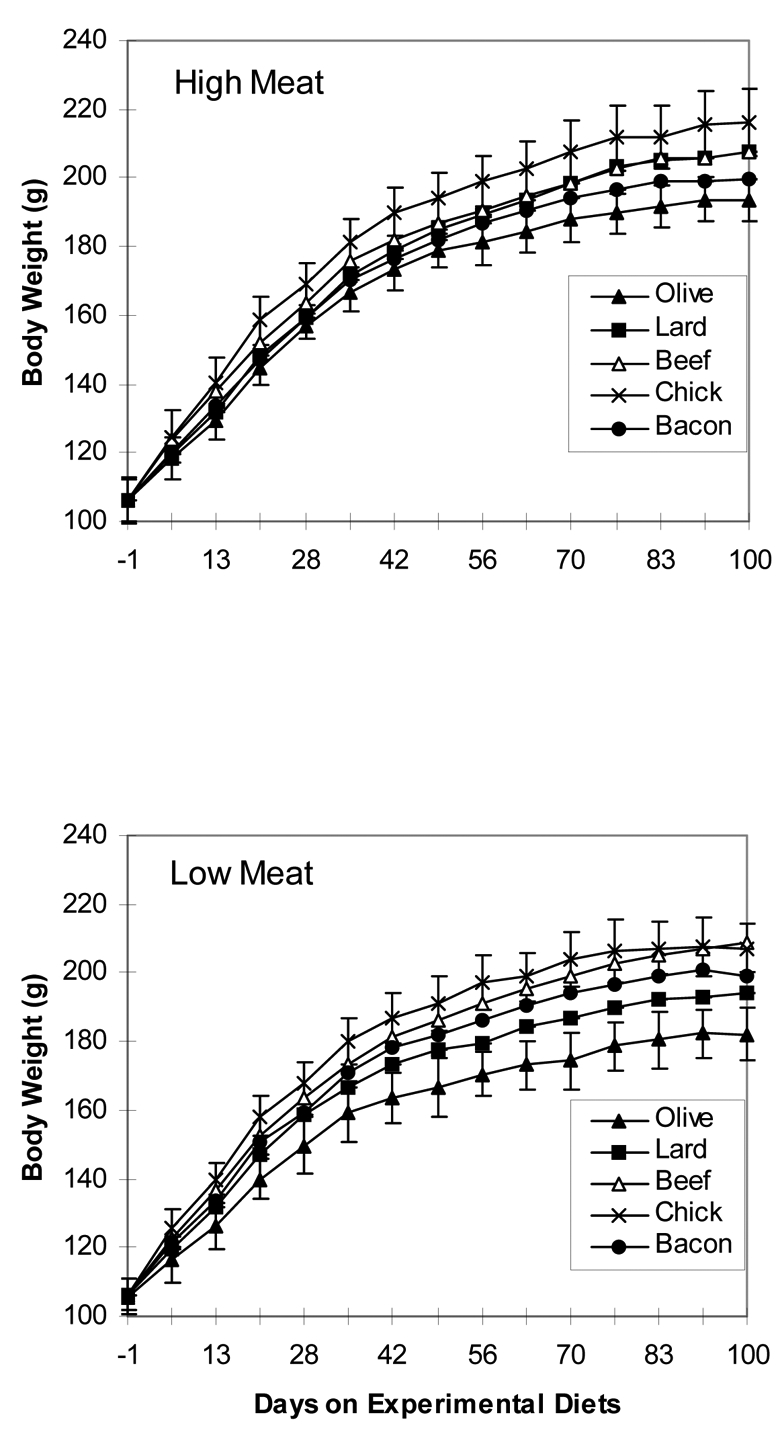
Effect of meat-based diets on the body weight gain of female F344 rats. Panel H shows the mean weight of 5 groups of 10 rats given high-meat and high-fat diets containing 60% cooked freeze-dried beef, chicken or bacon, or 28% olive oil or lard. Panel L shows the weigh rats given low-meat and low-fat diets containing 30% beef, chicken or bacon, or 14% olive oil or lard. Standard deviation bars are shown only for lightest and heaviest groups in each panel.
Table 3.
Body weight, food and water intake of female F344 rats, receiving diets containing 30 or 60% of meata
| Weightsb at d100 (g/rat) | Food Intakec (g/rat) | Water Intake c (ml/rat) | ||||
|---|---|---|---|---|---|---|
| Group | Low Meat | High Meat | Low Meat | High Meat | Low Meat | High Meat |
| Lard | 194 ± 9 | 208 ± 7 | 8.9 ± 0.8 | 7.3 ± 0.6 | 11.5 ± 1.1 | 13.3 ± 0.8 |
| Olive | 182 ± 8 | 194 ± 6*** | 7.9 ± 0.5 | 7.1 ± 0.5 | 11.0 ± 0.7 | 12.8 ± 0.4 |
| Beef | 209 ± 9 | 207 ± 7 | 8.9 ± 0.4 | 7.2 ± 0.3 | 12.2 ± 0.5 | 14.0 ± 0.7 |
| Chick | 207 ± 7 | 216 ± 10*** | 8.8 ± 0.2 | 7.5 ± 1.0 | 11.5 ± 0.3 | 13.4 ± 0.9 |
| Bacon | 199 ± 6 | 200 ± 6 | 8.3 ± 0.6 | 7.9 ± 0.3 | 15.4 ± 0.9 | 19.3 ± 0.9*** |
| ANOVA P values | ||||||
| meat level effect | 0.001 | 0.000 | 0.000 | |||
| meat type effect | 0.000 | 0.077 | 0.000 | |||
| level x type effect | NS | NS | NS | |||
Diets are defined in Table 1. Statistical significance is as follows:
p < 0.001 vs. lard-fed control by Dunnett’s test.
Mean ± SD for 10 rats/group, but 9 rats in the High Meat Beef group (in this group, one rat died on Day 75 because of aberrant teeth growth).
Mean ± SD (n=5 because rats were 2 per cage). Intake was measured between Day 63 and 65 of the study.
Fecal Weight and Moisture Content
Fecal weight was not significantly different between groups of rats (data not shown, p=0.15), but it was correlated with the water intake water (r = 0.7, p<0.001, n = 99). Feces of rats given the bacon diets were more humid than feces from the other groups. Feces from rats on a diet with 60% bacon contained twice more water than feces of lard-fed controls (28 ± 6 and 15 ± 2%, p<0.0001). The difference did not reach significance between rats on a diet with 30% bacon and lard-fed controls (19 ± 5 and 14 ± 2%, p=0.1). The percentages of water in feces were not different between the other groups.
Iron and Bile Acids in Fecal Water; Fatty Acids in Feces
The iron concentration in fecal water was higher in rats given the high-meat and high-fat diets than in rats given the low-fat diets (11.3 ± 3.5 and 9.04 ± 2.2 μg/g dry feces respectively, p< 0.001). As seen in Table 4, the fecal iron concentration was also affected by the nature of the diet. Fecal iron was high in rats fed on the control diets with olive oil, and low in chicken-fed rats. Rats on high-fat diets had more bile acid in fecal water than rats on “low-fat” diets (4.04± 2.26 and 2.73 ± 0.91 μmol/g dry feces, p<0.001). Bile acid concentrations differed significantly among groups with elevated values for rats given the chicken diets and rats given the olive oil diets (Table 4). Lard- and bacon-fed rats had similar fecal bile acids concentration (p=0.99).
Table 4.
Effect of diets containing 30 or 60% meat on concentrations of iron and bile acids in fecal water and of total fatty acids in fecesa.
| Iron b μg/g dry feces | Bile Acids b μmol/g dry feces | Total Fatty Acids c mg/g | ||||
|---|---|---|---|---|---|---|
| Groups | Low Meat | High Meat | Low Meat | High Meat | Low Meat | High Meat |
| Lard | 9.4 ± 1.2 | 10.3 ± 2.7 | 2.33 ± 0.64 | 2.29 ± 1.01 | 62.2 ± 13.4 | 84.5 ± 13.0 |
| Olive | 11.0 ± 0.7 | 15.5 ± 1.2** | 3.72 ± 0.98 ** | 5.55 ± 2.26** | 8.7 ± 3.5 | 12.7 ± 5.8*** |
| Beef | 6.7 ± 1.2* | 11.9 ± 3.1 | 2.09 ± 0.57 | 3.39 ± 0.57 | 51.9 ± 14.7 | 72.6 ± 23.3 |
| Chick | 7.3 ± 1.0 | 6.7 ± 0.7 | 3.44 ± 0.21* | 6.79 ± 2.08*** | 7.1 ± 0.8 | 9.6 ± 2.6*** |
| Bacon | 10.7 ± 2.3 | 12.0 ± 2.0 | 2.08 ± 0.43 | 2.44 ± 0.71 | 22.7 ± 0.1 | 26.9 ± 10.2*** |
| ANOVA P values | ||||||
| meat level effect | 0.000 | 0.000 | 0.058 | |||
| meat type effect | 0.000 | 0.000 | 0.000 | |||
| level x type effect | 0.003 | 0.008 | NS | |||
Diets are defined in Table 1. Statistical significance is as follows:
p < 0.001;
p < 0.01;
p < 0.05 vs. lard-fed control by Dunnett’s test. Separated ANOVA and Dunnet’s test were done for Low Meat and High Meat subsets, for iron and bile acids values, because the (level x type) interaction was significant.
Mean ± SD (n = 5 cages)
The pool of feces from10 rats in each group was assayed in duplicate, for total fatty acids.
Fecal fatty acids concentrations were slightly affected by the fat content of diets, with higher concentrations in rats given the high-meat and high-fat diets than in rats given the low-fat diets (p=0.06). The type of diet affected the fatty acids concentrations in feces (Table 4). With the same pattern in high-fat and low-fat fed groups, high concentrations of fatty acids were found in the feces of rats fed on lard or beef diets and low values in the feces of rats fed on chicken or olive oil diets. Fatty acids were lower by a factor of 3 in feces of rats fed bacon than in feces of control rats fed lard (p<0.001, Table 4), although both diets contained 28% of lard (Table 1).
Visceral Fat and Length of Colon
Visceral fat weight was higher in rats given the high-meat and high-fat diets than in rats given low-fat diets (14.2 ± 2.5 and 12.5 ± 2.8 g, p=0.01, Table 5). Also, body weight of rats was correlated with visceral fat weight (r=0.73, p<0.001, n=72). Among high-fat diets fed groups, rats given the diet containing 60% bacon had the least visceral fat. Their colons were shorter than the colons of the other rats (compared with lard-fed controls, p = 0.005, Table 5).
Table 5.
Length of colon and visceral fat of female F344 rats, after 100 d on diets containing 30 or 60% meata
| Colon Length(cm)b | Visceral Fat (g/rat)c | |||
|---|---|---|---|---|
| Group | Low Meat | High Meat | Low Meat | High Meat |
| Lard | 14.3 ± 1.6 | 14.2 ± 1.0 | 11.3 ± 3.1 | 14.8 ± 2.7 |
| Olive | 13.1 ± 1.7 | 13.6 ± 1.4 | 10.9 ± 1.4 | 13.0 ± 1.7 |
| Beef | 13.1 ± 2.0 | 13.2 ± 0.9 | 15.6 ± 3.2* | 15.2 ± 1.3 |
| Chick | 13.9 ± 0.7 | 13.8 ± 1.4 | 12.8 ± 2.7 | 17.1 ± 1.9 |
| Bacon | 13.1 ± 1.2 | 12.3 ± 2.0** | 12.9 ± 1.3 | 11.8 ± 1.3* |
| ANOVA P values | ||||
| meat level effect | NS | 0.001 | ||
| meat type effect | 0.011 | 0.000 | ||
| level x type effect | NS | 0.003 | ||
Promotion of ACF
The number of ACF per colon, which is considered as a marker for tumor initiation, but not for tumor promotion, was not affected by the type of diet (Table 6). In contrast, the multiplicity of ACF (number of crypt per ACF), which is considered as a marker for tumor promotion, was diet-dependent (p=0.002, Table 6). The multiplicity was nearly the same in all groups, except those on bacon diets, with no effect of fat and protein source (Table 6, ANOVA p=0.7 between 8 groups, without bacon-fed rats). Compared with lard-fed controls, olive oil, beef and chicken diets did not change the growth of ACF. There was no effect of dietary fat level on ACF multiplicity: the number of crypt per ACF was 3.12 ± 0.41 in rats on low-meat and low-fat diets and 3.03 ± 0.49 in rats on a high-fat diet (p = 0.34). By contrast, compared with lard-fed controls, the ACF multiplicity was reduced by 12% in rats fed 30% of bacon and by 20% in rats fed 60% bacon (significance of bacon effect p<0.001). Moreover, the ACF growth reduction by bacon seems dose-dependent, because Pearson correlation between multiplicity of ACF and bacon level in the diet was r = −0.51 (p=0.001, n=40 rats).
Table 6.
Effect of diets containing 30% or 60% of meat on the number and the multiplicity of aberrant crypt foci (ACF) in the colon of F344 female rats, 100 days after a single azoxymethane injections a
| Multiplicity (crypt/ACF) b | Number of ACF/rat b | |||
|---|---|---|---|---|
| Group | Low Meat | High Meat | Low Meat | High Meat |
| Lard | 3.21 ± 0.47 | 3.27 ± 0.38 | 65 ± 34 | 75 ± 44 |
| Olive | 3.11 ± 0.28 | 2.94 ± 0.30 | 83 ± 30 | 61 ± 43 |
| Beef | 3.25 ± 0.44 | 3.15 ± 0.59 | 69 ± 23 | 71 ± 25 |
| Chick | 3.16 ± 0.34 | 3.18 ± 0.32 | 76 ± 37 | 98 ± 30 |
| Bacon | 2.84 ± 0.45 | 2.62 ± 0.60*** | 86 ± 47 | 72 ± 37 |
| Factorial ANOVA P values | ||||
| meat level effect | NS | NS | ||
| meat type effect | 0.002 | NS | ||
| level x type effect | NS | NS | ||
The multiplicity of ACF was not associated with iron (r=−0.54, p=0.11, n=10 groups), with bile acids (r=0.08, p=0.83, n=10), or with fatty acids concentrations in feces (r=0.41, p=0.23, n=10). Similar non-significant correlations were obtained at the rat level (n = 99).
Discussion
The present study yielded five major findings. 1) The intake of a diet containing 60% dry cooked bacon decreased the multiplicity of preneoplastic lesions in the colon of rats, compared with control diets based on casein and lard. 2) Diets containing 30 or 60% dry cooked beef meat did not promote the growth of ACF in the colon of rats, compared with casein-based control diets balanced for fat level. 3) Diets containing 30 or 60% dry cooked chicken did not reduce the ACF growth in rats, compared with casein or beef-based diets balanced for fat. 5) High-fat and high-meat diets increased the rat body weight gains, but did not promote the ACF growth, compared with diets with less fat and less meat. 5) Fecal concentrations of iron, bile acids and total fatty acids were not correlated with the promotion of ACF. These five points are discussed below.
Bacon-based diet appears to protect rats against carcinogenesis, in a dose-dependant manner. This finding was in contrast with our starting hypothesis that bacon diets would promote carcinogenesis. Our hypothesis was based on epidemiological studies showing that intake of processed meat (mainly pork) is associated with risk. We also thought that nitrite and N-nitroso compounds found in bacon might increase carcinogenesis. However, bacon-fed rats drank more water than controls (Table 3), probably because bacon is salty. We thus propose the hypothesis that a high water intake, and not the bacon intake, can protect the rats against carcinogenesis (figure 2). The water intake is seldom measured in both animal and human studies, although it plays a vital role in gut functions. Recently, two case-control studies have analyzed the connection between the water intake and the risk of colorectal cancer (25,26). In Seattle, Shannon and colleagues reported a reduced risk of colon cancer associated with a high intake of water. The adjusted odd ratio (OR) for women drinking more than five glasses of water per day, versus those drinking less than two glasses, was 0.55, with a 95% confidence interval (95%CI) of 0.31–0.99 (25). Lubin and colleagues reported similar OR (0.5) and 95%CI (0.3–0.9) for adenoma associated with highest versus lowest tertiles of the mean daily water intake in Israel (26). Moreover, Stookey and colleagues reported a strong negative association between water drinking and breast cancer in women (OR=0.21, 95%CI=0.07–0.62) (27). In rats given dimethylhydrazine injections for 25 weeks, Uccheddu and colleagues limited the access to the drinking water to only one hour per day. The water restricted group of rats had more colonic tumors, and excreted fewer fecal pellets, than control rats given water ad libitum (both p=0.02) (28). Although these results were poorly reported, they suggest that a low water intake may increase colon cancer risk, possibly by delaying the bowel transit time, and by raising the concentration of toxic compounds in fecal water. Here, bacon-fed rats drank 45% more water (Table 3), and they had 90% more water in feces, than rats receiving the diets without bacon. Based on dry feces values, bacon-fed rats had low concentrations of bile acids and of total fatty acids in feces (Table 4). Moreover, bile acids and fatty acids were more diluted in wet feces of bacon-fed rats, than in less humid feces of rats in the other groups. Finally, bacon-fed rats had smaller ACF than rats fed on the other diets (Table 6). Thus, the protection afforded by the bacon-based diet might be explained by the dilution of promoting compounds in the gut content water phase.
Beef meat showed no promoting effect here. This is not a new finding, and it is consistent with published studies where meat was given to rats during and after dimethylhydrazine injections (review ref. 3). Reddy and colleagues reported that the incidence of colon tumors was the same in F344 rats given a diet containing 60% beef meat, and in rats given a diet similarly balanced with 40% soybean protein and 25% corn oil (tumor incidence beef/soy ratio =1.1, p=0.79) (10). Clinton and colleagues have also shown that the incidence of adenocarcinoma was the same in Sprague Dawley rats given balanced diets containing 20% beef or 20% soybean protein (tumor incidence beef/soy ratio=1.1, p=0.96) (11). Pence and colleagues have compared the effects of diets containing 50–60% beef with diets containing 17–21% casein on the promotion of colon carcinogenesis in Sprague Dawley rats. The colon tumor incidence and burden were reduced in the groups of beef-fed rats, compared with casein-fed controls (tumor incidence beef/casein ratio= 0.4, p<0.05) (15). Moreover, the study of beef meat effect in mice by Nutters, and the study of kangaroo meat effect in rats by McIntosh, do not support either that red meat promotes colon cancer in rodents (12,14). A recent study by Pence and colleagues shows that well-cooked beef meat, containing a high heterocyclic amine content, can enhance colon carcinogenesis in rats, only when given during DMH initiation, and only in a low-fat diet context (tumor incidence ratio=1.5, p<0.05) (16). The present study is the first where meat was not given during carcinogens injections, but only postinitiation. Its major limitation is that we stopped the experiment at the stage of ACF, which are putative precancerous lesions, but not true cancers (19). These experimental data are in contrast with epidemiological data. In people, most case-control studies and some prospective studies show an elevated risk of colorectal cancer associated with the red meat intake (3). It is possible that the rat model is not pertinent for human cancers, for instance because rodent diet is high in micronutrients., or because rat detoxifying enzymes are more efficient than human ones. Alternatively, it is possible that the epidemiological association between meat and colorectal cancer is entirely due to fat, and fat was fully balanced in this study, or to a compound that is sometimes present in meat, but not always (e.g., heterocyclic amines (16,17)).
Chicken meat diets were not protective against carcinogenesis, when compared with beef or casein control diets. This is the first experimental study to examine the effects of a chicken-based diet on rat colon carcinogenesis. Two cohort studies have examined the association of the chicken intake with the risk of colon cancer (4,5). Those who frequently eat chicken have a lower risk of developing colon cancer than those who consume no or very little chicken (the relative risk was 0.47 and 0.82 in men and women respectively). Similarly, in a recent case-control study, the intake of chicken without skin was negatively associated with risk in both genders (29). According to Giovannucci, chicken would protect people against colorectal cancer because it is high in methionine, but low in (saturated) fat. In this study, the fatty chicken skin was included in the chicken diets, to get identical fat levels in the five high-meat and high-fat diets, and in the five “low-fat” diets. Thus, the high fat content of chicken diets may have impaired the possible protective effect of lean chicken.
High-fat and high-meat diets did not promote the growth of ACF, when compared with the relatively “low-fat” diets. It is often assumed that fat promotes carcinogenesis. However, many rodent studies failed to show a colon cancer promoting effect of saturated fat (16,30). In addition, the “low-fat” diets used in this study contained a high 32% of energy from fat. It seems that above a 30% threshold value, a raise in fat calories in diet does not enhance the tumor yield in rats. Tang and colleagues showed that the colon and mammary cancer incidence increased when fat was raised from 15% to 30% calories in diet. There was no further increase of cancer risks above 30% of calories from fat (31). Similarly, Cohen and colleagues showed no increase in mammary carcinogenesis when the fat content in the diet of rats was raised over 30% calories (32).
Bile acids in fecal water, and fatty acids in colon content, are toxic to the colonic mucosa (20). High concentrations of these surfactants may explain the promoting effect of dietary fat (33,34). Here, for each meat type, rats given high fat diet had more bile acids in fecal water than rats given the “low fat” diet (p<0.001). Also, rats given high levels of olive oil or of chicken had high bile acid concentrations in fecal water (Table 4). The intake of unsaturated fatty acids, but not of saturated fat, may thus increase fecal bile acids (35–37). Lard- and beef-fed rats had high fecal fatty acids concentrations compared with olive oil and to chicken fed rats. It is thus likely that a high intake of saturated fatty acids (palmitic and stearic) enhances fat and fatty acid excretion in feces (20). However, we did not find any significant correlation between any specific fatty acid in diet, fecal fatty acids, bile acids concentration in fecal water, and the multiplicity of ACF. Because the variance in the ACF multiplicity was mainly due to the bacon diets, and may come from differences in the water intake, we also analyzed data after removing the two bacon-fed groups. Again, we found no correlation between data. However, a limitation of this study is that we did not specifically assay the secondary bile acids or the free fatty acids, which may be more potent promoters of colon carcinogenesis than total bile acids and fatty acids (33).
Iron might be a risk factor brought by beef meat. In the present study, the fecal iron level in beef-fed rats was not higher than in controls given the casein diets (Table 4). We did not measure, however, the serum iron levels. Yet, Lai and colleagues have observed that rats given a beef diet appeared to use and absorb the iron better than control rats given a casein diet, according to the serum iron levels. However, they did not show a promoting effect of increased dietary iron derived from cooked beef, on the development of colon tumors (38). We did not either show an association of the iron level in feces with the ACF multiplicity.
Dietary guidelines advise to reduce the intake of red meat and/or of saturated fat, to the benefit of “white” lean meat (39). The need to develop strong supporting data in animal models before conducting intervention trials has recently been stressed by De Luca and Ross, in a commentary on the failure of the recent alpha-tocopherol, beta-carotene prevention studies (40). The present experimental study does not support the belief that in a high-fat diet context, red meat consumption promotes, or that white meat protects against, colon carcinogenesis. This study has nevertheless two major limitations: 1) it was done in rodents, and we do not know if AOM-initiated rats are good models for human colon cancers, and 2) the endpoint was the development of putative precancerous lesions. The multiplicity of aberrant crypt foci correlates with the adenocarcinoma incidence in most rodents studies (41–43), but not all (44). In conclusion, this study has introduced a new and potentially important experimental finding concerning a possible beneficial effect of the water intake on colon cancer prevention.
Acknowledgments
This work was supported by the Direction Générale de l’Enseignement et de la Recherche, Ministère de l’Agriculture, France, and by a grant from the Ligue Nationale Contre le Cancer, comity of Gers. The authors thank Raymond Gazel and Annette Debrusse for taking care of the rats, Marie-Claude Nicot for doing fatty acids analysis of feces. Results were presented as a poster at the 89th meeting of the American Association for Cancer Research, New Orleans, LA, France, March 1998 (abstract: Proc.AACR 39, p.16, #105). Address reprint request to D. Corpet, ENVT, 23 Capelles, 31076 Toulouse, France. Phone : (33) 561 193 982. Fax : (33) 561 491 263. e-mail : d.corpet@envt.fr
References
- 1.Potter JD. Reconciling the epidemiology, physiology, and molecular biology of colon cancer. J Am Med Assoc. 1992;268:1573–1577. [PubMed] [Google Scholar]
- 2.Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries with special reference to dietary practices. Int J Cancer. 1975;15:617–631. doi: 10.1002/ijc.2910150411. [DOI] [PubMed] [Google Scholar]
- 3.Parnaud G, Corpet DE. Colorectal cancer : controversial role of meat consumption. Bull Cancer. 1997;84:899–911. [PubMed] [Google Scholar]
- 4.Willett WC, Stampfer MJ, Colditz GA, Rosner BA, Speizer FE. Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N Engl J Med. 1990;323:1664–1672. doi: 10.1056/NEJM199012133232404. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, et al. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. 1994;54:2390–2397. [PubMed] [Google Scholar]
- 6.Bostick RM, Potter JD, Kushi LH, Sellers TA, Steinmetz KA, et al. Sugar, meat, and fat intake, and non-dietary risk factors for colon cancer incidence in Iowa women (United States) Cancer Causes Control. 1994;5:38–52. doi: 10.1007/BF01830725. [DOI] [PubMed] [Google Scholar]
- 7.Thun MJ, Calle EE, Namboodiri MM, Flanders WD, Coates RJ, et al. Risk factors for fatal colon cancer in a large prospective study. JNCI. 1992;84:1491–1500. doi: 10.1093/jnci/84.19.1491. [DOI] [PubMed] [Google Scholar]
- 8.Goldbohm RA, Vandenbrandt PA, Vantveer P, Brants HAM, Dorant E, et al. A prospective cohort study on the relation between meat consumption and the risk of colon cancer. Cancer Res. 1994;54:718–723. [PubMed] [Google Scholar]
- 9.Cox BD, Whichelow MJ. Frequency consumption of red meat is not risk factor for cancer. Brit Med J. 1997;315:1018. doi: 10.1136/bmj.315.7114.1018a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy BS, Narisawa T, Weisburger JH. Effect of a diet with high levels of protein and fat on colon cancinogenesis in F344 rats treated with 1,2-dimethylhydrazine. JNCI. 1976;57:567–569. doi: 10.1093/jnci/57.3.567. [DOI] [PubMed] [Google Scholar]
- 11.Clinton SK, Destree RJ, Anderson DB, Truex CR, Imrey PB, et al. 1,2-dimethylhydrazine induced intestinal cancer in rats fed beef or soybean protein. Nutr Report Internat. 1979;20:335–342. [Google Scholar]
- 12.Nutter RL, Gridley DS, Kettering JD, Goude AG, Slater JM. BALB/c mice fed milk or beef protein : differences in response to 1,2-dimethylhydrazine carcinogenesis. JNCI. 1983;71:867–874. [PubMed] [Google Scholar]
- 13.Alink GM, Kuiper HA, Hollanders VMH, Koeman JH. Effect of heat processing and of vegetables and fruit in human diets on 1,2-dimethylhydrazine-induced colon carcinogenesis in rats. Carcinogenesis. 1993;14:519–524. doi: 10.1093/carcin/14.3.519. [DOI] [PubMed] [Google Scholar]
- 14.McIntosh GH, Regester GO, Leleu RK, Royle PJ, Smithers GW. Dairy proteins protect against dimethylhydrazine-induced intestinal cancers in rats. J Nutr. 1995;125:809–816. doi: 10.1093/jn/125.4.809. [DOI] [PubMed] [Google Scholar]
- 15.Pence BC, Butler MJ, Dunn DM, Miller MF, Zhao C, et al. Non-promoting effects of lean beef in the rat colon carcinogenesis model. Carcinogenesis. 1995;16:1157–1160. doi: 10.1093/carcin/16.5.1157. [DOI] [PubMed] [Google Scholar]
- 16.Pence BC, Landers M, Dunn DM, Shen CL, Miller MF. Feeding of a well-cooked beef diet containing a high heterocyclic amine content enhances colon and stomach carcinogenesis in 1,2-dimethylhydrazine-treated rats. Nutr Cancer. 1998;30:220–226. doi: 10.1080/01635589809514667. [DOI] [PubMed] [Google Scholar]
- 17.Layton DW, Bogen KT, Knize MG, Hatch FT, Johnson VM, et al. Cancer risk of heterocyclic amines in cooked foods: an analysis and implications for research. Carcinogenesis. 1995;16:39–52. doi: 10.1093/carcin/16.1.39. [DOI] [PubMed] [Google Scholar]
- 18.Corpet DE, Stamp D, Medline A, Minkin S, Archer MC, et al. Promotion of colonic microadenoma growth in mice and rats fed cooked sugar or cooked casein and fat. Cancer Res. 1990;50:6955–6958. [PubMed] [Google Scholar]
- 19.Bird RP. Role of aberrant crypt foci in understanding the pathogenesis of colon cancer. Cancer Lett. 1995;93:55–71. doi: 10.1016/0304-3835(95)03788-X. [DOI] [PubMed] [Google Scholar]
- 20.Lapré JA, De Vries HT, Koeman JH, Van der Meer R. The antiproliferative effect of dietary calcium on colonic epithelium is mediated by luminal surfactants and dependent on the type of dietary fat. Cancer Res. 1993;53:784–789. [PubMed] [Google Scholar]
- 21.Persijn JP, Van Der Slik W, Riethorst A. Determination of serum iron and latent binding capacity. Clin Chim Acta. 1971;35:91. doi: 10.1016/0009-8981(71)90298-1. [DOI] [PubMed] [Google Scholar]
- 22.Pritam SS, Palmquist DL. Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J Agric Food Chem. 1988;36:1202–1206. [Google Scholar]
- 23.Bird RP. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen : preliminary findings. Cancer Lett. 1987;37:147–151. doi: 10.1016/0304-3835(87)90157-1. [DOI] [PubMed] [Google Scholar]
- 24.Caderni G, Giannini A, Lancioni L, Luceri C, Biggeri A, et al. Characterisation of aberrant crypt foci in carcinogen-treated rats : association with intestinal carcinogenesis. Brit J Cancer. 1995;71:763–769. doi: 10.1038/bjc.1995.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shannon J, White E, Shattuck AL, Potter JD. Relationship of food groups and water intake to colon cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:495–502. [PubMed] [Google Scholar]
- 26.Lubin F, Rozen P, Arieli B, Farbstein M, Knaani Y, et al. Nutritional and lifestyle habits and water-fiber interaction in colorectal adenoma etiology. Cancer Epidemiol Biomarkers Prev. 1997;6:79–85. [PubMed] [Google Scholar]
- 27.Stookey JD, Belderson PE, Russell JM, Barker ME. Correspondence re : J. Shannon et al.: Relationship of food groups and water intake to colon cancer risk. Cancer Epidemiol, Biomarkers Prev. 1997;6:657–658. [PubMed] [Google Scholar]
- 28.Uccheddu A, Murgia C, Licheri S, Dessy E, Ghinami E, et al. Incidenza delle neoplasie coliche indotte da 1,2-dimetilidrazina nel ratto : influenza della stipsi. Giorn Chir. 1991;12:572–574. [PubMed] [Google Scholar]
- 29.Le-Marchand L, Wilkens LR, Hankin JH, Kolonel LN, Lyu LC. A case control study of diet and colorectal cancer in a multiethnic population in Hawaii (United States) : lipids and foods of animal origin. Cancer Causes Control. 1997;8:637–648. doi: 10.1023/a:1018406716115. [DOI] [PubMed] [Google Scholar]
- 30.Zhao LP, Kushi LH, Klein RD, Prentice RL. Quantitative review of studies of dietary fat and rat colon carcinoma. Nutr Cancer. 1991;15:169–177. doi: 10.1080/01635589109514124. [DOI] [PubMed] [Google Scholar]
- 31.Tang ZC, Shivapurkar N, Frost A, Alabaster O. The effect of dietary fat on the promotion of mammary and colon cancer in a dual-organ rat carcinogenesis model. Nutr Cancer. 1996;25:151–159. doi: 10.1080/01635589609514437. [DOI] [PubMed] [Google Scholar]
- 32.Cohen LA, Choi K, Weisburger JH, Rose DP. Effect of varying proportions of dietary fat on the development of N-nitrosomethylurea-induced rat mammary tumors. Anticancer Res. 1986;6:215–218. [PubMed] [Google Scholar]
- 33.Nagenstat FM, Grubben MJAL, Van Munster IP. Role of bile acids in colorectal carcinogenesis. Eur J Cancer. 1995;31A:1067–1070. doi: 10.1016/0959-8049(95)00216-6. [DOI] [PubMed] [Google Scholar]
- 34.Weisburger JH. Causes, relevant mechanism, and prevention of large bowel cancer. Seminars in Oncology. 1991;18:316–336. [PubMed] [Google Scholar]
- 35.Reddy BS, Simi B, Patel N, Aliaga C, Rao CV. Effect of amount and types of dietary fat on intestinal bacterial 7 α–dehydroxylase and phosphatidylinositol-specific phospholipase C and colonic mucosal diacylglycerol kinase and PKC activities during stages of colon tumor promotion. Cancer Res. 1996;56:2314–2320. [PubMed] [Google Scholar]
- 36.Reddy BS, Mangat S, Sheinfil A, Weisberger JH, Wynder E. Effect of type and amount of dietary fat and 1,2 dimethylhydrazine on biliary bile acids, fecal bile acids, and neutral sterols in rats. Cancer Res. 1977;37:2132–2137. [PubMed] [Google Scholar]
- 37.Gallaher DD, Chen CL. Beef tallow, but not corn bran or soybean polysaccharide, reduces large intestinal and fecal bile acid concentrations in rats. Nutr Cancer. 1995;23:63–75. doi: 10.1080/01635589509514362. [DOI] [PubMed] [Google Scholar]
- 38.Lai C, Dunn DM, Miller MF, Pence BC. Non-promoting effects of iron from beef in the rat colon carcinogenesis model. Cancer Lett. 1997;112:87–91. doi: 10.1016/S0304-3835(96)04549-1. [DOI] [PubMed] [Google Scholar]
- 39.World Cancer Research Fund, American Institute for Cancer Research. AICR. Whashington DC: 1997. Food nutrition, the prevention of cancer a global perspective; p. 670. [DOI] [PubMed] [Google Scholar]
- 40.De Luca LM, Ross SA. Beta-carotene increases lung cancer incidence in cigarette smokers. Nutr Rev. 1996;54:178–180. doi: 10.1111/j.1753-4887.1996.tb03926.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Stamp D, Minkin S, Medline A, Corpet DE, et al. Promotion of aberrant crypt foci and cancer in rat colon by thermolyzed protein. JNCI. 1992;84:1026–1030. doi: 10.1093/jnci/84.13.1026. [DOI] [PubMed] [Google Scholar]
- 42.Magnuson BA, Carr I, Bird RP. Ability of aberrant crypt foci characteristics to predict colonic tumor incidence in rats fed cholic acid. Cancer Res. 1993;53:4499–4504. [PubMed] [Google Scholar]
- 43.Pretlow TP, Oriordan MA, Somich GA, Amini SB, Pretlow TG. Aberrant crypts correlate with tumor incidence in F344 rats treated with azoxymethane and phytate. Carcinogenesis. 1992;13:1509–1512. doi: 10.1093/carcin/13.9.1509. [DOI] [PubMed] [Google Scholar]
- 44.Hardman WE, Cameron IL, Heitman DW, Contreras E. Demonstration of the need for end point validation of putative biomarkers - failure of aberrant crypt foci to predict colon cancer incidence. Cancer Res. 1991;51:6388–6392. [PubMed] [Google Scholar]


