Abstract
The existence of a sex difference in several chronic pain syndromes and the fluctuation of symptoms during the menstrual cycle strongly suggest sex hormones are involved in pain processing. The mechanisms underlying these changes are not well understood. Using the colorectal distention model in the rat, we previously reported a sex difference in the response to distention (Am J Phys 2006, 291:R037) and that ovariectomy decreased the responses to distention while estrogen replacement reversed the decrease (J Neurosci 2003, 23:3908), suggesting estrogen increases visceral nociception. In the present study we tested the hypothesis that the visceromotor response to colorectal distention fluctuates with the estrous cycle. Three measurements (vaginal smears, uterine tube weight and plasma estrogen concentration) were used to determine the estrous phase. Comparison of the visceromotor response threshold and magnitude was made between proestrus and metestrus/diestrus. Our experiment demonstrated that the distention threshold was significantly lower in proestrus (median: 15 mmHg) as compared with metestrus/diestrus (median: 25 mmHg); and the magnitude of the visceromotor response to graded intensities of colorectal distentions (20, 40, 60, 80 mmHg) was significantly higher in proestrus. The results indicate that the visceromotor response fluctuates with estrous phase, providing evidence for endogenous estrogen modulation of visceral nociceptive processing that could contribute to sex differences.
Keywords: nociception, visceral pain, gonadal hormone, estrogen, spinal cord, estradiol
Several chronic pain syndromes such as irritable bowel syndrome (IBS), migraine, fibromyalgia and temporomandibular disorders are 2-10 times more prevalent in women (Unruh, 1996; Berkley, 1997; Craft, 2007). In many cases, symptoms become more severe in certain phases of the menstrual cycle (Dao et al., 1998; Kane et al., 1998; Fillingim and Ness, 2000; Dao and LeResche, 2000; Fillingim and Edwards, 2001; Houghton et al., 2002). Experimental studies in humans suggest there is a sex difference in nociception (for review, see (Riley et al., 1998)) and noxious stimuli-induced painful sensations are influenced by the menstrual cycle (for review, see (Riley et al., 1999)), suggesting female gonadal hormones are involved in modulation of painful signal processing.
The mechanisms underlying sex differences and cyclic changes of pain sensation in humans are not well understood. As a first step to explore the mechanisms, multiple animal models have been employed in the lab (for review, see (Fillingim and Ness, 2000; Craft et al., 2004; Aloisi and Bonifazi, 2006)) to address two general issues: 1. Is there a sex difference in nociceptive behavior in a specific animal model? 2. Do sex hormones contribute to the sex difference? The latter was investigated by comparing nociceptive responses in gonadectomized and hormone replacement animals or across phases of the estrous cycle in intact cycling female animals.
It is generally accepted that the first stage of any sex difference study should be a comparison of gonadally intact adult females and males followed by studies of gonadal hormones (Becker et al., 2005; Greenspan et al., 2007). Using a model of visceral nociception, colorectal distention, we have reported that there exists a sex difference in nociceptive processing (Ji et al., 2006) and that ovariectomy decreased visceromotor as well as spinal dorsal horn neuronal responses to colorectal distention and estrogen replacement reversed this effect (Ji et al., 2003), suggesting estrogen modulates visceral nociceptive processing. It is of interest to know if fluctuation of endogenous estrogen also influences nociceptive processing.
We previously reported the visceromotor response to colorectal distention did not fluctuate with the estrous cycle at a fixed pressure (60 mmHg) in awake rats (Ji et al., 2006). However, a study in anesthetized rats showed the visceromotor response threshold to colorectal distention fluctuated across the estrous cycle, with the lowest threshold occurring in proestrus (Holdcroft et al., 2000). Based on our previous observations that there exists a sex difference in visceral nociceptive processing (Ji et al., 2006) and ovariectomy decreased the rats' response to distention (Ji et al., 2003), we hypothesized that there exists a cyclic change of the visceromotor response that is not readily discernible without extraordinary care. To confirm this, we used three measurements to help precisely determine phases of the estrous cycle, and observed a change of visceromotor response that paralleled changes in plasma estrogen concentration.
Methods
Animals
Intact female Sprague-Dawley rats (200-220 g, 9-10 weeks of age) were used in the present study. Rats were housed 2 per cage, with free access to food and water and maintained on a 12 hr -12 hr light-dark cycle. All protocols were approved by the University of Maryland Dental School Institutional Animal Care and Use Committee and adhered to the guidelines for experimental pain in animals published by the International Association for the Study of Pain.
Vaginal smear
Vaginal smears were taken once daily at 10:00 am to 11:00 am starting 4 days after the surgery (see below) for at least two estrous cycles (8-10 days) to determine the cycling pattern for each rat. The samples were placed on glass microscope slides and viewed under dark field illumination at 100× magnification to determine the estrous phase. Images were digitally recorded.
Visceromotor response
All rats were implanted with electromyogram (EMG) electrodes 12-14 days before testing. In isoflurane anesthetized rats, an electrode made from Teflon coated 32 g stainless steel wire (Cooner Wire Co., CA) was placed in the lateral abdominal wall, the electrode leads tunneled subcutaneously and exteriorized at the back of the neck. On the day of the experiment, rats were briefly sedated with isoflurane and a 5-6 cm balloon attached to Tygon tubing was inserted through the anus into the descending colon and rectum. The distal end of the balloon was maintained 1 cm proximal to the external anal sphincter by taping the tubing to the tail. Rats were loosely restrained in Plexiglas rodent restrainers and allowed 30 min to recover from the anesthetic. Colorectal distention (CRD) was produced by inflating the distention balloon with air. The pressure was monitored and kept constant by a pressure controller/timing device (Bioengineering, University of Iowa). The visceromotor response (the EMG in response to CRD) was recorded with a CED 1401plus and analyzed using Spike 2 for windows software (Cambridge Electronic Design, UK). The EMG was rectified offline and the area under the curve (AUC) determined. The baseline visceromotor response was defined as the AUC 10 seconds prior to distention.
Two sets of data were collected in this experiment: The threshold pressure to evoke a visceromotor response and the magnitude of response to supra-threshold distention pressures. To determine threshold, the visceromotor response to graded intensities of distention (5, 10, 15, 20, 25, 30, 35 mmHg, five distentions at each pressure, 10 seconds duration, 30 seconds inter-stimulus interval) was measured and the threshold was defined as the minimum pressure that elicited a response at least 60% of the time. A response to CRD was considered positive if the AUC during distention was greater than two times the standard deviation above the baseline AUC. The visceromotor response to supra-threshold stimuli was measured using 20, 40, 60, 80 mmHg CRD (five distentions at each pressure, 10 seconds duration, 30 seconds inter-stimulus interval).
Plasma estrogen concentration
On completing each experiment (1:00 to 2:00 pm), the rats were deeply anesthetized with Nembutal and blood samples were collected by cardiac puncture and put into tubes pretreated with heparin. The samples were let stand for one hour before centrifuging at 14000 rpm for 10 minutes. The blood serum was then collected and stored at -80 °C until use. Serum estradiol levels were measured using Estradiol EIA kit (Catalog No. 582251. Cayman Chemical Company, MI). The sensitivity was 19 pg/ml.
Uterine weight
After the blood was collected, the abdomen of the rat was cut open, both uterine horns were cut at the ovary and carefully dissected to where the uterine horns join the cervix and removed for weighing. Fluid in the lumen of the uterine horns was extruded before weighing.
Data analysis
The data are expressed as mean ± sem. Data were analyzed using t-test, Rank sum test, one or two-way ANOVA followed by Student-Newman-Keuls Method for multiple comparisons as appropriate. p< 0.05 was considered significant.
Results
Estrous phase
Three measurements were taken into account to identify rats in different stages of the estrous cycle. Intact female rats (n=29) were primarily classified as in metestrus (n=6), diestrus (n=6), proestrus (n=12) or estrus (n=5) on the day of the visceromotor recording according to the cellular characteristics of their vaginal smears (Figure 1). All rats had 4-5 day cycles. The weight of the uterus and plasma estrogen concentration were measured and used as the second and third index for determination of the estrous phase. Since metestrus only lasts for a short period of time (5-6 hours) and the plasma estrogen concentration in metestrus did not differ from that in diestrus, data from these two groups of rats were pooled. Of the 5 rats with an estrus vaginal smear pattern, two had either a markedly high plasma estrogen concentration or uterine weight. One rat that had a diestrus smear one day showed an estrus smear pattern the following day, suggesting it was in the early stage of estrus. Due to this observation and some other reasons (see discussion), rats in estrus were not included in the data analysis.
Figure 1.
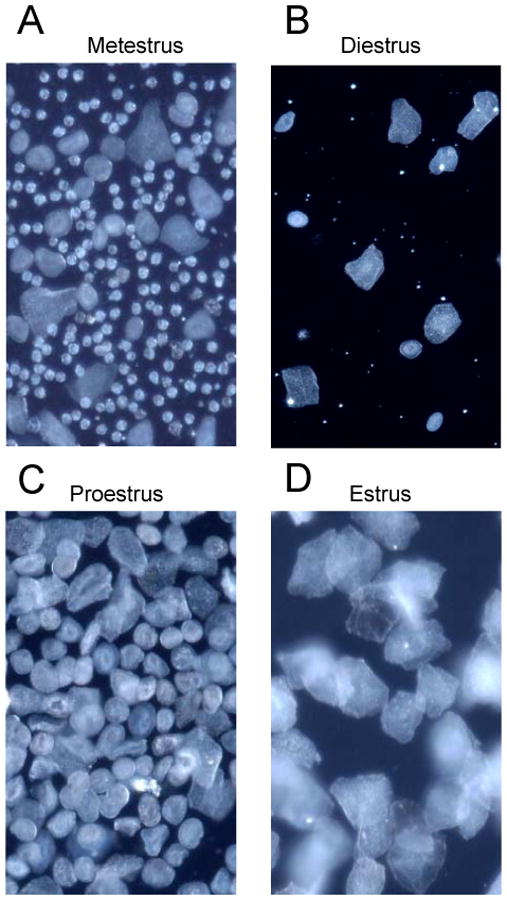
Representative photomicrographs showing smears from rats in metestrus/diestrus, proestrus and estrus. A: Metestrus is characterized by the appearance of small leukocytes, mixed with round or irregular shaped nucleated or squamous epithelial cells in a vaginal smear. B: Diestrus smear has fewer leukocytes mixed with cornified epithelial cells. C: The smear from a proestrus rat is characterized by a predominance of round nucleated cells of uniform size, with a complete absence of leucocytes. Cells are distributed individually or in clusters. D: Estrus, nonnucleated cornified epithelial cells are predominant.
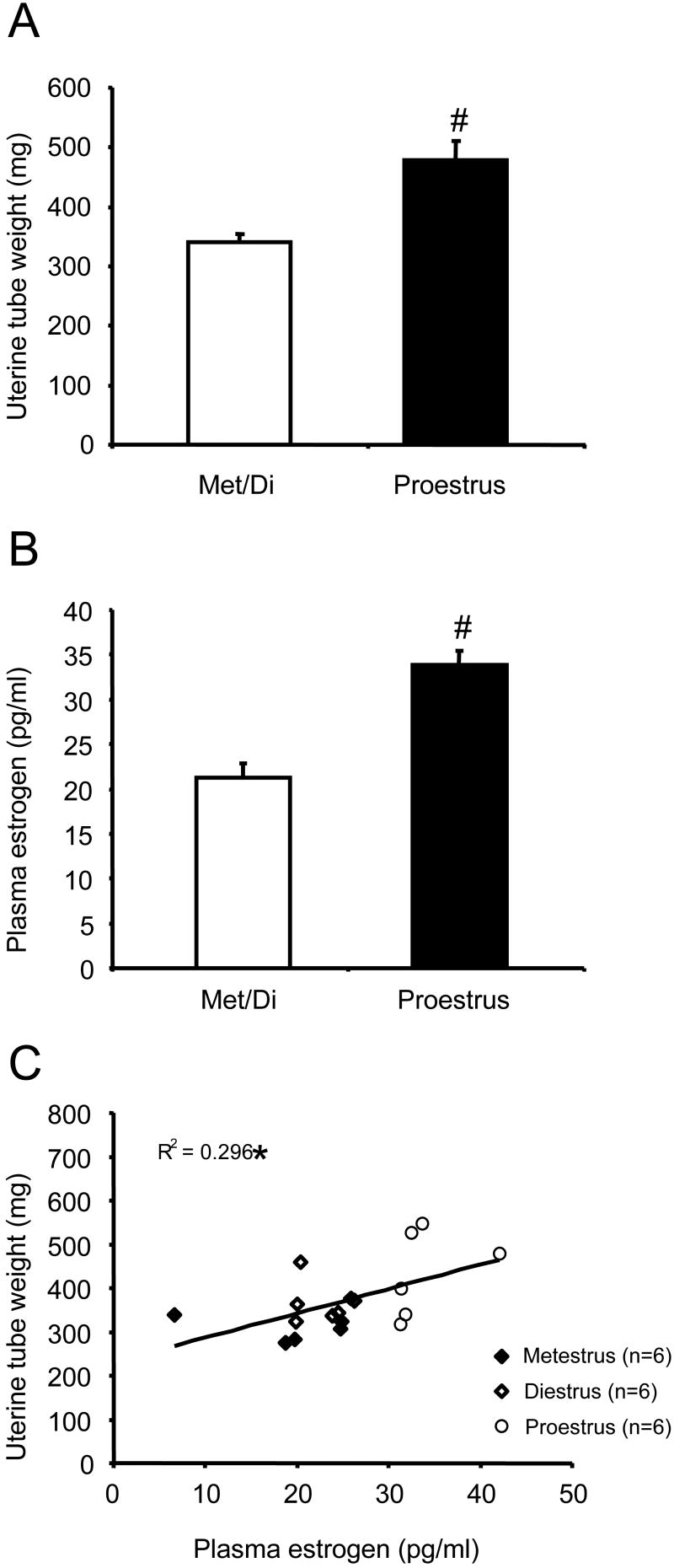
The classification of rats as in metestrus/diestrus or proestrus phase by vaginal smear was further supported by the measurement of their uterine weight and/or plasma estrogen concentration upon completion of each experiment. Overall, the uterus from rats in proestrus was thick and filled with fluid and weighed significantly more than rats in metestrus/diestrus (Figure 2A; t-test, p<0.001). Rats recognized as in proestrus had significantly higher plasma estrogen concentration than those in metestrus/diestrus (Figure 2B; t-test, p<0.001). The uterine weight was positively correlated with the plasma estrogen concentration (Figure 2C; Linear regression, p<0.05.).
Figure 2.
The uterine tube weight and plasma estrogen concentration are positively correlated. A: the uterine tube weight from rats in proestrus (n=12) is significantly greater than rats in metestrus/diestrus (n=12). B: Rats in proestrus (n=6) had significantly greater plasma estrogen concentration than rats in metestrus/diestrus (n=12). C: the uterine tube weight is positively correlated with plasma estrogen level. # t-test p<0.001 compared with met/di. * linear regression p<0.05.
Visceromotor response threshold
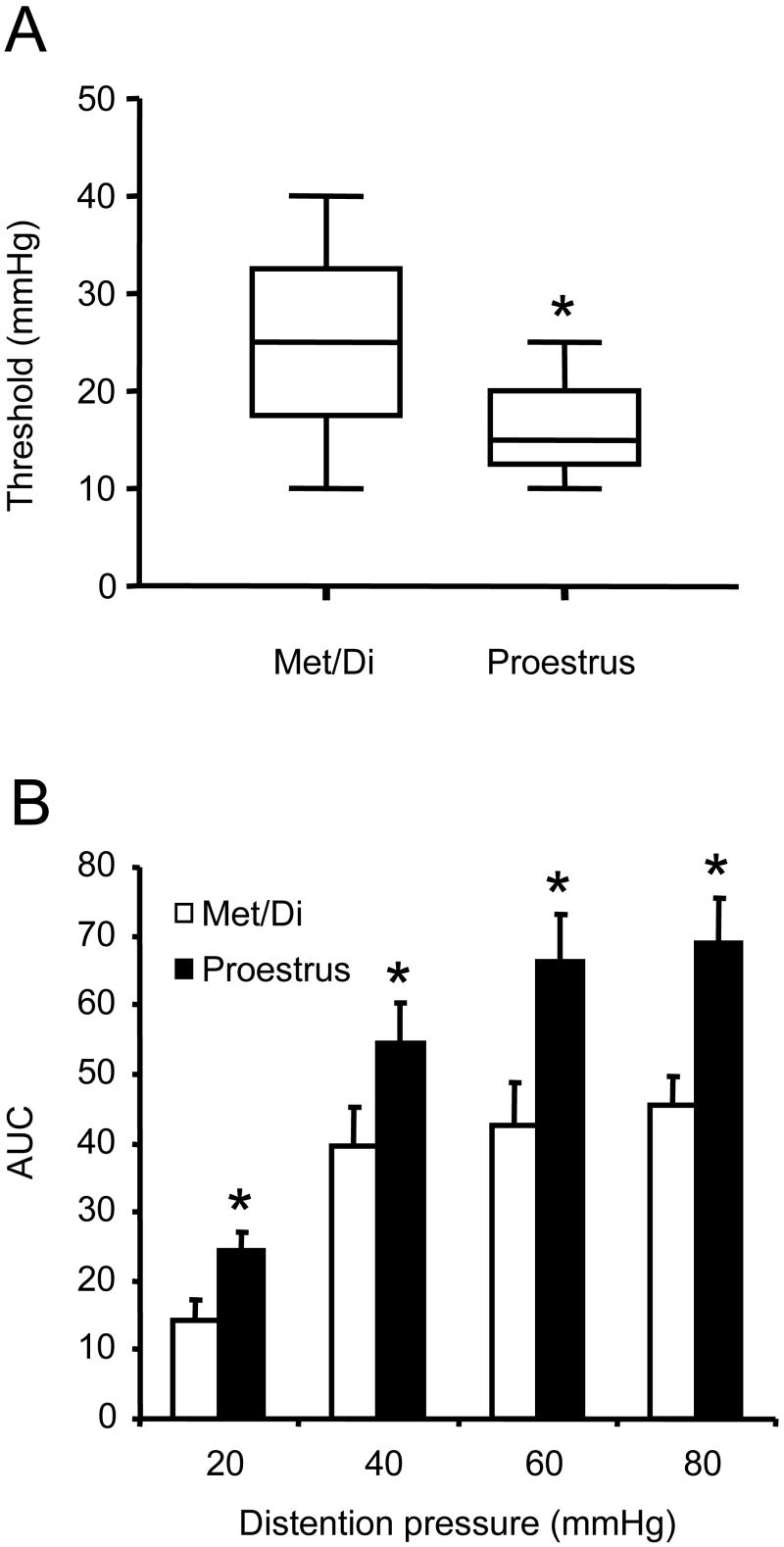
Reports from Holdcroft's group showed that in lightly anesthetized rats the distention threshold was the lowest in proestrus (Sapsed-Byrne et al., 1996; Holdcroft et al., 2000). To determine whether similar cyclic changes in threshold occur in awake rats, the visceromotor response to increasing intensities of colorectal distention was recorded to determine the threshold. As shown in figure 3A, the threshold to evoke a visceromotor response was significantly lower in proestrus (Range: 5-25mmHg; Median: 15 mmHg) as compared with metestrus/diestrus (Range: 10-40mmHg; Median: 25 mmHg; Rank sum test, p<0.05), suggesting rats in proestrus are more sensitive to visceral stimuli.
Figure 3.
Rats in proestrus are more sensitive to colorectal distention compared with rats in metestrus/diestrus (n=12 per group). A: the threshold for eliciting a visceromotor response in proestrus rats is significantly lower than metestrus/diestrus rats. * Rank sum test p<0.05; B: The magnitude of the visceromotor response to graded intensities of distention was significantly greater at each distention pressure in rats in proestrus compared to rats in metestrus/diestrus. * Two way RM ANOVA p<0.05.
Visceromotor response to graded intensities of CRD
We have reported previously that there was no cyclic fluctuation in the rats' visceromotor response to 60 mmHg of distention (Ji et al., 2005). Since other lines of evidence suggest rats with higher estrogen levels tend to have greater responses to distention (Ji et al., 2006) (Ji et al., 2003), we re-evaluated the effect of the estrous phase on the visceromotor response by comparing the rats' response to graded intensities of pressure in two phases: proestrus and metestrus/diestrus (see discussion for the rationale of excluding rats in estrus). The visceromotor response to CRD in both phases was pressure-dependent (Figure 3B; one-way RM ANOVA, p <0.001). Statistical analysis indicated a significant difference in the visceromotor response to 20, 40, 60 and 80 mmHg of distention between proestrus and metestrus/diestrus (two-way RM ANOVA, p <0.05).
Discussion
In the present study, we used three measurements to determine the phase of the estrous cycle and observed a fluctuation in the magnitude of the visceromotor response with estrous phase, by showing that rats in proestrus (when high plasma estrogen concentration was present) had significantly lower threshold and higher magnitude of response to colorectal distention as compared with rats in metestrus/diestrus (when plasma estrogen concentration was low), further supporting our previous observation that estrogen increases visceral pain.
Vaginal smears have been commonly used for determining the rats' estrous phase (Cooper et al., 1993; Hubscher et al., 2005). Since the estrous cycle in a laboratory rat is relatively short (4-5 days), and in a typical cycle proestrus (12-14 hours) and metestrus (5-6 hours) are much shorter than estrus (24-27 hours) and diestrus (55-57 hours), ideally, multiple smears should be taken each day to precisely monitor the estrous phase. This is not practical in behavioral studies since multiple handling could be extremely stressful to the rat and therefore impact the result of the study (Balcombe et al., 2004; Becker et al., 2005). Obviously, it is not appropriate to measure the plasma estrogen concentration without taking into consideration the estrous phase, since the same estrogen level when climbing would give different meaning than when it is decreasing. As reported by others (White et al., 1978), we found that the uterine weight fluctuates with the estrous phase and a positive correlation was found between the uterine weight and plasma estrogen concentration, suggesting this could be a useful index to help determine the estrous phase. For example, a high uterine weight with high estrogen concentration would strongly suggest the rats were in or close to proestrus. A combination of plasma estrogen level with vaginal smear and uterine weight should be more reliable in determining the estrous phase.
The plasma estrogen concentration fluctuates across the estrous cycle, with the lowest estrogen concentration occurring during estrus. Estrogen increases gradually during metestrus/diestrus and peaks during proestrus (Freeman, 1994; Becker et al., 2005). Although the onset of the effect of estrogen on neuronal activity is rapid, the effect lasts for a longer period of time due to its genomic effects (McEwen, 1991; McEwen and Alves, 1999; McEwen, 2001). For example, we have reported that subcutaneous injection of estradiol in ovariectomized rats caused an increase in behavioral as well as spinal dorsal horn neuronal responses to colorectal distention that occurred at 4 hours and persisted more than 48 hours (Ji et al., 2003). In addition, the threshold for activating uterine afferents in the hypogastric nerve was lowest during proestrus/estrus (Robbins et al., 1990). Therefore, in early estrus, although there is a sudden drop of estrogen, the prolonged effect of the elevated estrogen level from the previous phase on some physiological activities might persist. This could influence the results in certain tests especially when the impact of estrogen is determined by a comparison made between the proestrus (when blood estrogen is the highest) and estrus (when blood estrogen level is low). To avoid this, comparisons between the proestrus and metestrus/diestrus were made to give more explicit results (Bereiter, 2001; Bi et al., 2001; Bereiter et al., 2002; Okamoto et al., 2003).
We found a significant difference in the visceromotor response threshold between the proestrus and metestrus/diestrus in awake rats. This is consistent with the report from Holdcroft's lab showing rats in proestrus had significantly lower threshold compared with rats in other phases in lightly anesthetized rats (Sapsed-Byrne et al., 1996; Holdcroft et al., 2000), suggesting that rats in proestrus are more sensitive to colorectal distention. This is further supported by our present findings that the magnitude of the visceromotor response to graded intensities of CRD was significantly greater in proestrus rats compared to metestrus/diestrus rats. Our previous observations that ovariectomy decreased the visceromotor response and that estrogen replacement reversed the decrease (Ji et al., 2003; Ji et al., 2006) suggest estrogen increases visceral nociception and therefore is a key factor underlying cyclic changes in the visceromotor response during the estrous cycle.
The fluctuation of visceral nociception with estrous cycle is not likely due to changes in the tone of the colon wall based on our previous observation that estrogen does not change the compliance of the colon (Ji et al., 2003). Other cyclic changes that may occur in the colon might be reflected by changes in primary afferent activity. The descending colon and the rectum are innervated by both the hypogastric/lumbar colonic and pelvic nerves. These nerves also transmit sensory information from pelvic reproductive organs such as the uterus and vagina. The minimal necessary pressures in the vagina or uterus to elicit neuronal activity in the afferent fibers was significantly lower during proestrus (Robbins et al., 1992). Similarly, spinal dorsal root fibers are more sensitive to colorectal distention in proestrus (Wang and Al-Chaer, 2007). Ovariectomy decreased the response and estrogen replacement reversed this further indicating a facilitatory role of estrogen on primary afferents responding to colorectal distention (Wang and Al-Chaer, 2007). Primary afferent nerves innervating visceral tissues express P2X3, vanilloid receptor 1 (VR1), N-methyl-D-aspartate receptors (NMDARs), etc (McRoberts et al., 2001; Chaban et al., 2004; Brierley et al., 2005a; Brierley et al., 2005b). One of the mechanisms through which estrogen modulates primary afferent activity is the potentiation of NMDA currents in dorsal root ganglia (DRG) neurons, increasing their mechanical sensitivity (McRoberts et al., 2007).
Estrogen could act at multiple sites in the nervous system to modulate nociceptive signal processing. In addition to expression in DRG, estrogen receptors are expressed in the spinal cord and supraspinally (Williams and Papka, 1996; Alves et al., 1998; Ravizza et al., 2002; Chakraborty et al., 2005; Tang et al., 2007). Neuronal activity was the highest in proestrus, and the lowest in the metestrus in the lateral septal nucleus (Contreras et al., 2000), an area that is involved in the motivational-affective dimension of nociception (Khanna, 1997; Leite-Almeida et al., 2006). In the trigeminal subnucleus caudalis/upper cervical cord (Vc/C2) junction region, more Fos-positive neurons were produced after acute injury to the temporomandibular joint (TMJ) region and injection of bradykinin into the TMJ evoked greater magnitude and duration of responses in neurons from rats in proestrus compared with diestrus (Bereiter, 2001; Okamoto et al., 2003). Our previous experiment showed subcutaneous injection of estrogen increased dorsal horn neuronal response and Fos expression to CRD (Tang et al., 2006), however, it does not necessarily mean the effect was occurring in the spinal cord since increased primary afferent activity could induce an increase in dorsal horn neuronal activity. Intrathecal injection of estrogen caused an increase in the visceromotor response (Tang et al., 2006) supporting an effect of estrogen on the spinal cord that could contribute to the increased visceromotor response in proestrus. An increase in estrogen receptor binding sites in the lumbosacral spinal cord during proestrus could further the effect of estrogen (Williams et al., 1997). Our observation that estrogen increases spinal NMDAR subunit 1 phosphorylation and extracellular signal-regulated kinase (ERK) activation provides one possible mechanism underlying pronociceptive effect of estrogen (Traub et al., 2007; Tang et al., 2007).
In summary, the existence of a sex difference in the prevalence of human chronic pain syndromes such as IBS suggests gonadal hormones could influence visceral pain perception. The mechanisms underlying the sex difference in the clinical condition remain unclear. Our previous observations that there is a sex difference in behavioral and neuronal response to colorectal distention, along with the findings that ovariectomy decreased and estrogen replacement reversed the response to colorectal distention suggest the model could be useful in exploring sex differences in visceral pain. The present findings that fluctuations in the magnitude of the visceromotor response positively correlated to the plasma estrogen concentration in different phases of the estrous cycle provide evidence for endogenous estrogen modulation of visceral pain.
Acknowledgments
This work was supported by R01 NS 37424. Authors would like to thank Sangeeta Pandya for technical assistance.
Abbreviations
- CRD
colorectal distention
- EMG
electromyogram
- AUC
area under the curve
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aloisi AM, Bonifazi M. Sex hormones, central nervous system and pain. Horm Behav. 2006;50:1–7. doi: 10.1016/j.yhbeh.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Alves SE, Weiland NG, Hayashi S, McEwen BS. Immunocytochemical localization of nuclear estrogen receptors and progestin receptors within the rat dorsal raphe nucleus. J Comp Neurol. 1998;391:322–334. [PubMed] [Google Scholar]
- Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci. 2004;43:42–51. [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Bereiter DA. Sex differences in brainstem neural activation after injury to the TMJ region. Cells Tissues Organs. 2001;169:226–237. doi: 10.1159/000047886. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Shen S, Benetti AP. Sex differences in amino acid release from rostral trigeminal subnucleus caudalis after acute injury to the TMJ region. Pain. 2002;98:89–99. doi: 10.1016/s0304-3959(01)00476-6. [DOI] [PubMed] [Google Scholar]
- Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20:371–380. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. PNAS. 2001;98:13391–13395. doi: 10.1073/pnas.241507698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley SM, Carter R, Jones W, III, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol. 2005a;567:267–281. doi: 10.1113/jphysiol.2005.089714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley SM, Jones RC, III, Xu L, Gebhart GF, Blackshaw LA. Activation of splanchnic and pelvic colonic afferents by bradykinin in mice. Neurogastroenterol Motil. 2005b;17:854–862. doi: 10.1111/j.1365-2982.2005.00710.x. [DOI] [PubMed] [Google Scholar]
- Chaban VV, Li J, Ennes HS, Nie J, Mayer EA, McRoberts JA. N-methyl-D-aspartate receptors enhance mechanical responses and voltage-dependent Ca2+ channels in rat dorsal root ganglia neurons through protein kinase C. Neuroscience. 2004;128:347–357. doi: 10.1016/j.neuroscience.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Chaban VV, Mayer EA, Ennes HS, Micevych PE. Estradiol inhibits atp-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003;118:941–948. doi: 10.1016/s0306-4522(02)00915-6. [DOI] [PubMed] [Google Scholar]
- Chaban VV, Micevych PE. Estrogen receptor-alpha mediates estradiol attenuation of ATP-induced Ca(2+) signaling in mouse dorsal root ganglion neurons. J Neurosci Res. 2005;81:31–37. doi: 10.1002/jnr.20524. [DOI] [PubMed] [Google Scholar]
- Chakraborty TR, Rajendren G, Gore AC. Expression of estrogen receptor {alpha} in the anteroventral periventricular nucleus of hypogonadal mice. Exp Biol Med (Maywood) 2005;230:49–56. doi: 10.1177/153537020523000106. [DOI] [PubMed] [Google Scholar]
- Contreras CM, Molina M, Saavedra M, Martinez-Mota L. Lateral septal neuronal firing rate increases during proestrus-estrus in the rat. Physiol Behav. 2000;68:279–284. doi: 10.1016/s0031-9384(99)00169-9. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Goldman JM, Vandenbergh JG. In: Monitoring of estrus cyclicity in the laboratory rodent by vaginal lavage. Chapin REaJJ., editor. Academic Press; Orlando: 1993. pp. 45–56. [Google Scholar]
- Craft RM. Modulation of pain by estrogens. Pain. 2007;132 1:S3–12. doi: 10.1016/j.pain.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004;8:397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Dao TT, Knight K, Ton-That V. Modulation of myofascial pain by the reproductive hormones: a preliminary report. J Prosthet Dent. 1998;79:663–670. doi: 10.1016/s0022-3913(98)70073-3. [DOI] [PubMed] [Google Scholar]
- Dao TT, LeResche L. Gender differences in pain. J Orofac Pain. 2000;14:169–184. [PubMed] [Google Scholar]
- Fillingim RB, Edwards RR. The association of hormone replacement therapy with experimental pain responses in postmenopausal women. Pain 2001. 2001;92:229–234. doi: 10.1016/s0304-3959(01)00256-1. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Ness TJ. Sex-related hormonal influences on pain and analgesic responses. Neurosci Biobehav Rev. 2000;24:485–501. doi: 10.1016/s0149-7634(00)00017-8. [DOI] [PubMed] [Google Scholar]
- Freeman ME. The neuroendocrine control of the ovarian cycle of the rat. In: Knobil E, Neill JD, editors. The physiology of reproduction. Raven Press; New York: 1994. pp. 613–658. [Google Scholar]
- Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132 1:S26–S45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdcroft A, Sapsed-Byrne S, Ma D, Hammal D, Forsling ML. Sex and oestrous cycle differences in visceromotor responses and vasopressin release in response to colonic distention in male and female rats anesthetized with halothane. Br J Anaesth. 2000;85:907–910. doi: 10.1093/bja/85.6.907. [DOI] [PubMed] [Google Scholar]
- Houghton LA, Lea R, Jackson N, Whorwell PJ. The menstrual cycle affects rectal sensitivity in patients with irritable bowel syndrome but not healthy volunteers. Gut. 2002;50:471–474. doi: 10.1136/gut.50.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubscher CH, Brooks DL, Johnson JR. A quantitative method for assessing stages of the rat estrous cycle. Biotech Histochem. 2005;80:79–87. doi: 10.1080/10520290500138422. [DOI] [PubMed] [Google Scholar]
- Ji Y, Murphy AZ, Traub RJ. Estrogen Modulates the Visceromotor Reflex and Responses of Spinal Dorsal Horn Neurons to Colorectal Stimulation in the Rat. J Neurosci. 2003;23:3908–3915. doi: 10.1523/JNEUROSCI.23-09-03908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Murphy AZ, Traub RJ. Sex differences in morphine analgesia of visceral pain are supraspinally mediated. Society for Neuroscience Abstracts. 2005 doi: 10.1152/ajpregu.00824.2005. Abstract viewer, 294.5. [DOI] [PubMed] [Google Scholar]
- Ji Y, Murphy AZ, Traub RJ. Sex differences in morphine induced analgesia of visceral pain are supraspinally and peripherally mediated. Am J Physiol Regul Integr Comp Physiol. 2006;291:R307–R314. doi: 10.1152/ajpregu.00824.2005. [DOI] [PubMed] [Google Scholar]
- Kane SV, Sable K, Hanauer SB. The menstrual cycle and its effect on inflammatory bowel disease and irritable bowel syndrome: a prevalence study. Am J Gastroenterol. 1998;93:1867–1872. doi: 10.1111/j.1572-0241.1998.540_i.x. [DOI] [PubMed] [Google Scholar]
- Khanna S. Dorsal hippocampus field CA1 pyramidal cell responses to a persistent versus an acute nociceptive stimulus and their septal modulation. Neuroscience. 1997;77:713–721. doi: 10.1016/s0306-4522(96)00456-3. [DOI] [PubMed] [Google Scholar]
- Leite-Almeida H, Valle-Fernandes A, Almeida A. Brain projections from the medullary dorsal reticular nucleus: an anterograde and retrograde tracing study in the rat. Neuroscience. 2006;140:577–595. doi: 10.1016/j.neuroscience.2006.02.022. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Non-genomic and genomic effects of steroids on neural activity. Trends Pharmacol Sci. 1991;12:141–147. doi: 10.1016/0165-6147(91)90531-v. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Invited review: Estrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- McRoberts JA, Coutinho SV, Marvizon JC, Grady EF, Tognetto M, Sengupta JN, Ennes HS, Chaban VV, Amadesi S, Creminon C, Lanthorn T, Geppetti P, Bunnett NW, Mayer EA. Role of peripheral N-methyl-D-aspartate (NMDA) receptors in visceral nociception in rats. Gastroenterology. 2001;120:1737–1748. doi: 10.1053/gast.2001.24848. [DOI] [PubMed] [Google Scholar]
- McRoberts JA, Li J, Ennes HS, Mayer EA. Sex-dependent differences in the activity and modulation of N-methyl-d-aspartic acid receptors in rat dorsal root ganglia neurons. Neuroscience. 2007;148:1015–1020. doi: 10.1016/j.neuroscience.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Hirata H, Takeshita S, Bereiter DA. Response properties of TMJ units in superficial laminae at the spinomedullary junction of female rats vary over the estrous cycle. J Neurophysiol. 2003;89:1467–1477. doi: 10.1152/jn.00795.2002. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Galanopoulou AS, Veliskova J, Moshe SL. Sex differences in androgen and estrogen receptor expression in rat substantia nigra during development: an immunohistochemical study. Neuroscience. 2002;115:685–696. doi: 10.1016/s0306-4522(02)00491-8. [DOI] [PubMed] [Google Scholar]
- Riley JL, 3, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74:181–187. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- Riley JL, 3, Robinson ME, Wise EA, Price DD. A meta-analytic review of pain perception across the menstrual cycle. Pain. 1999;81:225–235. doi: 10.1016/S0304-3959(98)00258-9. [DOI] [PubMed] [Google Scholar]
- Robbins A, Berkley KJ, Sato Y. Estrous cycle variation of afferent fibers supplying reproductive organs in the female rat. Brain Res. 1992;596:353–356. doi: 10.1016/0006-8993(92)91572-v. [DOI] [PubMed] [Google Scholar]
- Robbins A, Sato Y, Hotta H, Berkley KJ. Responses of hypogastric nerve afferent fibers to uterine distension in estrous or metestrous rats. Neurosci Lett. 1990;110:82–85. doi: 10.1016/0304-3940(90)90791-7. [DOI] [PubMed] [Google Scholar]
- Sapsed-Byrne S, Ma D, Ridout D, Holdcroft A. Estrous cycle phase variations in visceromotor and cardiovascular responses to colonic distension in the anesthetized rat. Brain Res. 1996;742:10–16. doi: 10.1016/s0006-8993(96)00989-4. [DOI] [PubMed] [Google Scholar]
- Tang B, Ji Y, Traub RJ. Estrogen-induced facilitation of visceral nociceptive processing in the rat is spinally mediated. Soc for Neuroscience. 2006 abstract viewer, 346.5. [Google Scholar]
- Tang B, Ji Y, Traub RJ. Estrogen alters spinal NMDA receptor activity via a PKA signaling pathway in a visceral pain model in the rat. Pain. 2007 doi: 10.1016/j.pain.2007.10.017. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RJ, Ji Y, Tang B. Estrogen increases NMDA receptor expression and phosphorylation modulating visceral pain in the rat. American Pain Society abstracts. 2007 http://www.ampainsoc.org/db2/abstract/2007/view?poster_id=3080#655.
- Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- Wang J, Al-Chaer ED. Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience; 2007. Sex hormones modulate primary afferent responses to colorectal distension (CRD) in rats. Program No. 725.15. [Google Scholar]
- White JO, Thrower S, Lim L. Intracellular relationships of the oestrogen receptor in the rat uterus and hypothalamus during the oestrous cycle. Biochem J. 1978;172:37–47. doi: 10.1042/bj1720037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SJ, Chung K, Om AS, Papka RE. Cytosolic estrogen receptor concentrations in the lumbosacral spinal cord fluctuate during the estrous cycle. Life Sci. 1997;61:2551–2559. doi: 10.1016/s0024-3205(97)01009-6. [DOI] [PubMed] [Google Scholar]
- Williams SJ, Papka RE. Estrogen receptor-immunoreactive neurons are present in the female rat lumbosacral spinal cord. J Neurosci Res. 1996;46:492–501. doi: 10.1002/(SICI)1097-4547(19961115)46:4<492::AID-JNR11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]