Abstract
Anxious individuals show an attention bias towards threatening information. However, under conditions of sustained environmental threat this otherwise-present attention bias disappears. It remains unclear whether this suppression of attention bias can be caused by a transient activation of the fear system. In the present experiment, high socially-anxious and low socially-anxious individuals (HSA group, n = 12; LSA group, n = 12) performed a modified dot-probe task in which they were shown either a neutral or socially threatening prime word prior to each trial. EEG was collected and ERP components to the prime and faces displays were computed. The behavioral results indicated that HSA individuals show an attention bias to threat after a neutral prime, but no attention bias after a threatening prime, demonstrating that suppression of attention bias can occur after a transient activation of the fear system. Low Socially Anxious individuals showed an opposite pattern: no evidence of a bias to threat with neutral primes but induction of an attention bias to threat following threat primes. ERP results suggested differential processing of the primes and faces displays by HSA and LSA individuals. However, no group by prime interaction was found for any of ERP components.
Keywords: Social anxiety, attentional bias, threat, social information processing, priming, ERP
1. Introduction
The mechanisms underlying the fear system serve a normative function, allowing individuals to rapidly detect threat in the environment and appropriately respond to the threat (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007; Öhman, 2005). Hypervigilant processing of threat, a heightened tendency to direct attention preferentially to threatening stimuli is often found in anxious individuals (Mathews & MacLeod, 1985; MacLeod, Mathews, & Tata, 1986; Mogg, Mathews, & Eysenck, 1992; Mathews, Mackintosh, & Fulcher, 1997). This attention bias to threat has been shown in both clinically and non-clinically anxious individuals, in a wide variety of tasks and may be involved in both the development and maintenance of anxiety disorders (Bar-Haim et al., 2007).
However, accumulating evidence suggests that stressful circumstances alter anxious individuals’ typical pattern of attention to threat. In the emotional Stroop task, where participants are asked to name the color ink in which a set of words are printed, anxious individuals typically evidence greater interference, reflected in slower reaction times for emotionally evocative words compared to neutral words, an effect not seen in nonanxious individuals (Williams, Mathews, & MacLeod, 1996). Several studies have shown that this effect disappears when anxious participants are asked to perform the task while experiencing a larger threat or stressor (Mathews & Sebastian, 1993; Mogg, Kentish, & Bradley, 1993; Amir, McNally, Riemann, & Burns, 1996; Constans, McCloskey, Vasterling, Brailey, & Mathews, 2004). Mathews and Sebastian (1993), for example, showed that the slowed reaction times of snake phobics to both snake-related words and generally threatening words disappeared when told that they would be asked to touch a live boa constrictor after the task. Likewise, the attention bias effect for highly anxious participants disappeared when they were either told they would be taking an IQ test after the task (Mogg et al., 1993), or, in the case of social phobics, when they were told they would be asked to give a speech after the task (Amir et al., 1996). Vietnam veterans with PTSD also showed a suppression of an attention bias to threat when told they would be asked to watch combat videos after performing the emotional Stroop task (Constans et al., 2004).
Two hypotheses have been proposed for why anxious individuals’ slowed reaction time to threatening words disappears under conditions of high stress. Williams et al. (1996) have suggested that the anxiety produced by stressful conditions leads participants to increase task effort, thereby compensating for the interference effect. Alternatively, Mathews and Sebastian (1993) have suggested that the addition of a stressor might lead to a “shift in processing priorities”; a more imminent threat (e.g., giving a speech) inhibits attention to, and detection of, less threatening stimuli in the Stroop task.
Performance on the emotional Stroop cannot discriminate between these two hypotheses, because both increased effort and decreased attention to the words will lead to a decrease in response time for emotional words. An alternative paradigm for measuring attention bias to threat in anxious individuals is the dot-probe task (MacLeod et al., 1986). In this task, participants simultaneously view two stimuli, one threatening and one neutral. After a set time these stimuli disappear and a probe appears in the same location as one of the two stimuli. The participant is asked to respond to the probe as quickly and accurately as possible. In a large number of studies, anxious participants have been shown to respond more quickly when the probe appeared in the same location as the threatening stimulus compared to when it appeared in the location of the neutral stimulus, suggesting an attention bias toward threat. This finding is not seen in non-anxious participants (reviewed in Bar-Haim et al., 2007). The dot-probe task, thus, appears to have an advantage in examining the underlying processes involved in the suppression of the attention bias to threat in the presence of an environmental stressor. Specifically, if anxious participants increase effort when under stress, one would expect a general decrease in reaction times to probe detection on all trials (i.e., both when target probes appear in the threat location and in the neutral location), leaving the threat bias unchanged. By contrast, if anxious individuals diminish attention to threatening stimuli due to a change in processing priorities, one would expect to see a decrease in attention bias toward threat, with no overall decrease in reaction time.
Two recent studies have used modified dot probe tasks to examine aspects of anxious individuals’ performance under conditions of social threat. In Mansell, Clark, Ehlers, and Chen (1999), half the participants were told they would be asked to give a speech after performing the task, while the other half of the participants received no such instruction. On each trial, participants were presented with a picture of a household object paired with a picture of a face, with either a neutral, positive, or a negative expression. They found that when participants were not under conditions of stress, there was no reaction time difference in probe detection between high socially-anxious and low socially-anxious participants. However, when participants were told they would be giving a speech after the task, high socially-anxious participants showed bias scores away from both positive and negative faces, a pattern that was not found in non-anxious participants.
Garner, Mogg, and Bradley (2006), using a dot-probe paradigm, presented participants with neutral face–object pairs, neutral face–angry face pairs, and neutral face–happy face pairs. These authors used eye tracking to examine participants gaze during stimulus presentation. Similar to Mansell, et al. (1999), they found that under ordinary circumstances, high socially-anxious individuals were more likely to make an initial gaze shift towards the neutral face in a neutral face-object pairing. However, when participants were told they would be asked to give a speech after the task, their initial bias to orient to faces was reduced compared to the low socially-anxious group. Taken together, these two studies show that when a sustained threat is introduced to the task, anxious participants no longer show the typical attention bias to threat
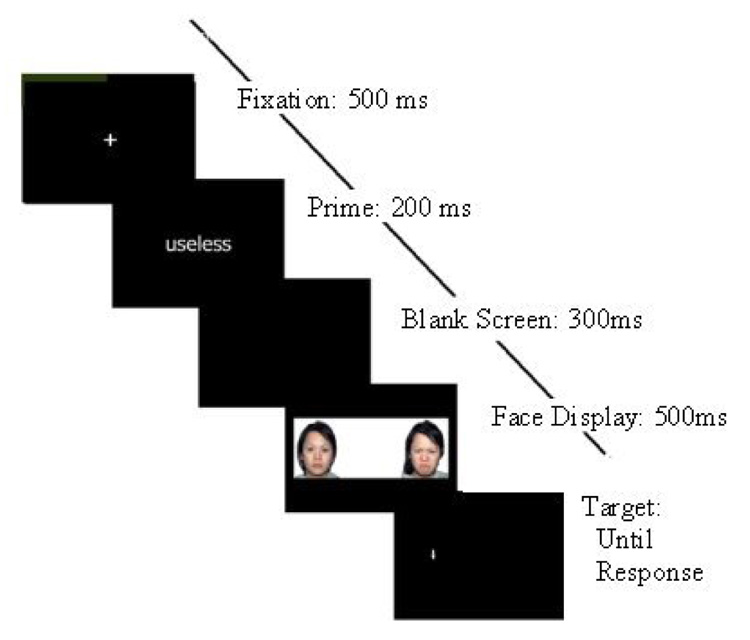
All of the studies discussed above used a sustained stressor—the threat of performing a stress inducing activity after the attention-testing task—to examine changes in patterns of attention allocation to threat under stress. It remains unknown whether transient stressors can produce similar effects on anxiety-related attention bias. In the present study, we used a modified version of the dot-probe task. Each trial began with the presentation of either a neutral or a socially threatening word. It was expected that the socially threatening words would briefly increase stress levels, affecting attention allocation patterns on the immediately subsequent trial of a typical dot-probe task (see Figure 1 for sequence of events in this task). Thus, each participant’s performance could be compared under conditions of greater or lesser social threat.
Figure 1.
Sequence of events in the modified dot-probe paradigm.
In addition to behavioral reaction time data, Event-Related Potentials (ERPs) can provide detailed information about the temporal structure of attention allocation. Many studies have used this approach to investigate attentional allocation during manual reaction time tasks (reviewed in Awh & Jonides, 1998). In addition, several studies have used ERP methodologies to study threat-related processing in anxious vs. non-anxious individuals (e.g., Rossignol, Anselme, Vermeulen, Philippot, & Campanella, 2007; Bar-Haim, Lamy, & Glickman, 2005; Holmes, Nielsen, & Green, 2008). Three ERP components are particularly relevant to the current study, the P1, N1, and P2. The amplitude of the first positivity (P1) and the first negativity (N1) appearing after presentation of a visual stimulus is influenced by the degree of visual attention the stimulus receives (Hillyard, Vogel, & Luck, 1998). Thus, in the dot-probe task, an increase in amplitude of the P1/N1 complex to the faces display is indicative of an increase in attention directed towards the face images, and would be expected to vary in response to presentation of the face stimuli in a dot-probe task as a function of participant attention. Thus, the ERP results could reveal whether anxiety-related modulations occur in the early attention processes indexed by the P1/N1 complex. Such findings would confirm an initial effect of the prime on attention.
Although relatively little is known about the posterior P2 component (Luck, 2005), it has been shown to vary in amplitude between anxious and non-anxious individuals while viewing angry faces in an attention disengagement task (Bar-Haim, Lamy, & Glickman, 2005). Hence, we expected that this component might differ across group and prime conditions in the present study, as well.
The current study presented participants either high or low in self-reported social anxiety with either a threat or neutral prime word prior to a typical dot-probe trial. ERPs in response to presentation of the prime were used to confirm the effectiveness of the threat prime manipulation independently of behavioral responses. Bernat, Bunce, and Shevrin (2001) have shown that participants display different patterns of ERP activation in frontal and parietal sites to negative vs. neutral words. Therefore, we expected similar findings in the current study.
To summarize, the objectives of the current study were three-fold. First, we hoped to determine if the changes in attention allocation seen under sustained threat occur under transient activation of the fear system. Second, by using a dot-probe paradigm, we hoped to discern whether this shift in attention allocation was due to an increase in task effort, or a shift in processing priorities. Finally, we complemented our behavioral reaction time data with ERP data to examine the chronometry of attention allocation during activation of the fear system.
2. Method
2.1 Participants
Participants were 24 females recruited from undergraduate developmental psychology courses at the University of Maryland, College Park. Mean age was 20.26 years (range = 19 to 27). Only females were recruited because the male to female ratio in these courses would not allow us to recruit a sizable enough sample of males. To ensure recruitment of participants who would be extreme on the traits of interest we used a double screen procedure. First, 153 participants were mass-screened with a composite questionnaire tapping into social anxiety and shyness. The questionnaire included the 13-item Revised Cheek and Buss Shyness Scale (RCBS) (Cheek, 1983) and the 6-item social anxiety scale from the Self-Consciousness Scale (SCS) (Fenigstein, 1975). Mean responses on the shyness and social anxiety scales of the screening questionnaire were summed to create a preliminary selection score (Cronbach’s alpha = 0.88). Only participants with selection scores in the top or bottom third of the distribution (n = 96) were considered to participate in the second selection stage. Of these, 24 participants were recruited for the experiment (12 HSA and 12 LSA).
To verify the social anxiety status of the particpants, they were asked to fill out two additional questionnaires prior to participating in the dot probe task, the Adult Temperament Questionnaire (ATQ) (version 1.3; Rothbart, Ahadi, & Evans, 2000), and the 18-item Social Anxiety Scale (SAS) for Adolescents (La Greca & Lopez, 1998). Participants’ scores on the social anxiety scale from the SAS and the 7-item fear scale from the ATQ were standardized within the sample, and the Z-scores from the two scales were summed to generate a measure of social anxiety that includes both temperamental aspects of fear of social situations as well as more general vigilance and anxious arousal. Participants were assigned to the high or low social-anxiety group using a median split of the composite score. The composite Z-scores correlated significantly with the preliminary screening scores (r = .69, p<.001)1. Table 1 presents participant characteristics for each group.
Table 1.
Participant Characteristics
| High anxious group, M (SD) (n = 12) | Low anxious group, M (SD) (n = 12) | Inferential statistics | |
|---|---|---|---|
| Age | 20.08 (1.38) | 20.25 (2.26) | t(1, 22) = 0.22, p = .829 |
| Cheek & Buss – shyness | 23.58 (7.57) | 15.08 (4.81) | t(1, 22) = −3.28, p = .003 |
| SCS - social anxiety | 14.17 (6.03) | 4.17 (3.07) | t(1, 22) = −5.12, p<.0005 |
| ATQ – fear | 33.17 (5.46) | 23.17 (4.51) | t(1, 22) = −4.89, p<.0005 |
| Social Anxiety Scale | 54.25 (9.06) | 36.83 (7.84) | t(1, 22) = −5.04, p<.0005 |
2.2 Stimuli
All stimuli were presented on a 17” monitor placed 0.5 m away from the participant. Prime, face, fixation, and probe images were all .tif files created on a black background.
2.2.1 Priming words
The primes were 64 neutral words and 64 social threat words (see Table 2), presented in pseudorandom order so that each word was seen twice over the course of the entire session. Social threat words were compiled from word lists used in previous studies of socially threatening stimuli (Wenzel, Jackson, & Holt, 2002; Heinrichs, Hofmann, & Barlow, 2004; Merwin & Wilson, 2005; Munafo, Hayward, & Harmer, 2006). Neutral words were chosen from the Affective Norms for English Words (ANEW) database, a list of over 1,000 words with ratings of pleasure, arousal, and dominance on scales from 1–9 (Bradley & Lang, 1999). The selected neutral words had pleasure scores within 0.5 points of the median pleasure score for the list. All the prime words were presented in white 72-pt Tahoma font centered vertically and horizontally on a black screen.
Table 2.
Priming Words
| Neutral Primes | Threat Primes | ||
|---|---|---|---|
| ankle | iron | ashamed | joke |
| appliance | item | blamed | judged |
| barrel | journal | blushing | loathed |
| blasé | jug | boring | lonely |
| board | kettle | class | loser |
| bowl | lawn | clumsy | manipulate |
| building | lock | conversation | mistake |
| cabinet | machine | criticized | naïve |
| cannon | mantel | dance | neglected |
| chair | material | date | offended |
| chin | metal | despised | party |
| clock | month | disgraced | pathetic |
| column | news | dull | piteous |
| contents | nun | embarrassed | presentation |
| context | office | failure | rejected |
| cord | paper | festivity | ridicule |
| cork | part | flawed | scorned |
| corridor | passage | foolish | scrutiny |
| curtains | pencil | game | shameful |
| door | rain | handshake | shy |
| egg | rattle | hated | silence |
| elbow | seat | hostile | snub |
| engine | statue | humiliated | speech |
| fabric | stove | ignorant | stare |
| finger | street | inadequate | stranger |
| foot | table | incompetent | stupid |
| fork | taxi | inept | tense |
| hammer | theory | inferior | uninvolved |
| hay | time | inhibited | unwelcome |
| history | tool | insult | unworthy |
| hydrant | trunk | intimidated | useless |
| ink | umbrella | invitation | worthless |
2.2.2 Dot-probe materials
The faces display consisted of two photographs of the same individual, one to the left and the other to the right of the central fixation point location. On the computer monitor, the two faces were separated by 11 cm of white space; each face was 11 cm tall by 8 cm wide. One image showed the face with an angry expression and the other showed the face with a neutral expression. The angry face appeared on each side of the screen on half of the trials. Face images were of 16 individuals (8 male, 8 female) taken from the NimStim Face Stimulus Set (Macarthur Research Network on Early Experience and Brain Development, 2002). Selected face images for both neutral and angry expressions had closed mouths. Faces were presented in a pseudorandom order. Probe arrows were white and were 2 cm tall X 1 cm wide on the monitor, and appeared in the center of the location previously occupied by the face. Angry face location, location of the probe, and orientation of the probe were fully balanced across face pairs.
2.3 Procedure
The dot-probe session consisted of 256 trials. Figure 1 describes the sequence of events in each trial. Each trial in the task consisted of a fixation cross, a prime, a blank screen, a faces display, a probe, and an intertrial interval. The white fixation cross was presented in the center of the screen for 500 ms. The word prime was then presented at the center of the screen in white text for 200 ms. The prime was then replaced with a blank screen for 300 ms before the faces display appeared. The faces remained on the screen for 500 ms, and then were replaced with the probe, which was a small white arrow that appeared in the same location as one of the two faces, and pointed up or down. The participant was asked to press one of two buttons on a button box to indicate which direction the arrow was pointing as quickly and accurately as possible. The trial ended once the participant pressed a button, or after the probe had remained on the screen for 1100 ms without a response. Participants’ accuracy and reaction time were recorded.
2.4 Apparatus and physiological recordings
During the task, EEG was recorded using an electrode cap manufactured by Electro-Cap Corporation (Eaton, OH). Recordings were made from 17 scalp locations: F3, F4, F7, F8, T7, T8 C3, C4, P3, P4, O1, O2, M1, M2, Fz, Pz, and Oz. Cz was used as a reference site. EOG electrodes were placed above and below the subject’s left eye to detect vertical eye movements and blinks. During recording, the EEG signal was amplified using a custom bioelectric amplifier (SA Instruments, San Diego, CA) with a gain of 1000 Hz and analog high-pass and low-pass filters at 0.1 Hz and 100 Hz, respectively. The amplified signal was digitized at 512 Hz using a 12-bit A/D converter (± 2.5 V input range) and Snap-Master acquisition software (HEM Data Corporation, Southfield, MI).
2.5 Data reduction and statistical analysis
The EEG signal was processed and analyzed using the EEG Analysis system from James Long Company (Caroga Lake, NY). EEG was re-referenced to the averaged Mastoid signal of M1 and M2 so that data collected at Cz could be analyzed. Blink artifacts were minimized using a regression-based algorithm (rise time 100 milliseconds, fall time 150 milliseconds, peak 125 µV) and data contaminated by motor artifacts (200 µV cutoff) were removed from analysis. The data were low-pass filtered at 30 Hz.
Event marks for faces onset and of participant button press on each trial were collected in synchrony with EEG acquisition. Trials where the participant did not press a button in response to the arrow were excluded. Using averaging techniques separate ERP waveforms were derived time locked to the onset of the word-prime display, the faces display, and the target display. Within each ERP type, trials were divided into threat-prime and neutral-prime trials to create two distinct ERP waveforms. The waveform to the word prime extended from 0 to 500ms after prime onset. 2584 epochs from 22 subjects were used to create the waveform for threat prime trials (8.2% of epochs were excluded due to artifact); 2494 epochs from 22 subjects were used for neutral prime trials (11.4% of epochs were excluded). The waveform to the faces display extended from 0 to 500ms after faces onset. 2604 epochs from 22 subjects were used to create a waveform for threat prime trials (7.5% of epochs were excluded due to artifact); 2522 epochs from 22 subjects were used for neutral prime trials (10.4% of epochs were excluded). The waveform to the target display extended from 0 to 500ms after target onset (2615 epochs from 22 subjects for threat prime trials; 2545 epochs from 22 subjects for neutral prime trials). Each waveform was calculated relative to the 100ms immediately prior to that segment as a baseline. Individual data points were plotted on a box plot and outliers were removed.
Windows for individual components were set after viewing the grand mean ERPs. The slow wave component to the prime words was analyzed at Cz, 300–500ms after prime onset. ERPs for the faces onset were analyzed as follows: P1 (averaged across O1 and O2, 95–140ms after face onset), N1 (averaged across P3 and P4, 155–200ms after face onset), and P2 (averaged across P3 and P4, 185–320ms after faces onset). All analyses examined mean amplitudes of each component. The mean amplitude for each component was examined for outliers, and the sole outlier (one participant in the slow wave to the prime words) was removed from this particular analysis.
3. Results
3.1 Behavioral findings
3.1.1 Accuracy rates to target detection
Across all participants, the mean accuracy rate for correctly identifying the direction of the arrow probe was 91.06 % (SD = .08 or 7.8%). A repeated measures ANOVA was conducted with Prime (neutral, threat) X Congruency (target location congruent to angry face, target location incongruent to angry face) as within subjects factors, and group (HSA, LSA) as a between subjects factor. Results revealed no differences in participants’ accuracy rate between neutral prime trials (M=91.3%, SD=.09 or 8.8%) and threat prime trials (M=91.8%, SD=.07 or 7.3%), F(1,22)<1. Participants were marginally less accurate on incongruent trials (defined as trials where the probe appeared in the location of the neutral face; M=91.0%, SD= .08 or 7.8%) compared to congruent trials (trials where the probe appeared in the location of the angry face; M=92.2%, SD = .08 or 7.8%), F(1,22)=3.52, p=.074. There was no difference between groups in participants’ accuracy rate, F(1,22)<1. There was a trend towards a target location by group interaction, F(1,22)=3.51, p=.074. The high socially-anxious (HSA) group was significantly less accurate on incongruent trials (M=88.3%, SD=.11 or 11.3%) than congruent trials (M= 91.1% , SD=.11 or 11.3%), t(11) =3.32, p<.01; there were no differences in the low socially-anxious (LSA) group’s accuracy rate between incongruent (M= 93.6% , SD=.11 or 10.8%) and congruent trials (M= 93.3%, SD=.11 or 10.8%), t(11)<1. The Prime by Group, Prime by Congruency, and Prime by Group by Congruency interactions were not significant, F(1,22)<1, F(1,22)<1, and F(1,22)=1.02, p=.32, respectively.
3.1.2 Reaction times and attention bias
Incorrect trials were excluded from further analyses, as were trials where the participant responded faster than 200 ms after the target presentation. To examine reaction times to target detection a repeated measures ANOVA was conducted with Prime (neutral, threat) X Congruency (target location congruent to angry face, target location incongruent to angry face) as within subjects factors, and group (HSA, LSA) as a between subjects factor. Results revealed no significant differences between groups in overall reaction time, F(1,22)<1. Also, participants did not differ on reaction time to target detection between neutral and threat prime trials, F(1, 22)<1. There was a significant main effect of Congruency, F(1,22)=6.31, p<.05; participants were faster to respond to targets appearing at the location of the angry face (M=583ms, SD=99ms) than targets appearing at the location opposite the angry face (M=589ms, SD=97ms). The Group X Prime and Group X Congruency interactions were non-significant, Fs(1,22)<1. However, there was a significant three way interaction, F(1,22)=8.09, p<.01. A set of two-way repeated measures ANOVAs were conducted to explore this three-way interaction. These ANOVAs revealed that the HSA group was significantly faster to detect targets congruent to the angry face location (M=574ms, SD=94ms) than targets incongruent to the angry face location (M=580ms, SD=90ms), F(1,11)=6.88, p<.05. Both the HSA and LSA groups showed trends towards a Prime X Congruency interaction, LSA: F(1,11)=4.13, p=.067; HSA: F(1,11)=4.317, p=.062. However, these interactions were in opposite directions. The HSA group showed a significant difference between congruent and incongruent trials for the neutral prime condition, t(11)=−3.20, p=.01, but not for the threat prime condition t(11)<1. By contrast, the LSA group differed in reaction time between congruent and incongruent trials for the threat prime condition, t(11)=− 2.23, p<.05, but not neutral, t(11)<1.
Bias scores were calculated by subtracting the reaction times on trials in which the target appeared in the location of the angry face from trials in which the target appeared in the location opposite to the angry face. The bias scores provide a measure of participants’ spatial attention allocation; positive scores reflect a greater allocation of attention to the location of the angry faces, whereas negative scores reflect greater allocation of attention to the location of the neutral faces. Mean reaction times and bias scores are presented in Table 3.
Table 3.
Reaction Times, Bias Scores, and Accuracy Rates by group and trial type
| neutral congruent trial (SD) | neutral incongruent trial (SD) | threat congruent trial (SD) | threat incongruent trial (SD) | bias score for neutral trials (SD) | bias score for threat trials (SD) | |
|---|---|---|---|---|---|---|
| LSA | 598 ms (115 ms) | 595 ms (106 ms) | 587 ms (101 ms) | 600 ms (106 ms) | 0 ms (18 ms) | 13 ms (20 ms) |
| LSA | 90.6% (10.3%) | 90.9% (9.5%) | 91.3% (7.3%) | 91.0% (8.6%) | ||
| HSA | 567 ms (90 ms) | 586 ms (97 ms) | 580 ms (98 ms) | 574 ms (87 ms) | 19 ms (20 ms) | −1 ms (24 ms) |
| HSA | 93.5% (8.0%) | 90.2% (8.6%) | 93.3% (6.6%) | 91.8% (7.2%) |
Note: Bias scores were calculated by subtracting the reaction times on trials in which the target appeared in the location of the angry face from trials in which the target appeared in the location opposite to the angry face.
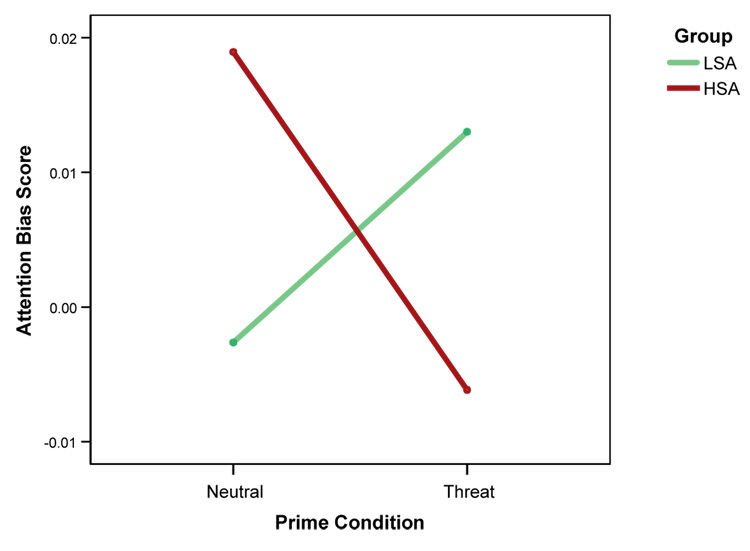
A repeated measures ANOVA comparing participant’s bias scores revealed no main effects for Prime, F(1, 22)<1, or Group F(1,22)<1. There was a significant Group X Prime interaction, F(1, 22)=8.09, p=.01. The nature of this interaction effect (Fig. 2) suggests that the HSA group had a larger attention bias to threat on neutral prime trials than the LSA group. However, in the threat prime trials the LSA group showed a bias to threat faces, while the HSA group did not.
Figure 2.
Mean attention bias scores on neutral prime trials and socially threatening prime trials for the low socially-anxious group (LSA) and the high socially-anxious group (HSA).
To determine the conditions under which participants showed a significant bias towards or away from the angry face one sample t-tests were computed for each group’s bias scores in both prime conditions. The bias scores were significantly different from zero in both the neutral Prime X HSA Group condition, t(11)=3.20, p=.01, and the threat Prime X LSA Group condition, t(11)=2.23, p=.05, indicating that HSA participants show greater attention allocation to the angry face after viewing a neutral prime, while LSA participants show greater attention allocation to the angry face only after viewing a socially threatening prime. The bias scores were not significant in the other conditions, indicating no preferential attention allocation on these trials.
3.2 ERP analyses and results
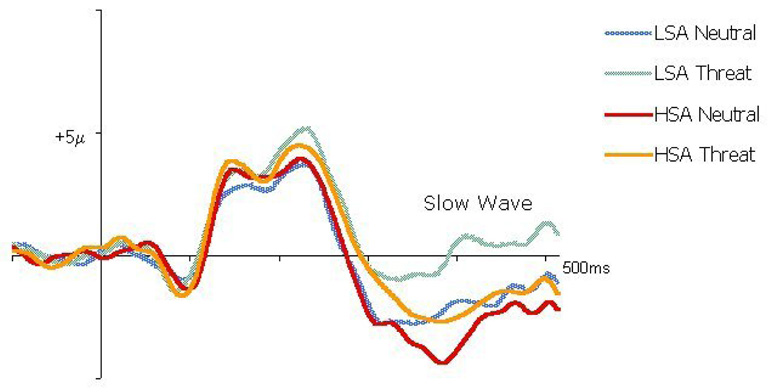
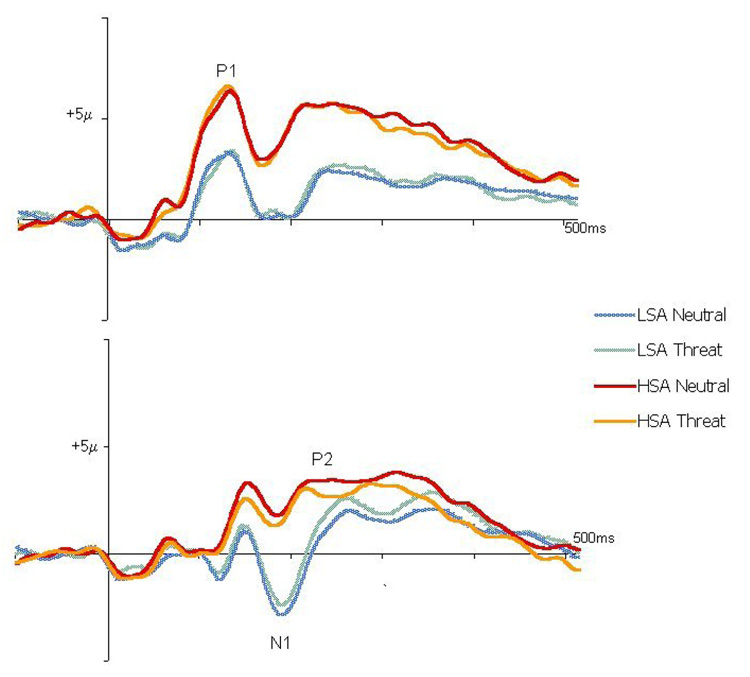
Two participants, one from the HSA group and one from the LSA group, were excluded from EEG analysis: one declined EEG collection, and the other was excluded due to poor signal. Grand-averaged ERPs by Group and Prime condition to prime onset are presented in Fig. 3. Grand-averaged ERPs by Group and Prime condition to faces onset are presented in Fig. 4.
Figure 3.
Grand-averaged ERP waveforms by Prime condition (neutral, threat) and Group (HSA, LSA) elicited at Cz to prime onset.
Figure 4.
The top display shows grand-averaged ERP waveforms to the faces display by Prime condition (neutral, threat) and Group (HSA, LSA) taken as the average of the O1 and O2 electrode sites. The bottom display shows grand-averaged ERP waveforms to faces display onset by Prime condition (neutral, threat) and Group (HSA, LSA) taken as the average of the P3 and P4 electrode sites.
3.2.1 Prime onset
To examine the neural correlates of the impact of the prime words' valence on HSA vs. LSA participants, an ANOVA on mean amplitude of the ERP slow wave (300–500 msec following prime onset) was conducted. Prime (neutral, threat) served as a within subjects factor and Group (HSA, LSA) as a between subjects factor. This ANOVA revealed a significant main effect for both Prime, F(1, 20) = 24.94, p<.001, and Group, F(1, 20) = 7.926, p = .01. Neutral primes (M= −2.01µV, SD = 2.25) produced a greater overall negative slow wave amplitude than threat primes (M=−.61µV, SD=2.01). HSA individuals also produced a greater negative slow wave amplitude, F(1,20) = −2.39µV, SD=2.42, than LSA individuals, F(1,20) = −.23µV, SD=2.54. There was no significant Group X Prime interaction, F(1,20)=1.58, p=.22. These ERP findings to prime onset confirm that our priming manipulation was effective in eliciting differential responses to the threat and neutral words, and that HSA and LSA individuals were showing differential electrophysiological responses to the primes.
3.2.2 Faces Display Onset
3.2.2.1 P1 Mean Amplitude
To examine mean amplitude of P1 to faces onset an ANOVA was conducted using Prime (neutral, threat) as the within subjects factor and Group (HSA, LSA) as the between subjects factor. Results revealed a significant main effect for group, F(1,21)=7.34, p<.01, with HSA individuals showing higher P1 amplitude (M=5.66µV, SD=3.68) than LSA individuals (M=2.59µV, SD=3.68). The Group X Prime interaction was not significant, F(1,21)<1.
3.2.2.2 N1 Mean Amplitude
To examine mean amplitude of N1 to faces onset an ANOVA was conducted using Prime (neutral, threat) and Group (HSA, LSA) as the between groups factor. The LSA group (M= −1.74µV, SD=3.83) had a more negative N1 mean amplitude than the HSA group (M= 2.08µV, SD=3.83), F(1,20)= 10.49, p=.005. There was also an effect at the trend level for Prime, F(1,20)=4.22, p=.053. N1 mean amplitude was more negative for threat primes than for neutral primes (M= −0.09µV, SD= 3.50 and M=.44µV, SD=3.28, respectively). The Group X Prime interaction was not significant, F(1,20)<1.
3.2.2.3 P2 Mean Amplitude
To examine mean amplitude of the P2 component to face onset an ANOVA was conducted using Prime (neutral, threat) as the within subjects factor and Group (HSA, LSA) as the between subjects factor. Mean amplitude was overall greater for neutral primes (M=2.21µV, SD=2.96) than for threat primes (M=1.8µV, SD=2.87), F(1,21)=7.66, p<.01. There was a group effect at the trend level, F(1,21)=3.69, p=.07, with HSA individuals showing a more positive P2 (M=3.137µV, SD=3.86) than LSA individuals (M=.90, SD=3.86). The Group X Prime interaction was not significant, F(1,21)<1.
3.2.2.4 Summary
There were significant main effects for group in both the P1 and the N1 components of the ERP. HSA individuals showed a larger (more positive) P1 and a smaller (more positive) N1 than HSA individuals. The HSA individuals also showed a trend towards a larger (more positive) P2 than LSA individuals. Additionally, regardless of anxiety group, participants showed a trend towards a greater (more negative) N1 and showed a smaller (more negative) P2 in response to the faces display on threat prime trials compared to neutral prime trials.
4. Discussion
By presenting social threat or neutral prime words immediately prior to standard dot-probe trials, we replicated and extended previous research reporting that when anxious individuals are presented with a more general stressor prior to an attention task, this initial stressor modulates their performance (Mathews & Sebastian, 1993; Amir, et al., 1996; Constans, et al., 2004). In our study, when high socially-anxious participants were presented with a neutral word prior to dot-probe trials they showed an attention bias toward threat. However, when these same participants were primed with a social threat word prior to the dot-probe trials, their attention bias disappeared. Thus, it appears that transient activation of the fear system influences attention allocation to threat similarly to conditions of sustained activation of the fear system.
Additionally, we found that transient exposure to a socially threatening word induces an attention bias to threat in low socially-anxious individuals. This finding has not been highlighted in the literature. However, previous research has shown a similar, but nonsignificant, pattern of results in non-anxious individuals (see Amir, et al., 1996; Mathews & Sebastian, 1993). This induction of bias in low-anxious individuals suggests that brief exposure to threat (i.e., priming with a threat word) increases their threat vigilance. This pattern may be viewed as an adaptive mechanism that functions to increase alertness in situations in which threat has already been detected.
ERP measures were collected to better understand group differences in the patterns of attention to threat and to detect differences between high and low socially-anxious participants’ processing during both threat and neutral conditions. In response to the presentation of the word primes, all participants showed greater negative slow wave amplitude after viewing a neutral word compared to a threat word. The ERP differences to the prime type (social threat vs. neutral) serves as a manipulation check confirming that the participants were processing the neutral and threat words differently (Bernat et al., 2001; Kuchinke et al., 2005). There was also an overall group difference in the slow-wave response to the primes suggesting that high and low anxious participants had differential responses to the prime. Interestingly, despite a significant group X prime interaction in the behavioral reaction time data, no such effect emerged in the ERP data time-locked to primes onset. The differential processing of the threat word by the anxious and nonanxious groups may emerge later in processing, and therefore is not picked up during the 500ms immediately following word presentation. Alternatively, the processing differences producing the group by prime behavioral interaction may occur in subcortical regions, such as the amygdala, regions that are not picked up in EEG recordings (Luck, 2005).
High socially-anxious and low socially-anxious individuals showed differential processing of the faces stimuli. On both the P1 and the N1 components, high socially-anxious participants showed more positive amplitudes than low socially-anxious participants, a pattern that continued at the trend level in the P2 component. These findings are consistent with those from Holmes, Nielsen, and Green (2008), in which event related potentials from high- and low-anxious individuals were recorded while participants were viewing centrally presented angry faces. High-anxious individuals showed greater P1 amplitude when viewing an angry face than did low-anxious individuals. These authors also found that when viewing angry faces, low-anxious individuals showed an Early Posterior Negativity (EPN) in lateral parietal sites around 220ms post-stimulus, an effect that was attenuated in high-anxious individuals. In the present study, all trials included angry faces. It is therefore possible that like the findings of Holmes, et al. (2008), high-anxious individuals in the current study showed an enhanced P1 and a reduced N1, a pattern similar to the P1 and EPN findings in the Holmes, et al. study. The enhanced P1 seen in high-anxious individuals may be taken as a sign of increased sensory processing of the faces, most likely due to projections from the amygdala or other motivational centers to the visual cortex (Lang, Bradley, et al., 1998; Pourtois, Grandjean, Sander, and Vuilleumier, 2004).
The lack of a Group X Prime interaction in the ERP findings of the current study may reflect differential sensitivities to task demands in the ERP and the behavioral measures. While response time measures reflect a global index of task performance, encompassing influences of all the different processing aspects related to performance, modulation of specific ERP components typically reflects more refined and specific stages of processing.
Previous research has generated two main hypotheses as to why anxious individuals’ attention bias toward threatening information is suppressed under conditions of high stress. First, threat could disproportionately increase task effort in high-anxious individuals. That is, due to the increased effort needed to perform the task, the activity of the threat detection system may be attenuated (Williams et al., 1996; Mathews & Mackintosh, 1998). Second, a larger or sustained threat may shift processing priorities away from subsequent information; thus the attention to, and detection of, the subsequently presented threatening stimuli in the task is suppressed.
It appears that the suppression of attention bias to threat in the current study was not a result of an increase in task effort by the high socially-anxious participants. Previous research suggests that increased effort on a task is accompanied by a general increase in task performance, reflected in overall faster reaction times and greater accuracy (Mathews & Sebastian, 1993; Amir et al., 1996; Williams et al., 1996; Mathews & Mackintosh, 1998; Constans et al., 2004). However, in the current study high socially-anxious individuals did not show an overall speeding in performance or increased accuracy on threat prime trials relative to neutral prime trials.
The initial threat prior to task performance may still serve as a type of distractor, suppressing attention bias to threat (Mathews & Mackintosh, 1998), even if it does not necessarily increase task demands. A person’s current level of fear or anxiety can temporarily change the threshold of their threat detection system, the system that allocates the resources needed to process threatening stimuli (Williams et al., 1996; Mathews & Mackintosh, 1998; Bar-Haim et al., 2007). In the current study the threat word may have modulated the threat threshold for incoming stimuli, diminishing the attentional resources available for subsequent threat face processing. The ERP data reveal that high socially-anxious participants had overall more positive amplitudes for both the P1 and N1 components to the faces display compared to the low-anxious group. These findings suggest that high-anxious participants processed the faces display differently from low-anxious participants, regardless of prime condition.
In the current study it may be that the social threat word altered the threat detection system in high socially-anxious individuals such that ambiguous or neutral stimuli were interpreted as a source of threat leading to an apparent suppression of bias to threat in the attention resource allocation system. Previous research has shown that anxious individuals have a tendency to interpret ambiguous information as negative (Eysenck, Mogg, May, Richards, & Mathews, 1991; MacLeod & Cohen, 1993; Bar-Haim et al., 2007) and the presentation of the social threat word may increase this propensity. This could explain the lack of change in the ERPs of high socially-anxious individuals between threat prime trials and neutral prime trials. While the electrophysiological response to a stimulus with a threat cue will be different than the response to a stimulus with only safety cues (Holmes, Nielsen, & Green, 2008), there may not be large differences between the response to a stimulus with one threat cue vs. multiple threat cues. Thus, if threat primes are leading high socially-anxious individuals to interpret both the threat face and neutral face as threatening, their bias scores would disappear, but their ERPs to the faces stimulus would remain the same as on neutral prime trials.
Another possibility is that the threat word could create more general processing interference, not associated with the level of perceived threat within subsequent incoming information. In the case of either of the abovementioned pathways (increased effort or modulation of attentional patterns in socially anxious individuals), the stage of processing that is affected by the threat word, whether early automatic or more downstream strategic, remains unclear. Furthermore, the stage of processing that is affected by the presentation of the threat prime may be different for anxious and non-anxious individuals. It has been shown that a Stimulus Onset Asynchrony (SOA) of 300ms between a prime and subsequent stimulus is at the edge of the activation curve (e.g., Hermans, De Houwer, and Eelen, 2001), and affective priming results are most consistently found with SOA between 0 and 100ms (Klauer, 1998) or 150ms (Hermans et al., 2001). This means that the rather long Stimulus Onset Asynchrony between word and face onset (500ms) in the present study may have allowed participants to reflect on the meaning of the word, recruiting late-stage processes leading to the suppression of attention bias in high socially anxious participants and the induction of bias in low-socially anxious participants.
Recent research by Vermeulen, Corneille, and Luminet (2007) suggests that an individual’s mood state may influence their strategy for processing affective information. The current study focused on trait anxiety. However, since attention bias has also been found with state anxiety (see Bar-Haim et al, 2007), future studies could attempt to parse out the separate contributions of state and trait anxiety to the effect of transient priming on attention allocation patterns.
In conclusion, the current study is the first to show suppression of attention bias toward threat in socially anxious individuals using priming preceding typical dot-probe trials. The current study is also the first to show that threat priming induces an attention bias in non-anxious individuals (but see Eldar, Ricon, & Bar-Haim, in press; and MacLeod, Rutherford, Campbell, Ebsworthy, & Holker, 2002). It appears that presenting a threat word immediately prior to a dot-probe trial creates the same contextual conditions seen in stress-manipulation studies that induce a more general stress prior to task performance. These behavioral findings along with the ERP data suggest that several levels of processing may be involved in the reported group and condition differences. Specifically, the current findings suggest that the suppression of attention bias to threat in anxious individuals is more likely a result of a shift in processing priorities rather than an increase in task effort on the part of anxious participants.
5. Acknowledgements
This research was supported in part by a Grant from the National Institute of Health (HD17899) and by a NARSAD Distinguished Investigator Award awarded to N. A. Fox, and a National Science Foundation Graduate Research Fellowship awarded to S. M.Helfinstein.
Footnotes
The behavioral interaction reported below was not present when participants were divided according to preliminary screening scores alone.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- Amir N, Mcnally R, Riemann B, Burns J. Suppression of the emotional Stroop effect by increased anxiety in patients with social phobia. Behaviour Research and Therapy. 1996;34:945–948. doi: 10.1016/s0005-7967(96)00054-x. [DOI] [PubMed] [Google Scholar]
- Awh E, Jonides J. Spatial working memory and spatial selective attention. Cambridge, MA, US: The MIT Press; 1998. [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg M, Van Ijzendoorn M. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Glickman S. Attentional bias in anxiety: A behavioral and ERP study. Brain and Cognition. 2005;59:11–22. doi: 10.1016/j.bandc.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Bernat E, Bunce S, Shevrin H. Event-related brain potentials differentiate positive and negative mood adjectives during both supraliminal and subliminal visual processing. International Journal of Psychophysiology. 2001;42:11–34. doi: 10.1016/s0167-8760(01)00133-7. [DOI] [PubMed] [Google Scholar]
- Bradley M, Lang P. Fearfulness and affective evalutaions of pictures. Motivation and Emotion. 1999;23:1–13. [Google Scholar]
- Cheek J. The revised Cheek and Buss shyness scale. Wellesley, MA: 1983. [Retrieved February 13, 2008]. www.wellesley.edu/Psychology/Cheek/research.html. [Google Scholar]
- Constans J, Mccloskey M, Vasterling J, Brailey K, Mathews A. Suppression of attentional bias in PTSD. Journal of Abnormal Psychology. 2004;113:315–323. doi: 10.1037/0021-843X.113.2.315. [DOI] [PubMed] [Google Scholar]
- Eldar S, Ricon T, Bar-Haim Y. Plasticity in attention: Implications for stress response in children. Behavior Research and Therapy. doi: 10.1016/j.brat.2008.01.012. (in press) [DOI] [PubMed] [Google Scholar]
- Eysenck M, Mogg K, May J, Richards A, Mathews A. Bias in interpretation of ambiguous sentences related to threat in anxiety. Journal of Abnormal Psychology. 1991;100:144–150. doi: 10.1037//0021-843x.100.2.144. [DOI] [PubMed] [Google Scholar]
- Fenigstein A. Self-awareness, self-consciousness and rejection. The University of Texas at Austin; 1975. [Google Scholar]
- Fenigstein A, Scheier M, Buss A. Public and private self-consciousness: Assessment and theory. Journal of Consulting and Clinical Psychology. 1975;43:522–527. [Google Scholar]
- Garner M, Mogg K, Bradley B. Orienting and maintenance of gaze to facial expressions in social anxiety. Journal of Abnormal Psychology. 2006;115:760–770. doi: 10.1037/0021-843X.115.4.760. [DOI] [PubMed] [Google Scholar]
- Heinrichs N, Hofmann S, Barlow D. Non-specific encoding of threat in social phobia and panic disorder. Cognitive Behaviour Therapy. 2004;33:126–136. doi: 10.1080/16506070410021692. [DOI] [PubMed] [Google Scholar]
- Hermans D, De Houwer J, Eelen P. A time course analysis of the affective priming effect. Cognition & Emotion. 2001;15:143–165. [Google Scholar]
- Hillyard S, Vogel E, Luck S. Sensory gain control (amplification) as a mechanism of selective attention: Electrophysiological and neuroimaging evidence. Philosophical Transactions of the Royal Society: Biological Sciences. 1998;353:1257–1270. doi: 10.1098/rstb.1998.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Nielsen M, Green S. Effects of anxiety on the processing of fearful and happy faces: An event-related potential study. Biological Psychology. 2008;77:159–173. doi: 10.1016/j.biopsycho.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Klauer K. Affective priming. European Review of Social Psychology. 1998;8:67–103. [Google Scholar]
- Kolassa I, Musial F, Mohr A, Trippe R, Miltner W. Electrophysiological correlates of threat processing in spider phobics. Psychophysiology. 2005;42:520–530. doi: 10.1111/j.1469-8986.2005.00315.x. [DOI] [PubMed] [Google Scholar]
- Kuchinke L, Jacobs A, Grubich C, Vo M, Conrad M, Herrmann M. Incidental effects of emotional valence in single word processing: An fMRI study. NeuroImage. 2005;28:1022–1032. doi: 10.1016/j.neuroimage.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Lang P, Bradley M, et al. Emotional arousal and activation of the visual cortex: An fMRI analysis. Psychophysiology. 1998;35:199–210. [PubMed] [Google Scholar]
- Larson C, Schaefer H, Siegle G, Jackson C, Anderle M, Davidson R. Fear is fast in phobic individuals: Amygdala activation in response to fear-relevant stimuli. Biological Psychiatry. 2006;60:410–417. doi: 10.1016/j.biopsych.2006.03.079. [DOI] [PubMed] [Google Scholar]
- La Greca A, Lopez N. Social anxiety among adolescents: Linkages with peer relations and friendships. Journal of Abnormal Child Psychology. 1998;26:83–94. doi: 10.1023/a:1022684520514. [DOI] [PubMed] [Google Scholar]
- Luck S. An introduction to the event-related potential technique. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Macarthur Research Network on Early Experience and Brain Development. NimStim face stimulus set. 2002 [Google Scholar]
- Macleod C, Cohen I. Anxiety and the interpretation of ambiguity: Text comprehension study. Journal of Abnormal Psychology. 1993;102:238–247. doi: 10.1037//0021-843x.102.2.238. [DOI] [PubMed] [Google Scholar]
- Macleod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L. Selective attention and emotional vulnerability: Assessing the causal basis of their association through the experimental manipulation of attentional bias. Journal of Abnormal Psychology. 2002;111:107. [PubMed] [Google Scholar]
- Mansell W, Clark D, Ehlers A, Chen Y. Social anxiety and attention away from emotional faces. Cognition & Emotion. 1999;13:673–690. [Google Scholar]
- Mathews A, Mackintosh B. A cognitive model of selective processing in anxiety. Cognitive Therapy and Research. 1998;22:539–560. [Google Scholar]
- Mathews A, Mackintosh B, Fulcher E. Cognitive biases in anxiety and attention to threat. Trends in Cognitive Sciences. 1997;1:340–345. doi: 10.1016/S1364-6613(97)01092-9. [DOI] [PubMed] [Google Scholar]
- Mathews A, Macleod C. Selective processing of threat cues in anxiety states. Behaviour Research and Therapy. 1985;23:563–569. doi: 10.1016/0005-7967(85)90104-4. [DOI] [PubMed] [Google Scholar]
- Mathews A, Sebastian S. Suppression of emotional Stroop effects by fear-arousal. Cognition & Emotion. 1993;7:517–530. [Google Scholar]
- Merwin R, Wilson K. Preliminary findings on the effects of self-referring and evaluative stimuli on stimulus equivalence class formation. Psychological Record. 2005;55:561–575. [Google Scholar]
- Mogg K, Kentish J, Bradley B. Effects of anxiety and awareness on colour-identification latencies for emotinoal words. Behaviour Research and Therapy. 1993;31:559–567. doi: 10.1016/0005-7967(93)90107-6. [DOI] [PubMed] [Google Scholar]
- Mogg K, Mathews A, Eysenck M. Attentional bias to threat in clinical anxiety states. Cognition & Emotion. 1992;6:149–159. [Google Scholar]
- Munafo M, Hayward G, Harmer C. Selective processing of social threat cues following acute tryptophan depletion. Journal of Psychopharmacology. 2006;20:33–39. doi: 10.1177/0269881105056667. [DOI] [PubMed] [Google Scholar]
- Öhman A. The role of the amygdala in human fear: Automatic detection of threat. Psychoneuroendocrinology. 2005;30:953–958. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Grandjean D, Sander D, Vuilleumier P. Electrophysiological correlates of rapid spatial orienting towards fearful faces. Cerebral Cortex. 2004;14:619–633. doi: 10.1093/cercor/bhh023. [DOI] [PubMed] [Google Scholar]
- Rossignol M, Anselme C, Vermeulen N, Philippot P, Campanella S. Categorical perception of anger and disgust facial expression is affected by non-clinical social anxiety: An ERP study. Brain Research. 2007;1132:166–176. doi: 10.1016/j.brainres.2006.11.036. [DOI] [PubMed] [Google Scholar]
- Rothbart M, Ahadi S, Evans D. Temperament and personality: Origins and outcomes. Journal of Personality and Social Psychology. 2000;78:122–135. doi: 10.1037//0022-3514.78.1.122. [DOI] [PubMed] [Google Scholar]
- Vermeulen N, Corneille O, Luminet O. A mood moderation of the extrinsic affective Simon task. The European Journal of Personality. 2007;21:359–369. [Google Scholar]
- Wenzel A, Jackson L, Holt C. Social phobia and the recall of autobiographical memories. Depression and Anxiety. 2002;15:186–189. doi: 10.1002/da.10053. [DOI] [PubMed] [Google Scholar]
- Williams J, Mathews A, Macleod C. The emotional Stroop task and psychopathology. Psychological Bulletin. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]