Summary
Type II deiodinase (D2) plays a critical role in controlling intracellular T3 concentration and early studies indicated a follicular but not a parafollicular C-cell origin of D2 activity in the thyroid gland. Here, we show that D2 is highly expressed in human medullary thyroid carcinoma (MTC), a tumour that arises from the C-cells. D2 transcripts were detected in all MTC samples obtained from 12 unselected MTC patients and the levels of D2 activity were comparable to those found in surrounding normal follicular tissue (0.41±0.10 vs 0.43±0.41 fmol.min.mg.protein, P=0.91). Additional analysis in the TT cells, a human MTC cell line, demonstrated that the D2 expression is down regulated by thyroid hormones and enhanced by cAMP analogs and dexamethasone. The thyroid hormone receptor α1 and β isoforms were also detected in all MTC samples and in TT cells, thus suggesting a potential role of T3 locally produced by D2 in this neoplastic tissue.
Keywords: type 2 deiodinase, medullary thyroid carcinoma, gene expression
Introduction
Medullary thyroid carcinoma (MTC) is an uncommon thyroid tumor that arises from the parafollicular C-cells of the thyroid, embryologically unrelated to the follicular epithelium. In addition to calcitonin gene products, MTC cells express several biochemical markers that typify secretory cells of the diffuse neuroendocrine system (Leboulleux et al., 2004). This tumor may be sporadic or may occur on a hereditary basis caused by germline mutations in the RET protooncogene (Ponder, 1999). The RET proto-oncogene is expressed in cells of neuronal and neuroepithelial origin and encodes a receptor tyrosine kinase (Mulligan et al., 1993). In patients with familial MTC (FMTC) only the thyroid is affected. Patients with multiple endocrine neoplasia (MEN) 2A develop MTC, pheochromocytoma (pheo) and/or primary hyperparathyroidism. In addition, MEN2B patients have MTC, pheo, ganglioneuromas of the digestive tract, mucosal neuromas, and/or skeletal abnormalities (Ponder, 1999).
The type 1 and type 2 iodothyronine deiodinases (D1 and D2) enzymes are responsible for catalyzing deiodination of T4 to T3. D2 plays a critical role in providing local T3 to regulate intracellular T3 concentration and recent studies suggest that this enzyme also contributes to a significant fraction of plasma T3 in rodents and humans (Maia et al., 2005; Streckfuss et al., 2005). D2 is expressed in normal and stimulated human thyroid gland and has been evaluated as a possible marker of thyroid follicular cell differentiation (Arnaldi et al., 2005; De Souza Meyer et al., 2005; Kim et al., 2003; Murakami et al., 2001a; Salvatore et al., 1996; Takano et al., 2006). Previous studies have described underexpression of D2 in papillary thyroid carcinoma (PTC) (Arnaldi et al., 2005; Murakami et al., 2001a;). In contrast, D2 are reported to be significant increased in follicular carcinoma (De Souza Meyer et al., 2005; Kim et al., 2003; Takano et al., 2006). Little is known about the metabolism of iodothyronine in MTC. Early studies have suggested that only differentiated thyroid neoplasias contain 5’-deiodinase activity, whereas medullary carcinomas do not, suggesting a follicular but not a C-cell origin of enzyme activity (Boye and Laurberg, 1984; Ishii et al., 1981). Here, we demonstrated that the D2 enzyme is also highly expressed in human MTC samples.
Materials and Methods
Patients and tissues
Samples of MCT were collected from 12 consecutive unselected patients attending the Endocrine or the Head and Neck Surgery Divisions at Hospital de Clínicas de Porto Alegre. Surgery was independently indicated by attending physicians based on clinical indications. Tumors were histologically classified according to WHO recommendations (Hedinger et al., 1988). Immunohistochemical staining for calcitonin was positive in all CMT samples. Identification of RET germline mutations was performed by standard procedures (Puñales et al., 2003). Clinical data were retrospectively reviewed in medical records. The clinical stage was determined by the Tumor/Node/Metastases (TMN) system (Greene and Sobin, 2002). Serum TSH was measured by a double antibody—sensitive assay (Immulite, Diagnostic Products Corporation, EUA). For determination of preoperative serum calcitonin levels, a sensitive immunoradiometric assay was performed (Calcitonin IRMA-DSL 7700, Diagnostic Systems Laboratories, Inc., Webster, TX; reference range < 12 pg/ml).
Thyroid tissue was obtained from both tumor and adjacent normal tissue at the time of surgery. All tissues obtained at surgery were immediately frozen in liquid nitrogen and stored at−70° C until analysis.
The information obtained from this study did not influence or affect the patients’ diagnosis or treatment. The Ethics Committee at the Hospital approved the study protocol and all patients gave their informed consent.
Cell culture
The TT cells, a human MTC cell line (American Type Culture Collection, number CRL-1803), were cultured in F-12K medium supplemented with 10% fetal bovine serum (Invitrogen Life Technologies Inc., NY, USA). Cells were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air, and the culture medium was changed twice a week. TT cells were seeded in six-well or in 60-mm plastic culture plates for total RNA extraction and measurement of deiodinase activity, respectively. To test the effects of thyroid hormones, the cells were cultured for 18-24 hours in serum-free 0.1% BSA in F-12K medium before the experiments. T3 (10-7M), T4 (10-7M), rT3 (10-7M), dibutyril cAMP ((Bu)2cAMP, 10-3 M ), Foskolin (10-5M) or dexamethasone (DEX, 10-6M) were added to the medium at the times indicated. All reagents were obtained from Sigma-Aldrich (St. Louis, MO). TT cells were incubated with medium + vehicle (2% DMSO, control), or medium containing the specified compound for ∼16 h. Each experiment was performed with duplicate dishes for each condition. At the appropriate times, cells were harvested and processed for total RNA extraction or measurement of D2 activity.
RNA isolation and reverse transcription
Total RNA was isolated from 50 - 100 mg of thyroid tumor and surrounding nontumor tissues using TRIzol® reagent (Invitrogen Life Technologies Inc., NY, USA) according to the manufacturer’s instructions. To prepare total RNA from TT cells, medium was removed and cells washed twice with cold PBS (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4.7H20, 1.4 mM KH PO4). Total RNA was isolated with the use of the Rneasy Mini Kit (Qiagen GmBH, Hilden, Germany) according to the manufacturer’s protocol. The purity and integrity of the total RNA of all samples was assessed by UV spectrophotometry (GeneQuant II®, Amersham Pharmacia Biotech, Uppsala, Sweden). RNA was reverse transcribed using the Superscript Preamplification System for First Strand cDNA Synthesis (Invitrogen Life Technologies Inc., NY, USA) with 3 μg of total RNA as template.
Reverse transcription polymerase chain reaction (RT-PCR)
A RT-PCR technique was used to determine the expression levels of D2, TSH receptor (TSH-R), thyroid hormone receptor TRα1 and TRβ genes in RNA samples isolated from thyroid tissues. Primer sequences are given in Table 1. PCR reactions included 1 μl of RT products, and were carried out with Taq DNA polymerase (InvitrogenTM Life Technologies Inc., NY, USA) in a final 50 -μl volume. The amplification profile was an initial denaturation step at 94°C for 3 min, followed by 94°C for 1 min, annealing at 60°C (D2 and TSH-R) or 55°C (TRα1 and TRβ), and extension at 72°C for 2 min. Thirty amplification cycles were used for all genes, with a final additional extension step at 72°C for 4 min. The β2-microglobulin primers were used as an internal control. RT-PCR reactions without cDNA samples were carried out as negative control. After amplification, 10 μl of the PCR products were analyzed on a 1.5 % ethidium bromide agarose gel and the intensity of each band was determined by optic densitometry (arbitrary units - AU) (ImageMaster® VDS, Amersham Pharmacia Biotech, Uppsala, Sweden).
Table 1.
Oligonucleotide sequences used for RT-PCR and qPCR reactions
| Gene | Primers | PCR product size (bp) |
|---|---|---|
| DIO 2 | F: 5’-ACTCGGTCATTCTGCTCAAG-3’ R: 5’-GAGAACTCTTCCACCAGTTTTG-3’ |
368 |
| TSH-R | F: 5’-GTCCAGAATGTATAGCGGCTC-3’ R: 5’-GCTTTTCAGGGACTATGCAATGAA-3’ |
239 |
| TRα1 | F: 5’-TCGAGCACTACGTCAACCAC-3’ R: 5’-TCGACTTTCATGTGGAGGAA-3’ |
127 |
| TRβ | F: 5’-ACCAGAGTGGTGGATTTTGC R: 5’-AAGGGACATGATCTCCATGC-3’ |
105 |
| β2-microglobulin | F: 5’-ATCCAGCGTACTCCAAAGATTCAG-3’ R: 5’- AAATTGAAAGTTAACTTATGCACGC- 3’ |
623 |
| DIO 2 * | F: 5’-ACTTCCTGCTGGTCTACATTGATG-3’ R: 5’-CTTCCTGGTTCTGGTGCTTCTTC-3’ |
58 |
| Cyclophilin * | F: 5’-GCCGATGACGAGCCCTTG-3 R: 5’TGCCGCCAGTGCCATTATG-3’ |
156 |
primers used in quantitative polymerase chain reaction
Quantitative PCR (qPCR)
Reactions for the quantification of D2 mRNA in TT cells experiments were performed in an ABI Prism 7500 Sequence Detection System (Applied Biosystems, Warrington, UK) using the SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK) and cyclophilin as a housekeeping internal control. Primer sequences are given in Table 1. Samples were run in duplicate. The cycle conditions were 94°C × 5 min (Hot Start), 40 cycles of 94°C × 30 sec; 58°C × 30 sec; 72°C × 45 sec and a final 1 min extension period. Initially, standard curves representing 5-point serial dilution of mixed cDNAs of the control and experimental groups were analyzed and used as calibrators to determine the relative quantification of product generated in the exponential phase of the amplification curve. Comparable efficiency was observed presenting r2 greater than 0.99. Sample quantification was calculated by the standard curve and corrected by the internal control cyclophilin in all experiments.
Iodothyronine deiodinase activity assays
Deiodinase assays were performed as described previously (Wagner et al., 2003). In brief, tissue samples were individually homogenized on ice in PE buffer (0.1 M potassium phosphate and 1 mM EDTA) containing 10 mM dithiothreitol (DTT) and 0.25 M sucrose (pH 6.9). Protein concentration was quantitated by Bradford assay using BSA as a standard. D2 assays were performed using 100-300 μg tissue protein and 1nM unlabeled T4 in a total volume of 300 μl PE buffer containing 20 mM DTT, 1mM propylthiouracil (PTU), and approximately 100.000 cpm [125I] T4 (Amersham Biosciences, Piscataway, NJ, USA). The apparent Km and Vmax for D2 enzymes were determined using various amounts of unlabelled T4 (0.25, 0.5, 1, 2 and 6 nM). Incubations were carried out at 37°C for 60-120 min. The reaction was terminated by adding 200 μl of horse serum and 100 μl of 50% trichloroacetic acid (TCA).
For measurements of activity in cell sonicates, the medium was removed and the cells washed twice with PBS, harvested, and sonicated in 0.25 M sucrose in PE buffer (0.1 M potassium phosphate and 1 mM EDTA) with 10 mM DTT. In deiodinase assays, we used 150-250 μg cell sonicate; 1nM unlabeled T4, and 20 mM DTT in a final volume of 300 μl PE. Incubation was for 60-120 minutes at 37°C, and 125I– was separated from labeled T4 by TCA precipitation. Results are the mean of values derived from at least two separate experiments.
Statistical analysis
Results are presented as mean ± SD. Statistical differences were evaluated by t test. P values ≤0.05 were considered statistically significant.
Results
Patients
Table 2 shows the clinical and laboratorial characteristics of the 12 patients studied. Thyroid samples covered patients aged 15 – 65 years. Nine patients had hereditary MTC with RET germline mutations in codon 634 with bilateral disease and C-cell hyperplasia on pathological examination. Three patients had a sporadic form of MTC. All patients present serum TSH levels within the reference range (0.4 - 4.0 UI/L).
Table 2.
Clinical characteristics of the patients with medullary thyroid carcinoma.
|
Patient N° |
Sex / age (yr.) |
RET mutations |
Preoperative basal calcitonin (pg/mL) |
Tumor histology |
Size (cm) |
Stagea | Invaded organ |
|---|---|---|---|---|---|---|---|
| 1 | F/54 | - | 93 | Unilateral | 2.2 | 2 | |
| 2 | F/50 | - | 50 | Unilateral | 5.0 | 2 | |
| 3 | F/60 | - | 431 | Unilateral | 2.4 | 2 | |
| 4 | F/65 | C634Y | 1100 | Bilateral; C-cell hyperplasia |
2.4 | 2 | |
| 5 | M/15 | C634Y | 411 | Bilateral; C-cell hyperplasia |
1.2 | 2 | |
| 6 | F/35 | C634Y | 43 | Bilateral; C-cell hyperplasia |
2.5 | 2 | |
| 7 | F/37 | C634Y | 125 | Bilateral; C-cell hyperplasia |
3.0 | 3 | Cervical lymph nodes |
| 8 | F/30 | C634Y | 882 | Bilateral; C-cell hyperplasia |
1.2 | 3 | Cervical lymph nodes |
| 9 | F/62 | C634Y | 2258 | Bilateral; C-cell hyperplasia |
3.0 | 3 | Cervical lymph nodes |
| 10 | F/35 | C634R | 1000 | Bilateral; C-cell hyperplasia |
1.3 | 2 | |
| 11 | F/33 | C634Y | 3309 | Bilateral; C-cell hyperplasia |
1.3 | 2 | |
| 12 | F/22 | C634R | 2200 | Bilateral; C-cell hyperplasia |
1.3 | 2 |
Stage according to the TNM system.
D2 expression in human medullary thyroid carcinoma
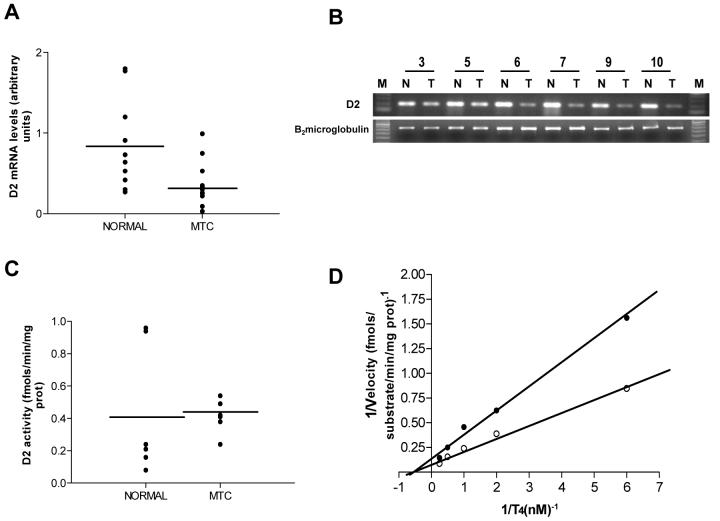
D2 mRNA was detected in all 12 MTC samples, though in lower levels than normal surrounding follicular tissues (0.37±0.28 vs 0.83±0.54 AU, P=0.03) (Fig.1A, B). Because of the limited amount of tissue available, D2 activities were measured in 7 MTC samples (n° 3, 4, 6 -10 of table 2). Enzyme activities in MTC tissues were comparable to those corresponding to normal thyroid tissues (0.41±0.10 vs 0.43±0.41 fmol.min.mg.protein, P=0.913) (Fig.1C). Further studies performed to determine the D2 kinectics in the neoplastic tissue demonstrated that the apparent Km (T4) value of 2.3 nM in the tumor homogenates is typical for this enzyme (Fig.1D). The Vmax for D2 in tumor was comparable to that of normal thyroid tissue (0.86 vs. 1.59 fmol.min.mg.protein). Interestingly, in a sample of a sporadic CMT (n° 3 of table 2), D2 activity was significantly higher than in surrounding normal follicular tissue (6.34 vs. 0.09 fmol.min.mg.protein, respectively).
Fig. 1.
D2 expression in human medullary thyroid carcinoma (MTC). A. D2 mRNA levels in MTC and corresponding normal tissue. Individual data points for each sample are depicted. Results are expressed in arbitrary units. B. An illustrative RT-PCR of D2 products in tumor (T) and surrounding normal tissue (N) from cases 3, 5-7, 9 and 10 (Table 2; 1.5% agarose gel stained by ethidium bromide). C. D2 enzyme activity in MTC and corresponding normal tissue. Individual data points for each sample are depicted. D2 activity was measured as the release of I- from 1nM T4 as substrate. D. Double-reciprocal plot of D2 activity in normal thyroid tissue (solid circles) and MTC (open circles). D2 activity was measured using different concentrations of 125I-T4 as described in Materials and Methods.
D2 expression in TT cell line
To assess whether the D2 expression found in MTC was truly expressed in this neoplastic tissue, we performed enzyme activities in TT cells, a human MTC cell line (Carlomagno et al, 1995). The results obtained confirmed those observed in MTC samples (Fig. 2A). The Km (T4) for D2 in the TT cell sonicates was 2.59 nM and D2 Vmax was 1.22 fmol.min.mg.protein.
Fig. 2.
D2 expression in TT cells. A. Double-reciprocal plot of D2 activity in TT cells. D2 activity was measured using different concentrations of T4 as described in Materials and Methods. B. Effects of thyroid hormones on D2 activity in TT cells. TT cells were cultured with thyroid-hormone-depleted medium for 24 h, and then incubated with thyroid hormones for 16 h. C Effects of (Bu)2cAMP and forskolin on D2 activity in TT cells. D. Effects of DEX (10-6M) on D2 mRNA and D2 activity in TT cells. For C and D, TT cells were incubated with medium containing vehicle (2% DMSO, control), (Bu)2cAMP (10-3 M), forskolin (10-5 M), or DEX (10-6M) for 16 h. *, P < 0.05; **, P < 0.001.
Effects of thyroid hormones on D2 expression in TT cells
To study the effects of thyroid hormones on D2 expression in TT cells, T3, T4 or rT3 was added to the culture medium for 16h before harvesting the cells. The addition of T3 (10-7M) significantly decreases D2 mRNA levels (1.55± 0.38 vs. 0.89±0.32 AU, P=0.010). Addition of T4 (10-7M) or rT3 (10-7M) to the incubation medium decreased significantly the deiodinating activity in TT cells (0.12±0.02 vs. 0.03±0.03 vs. 0.06±0.02 fmol.min.mg.protein, P<0.001 and P=0.01 respectively). However, T3 (10-7M) treatment has no effect on D2 activity (0.12±0.02 vs. 0.12±0.02 fmol.min.mg.protein, P= 0.26; Fig.2B).
Effects of cAMP and DEX on D2 expression in TT cells
Since previous studies have shown that D2 expression is regulated by a cAMP-dependent mechanism at pretranscriptional level (Hosoi, et al., 1999), we also investigated the effects of [(Bu)2cAMP] and forskolin on D2 expression in TT cells. By 16h of incubation, (Bu)2cAMP (10-3 M) nearly doubled D2 mRNA levels (1.08±0.09vs1.78±0.17 AU, P<0.001). In addition, (Bu)2cAMP and forskolin(10-5M) significantly stimulated deiodinating activity in TT cells (0.06±0.03 vs. 0.13 ±0.03 vs. 0.12±0.03 fmol.min.mg.protein, P=0.002 and P=0.03, respectively; Fig.2C).
In the next experiment, the effects of DEX on D2 expression were tested. We observed that the addition of DEX (10-6M) stimulated both D2 mRNA (1.05±0.09 vs.2.18±1.34 AU, P=0.055) and D2 activity (0.12±0.02 vs. 0.21±0.02 fmol.min.mg.protein, P=0.001) (Fig.2D).
Thyroid hormone receptor isoform transcripts in MTC samples and TT cell line
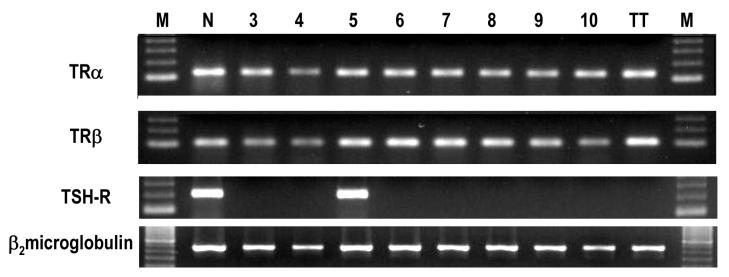
We evaluated TR α1 and β isoforms in MTC samples and TT cells by RTPCR. Because the limited amount of total RNA obtained from some samples, the TR isoforms were analyzed in 8 of the 12 MTC samples. We found transcripts of the TR isoforms in all MTC samples analyzed (cases 3-10 of table 2) and also in the TT cells (Fig. 3). We also evaluated the TSH-R expression in 7 MTC samples (cases 4-10 of table 2). TSH-R transcripts were found only in one sample (case 5 of table 2).
Fig. 3.
TRα, TRβ, and TSH-R mRNA expression in MTC. RT-PCR analysis of PCR products from 8 samples of MTC (patients 3 – 10 of Table 2). M, Marker; N, Normal thyroid tissue (positive control); TT, TT cells.
Discussion
In the present study, we demonstrated that D2 enzyme is expressed in MTC tissues at levels comparable with normal human follicular thyroid cells. The biochemical and molecular properties of D2 enzyme in TT cells, a human MTC cell line, seem to be preserved despite C cell dedifferentiation. Furthermore, TRs were demonstrated in all MTC samples and in TT cells, suggesting a potential role of T3 locally produced by D2 in this neoplastic tissue.
The MTC arises from the C cells of the thyroid gland and represents 5% to 8% of all thyroid cancers. Unlike the endoderm-derived thyroid follicular epithelial cells, the C cells originate in embryonic neural crest and account for only 0.1% of thyroid cells. Several biochemical features including the production of calcitonin and other neuroendocrine markers such as chromogranin A, neuron-specific enolase, and the neural cell adhesion molecule (NCAM) characterize these cells (Leboulleux et al., 2004). Expression of thyroid-specific genes involved in the complex process of thyroid hormone synthesis, such as NIS, thyroglobulin, and TSH-R genes has been demonstrated in MTC samples (Dohán et al., 2001; Elisei et al., 1994; Pacini et al., 1991). However, little is known about the metabolism of iodothyronine in C- cells or MTC.
Previous data have indicated that microsomes prepared from thyroidal C-cell neoplasms did not contain 5’-deiodinases, suggesting that the source of thyroid deiodinases in the thyroid tissue was exclusive of the follicular cells (Boye and Laurberg, 1984). Here, we show that functional D2, an enzyme that catalyzes T4 activation, is expressed in both sporadic and hereditary MTC samples. D2 mRNA and activities were detected in all MTC samples analyzed. Interestingly, D2 mRNA levels in MTC samples were lower than those corresponding to the follicular thyroid cells whereas D2 activity levels were comparable to those found in surrounding normal tissue. These apparently discrepant results can be explained by the complex process of the D2 regulation, which involves both transcriptional and post-transcriptional mechanisms. The potential explanation for the unexpected presence of D2 mRNA and activity in MTC samples would be the presence of RNA and protein from follicular cells, contaminating the medullary tumor. However, the results obtained in MTC samples were further confirmed in the TT cells, the best-known stabilized cell line derived from the human MTC. This cell line was established from a specimen obtained by needle biopsy from a 77 year old female with MTC that harbors a codon 634 mutation (Cys→Trp) in RET proto-oncogene which constitutively activates RET tyrosine kinase (Carlomagno et al., 1995).
The physiological importance of intracellular thyroid hormone activation by D2 has been clearly demonstrated in certain tissues. Adenohypophyseal T3 production by D2 plays an important role in feedback regulation of TSH secretion by thyroid hormones and also regulates pituitary cell growth and differentiation (Barrera-Hernandez et al., 1999; Bianco et al; 2002; Schneider et al., 2001; Stahl et al., 1999). Interestingly, a variable expression of D2 has been described in a number of subtypes of pituitary adenomas, implying that this enzyme is still active in tumor tissues (Baur et al., 2002; Tannahill et al., 2002). It is well established that thyroid hormones play an important role at multiple steps in the development of glial cells that, like C-cells, is of neuroectodermic origin (Rodríguez-Peña et al., 1999) and high levels of D2 activity was noted in oligodendrogliomas, a tumor derived from the glial cells (Mori et al., 1993; Murakami et al., 2000). Although D2 expression has not been accessed in normal C cells, the presence of this enzyme in the TT cell line, considered a reliable model system for studies of human parafollicular cells, might also suggest a possible role of thyroid hormones in human C-cell metabolism (Zabel et al., 1995; Zabel and Grzeszkowiak, 1997). In agreement with this hypothesis, transcripts of both TR α1 and β isoforms were observed in all MTC samples analyzed and in TT cells. Further studies to evaluate the possible role of T3 locally produced by D2 in the regulation of C cell-specific gene expression are warranty.
Abnormal D2 expression and function have been described previously in other human neoplasms (Curcio et al., 2001; Dentice et al., 2007; Meyer et al., 2007; Morimura et al., 2005). Indeed, it is conceivable that thyroid status is linked to the development and growth of neoplastic tissues (Lemaire et al., 1981; Leuthauser et al., 1987). By activating T3, a differentiating agent, as well as blocking its production, changes in deiodination can influence the balance between cell proliferation and differentiation in tissue microenvironment. Interestingly, it was reported that D2 is down regulated and D3 is up regulated in human basal cell carcinomas, supporting a potential therapeutic application for T3 in this malignant neoplasia (Dentice et al., 2007). On the other hand, high D2 activity has been demonstrated in the human mesothelioma cell line MSTO-211H (Curcio et al., 2001). This is an example of high levels of D2 in a human tumor cells derived from a tissue that normally does not express this enzyme.
D2 regulation in neoplastic cells is also of interest. Although several factors such as hormones, growth factors, adrenergic agents, and environmental and nutritional conditions influence iodothyronine deiodinase activities, these enzymes are mainly regulated by thyroid hormones. D1 activity is regulated by thyroid hormones almost exclusively at the transcriptional level (Maia et al., 1995). In contrast, the control of the D2 expression is more complex, occurring by transcriptional, posttranscriptional, and posttranslational mechanisms. At transcriptional level, D2 is downregulated by its end product T3, whereas its substrate, T4, controls enzyme activity at posttranslational level. Of note, D2 activity seems to be regulated mainly at posttranslational level in a tissue-specific manner (Wagner et al., 2007). In the TT cells, the D2 expression was inhibited by thyroid hormones. T4 and rT3 suppress D2 activity mainly at the posttranslational level through acceleration of the degradation rate D2 protein (Steinsapir et al., 1998). However, T3 was more potent than T4 to inhibit the synthesis of D2 mRNA, indicating that pretranscriptional mechanisms are also involved in the regulation of D2 expression in TT cells.
Several studies have demonstrated that D2 expression is regulated by a cAMP-dependent mechanism at pretranscriptional level (Araki, et al., 2003; Hosoi, et al., 1999; Kamiya, et al., 1999; Murakami, et al., 2001a). Moreover, a cAMP-response element (CRE) was identified in the human D2 promoter region (Bartha et al., 2000; Murakami, et al., 2001a). Accordingly, in this study, (Bu)2cAMP and forskolin stimulated both D2 mRNA and activities in the TT cells. These results indicate that D2 expression in C-cells is also regulated by a cAMP-dependent mechanism at the pretranslational level, as previously reported in human thyroid follicular cells, human skeletal muscle cells, rat brown adipocytes, and rat pineal glands (Hosoi et al., 1999; Kamiya et al., 1999; Murakami et al., 2001a; Murakami et al., 2001b). In follicular thyroid cell, D2 gene is regulated via TSH-R-cAMP-mediated mechanism (Murakami et al., 2001a). Nevertheless, since MTC samples or TT cells do not express TSH-R other factors might be involved in cAMP activation in these cells (Elisei et al., 2005).
The reported effects of DEX on D2 expression in various tissues and cell lines have been conflicting (Kim et al., 1998; St Germain, 1986). Previous studies performed in GH4C1 and GH3 rat pituitary tumors cells showed that glucocorticoids (DEX) significantly stimulated D2 expression (Araki et al., 2003; Kim et al., 1998) in agreement with the results obtained here in the TT cells. In contrast, DEX was reported to decrease D2 activity in cultured mouse neuroblastoma cells (St Germain, 1986). In the MC3T3-E1 osteoblastic cell line, DEX treatment has no effect on D2 expression (Gouveia et al., 2005). Therefore, the effect of glucocorticoids on D2 expression seems to differ among the various neoplastic tissues.
An interesting observation in this study came from the analysis of a sporadic MTC sample. In this tumor, the level of D2 activity was much higher than the normal surrounding epithelial tissue. The possibility of a mixed follicular-medullary tumor was ruled out by negative immunohistochemical analysis for thyroglobulin. Overexpression of D2 was also described in follicular carcinoma as a cause of low circulating free thyroxine levels (Kim et al., 2003). Both high serum TSH and/or low T4 levels up-regulate D2 activity, however, no changes were observed in thyroid function tests in this case.
In conclusion, the present results demonstrate that the D2 is expressed in samples of MTC, as well as in a human MTC derived cell line. Furthermore, TRs were demonstrated in MTC and in TT cells suggesting a potential role of T3 in this neoplastic tissue and presenting novel perspectives for thyroid hormone metabolism in human parafollicular C-cells.
Acknowledgments
We thank Dr. Alceu Migliavacca and Dr. José Ricardo Guimarães for surgical management of our patients. The authors are indebted to Mary Cleide Sogayar from Instituto de Ciências Básicas and Edna Kimura from Instituto de Ciências Biomédicas da Universidade de São Paulo (USP) for supplying cell line for the experiments. We are grateful to Gabriella Rejane dos Santos, Mateus Barbosa Vieira and Ursula Matte from Centro de Terapia Gênica do Hospital de Clínicas de Porto Alegre for their technical assistance in cell culture.
Grant support: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Apoio a Pesquisa do Rio Grande do Sul (FAPERGS), Fundo de Incentivo a Pesquisa (FIPE), Brazil and NIH grant FIC TW007559.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araki O, Morimura T, Ogiwara T, Mizuma H, Mori M, Murakami M. Expression of type 2 iodothyronine deiodinase in corticotropin-secreting mouse pituitary tumor cells is stimulated by glucocorticoid and corticotropin-releasing hormone. Endocrinology. 2003;144:4459–4465. doi: 10.1210/en.2003-0419. [DOI] [PubMed] [Google Scholar]
- Arnaldi LAT, Borra RC, Maciel RMB, Cerutti JM. Gene expression profiles reveal that DCN, DIO1, and DIO2 are underexpressed in benign and malignant thyroid tumors. Thyroid. 2005;15:210–221. doi: 10.1089/thy.2005.15.210. [DOI] [PubMed] [Google Scholar]
- Barrera-Hernandez G, Soo Park, K., Dace A, Zhan Q, Cheng S. Thyroid hormone-induced cell proliferation in GC cells is mediated by changes in G1 cyclin/cyclin-dependent kinase levels and activity. Endocrinology. 1999;140:5267–5274. doi: 10.1210/endo.140.11.7145. [DOI] [PubMed] [Google Scholar]
- Bartha T, Kim SW, Salvatore D, Gereben B, Tu HM, Harney JW, Rudas P, Larsen PR. Characterization of the 5’-flanking and 5’-untranslated regions of the cyclic adenosine 3’,5’-monophosphate-responsive human type 2 iodothyronine Deiodinase gene. Endocrinology. 2000;141:229–237. doi: 10.1210/endo.141.1.7282. [DOI] [PubMed] [Google Scholar]
- Baur A, Buchfelder M, Kȯhrle J. Expression of 5’-deiodinase enzyme in normal pituitaries and in various human pituitary adenomas. Eur. J. Endocrinol. 2002;147:263–268. doi: 10.1530/eje.0.1470263. [DOI] [PubMed] [Google Scholar]
- Boye N, Laurberg P. Deiodination of T4 to T3 and rT3 by microsomes from normal human thyroid tissue. Mol. Cell. Endocrinol. 1984;37:295–299. doi: 10.1016/0303-7207(84)90099-6. [DOI] [PubMed] [Google Scholar]
- Carlomagno F, Salvatore D, Santoro M, de Franciscis V, Quadro L, Panariello L, Colantuoni V, Fusco A. Point mutation of the RET proto-oncogene in the TT human medullary thyroid carcinoma cell line. Biochem. Biophys. Res. Comm. 1995;207:1022–1028. doi: 10.1006/bbrc.1995.1287. [DOI] [PubMed] [Google Scholar]
- Curcio C, Baqui MMA, Salvatore D, Rihn BH, Mohr S, Harney JW, Larsen PR, Bianco AC. The human type 2 iodothyronine deiodinase is a seleno protein highly expressed in a mesothelioma cell line. J. Biol. Chem. 2001;276:30183–30187. doi: 10.1074/jbc.C100325200. [DOI] [PubMed] [Google Scholar]
- Dentice M, Luongo C, Huang S, Ambrosio R, Elefante A, Mirebeau-Prunier D, Zavacki AM, Fenzi G, Grachtchouk M, Hutchin M, Dlugosz AA, Bianco AC, Missero C, Larsen PR, Salvatore D. Sonic hedgehog-induced type 3 deiodinase blocks thyroid hormone action enhancing proliferation of normal and malignant keratinocytes. Proc. Nat. Acad. Sci. 2007;104:14466–14471. doi: 10.1073/pnas.0706754104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza Meyer EL, Dora JM, Wagner MS, Maia AL. Decreased type 1 iodothyronine deiodinase expression might be an early and discrete event in thyroid cell dedifferentiation towards papillary carcinoma. Clin. Endocrinol. 2005;62:672–678. doi: 10.1111/j.1365-2265.2005.02277.x. [DOI] [PubMed] [Google Scholar]
- Dohán O, Baloch Z, Bánrévi Z, Livolsi V, Carrasco N. Rapid communication: predominant intracellular overexpression of the Na(+)/I(-) symporter (NIS) in a large sampling of thyroid cancer cases. J. Clin. Endocrinol. Metab. 2001;86:2697–2700. doi: 10.1210/jcem.86.6.7746. [DOI] [PubMed] [Google Scholar]
- Elisei R, Pinchera A, Romei C, Gryczynska M, Pohl V, Maenhaut C, Fugazzola L, Pacini F. Expression of thyrotropin receptor (TSH-R), thyroglobulin, thyroperoxidase, and calcitonin messenger ribonucleic acids in thyroid carcinomas: evidence of TSH-R gene transcript in medullary histotype. J. Clin. Endocrinol. Metab. 1994;78:867–871. doi: 10.1210/jcem.78.4.8157713. [DOI] [PubMed] [Google Scholar]
- Elisei R, Vivaldi A, Agate L, Ciampi R, Molinaro E, Piampiani P, Romei C, Faviana P, Basolo F, Miccoli P, Capodanno A, Collecchi P, Pacini F, Pinchera A. All-trans-retinoic acid treatment inhibits the growth of retinoic acid receptor ß messenger ribonucleic acid expressing thyroid cancer cell lines but does not reinduce the expression of thyroid-specific genes. J. Clin. Endocrinol. Metab. 2005;90:2403–2411. doi: 10.1210/jc.2004-0969. [DOI] [PubMed] [Google Scholar]
- Gouveia CH, Christoffolete MA, Zaitune CR, Dora JM, Harney JW, Maia AL, Bianco AC. Type 2 iodothyronine selenodeiodinase is expressed throughout the mouse skeleton and in the MC3T3-E1 mouse osteoblastic cell line during differentiation. Endocrinology. 2005;146:195–200. doi: 10.1210/en.2004-1043. [DOI] [PubMed] [Google Scholar]
- Greene FL, Sobin LH. The TNM system: our language for cancer care. J. Surg. Oncol. 2002;80:119–120. doi: 10.1002/jso.10114. [DOI] [PubMed] [Google Scholar]
- Hedinger CE, Williams ED, Sobin LH. WHO International Histological Classification of Tumours. 2nd Springer-Verlag; Berlin: 1988. Histological typing of thyroid tumors; pp. 5–6. [Google Scholar]
- Hosoi Y, Murakami M, Mizuma H, Ogiwara T, Imamura M, Mori M. Expression and regulation of type II iodothyronine deiodinase in cultured human skeletal muscle cells. J. Clin. Endocrinol. Metab. 1999;84:3293–3300. doi: 10.1210/jcem.84.9.5969. [DOI] [PubMed] [Google Scholar]
- Ishii H, Inada M, Tanaka K, Mashio Y, Naito K, Nishikawa M, Imura H. Triiodothyronine generation from thyroxine in human thyroid: enhanced conversion in Graves’ thyroid tissue. J. Clin. Endocrinol. Metab. 1981;52:1211–1217. doi: 10.1210/jcem-52-6-1211. [DOI] [PubMed] [Google Scholar]
- Kamiya Y, Murakami M, Araki O, Hosoi Y, Ogiwara T, Mizuma H, Mori M. Pretranslational regulation of rhythmic type II iodothyronine deiodinase expression by β-adrenergic mechanism in the rat pineal gland. Endocrinology. 1999;140:1272–1278. doi: 10.1210/endo.140.3.6594. [DOI] [PubMed] [Google Scholar]
- Kim BW, Harney JW, Larsen PR. Studies of the hormonal regulation of type 2 5’-iodothyronine deiodinase messenger ribonucleic acid in pituitary tumor cells using semiquantitative reverse transcription-polymerase chain reaction. Endocrinology. 1998;139:4895–4905. doi: 10.1210/endo.139.12.6334. [DOI] [PubMed] [Google Scholar]
- Kim BW, Daniels GH, Harrison BJ, Price A, Harney JW, Larsen PR, Weetman AP. Overexpression of type 2 iodothyronine deiodinase in follicular carcinoma as a cause of low circulating free thyroxine levels. J. Clin. Endocrinol. Metab. 2003;88:594–598. doi: 10.1210/jc.2002-020921. [DOI] [PubMed] [Google Scholar]
- Leboulleux S, Baudin E, Travagli JP, Schlumberger M. Medullary thyroid carcinoma. Clin. Endocrinol. 2004;61:299–310. doi: 10.1111/j.1365-2265.2004.02037.x. [DOI] [PubMed] [Google Scholar]
- Lemaire M, Bayens W, de Saint-Georges L, Baugnet-Mahieu L. Thyroid hormone influence on the growth of hepatoma HW-165 in Wistar rats. Biomedicine. 1981;34:133–139. [PubMed] [Google Scholar]
- Leuthauser SWC, Guernsey DL. Thyroid hormone affects the expression neoplastic transformation induced by DNA-transfection. Cancer Lett. 1987;35:321–326. doi: 10.1016/0304-3835(87)90134-0. [DOI] [PubMed] [Google Scholar]
- Maia AL, Harney JW, Larsen PR. Pituitary cells respond to thyroid hormone by discrete, gene-specific pathways. Endocrinology. 1995;136:1488–1494. doi: 10.1210/endo.136.4.7534701. [DOI] [PubMed] [Google Scholar]
- Maia AL, Kim BW, Huang SA, Harney JW, Larsen PR. Type 2 iodothyronine deiodinase is the major source of plasma T3 in euthyroid humans. J. Clin. Invest. 2005;115:2524–2533. doi: 10.1172/JCI25083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer ELS, Wagner MS, Maia AL. Expressão das iodotironinas desiodases nas neoplasias tireoidianas. Arq. Bras. Endocrinol. Metabol. 2007;51:690–700. doi: 10.1590/s0004-27302007000500006. [DOI] [PubMed] [Google Scholar]
- Mori K, Yoshida K, Kayama T, Kaise N, Fukazawa H, Kiso Y, Kikuchi K, Aizawa Y, Abe K. Thyroxine 5-deiodinase in human brain tumors. J. Clin. Endocrinol. Metab. 1993;77:1198–1202. doi: 10.1210/jcem.77.5.8077312. [DOI] [PubMed] [Google Scholar]
- Morimura T, Tsunekawa K, Kasahara T, Seki K, Ogiwara T, Mori M, Murakami M. Expression of type 2 iodothyronine deiodinase in human osteoblast is stimulated by thyrotropin. Endocrinology. 2005;146:2077–2084. doi: 10.1210/en.2004-1432. [DOI] [PubMed] [Google Scholar]
- Mulligan LM, Kwok JB, Healey CS, Elsdon MJ, Eng C, Gardner E, Love DR, Mole SE, Moore JK, Papi L, Ponder MA, Telenius H, Tunnacliffe A, Ponder BA. Germ-line mutation of the RET proto-oncogene in multiple endocrine neoplasia type 2 A. Nature. 1993;363:458–460. doi: 10.1038/363458a0. [DOI] [PubMed] [Google Scholar]
- Murakami M, Araki O, Morimura T, Hosoi Y, Mizuma H, Yamada M, Kurihara H, Ishiuchi S, Tamura M, Sasaki T, Mori M. Expression of Type II iodothyronine deiodinase in brain tumors. J. Clin. Endocrinol. Metab. 2000;85:4403–4406. doi: 10.1210/jcem.85.11.6952. [DOI] [PubMed] [Google Scholar]
- Murakami M, Araki O, Hosoi Y, Kamiya Y, Morimura T, Ogiwara T, Mizuma H, Mori M. Expression and regulation of type II iodothyronine deiodinase in human thyroid gland. Endocrinology. 2001a;142:2961–2967. doi: 10.1210/endo.142.7.8280. [DOI] [PubMed] [Google Scholar]
- Murakami M, Kamiya Y, Morimura T, Araki O, Imamura M, Ogiwara T, Mizuma H, Mori M. Thyrotropin receptors in brown adipose tissue: thyrotropin stimulates type II iodothyronine deiodinase and uncoupling protein-1 in brown adipocytes. Endocrinology. 2001b;142:1195–1201. doi: 10.1210/endo.142.3.8012. [DOI] [PubMed] [Google Scholar]
- Pacini F, Basolo F, Elisei R, Fugazzola L, Cola A, Pinchera A. Medullary thyroid cancer. An immunohistochemical and humoral study using six separate antigens. Am. J. Clin. Pathol. 1991;95:300–308. doi: 10.1093/ajcp/95.3.300. [DOI] [PubMed] [Google Scholar]
- Ponder BA. The phenotypes associated with RET mutations in the multiple endocrine neoplasia type 2 syndromes. Cancer Res. 1999;59:1736–1742. [PubMed] [Google Scholar]
- Puñales MK, Graf H, Gross JL, Maia AL. RET codon 634 mutations in multiple endocrine neoplasia type 2: variable clinical features and clinical outcome. J. Clin. Endocrinol. Metab. 2003;88:2644–2649. doi: 10.1210/jc.2002-021422. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Peña A. Oligodendrocyte development and thyroid hormone. J. Neurobiol. 1999;40:497–512. doi: 10.1002/(sici)1097-4695(19990915)40:4<497::aid-neu7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Salvatore D, Tu H, Harney JW, Larsen PR. Type 2 iodothyronine deiodinase is highly expressed in human thyroid. J. Clin. Invest. 1996;98:962–968. doi: 10.1172/JCI118880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MJ, Fiering SN, Pallud SE, Parlow AF, Germain DL, Galton VA. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol. Endocrinol. 2001;15:2137–2148. doi: 10.1210/mend.15.12.0740. [DOI] [PubMed] [Google Scholar]
- Stahl JH, Kendall SK, Brinkmeier ML, Greco TL, Watkins-Chow DE, Campos-Barros A, Lloyd RV, Camper SA. Thyroid hormone is essential for pituitary somatotropes and lactotropes. Endocrinology. 1999;140:1884–1892. doi: 10.1210/endo.140.4.6627. [DOI] [PubMed] [Google Scholar]
- Steinsapir J, Harney J, Larsen PR. Type 2 iodothyronine deiodinase in rat pituitary tumor cells is inactivated in proteasomes. J. Clin. Invest. 1998;102:1895–1899. doi: 10.1172/JCI4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streckfuss F, Hamann I, Schomburg L, Michaelis M, Sapin R, Klein MO, Kȯhrle J, Schweizer U. Hepatic Deiodinase activity is dispensable for the maintenance of normal circulating thyroid hormone levels in mice. Biochem. Biophys. Res. Commun. 2005;18:739–745. doi: 10.1016/j.bbrc.2005.09.102. [DOI] [PubMed] [Google Scholar]
- Germain DL. hormonal control of a low Km (typeII) iodothyronine 5’-deiodinase in cultured NB41A3 mouse neuroblastoma cells. Endocrinology. 1986;119:840–846. doi: 10.1210/endo-119-2-840. [DOI] [PubMed] [Google Scholar]
- Takano T, Miyauchi A, Ito Y, Amino N. Thyroxine to triiodothyronine hyperconversion thyrotoxicosis in patients with large metastases of follicular thyroid carcinoma. Thyroid. 2006;16:615–618. doi: 10.1089/thy.2006.16.615. [DOI] [PubMed] [Google Scholar]
- Tannahill LA, Visser TJ, McCabe CJ, Kachilele S, Boelaert K, Sheppard JA, Franklyn & Gittoes NJL. Dysregulation of iodothyronine deiodinase enzyme expression and function in human pituitary tumours. Clin. Endocrinol. 2002;56:735–743. doi: 10.1046/j.1365-2265.2002.01541.x. [DOI] [PubMed] [Google Scholar]
- Wagner MS, Morimoto R, Dora JM, Benneman A, Pavan R, Maia AL. Hypothyroidism induces type 2 iodothyronine deiodinase expression in mouse heart and testis. J. Mol. Endocrinol. 2003;31:541–550. doi: 10.1677/jme.0.0310541. [DOI] [PubMed] [Google Scholar]
- Wagner MS, Wajner SM, Dora JM, Maia AL. Regulation of Dio 2 gene expression by thyroid hormones in normal and type 1 deiodinase-deficient C3H mice. J. Endocrinol. 2007;193:435–444. doi: 10.1677/JOE-07-0099. [DOI] [PubMed] [Google Scholar]
- Zabel M, Seidel J, Kaczmarek A, Surdyk-Zasada J, Grzeszkowiak J, Gorny A. Immunocytochemical characterization of two thyroid medullary carcinoma cell lines in vitro. Histochem. J. 1995;27:859–868. [PubMed] [Google Scholar]
- Zabel M, Grzeszkowiak J. Characterization of thyroid medullary carcinoma TT cell line. Histol. Histopathol. 1997;12:283–289. [PubMed] [Google Scholar]