Abstract
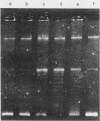
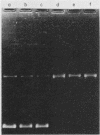
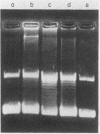
The time course of appearance of type I topoisomerase activity after the infection of mouse L cytoplasts by vaccinia virus was determined. When the enucleation procedure was carried out with unsynchronized cell cultures, a high level of host-cell-specific type I topoisomerase activity was found associated with the resulting cytoplasts. If cells were first synchronized by the two-cycle thymidine block method and then enucleated after release, the level of host type I topoisomerase activity was also high for S-phase-enucleated cells but was very low for cytoplasts prepared from cells previously synchronized and enucleated during either the G1 or the G2 phase. After the infection of G1-phase-enucleated cytoplasts with vaccinia virus, newly synthesized type I topoisomerase activity first appeared at about 3 h postinfection. Virosomes were isolated from the infected, synchronized cytoplasts and assayed for the presence of type I topoisomerase activity. The activity remained at the top of a sucrose gradient, well resolved from the virosome fraction, at the low salt levels (0.01 M KCl, 0.01 M Tris hydrochloride, pH 8.0) normally used in the course of virosome purification. If the sedimentation was at the higher salt concentration (0.15 M KCl) at which the enzyme shows optimal activity, type I topoisomerase cosedimented with the virosome fraction onto the sucrose gradient cushion. These results show that the type I topoisomerase activity dependent upon vaccinia virus infection may be detected with high sensitivity in G1-phase-enucleated cytoplasts. The association with virosomes is consistent with an involvement of topoisomerase activity either in DNA replication or in late transcription.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W. R., Ressner E. C., Kates J., Patzke J. V. A DNA nicking-closing enzyme encapsidated in vaccinia virus: partial purification and properties. Proc Natl Acad Sci U S A. 1977 May;74(5):1841–1845. doi: 10.1073/pnas.74.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakel C., Kates J. R. Poly(A) polymerase from vaccinia virus-infected cells. I. Partial purification and characterization. J Virol. 1974 Oct;14(4):715–723. doi: 10.1128/jvi.14.4.715-723.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter S. B. Effects of cytochalasins on mammalian cells. Nature. 1967 Jan 21;213(5073):261–264. doi: 10.1038/213261a0. [DOI] [PubMed] [Google Scholar]
- Champoux J. J., Young L. S., Been M. D. Studies on the regulation and specificity of the DNA-untwisting enzyme. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):53–58. doi: 10.1101/sqb.1979.043.01.009. [DOI] [PubMed] [Google Scholar]
- Fleischmann G., Pflugfelder G., Steiner E. K., Javaherian K., Howard G. C., Wang J. C., Elgin S. C. Drosophila DNA topoisomerase I is associated with transcriptionally active regions of the genome. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6958–6962. doi: 10.1073/pnas.81.22.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foglesong P. D., Bauer W. R. Effects of ATP and inhibitory factors on the activity of vaccinia virus type I topoisomerase. J Virol. 1984 Jan;49(1):1–8. doi: 10.1128/jvi.49.1.1-8.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett E. A. A convenient method for enucleating cells in quantity. Exp Cell Res. 1974 Mar 15;84(1):72–78. doi: 10.1016/0014-4827(74)90381-4. [DOI] [PubMed] [Google Scholar]
- Geshelin P., Berns K. I. Characterization and localization of the naturally occurring cross-links in vaccinia virus DNA. J Mol Biol. 1974 Oct 5;88(4):785–796. doi: 10.1016/0022-2836(74)90399-4. [DOI] [PubMed] [Google Scholar]
- Holowczak J. A. Poxvirus DNA. Curr Top Microbiol Immunol. 1982;97:27–79. doi: 10.1007/978-3-642-68318-3_2. [DOI] [PubMed] [Google Scholar]
- Hruby D. E., Guarino L. A., Kates J. R. Vaccinia virus replication. I. Requirement for the host-cell nucleus. J Virol. 1979 Feb;29(2):705–715. doi: 10.1128/jvi.29.2.705-715.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Lynn D. L., Kates J. R. Vaccinia virus replication requires active participation of the host cell transcriptional apparatus. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1887–1890. doi: 10.1073/pnas.76.4.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Gershowitz A. Characterization of a polyriboadenylate polymerase from vaccinia virions. J Biol Chem. 1975 Jun 25;250(12):4722–4729. [PubMed] [Google Scholar]
- Pennington T. H., Follett E. A. Vaccinia virus replication in enucleate BSC-1 cells: particle production and synthesis of viral DNA and proteins. J Virol. 1974 Feb;13(2):488–493. doi: 10.1128/jvi.13.2.488-493.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prem veer Reddy G., Pardee A. B. Multienzyme complex for metabolic channeling in mammalian DNA replication. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3312–3316. doi: 10.1073/pnas.77.6.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P. N., Johnson R. T. Mammalian cell fusion: studies on the regulation of DNA synthesis and mitosis. Nature. 1970 Jan 10;225(5228):159–164. doi: 10.1038/225159a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg B. H., Ungers G., Deutsch J. F. Variation in DNA swivel enzyme activity during the mammalian cell cycle. Nucleic Acids Res. 1976 Dec;3(12):3305–3311. doi: 10.1093/nar/3.12.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal E. C., Roseman N. A., Hruby D. E. Isolation of vaccinia virus mutants capable of replicating independently of the host cell nucleus. J Virol. 1984 Aug;51(2):359–366. doi: 10.1128/jvi.51.2.359-366.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M. H., Weinstein I. B. A preparative method for obtaining enucleated mammalian cells. Biochem Biophys Res Commun. 1975 Apr 7;63(3):669–674. doi: 10.1016/s0006-291x(75)80436-0. [DOI] [PubMed] [Google Scholar]
- Wittek R. Organization and expression of the poxvirus genome. Experientia. 1982 Mar 15;38(3):285–297. doi: 10.1007/BF01949349. [DOI] [PubMed] [Google Scholar]