Abstract
BALB/c mice were immunized with HeLa cells, and their spleen cells were fused with myeloma cells to produce hybridomas. Initial screening of culture fluids from 800 fusion products in a cell protection assay against coxsackievirus B3 (CB3) and the CB3-RD virus variant yielded five presumptive monoclonal antibodies with three specificities: protection against CB3 on HeLa, protection against CB3-RD on rhabdomyosarcoma (RD) cells, and protection against both viruses on the respective cells. Only one of the monoclonal antibodies (with dual specificity) survived two subclonings and was studied in detail. The antibody was determined to have an immunoglobulin G2a isotype and protected cells by blockade of cellular receptors, since attachment of [35S]methionine-labeled CB3 was inhibited by greater than 90%. The monoclonal antibody protected HeLa cells against infection by CB1, CB3, CB5, echovirus 6, and coxsackievirus A21 and RD cells against CB1-RD, CB3-RD, and CB5-RD virus variants. The monoclonal antibody did not protect either cell type against 16 other immunotypes of picornaviruses. The monoclonal antibody produced only positive fluorescence on those cells which were protected against infection, and 125I-labeled antibody confirmed the specific binding to HeLa and RD cells. The results suggest that this monoclonal antibody possesses some of the receptor specificity of the group B coxsackieviruses.
Full text
PDF

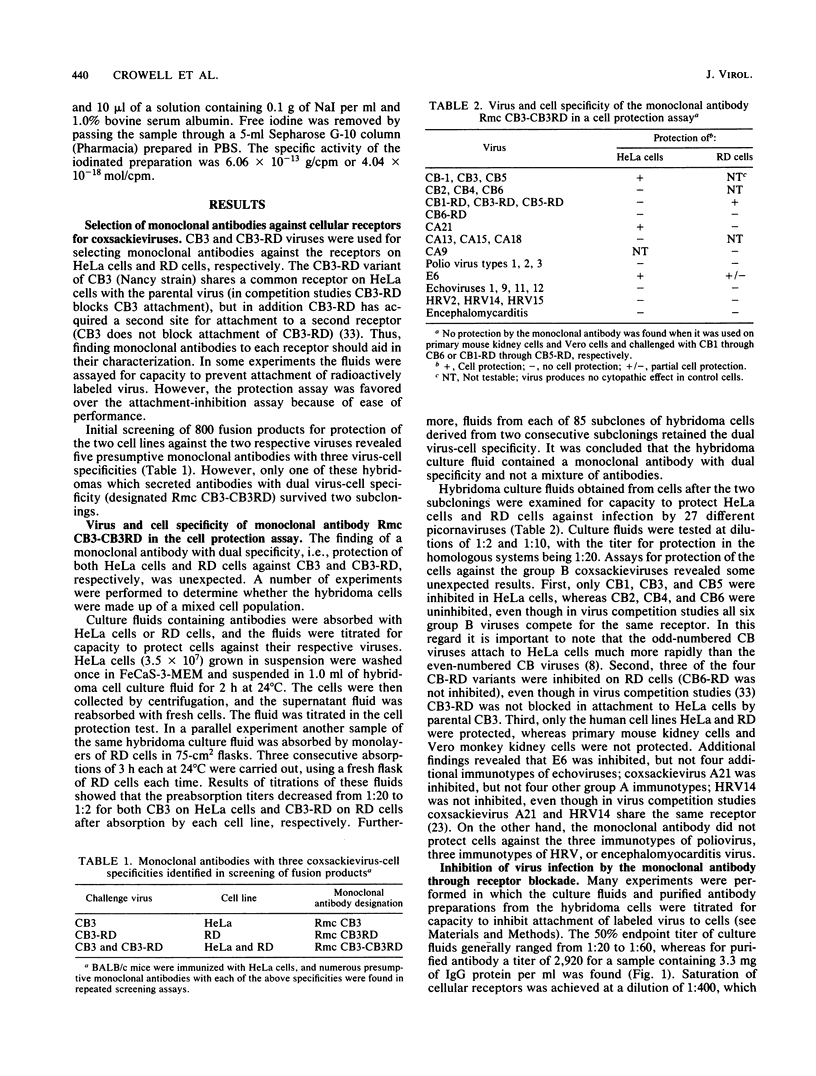
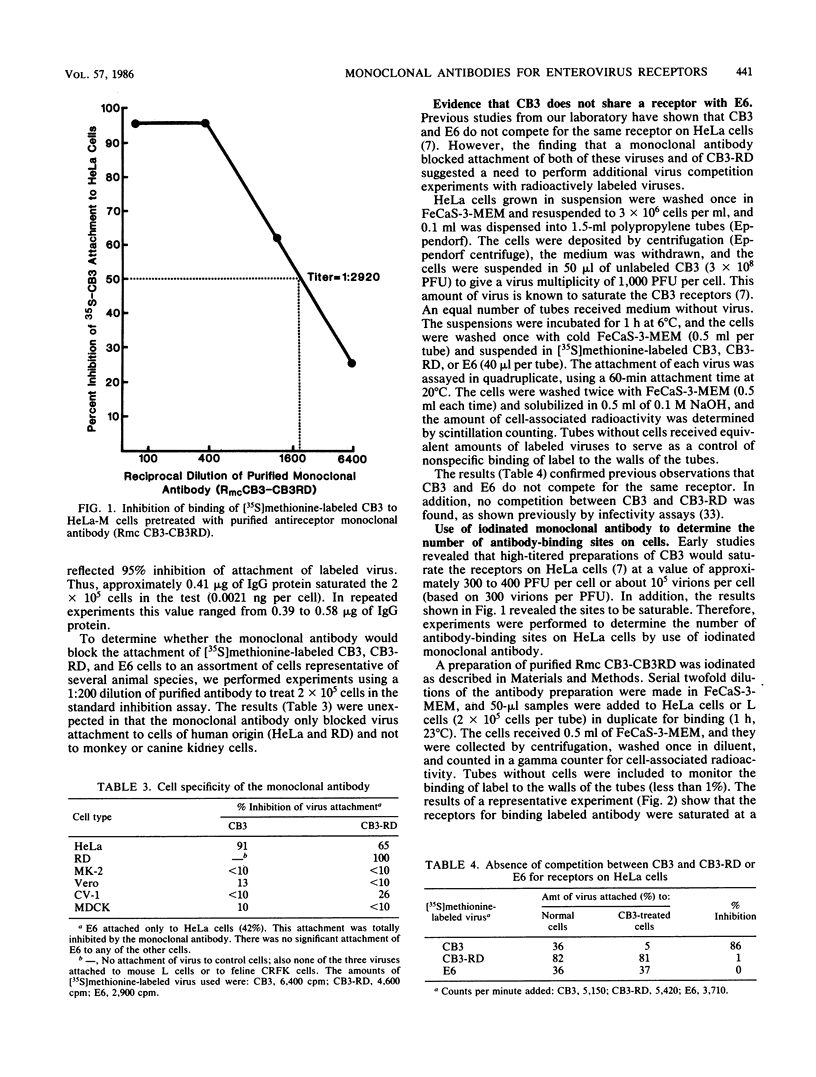
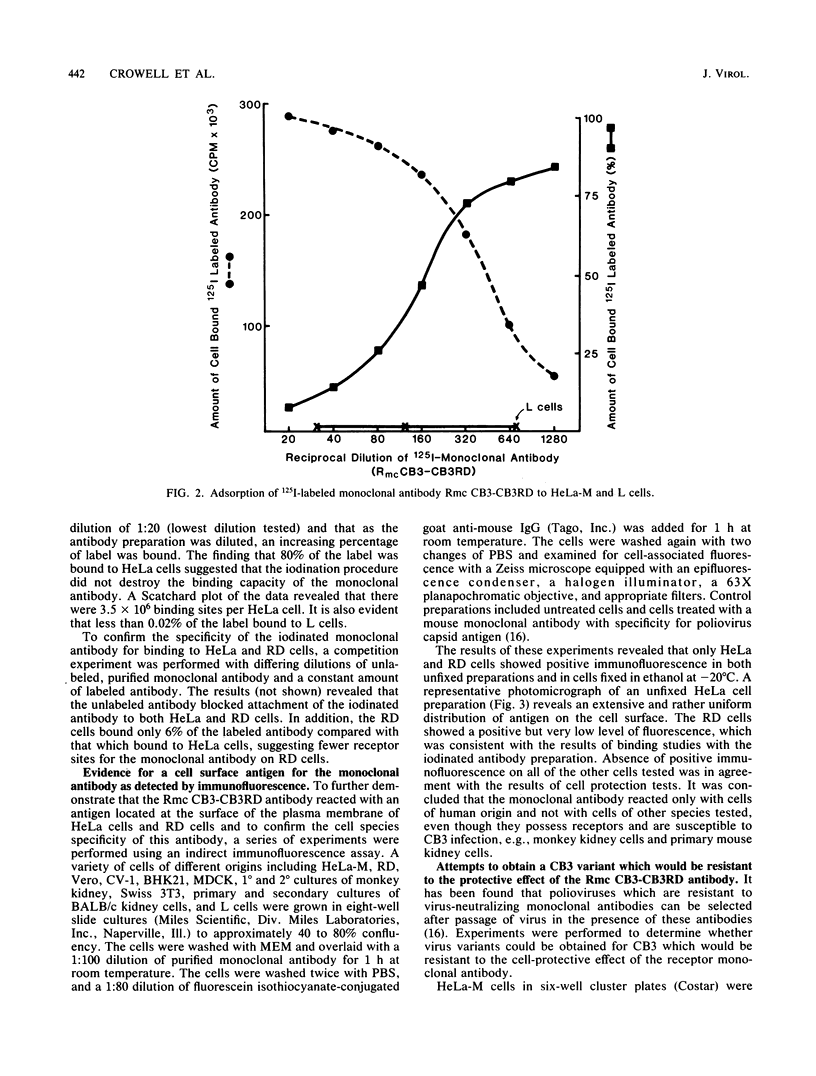
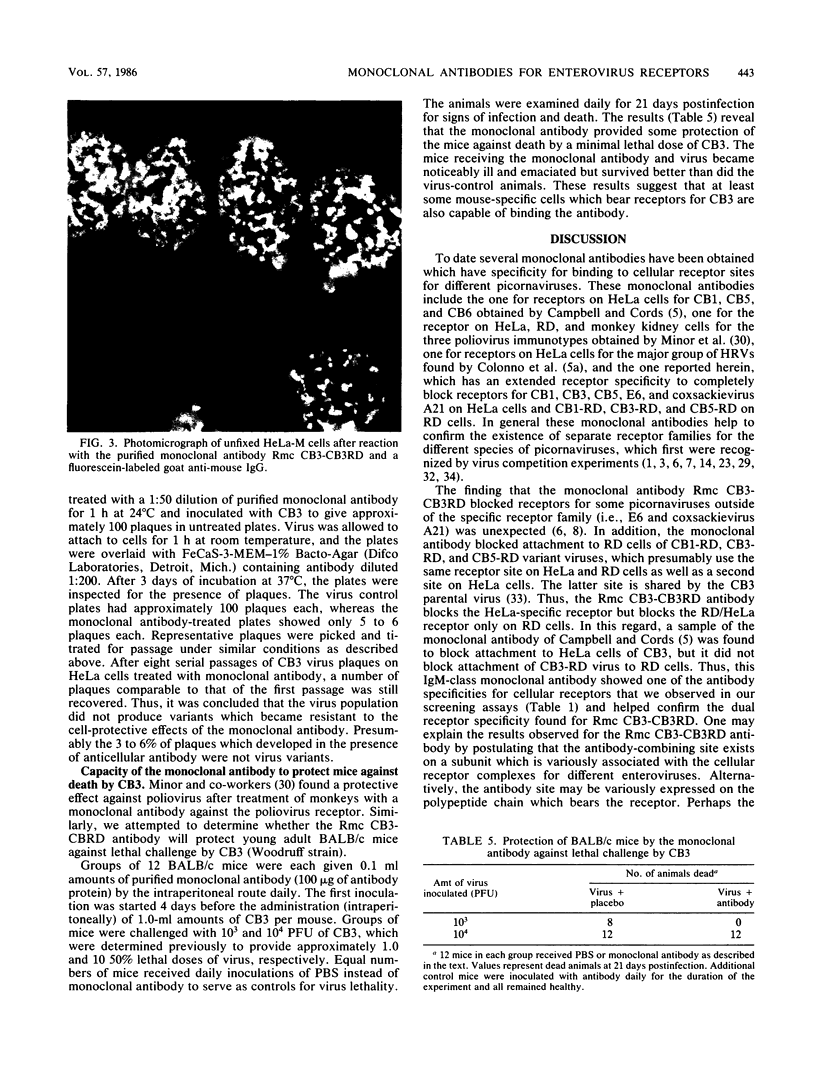


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Colonno R. J. Many rhinovirus serotypes share the same cellular receptor. J Virol. 1984 Aug;51(2):340–345. doi: 10.1128/jvi.51.2.340-345.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axler D. A., Crowell R. L. Effect of anticellular serum on the attachment of enteroviruses to HeLa cells. J Virol. 1968 Aug;2(8):813–821. doi: 10.1128/jvi.2.8.813-821.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxt B., Bachrach H. L. Early interactions of foot-and-mouth disease virus with cultured cells. Virology. 1980 Jul 15;104(1):42–55. doi: 10.1016/0042-6822(80)90364-5. [DOI] [PubMed] [Google Scholar]
- Bukrinskaya A. G. Penetration of viral genetic material into host cell. Adv Virus Res. 1982;27:141–204. doi: 10.1016/s0065-3527(08)60435-2. [DOI] [PubMed] [Google Scholar]
- CROWELL R. L. SPECIFIC VIRAL INTERFERENCE IN HELA CELL CULTURES CHRONICALLY INFECTED WITH COXSACKIE B5 VIRUS. J Bacteriol. 1963 Sep;86:517–526. doi: 10.1128/jb.86.3.517-526.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWELL R. L., SYVERTON J. T. The mammalian cell-virus relationship. VI. Sustained infection of HeLa cells by Coxsackie B3 virus and effect on superinfection. J Exp Med. 1961 Feb 1;113:419–435. doi: 10.1084/jem.113.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWELL R. L., SYVERTON J. T. The viral range in vitro of a malignant, human epithelial cell (strain HeLa, Gey). IV. The cytopathogenicity of C viruses. J Immunol. 1955 Feb;74(2):169–177. [PubMed] [Google Scholar]
- Campbell B. A., Cords C. E. Monoclonal antibodies that inhibit attachment of group B coxsackieviruses. J Virol. 1983 Nov;48(2):561–564. doi: 10.1128/jvi.48.2.561-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno R. J., Callahan P. L., Long W. J. Isolation of a monoclonal antibody that blocks attachment of the major group of human rhinoviruses. J Virol. 1986 Jan;57(1):7–12. doi: 10.1128/jvi.57.1.7-12.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell R. L., Philipson L. Specific alterations of coxsackievirus B3 eluted from HeLa cells. J Virol. 1971 Oct;8(4):509–515. doi: 10.1128/jvi.8.4.509-515.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell R. L. Specific cell-surface alteration by enteroviruses as reflected by viral-attachment interference. J Bacteriol. 1966 Jan;91(1):198–204. doi: 10.1128/jb.91.1.198-204.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmock N. J. Review article initial stages in infection with animal viruses. J Gen Virol. 1982 Mar;59(Pt 1):1–22. doi: 10.1099/0022-1317-59-1-1. [DOI] [PubMed] [Google Scholar]
- Emini E. A., Jameson B. A., Lewis A. J., Larsen G. R., Wimmer E. Poliovirus neutralization epitopes: analysis and localization with neutralizing monoclonal antibodies. J Virol. 1982 Sep;43(3):997–1005. doi: 10.1128/jvi.43.3.997-1005.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C., SYVERTON J. T. The mammalian cell-virus relationship. IV. Infection of naturally insusceptible cells with enterovirus ribonucleic acid. J Exp Med. 1959 Jul 1;110(1):65–80. doi: 10.1084/jem.110.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. V., Stanton L. W., Tomassini J. E., Long W. J., Scolnick E. M. Neutralizing monoclonal antibodies to hepatitis A virus: partial localization of a neutralizing antigenic site. J Virol. 1984 Nov;52(2):465–473. doi: 10.1128/jvi.52.2.465-473.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn A. Early interactions of viruses with cellular membranes. Adv Virus Res. 1979;24:223–276. doi: 10.1016/s0065-3527(08)60395-4. [DOI] [PubMed] [Google Scholar]
- Krah D. L., Crowell R. L. A solid-phase assay of solubilized HeLa cell membrane receptors for binding group B coxsackieviruses and polioviruses. Virology. 1982 Apr 15;118(1):148–156. doi: 10.1016/0042-6822(82)90328-2. [DOI] [PubMed] [Google Scholar]
- Krah D. L., Crowell R. L. Properties of the deoxycholate-solubilized HeLa cell plasma membrane receptor for binding group B coxsackieviruses. J Virol. 1985 Mar;53(3):867–870. doi: 10.1128/jvi.53.3.867-870.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lonberg-Holm K., Crowell R. L., Philipson L. Unrelated animal viruses share receptors. Nature. 1976 Feb 26;259(5545):679–681. doi: 10.1038/259679a0. [DOI] [PubMed] [Google Scholar]
- Mapoles J. E., Krah D. L., Crowell R. L. Purification of a HeLa cell receptor protein for group B coxsackieviruses. J Virol. 1985 Sep;55(3):560–566. doi: 10.1128/jvi.55.3.560-566.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeady M. L., Crowell R. L. Proteolytic cleavage of VP1 in 'A' particles of coxsackievirus B3 does not appear to mediate virus uncoating by HeLa cells. J Gen Virol. 1981 Aug;55(Pt 2):439–450. doi: 10.1099/0022-1317-55-2-439. [DOI] [PubMed] [Google Scholar]
- McLaren L. C., Scaletti J. V., James C. G. Isolation and properties of enterovirus receptors. Wistar Inst Symp Monogr. 1968;8:123–135. [PubMed] [Google Scholar]
- Minor P. D., Pipkin P. A., Hockley D., Schild G. C., Almond J. W. Monoclonal antibodies which block cellular receptors of poliovirus. Virus Res. 1984;1(3):203–212. doi: 10.1016/0168-1702(84)90039-x. [DOI] [PubMed] [Google Scholar]
- Much D. H., Zajac I. The effect of an antireceptor serum on mammalian cell lines. J Gen Virol. 1974 May;23(2):205–208. doi: 10.1099/0022-1317-23-2-205. [DOI] [PubMed] [Google Scholar]
- Reagan K. J., Goldberg B., Crowell R. L. Altered receptor specificity of coxsackievirus B3 after growth in rhabdomyosarcoma cells. J Virol. 1984 Mar;49(3):635–640. doi: 10.1128/jvi.49.3.635-640.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz M., Crowell R. L. Eclipse of coxsackievirus infectivity: the restrictive event for a non-fusing myogenic cell line. J Gen Virol. 1983 Aug;64(Pt 8):1725–1734. doi: 10.1099/0022-1317-64-8-1725. [DOI] [PubMed] [Google Scholar]
- Sharpe A. H., Fields B. N. Pathogenesis of viral infections. Basic concepts derived from the reovirus model. N Engl J Med. 1985 Feb 21;312(8):486–497. doi: 10.1056/NEJM198502213120806. [DOI] [PubMed] [Google Scholar]
- Tardieu M., Epstein R. L., Weiner H. L. Interaction of viruses with cell surface receptors. Int Rev Cytol. 1982;80:27–61. doi: 10.1016/S0074-7696(08)60366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F. H., Lomax N. B. Host range mutants of human rhinovirus in which nonstructural proteins are altered. J Virol. 1983 Nov;48(2):410–418. doi: 10.1128/jvi.48.2.410-418.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAJAC I., CROWELL R. L. EFFECT OF ENZYMES ON THE INTERACTION OF ENTEROVIRUSES WITH LIVING HELA CELLS. J Bacteriol. 1965 Mar;89:574–582. doi: 10.1128/jb.89.3.574-582.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac I., Crowell R. L. Differential inhibition of attachment and eclipse activities of HeLa cells for enteroviruses. J Virol. 1969 Apr;3(4):422–428. doi: 10.1128/jvi.3.4.422-428.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]