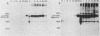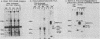Abstract
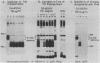
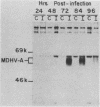
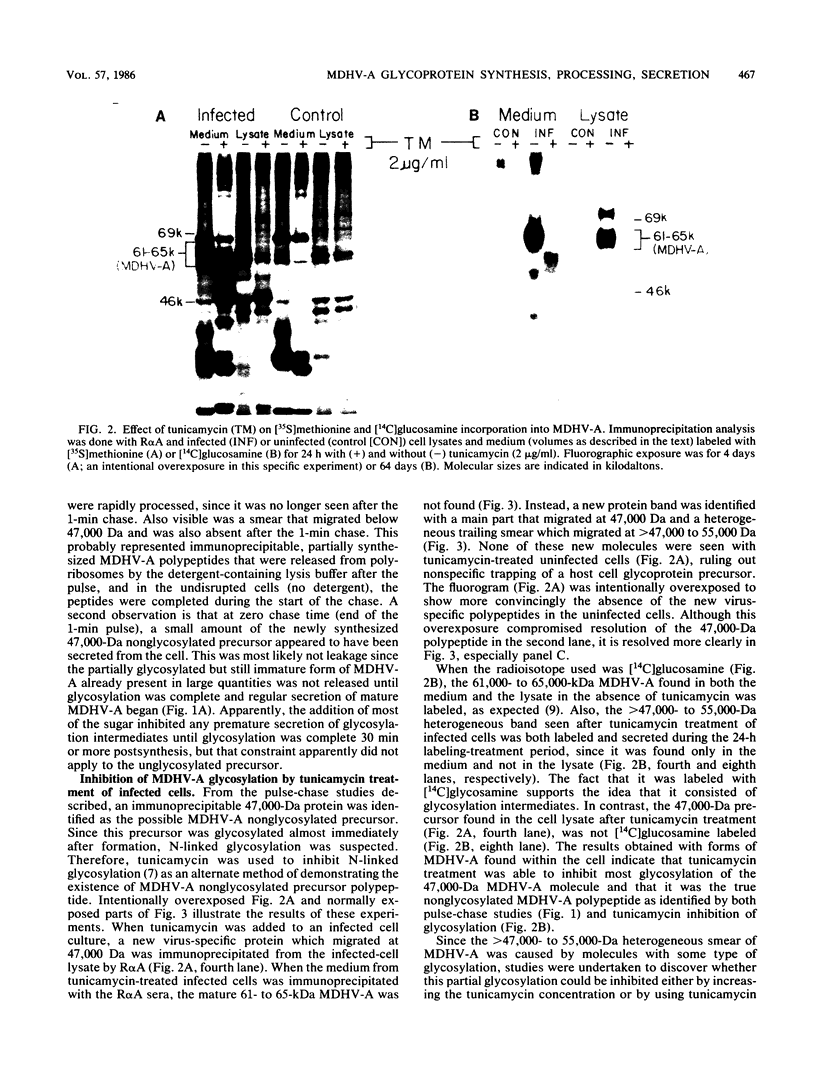
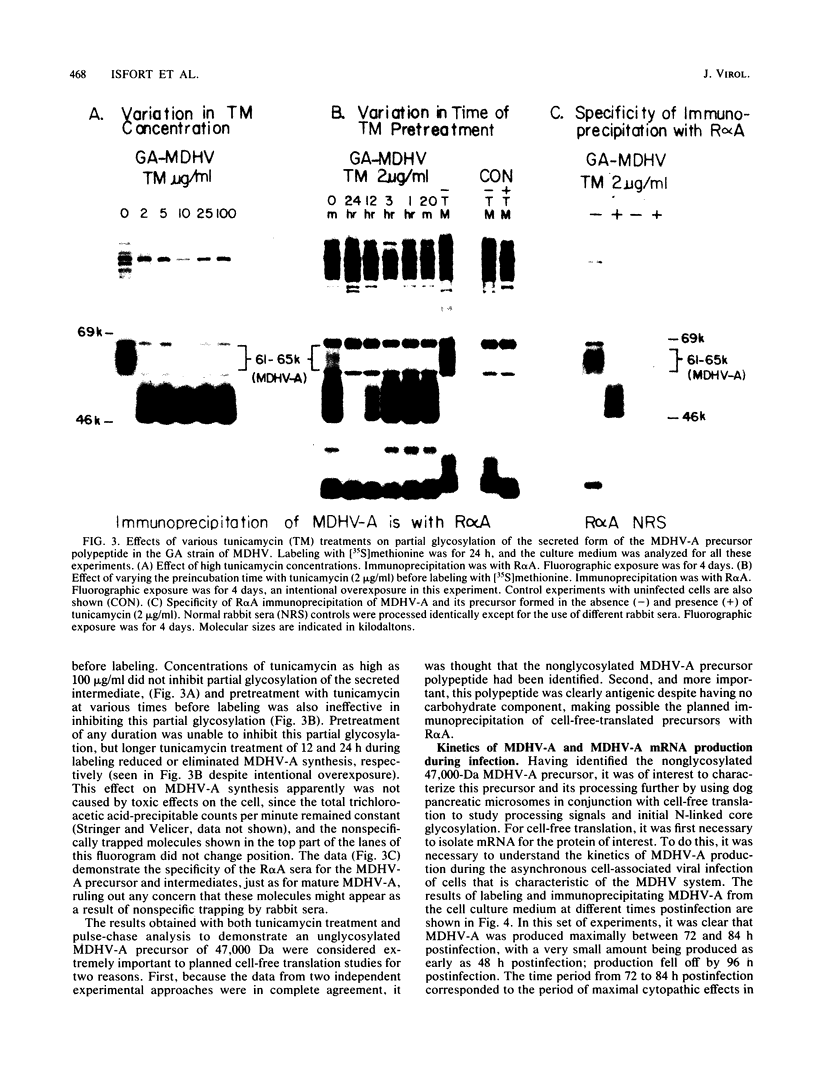
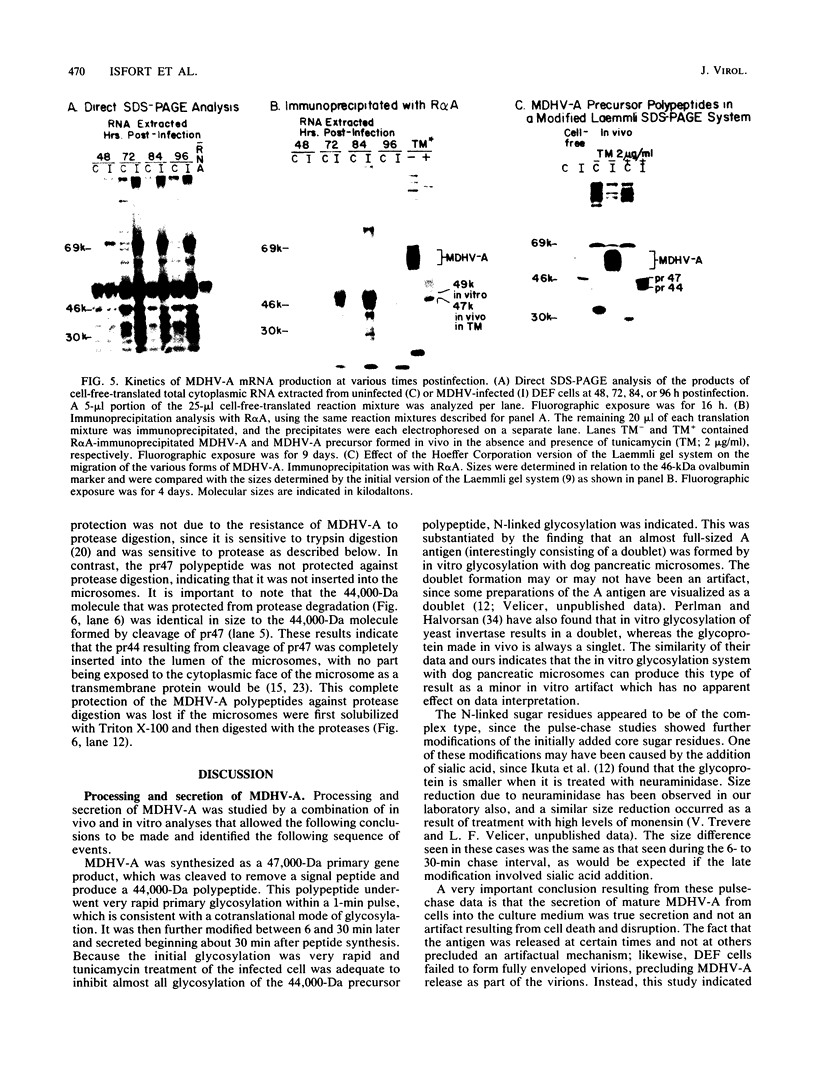
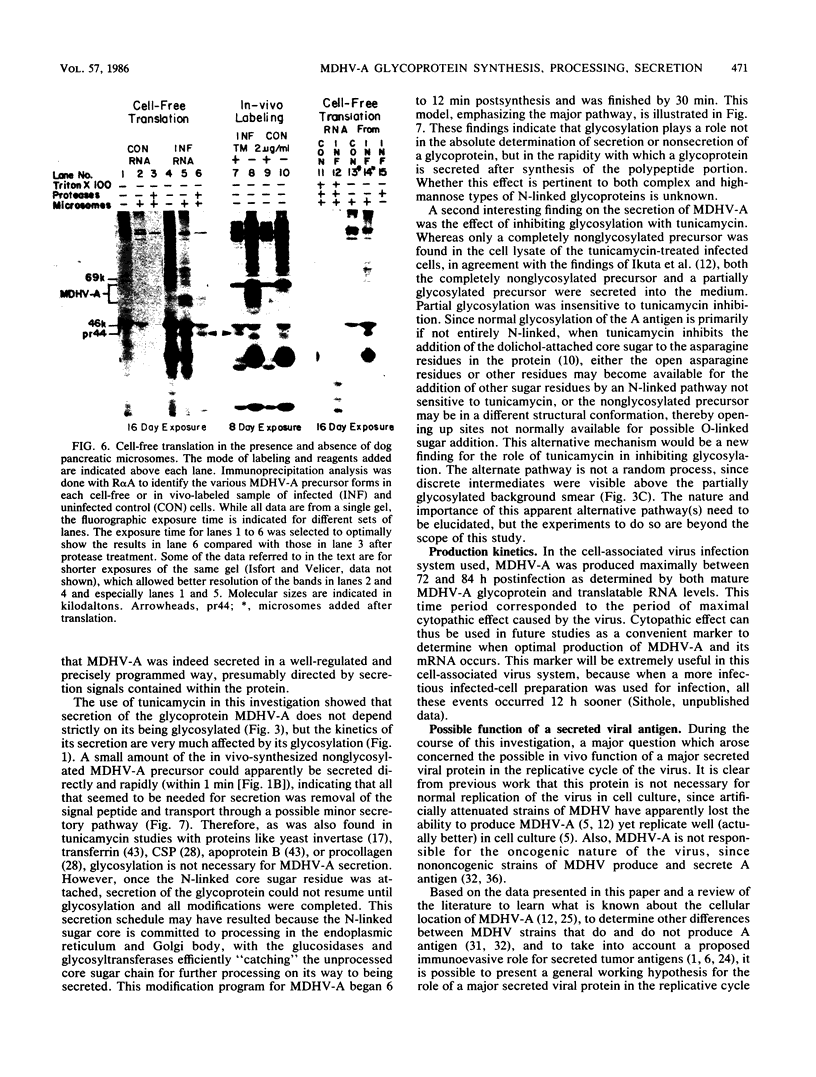
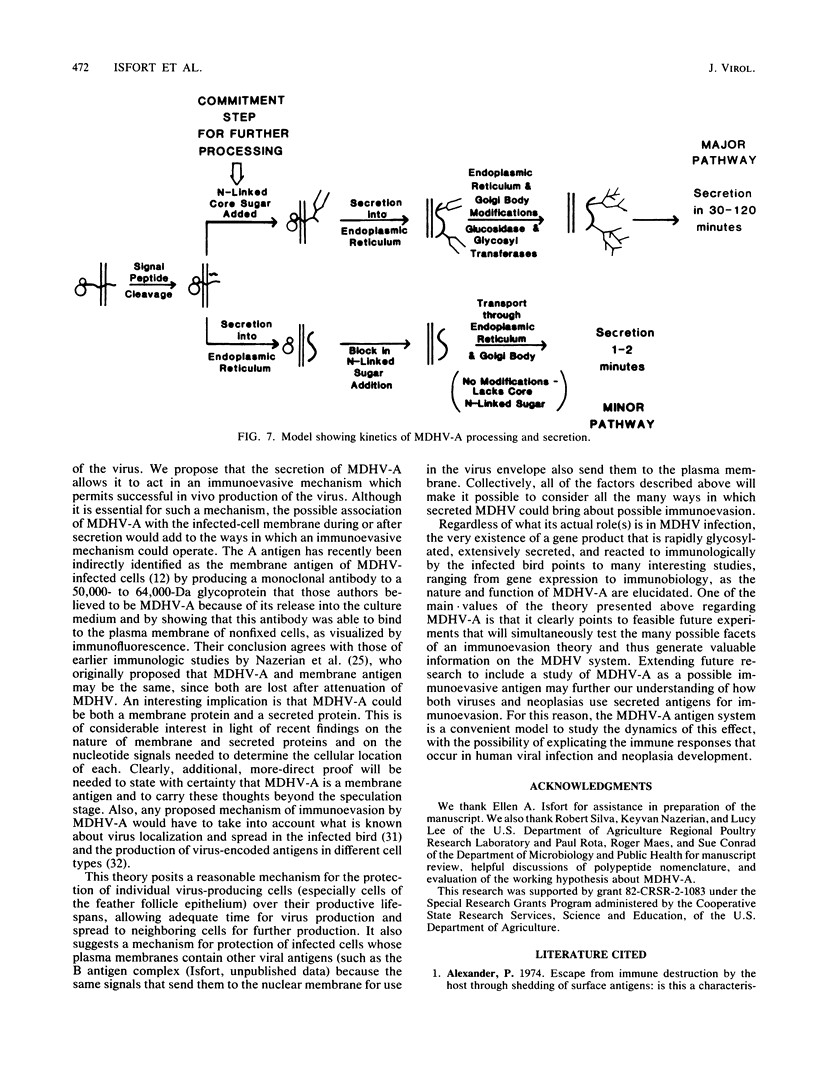
The 57,000- to 65,000-dalton (Da) Marek's disease herpesvirus A (MDHV-A) antigen glycoprotein (gp57-65) has a 47,000-Da unglycosylated precursor polypeptide (pr47), as determined by immunological detection after cell-free translation of infected-cell mRNA. Cleavage of its signal peptide yielded a 44,000-Da precursor polypeptide molecule (pr44), detected both in vivo after tunicamycin inhibition of glycosylation and in vitro after dog pancreas microsome processing of pr47. High-resolution pulse-chase studies showed that pr44 was quickly glycosylated (within 1 min) to nearly full size, a rapid processing time consistent with a cotranslational mode of glycosylation. This major glycosylation intermediate was further modified 6 to 30 min postsynthesis (including the addition of sialic acid), and mature MDHV-A was secreted 30 to 120 min postsynthesis. Limited apparent secretion of pr44 occurred only in the first minute postsynthesis, in contrast to the later secretion of most of the MDHV-A polypeptide as the fully glycosylated form described above. In addition, in the presence of tunicamycin a small fraction of the newly synthesized MDHV-A protein appeared as a secreted, partially glycosylated, heterogeneously sized precursor larger than pr44. pr44 constituted the major fraction of the new MDHV-A made in the presence of the inhibitor but the precursor was smaller than mature MDHV-A. These data indicate that there is a minor glycosylation pathway not sensitive to tunicamycin and that "normal" glycosylation is not necessary for secretion. Collectively, the data demonstrate that the rapid release of most of the fully glycosylated form of MHDV-A from the cell shortly after synthesis is true secretion in a well-regulated and precisely programmed way and not the result of cell death and disruption.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander P. Proceedings: Escape from immune destruction by the host through shedding of surface antigens: is this a characteristic shared by malignant and embryonic cells? Cancer Res. 1974 Aug;34(8):2077–2082. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Calnek B. W., Carlisle J. C., Fabricant J., Murthy K. K., Schat K. A. Comparative pathogenesis studies with oncogenic and nononcogenic Marek's disease viruses and turkey herpesvirus. Am J Vet Res. 1979 Apr;40(4):541–548. [PubMed] [Google Scholar]
- Churchill A. E., Chubb R. C., Baxendale W. The attenuation, with loss of oncogenicity, of the herpes-type virus of Marek's disease (strain HPRS-16) on passage in cell culture. J Gen Virol. 1969 Jun;4(4):557–564. doi: 10.1099/0022-1317-4-4-557. [DOI] [PubMed] [Google Scholar]
- Coggin J. H., Jr, Ambrose K. R., Dierlam P. J., Anderson N. G. Proceedings: Proposed mechanisms by which autochthonous neoplasms escape immune rejection. Cancer Res. 1974 Aug;34(8):2092–2101. [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Glaubiger C., Nazerian K., Velicer L. F. Marek's disease herpesviruses. IV. Molecular characterization of Marek's disease herpesvirus A antigen. J Virol. 1983 Mar;45(3):1228–1234. doi: 10.1128/jvi.45.3.1228-1234.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Ikuta K., Ueda S., Kato S., Hirai K. Monoclonal antibodies reactive with the surface and secreted glycoproteins of Marek's disease virus and herpesvirus of turkeys. J Gen Virol. 1983 Dec;64(Pt 12):2597–2610. doi: 10.1099/0022-1317-64-12-2597. [DOI] [PubMed] [Google Scholar]
- Ikuta K., Ueda S., Kato S., Hirai K. Most virus-specific polypeptides in cells productively infected with Marek's disease virus or herpesvirus of turkeys possess cross-reactive determinants. J Gen Virol. 1983 Apr;64(Pt 4):961–965. doi: 10.1099/0022-1317-64-4-961. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- Kaaden O. R., Dietzschold B. Alterations of the immunological specificity of plasma membranes from cells infected with Marek's disease and turkey herpes viruses. J Gen Virol. 1974 Oct;25(1):1–10. doi: 10.1099/0022-1317-25-1-1. [DOI] [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Lingappa V. R., Blobel G., Lodish H. F. Membrane assembly in vitro: synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3278–3282. doi: 10.1073/pnas.74.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kuo S. C., Lampen J. O. Tunicamycin--an inhibitor of yeast glycoprotein synthesis. Biochem Biophys Res Commun. 1974 May 7;58(1):287–295. doi: 10.1016/0006-291x(74)90925-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Long P. A., Clark J. L., Velicer L. F. Marek's Disease Herpesviruses II. Purification and Further Characterization of Marek's Disease Herpesvirus A Antigen. J Virol. 1975 May;15(5):1192–1201. doi: 10.1128/jvi.15.5.1192-1201.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long P. A., Kaveh-Yamini P., Velicer L. F. Marek's Disease Herpesviruses I. Production and Preliminary Characterization of Marek's Disease Herpesvirus A Antigen. J Virol. 1975 May;15(5):1182–1191. doi: 10.1128/jvi.15.5.1182-1191.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J. T., Cohen G. H., Eisenberg R. J. Synthesis and processing of glycoprotein D of herpes simplex virus types 1 and 2 in an in vitro system. J Virol. 1983 Nov;48(2):521–533. doi: 10.1128/jvi.48.2.521-533.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T., Mori E., Sudo T., Kano K. Expression of heterophil Forssman antigen as glycoprotein on transformed rat cell lines: shedding of the antigen from the cells. J Natl Cancer Inst. 1984 Nov;73(5):1179–1186. [PubMed] [Google Scholar]
- Nazerian K. Studies on intracellular and membrane antigens induced by Marek's disease virus. J Gen Virol. 1973 Oct;21:193–195. doi: 10.1099/0022-1317-21-1-193. [DOI] [PubMed] [Google Scholar]
- Okazaki W., Purchase H. G., Burmester B. R. Protection against Marek's disease by vaccination with a herpesvirus of turkeys. Avian Dis. 1970 May;14(2):413–429. [PubMed] [Google Scholar]
- Olden K., Pratt R. M., Yamada K. M. Role of carbohydrates in protein secretion and turnover: effects of tunicamycin on the major cell surface glycoprotein of chick embryo fibroblasts. Cell. 1978 Mar;13(3):461–473. doi: 10.1016/0092-8674(78)90320-3. [DOI] [PubMed] [Google Scholar]
- Olden K., Pratt R. M., Yamada K. M. Role of carbohydrates in protein secretion and turnover: effects of tunicamycin on the major cell surface glycoprotein of chick embryo fibroblasts. Cell. 1978 Mar;13(3):461–473. doi: 10.1016/0092-8674(78)90320-3. [DOI] [PubMed] [Google Scholar]
- Payne L. N., Frazier J. A., Powell P. C. Pathogenesis of Marek's disease. Int Rev Exp Pathol. 1976;16:59–154. [PubMed] [Google Scholar]
- Payne L. N., Rennie M. Pathogenesis of Marek's disease in chicks with and without maternal antibody. J Natl Cancer Inst. 1973 Nov;51(5):1559–1573. doi: 10.1093/jnci/51.5.1559. [DOI] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. Distinct repressible mRNAs for cytoplasmic and secreted yeast invertase are encoded by a single gene. Cell. 1981 Aug;25(2):525–536. doi: 10.1016/0092-8674(81)90071-4. [DOI] [PubMed] [Google Scholar]
- Purchase H. G., Burmester B. R., Cunningham C. H. Responses of cell cultures from various avian species to Marek's disease virus and herpesvirus of turkeys. Am J Vet Res. 1971 Nov;32(11):1811–1823. [PubMed] [Google Scholar]
- Purchase H. G., Okazaki W., Burmester B. R. Long-term field trials with the herpesvirus of turkeys vaccine against Marek's disease. Avian Dis. 1972 Apr;16(1):57–71. [PubMed] [Google Scholar]
- Ross L. J., Basarab O., Walker D. J., Whitby B. Serological relationship between a pathogenic strain of Marek's disease virus, its attenuated derivative and herpes virus of turkeys. J Gen Virol. 1975 Jul;28(1):37–47. doi: 10.1099/0022-1317-28-1-37. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Lodish H. F. Synchronised transmembrane insertion and glycosylation of a nascent membrane protein. Nature. 1977 Oct 27;269(5631):775–780. doi: 10.1038/269775a0. [DOI] [PubMed] [Google Scholar]
- Scheele G., Jacoby R., Carne T. Mechanism of compartmentation of secretory proteins: transport of exocrine pancreatic proteins across the microsomal membrane. J Cell Biol. 1980 Dec;87(3 Pt 1):611–628. doi: 10.1083/jcb.87.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele G. Methods for the study of protein translocation across the RER membrane using the reticulocyte lysate translation system and canine pancreatic microsomal membranes. Methods Enzymol. 1983;96:94–111. doi: 10.1016/s0076-6879(83)96011-1. [DOI] [PubMed] [Google Scholar]
- Silva R. F., Lee L. F. Monoclonal antibody-mediated immunoprecipitation of proteins from cells infected with Marek's disease virus or turkey herpesvirus. Virology. 1984 Jul 30;136(2):307–320. doi: 10.1016/0042-6822(84)90167-3. [DOI] [PubMed] [Google Scholar]
- Van Zaane D., Brinkhof J. M., Westenbrink F., Gielkens A. L. Molecular-biological characterization of Marek's disease virus. I. Identification of virus-specific polypeptides in infected cells. Virology. 1982 Aug;121(1):116–132. doi: 10.1016/0042-6822(82)90122-2. [DOI] [PubMed] [Google Scholar]
- Velicer L. F., Yager D. R., Clark J. L. Marke's disease herpesviruses. III. Purification and characterization of Marek's disease herpesvirus B antigen. J Virol. 1978 Jul;27(1):205–217. doi: 10.1128/jvi.27.1.205-217.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 1983;96:84–93. doi: 10.1016/s0076-6879(83)96010-x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Witte O. N., Wirth D. F. Structure of the murine leukemia virus envelope glycoprotein precursor. J Virol. 1979 Feb;29(2):735–743. doi: 10.1128/jvi.29.2.735-743.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter R. L., Nazerian K., Purchase H. G., Burgoyne G. H. Isolation from turkeys of a cell-associated herpesvirus antigenically related to Marek's disease virus. Am J Vet Res. 1970 Mar;31(3):525–538. [PubMed] [Google Scholar]