Abstract
To evaluate the potential for neuronal replacement following destruction of vagal afferent neurons, we examined nodose ganglia following intraperitoneal capsaicin treatment of adult rats. Rats received capsaicin or vehicle followed by a regimen of 5'-bromo-2'-deoxyuridine injections (BrdU) to reveal DNA replication. Nodose ganglia were harvested at various times post-treatment and processed for DAPI nuclear staining and immunofluorescence to estimate neuronal numbers and to determine vanilloid receptor, cleaved caspase 3, TUNEL, BrdU, the neuron-selective marker PGP-9.5 and neurofilament-M-immunoreactivity. Twenty-four hours after capsaicin approximately 40% of nodose ganglion neurons expressed cleaved caspase 3-immunoreactivity and 16% revealed TUNEL staining, indicating that primary sensory neurons are killed by the capsaicin treatment of adult rats. The occurrence of neuronal death was confirmed by counts of DAPI-stained neuronal nuclei, which revealed ≥50% reduction of nodose neuron number by 30 days post-capsaicin. However, by 60 days post-capsaicin, the total numbers of neuronal nuclei in nodose ganglia from capsaicin-treated rats were not different from controls, suggesting that new neurons had been added to the nodose ganglia. Neuronal proliferation was confirmed by significant BrdU incorporation in nuclei of nodose ganglion cells immunoreactive for the neuron-specific antigen PGP-9.5 revealed 30 and 60 days post-capsaicin. Collectively, these observations suggest that in adult rats massive scale neurogenesis occurs in nodose ganglia following capsaicin-induced neuronal destruction. The adult nodose ganglion, therefore, provides a novel system for studying neural plasticity and adult neurogenesis after peripheral injury of primary sensory neurons.
Keywords: nodose ganglion, capsaicin, neurogenesis, neuronal death, rat, sensory neurons
Introduction
The occurrence of neurogenesis in adult mammals now is well established. Although, the numbers of new neurons generated in adult mammalian nervous systems appear relatively small, the rate at which new neurons appear is not constant but can be increased or decreased in response to stress (Mirescu and Gould, 2006), activity (Bordey, 2006) drugs (Huang and Herbert, 2006; Perera et al., 2007) or neuronal injury (Groves et al., 2003; Kokaia and Lindvall, 2003; Zhang et al., 2006). Nevertheless, an increase in neurogenesis that results in addition to or replacement of a large proportion of existing neurons has not been reported in adult mammals, although it does occur in some non-mammalian vertebrates (Chapouton et al., 2007).
In the experiments reported here, we tested the hypothesis that destruction of primary vagal afferent neurons would trigger neuronal death and subsequent neurogenesis in the nodose ganglia (NG) of adult rats. We utilized the neurotoxin capsaicin to selectively destroy unmyelinated axons of small primary afferent neurons (Hiura, 2000). While direct examination of the adult NG to assess capsaicin-induced destruction of NG perikarya has not previously been reported, the vast majority of NG neurons express the capsaicin (vanilloid) receptor (VR1) (Ichikawa and Sugimoto, 2003), and are capsaicin sensitive (Simasko and Ritter, 2003). Moreover, capsaicin treatment of adult rats produces extensive degeneration of vagal afferent axons and terminals (Ritter and Dinh, 1988). These observations, suggested to us that capsaicin is likely to destroy the NG perikarya, thereby providing a system in which examine the effects of large scale neuronal destruction on neurogenesis.
To assess capsaicin-induced neuronal death we monitored expression of cleaved caspase-3 and TUNEL as indicators of apoptotic and necrotic destruction of NG neurons. We also confirmed the extent of subsequent neuronal loss by counting neuronal nuclei at various times after capsaicin treatment. We utilized bromodeoxyuridine (BrdU) incorporation as an indicator of proliferating cells (Nowakowski et al., 1989) and identified NG cells as having a neuronal phenotype according to the shape of their nuclei and their expression of protein gene product (PGP) 9.5, a neuron-specific protein (Thompson et al., 1983) or neurofilament-M (NfM). To determine whether neuronal phenotypes are altered following capsaicin treatment, we also surveyed the expression of VR1 in NG neurons pre- and post-capsaicin.
We observed that capsaicin treatment of adult rats resulted in rapid degeneration and death of NG neurons. We also observed that neuronal death was followed by an increase in neurogenesis sufficient to restore NG neuronal numbers to near pre-capsaicin levels. Our results indicate that the NG of capsaicin-treated animals constitutes a new system for studying neural plasticity and adult neurogenesis after primary sensory neurons destruction.
Materials and Methods
Animals
Male Sprague-Dawley rats (6 weeks old, Simonsen Labs, Gilroy, CA) were individually housed in a temperature-controlled vivarium with ad libitum access to food (Harlan Teklad F6 Rodent Diet W, Madison, WI) and water. Rats were maintained on a 12:12-h light-dark schedule and were habituated to laboratory conditions for 7 days prior to capsaicin injections. All animal procedures were approved by the Washington State University Institutional Animal Care and Use Committee and conform to National Institutes of Health guidelines for the use of vertebrate animals (publication No. 86-23, revised 1985).
Capsaicin treatment
Sixty-four rats were injected intraperitoneal with capsaicin (Sigma M2028). The total capsaicin dose (125 mg/kg) was administered as a series of three injections (25, 50, 50 mg/kg) at an injection volume of 1 ml/kg. All three injections were made within a 24-h period (0, 6, and 24 h respectively). Additionally, sixty-four rats were treated with the vehicle injections (10% ethanol in 10% Tween-80 in 0.9% saline) at the same injection volumes and schedule. Rats were given a subcutaneous injection of atropine sulfate (3 mg) twenty minutes before the first capsaicin or vehicle injection. Rats were under general inhalation anesthesia during the capsaicin or vehicle treatment. Anesthesia was maintained with isoflurane in oxygen at the end-tidal concentration 3.0% reduced after 5 min to 1.5%. Artificial ventilation was provided, as required, during the 3 – 5 minute period of respiratory arrest that typically occurred after the first capsaicin injection. The eye wipe response to mild corneal stimulation, a response mediated by the capsaicin-sensitive trigeminal innervation of the cornea (Gamse et al., 1981), was tested to screen the effectiveness of capsaicin treatment. Briefly, a drop of 1 % ammonium hydroxide was placed on the corneal surface of one eye. All control rats immediately wipe the stimulated eye about five times in the first 10 seconds post stimulation. None of the capsaicin-treated rats exhibited any eye wipe response. Therefore, all eighty-four capsaicin-treated rats were included in the study.
BrdU injections
Twenty four capsaicin-treated and twenty four vehicle-treated rats were used to determine the effect of capsaicin treatment on NG BrdU incorporation. BrdU (Sigma-Aldrich, USA) was dissolved in 0.9% NaCl (10 mg/ml). In brief, BrdU (50 mg/kg, 12 capsaicin and 12 vehicle-treated rats) or saline (5 ml/kg, 12 capsaicin and 12 vehicle-treated rats) were injected intraperitoneal 2 h after third capsaicin or vehicle injection and then every other day until euthanasia and collection of tissues. On days 3, 30 and 60 after treatment, 4 capsaicin/BrdU, 4 vehicle/BrdU, 4 capsaicin/saline and 4 vehicle-/saline-injected rats respectively were anesthetized and perfused transcardially with saline followed by 4% paraformaldehyde in buffered saline (pH 7.4).
Tissue fixation and sectioning
Immediately following the perfusion, nodose ganglia were collected, post-fixed (4% paraformaldehyde, 30 min), and immersed overnight in a cryoprotectant solution of 18% sucrose in PBS and NaN3. The ganglia were cut longitudinally into 20-µm serial sections, mounted in four series on glass slides (Superfrost Plus), with every fourth section on the same slide. Generally this procedure yielded 28–32 20-µm thick sections per ganglion, with each slide containing 7–8 20-µm sections with an interval of 60 microns between adjacent sections. After drying on the slides, the sections were rehydrated and processed for DAPI staining and detection of selected antigens.
Immunohistochemistry and DAPI staining
The slide-mounted sections were immersed for 15 min in a 0.1% sodium borohydride solution to reduce auto fluorescence (Clancy and Cauller, 1998). Hydrochloric acid or citrate buffer antigen retrieval methods were used in the study. Subsequently, the sections were incubated overnight in a blocking solution consisting of 10% normal horse serum in Tris sodium phosphate buffer (TPBS, pH 7.4). The blocking solution was washed from the tissues, and each section was incubated for an appropriate period of time at room temperature in primary antisera against VR1 (12h, 24 rats) or caspase 3 (cas-3; 12h, 16 rats). For double labeling (48 rats), sections were incubated in mixture of primary antisera against BrdU and PGP-9.5 (48h) or stained with In Situ Cell Death Detection Kit (TUNEL, Roche Applied Sciences, IN) and co-labeled with primary antibody against neurofilament M (Nf-M). BrdU/PGP-9.5 staining protocol was a combination of standard Labeled Streptavidin Biotin (LSAB) and standard immunofluorescence (IF) methods. Steamer/citrate antigen retrieval method was used for BrdU staining (Tang et al., 2007). Primary antibodies used in the study are listed and characterized in table 1. Subsequently, the sections were incubated 2h at room temperature in an appropriate secondary antibody for single staining or in an appropriate mixture of secondary antisera for double staining (Alexa 488, Alexa 555, dilution 1:400, Invitrogen, CA; HRP Rabbit Anti-Mouse IgG, dilution 1:400, Invitrogen, CA). Sections were counter-stained with DAPI (Molecular Probes, Eugene, OR), to reveal neuronal and glia nuclei, then mounted in ProLong (Molecular Probes, Eugene, OR) to reduce photo-bleaching. Nodose ganglia from sixteen capsaicin-treated rats and sixteen vehicle-treated rats were collected 3, 11, 30 or 60 days post treatment (4 animals in each group respectively) and processed only for DAPI staining.
Table 1.
Primary antibodies
| Target | Source | Cat. Nr. | Host | Dillution | Immunogen |
|---|---|---|---|---|---|
| VR1 | Neuromics | GT15129 | Goat | 1:200 | KLH-coupled peptide corresponding to amino acid residues 4 – 21 of VR1 |
| Cleaved cas-3 | Cell Signaling | Asp175 | Rabbit | 1:100 | fragment (17/19 kDa) of caspase-3 resulting from cleavage adjacent to Asp175 |
| BrdU | Sigma | B8434 | Mouse | 1:100 | bromodeoxyuridine conjugated to KLH |
| PGP 9.5 | Neuromics | GP14104 | Guinea Pig | 1:500 | corresponding to residues 175–191 of soluble cytoplasmic protein human PGP 9.5 |
| NfM | Chemicon | AB1981 | Rabbit | 1:250 | highly purified neurofilament (bovine) polypeptide (150 kDa) |
Controls of immunohistochemistry and DAPI staining
The primary antibodies used in the study were chosen based on the western blot confirmed specificity by the supplier. Additionally, 20 µm cryostat sections of the proximal duodenum were used as positive controls for cas-3, TUNEL and BrdU labeling. Significant number of epithelial cells nuclei was labeled with primary antibody against cas-3 or TUNEL kit. All the duodenum sections from capsaicin/BrdU and vehicle/BrdU treated rats revealed significant numbers of epithelial nuclei stained with primary antibody against BrdU while there was no staining in capsaicin/saline and vehicle-/saline-injected rats. Negative controls for the single and double immunohistochemistry trials were performed by omission of primary antibodies in original reaction sequences. Separate sets of control sections were pre-incubated with the immune serum plus the peptide antigen used to raise the antisera prior to application to the tissue. Both of these control procedures eliminated immunostaining in the nodose ganglia and duodenum for all of the antisera we used. Identification of TUNEL and BrdU labeled nuclei was confirmed by the analysis of Z-stack/ApoTome images of TUNEL/NfM and BrdU/PGP-9.5 captured on AxioPlan 2 and processed with AxioVision 4.6 software (Carl Zeiss Vision, Oberkochen, Germany). X and Y sliders were placed on the positive nuclei and “cut views” of the Z-stacks revealed TUNEL or BrdU stained nuclei surrounded by NfM or PGP-9.5 stained cytoplasm. DAPI labeled neuronal nuclear profiles were easily discriminated from glia nuclei by virtue of their large size and spherical shape and one or two nucleoli shadows. Control of DAPI staining was based on our previous study of NG neurons (Czaja et al., 2006).
Counting of nodose ganglion neurons
Images of the nodose ganglia were viewed and captured with a Zeiss Axioplan 2 imaging photomicroscope equipped with a digital camera (Axio Cam MRc) and appropriate filters for DAPI, CY3 and Alexa 488. With the aid of a computer, the captured images were evaluated with the Axio Vision 4.6 Imaging system. For preparation of microscopic illustrations, Corel Graphic Suite 11 was used only to adjust brightness and contrast and sharpness and to make composite plates.
In order to determine the total number of neurons in the NG, we counted DAPI labeled neuronal nuclear profiles under × 200 magnification in all of the sections for all series from a given ganglion and numbers were corrected for split nuclei as previously described (Czaja et al., 2006). The number of positive-stained neuronal profiles for particular antigen was counted using Axio Vision 4.6 Imaging system in every third section of the ganglion to eliminate the likelihood of counting the same neuron twice. Only neurons that exhibited a clear nuclear shadow or nuclear profile were counted and the percentage of immunopositive cells was calculated. Differences in cell number or expression of particular antigen immunoreactivity were tested for statistical significance using repeated measures analysis of variance (ANOVA) and Bonferroni’s post-hoc test. Data are expressed as means ±SEM.
Electron microscopy
Four capsaicin-treated rats and four vehicle-treated rats underwent ultrastructural examination of the vagi 180 days after capsaicin or vehicle treatment. Segments of cervical vagi from these rats were processed for transmission electron microscopy according to the procedure of Hulsebosch and Coggeshall (Hulsebosch and Coggeshall, 1982). Briefly, the rats were perfused with buffered NaCl, followed by a solution containing 3% paraformaldehyde, 3% glutaraldehyde and 0.1% picric acid in 0.1M caclodylate buffer (pH7.4). One-cm segments of the left cervical vagi were collected, post-fixed in the same fixative overnight, and then placed in 1% osmic acid and 1.5% potassium ferricyanide in 0.1M cacodylate (pH 7.4) for an additional 2h. After rinsing in 0.1M maleate buffer (pH5.2), the tissue was soaked overnight in 0.5% uranyl acetate in maleate buffer, then dehydrated through a series of ethanols, and embedded in araldite/epon. Thin sections were prepared with a diamond knife, placed on grids, stained with 0.1% lead acetate and examined with a Zeiss transmission electron microscope.
Results
There were no significant differences between the left and right NG in the expression of VR1-, cas-3-, TUNEL-, BrdU-, PGP-9.5 and NfM-immunoreactivity for particular time points after treatment. Additionally there were no significant differences in the immunoreactivity of studied antigens between NG from rats collected at different time points after vehicle-treatment. Therefore, the remaining results represent pooled data for both the left and the right NG and for all post-vehicle time points. We found that 59.3% ± 1.7% of the total population of NG neurons in vehicle-treated rats expressed VR1 immunoreactivity, while in NG collected 3 days after capsaicin treatment, only 10.8% ± 0.5% of the total NG population expressed VR1 immunoreactivity (Fig. 1). However, NG collected 30 days after capsaicin treatment revealed significantly higher VR1 immunoreactivity (46.8% ± 2.4%) than NG collected 3 days post capsaicin. There was no statistically significant difference between the proportions of VR1-IR neurons in NG collected 30 and 60 days post capsaicin (46.8% ± 2.4% vs. 49.4% ± 0.9%).
Fig. 1.
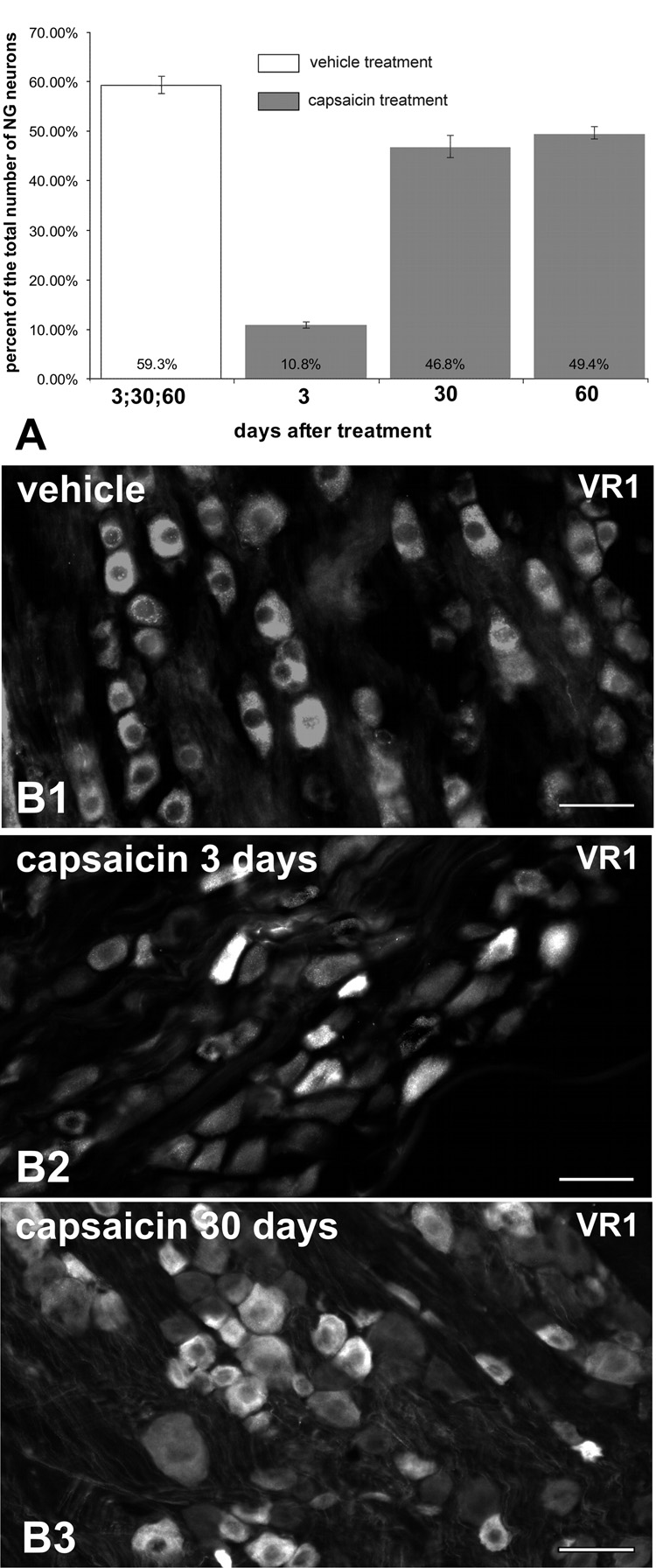
Decline and recovery of VR1-IR in NG after capsaicin treatment. Proportion of NG neurons expressing VR1-IR ± SEM (A; n=4 per time point per treatment). Immunofluorescence revealed VR1-IR in small and medium size neurons in the NG ganglia of vehicle- (B1) and capsaicin-treated rats (B2–3). However the proportion of VR1-IR neurons was decreased 3 days post-capsaicin (B2) but increased again by 30 days post-capsaicin (B3). Note, however, that the total number of NG neurons in capsaicin treated rats, as estimated by counts of neuronal nuclei (Fig. 5), was approximately 50% of the total for control. Therefore, the total number of VR1-IR neurons did not approach control levels until 60 days after capsaicin. Scale bars = 50 µm.
Twenty-four hours post-capsaicin, a significant proportion of NG neurons (39.2% ± 2.8%) exhibited cleaved cas-3 immunoreactivity (cas-3-IR); whereas NG collected 30 and 60 days post capsaicin as well as NG from vehicle-treated rats revealed only one to ten cas-3-IR neurons per ganglion (0.1 ± 0.02%, 0.1 ± 0.02%, and 0.1 ± 0.02% respectively; Fig. 2). Significant populations of TUNEL labeled nuclei of NfM-IR neurons were found 24h after capsaicin treatment (16.1% ± 3.2%; Fig. 3a–c) while very few labeled nuclei were found after vehicle treatment or 30 and 60 days post-capsaicin (4.0 ± 0.9%, 1.7 ± 0.4%, and 2.9 ± 0.8% respectively; Fig. 3d–f). Significant number of BrdU-labeled cells expressing simultaneously PGP-9.5-IR were found in NG collected 30 (9.2% ± 1.3%) or 60 (11.9% ± 0.4%) days after capsaicin treatment (Fig. 4a–c). Very few BrdU-immunoreactive nuclei were present in PGP-9.5-IR cells of the vehicle treated rats (0.4% ± 0.2%) and in the NG from rats perfused 3 days after capsaicin treatment (0.5% ± 0.2%; Fig. 4d–f). No BrdU immunoreactive nuclei were ever observed in the negative control sections obtained from rats that did not receive BrdU injections.
Fig. 2.
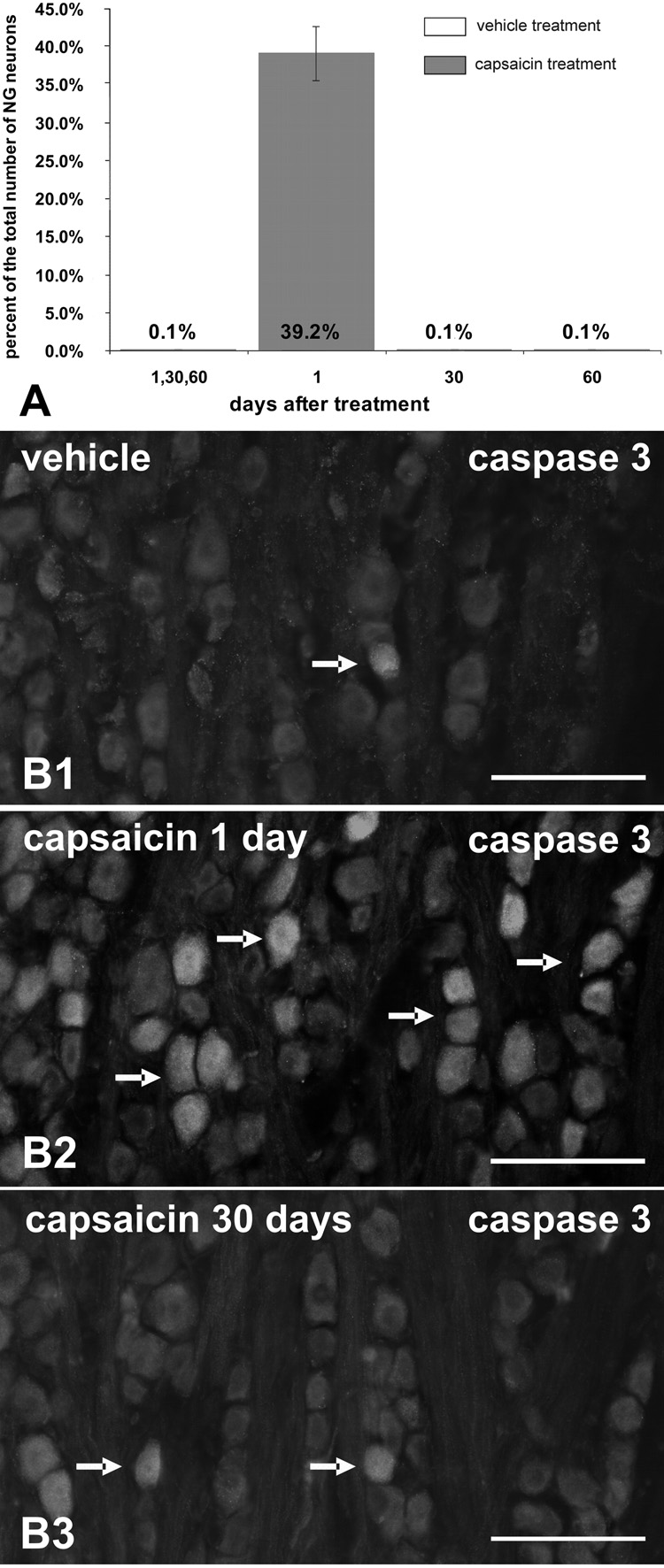
Caspase-3-IR in NG neurons 24h after systemic treatment with capsaicin or vehicle (A; n = 4 per time point per treatment). Note the numerous cas-3-IR neurons in NG one day postcapsaicin treatment (B2; arrows). Very few NG neurons were cas-3-IR at any time point in vehicle-treated rats (B1) or capsaicin-treated rats 30 (B3) and 60 days post-treatment (arrows). Scale bars = 50 µm.
Fig. 3.
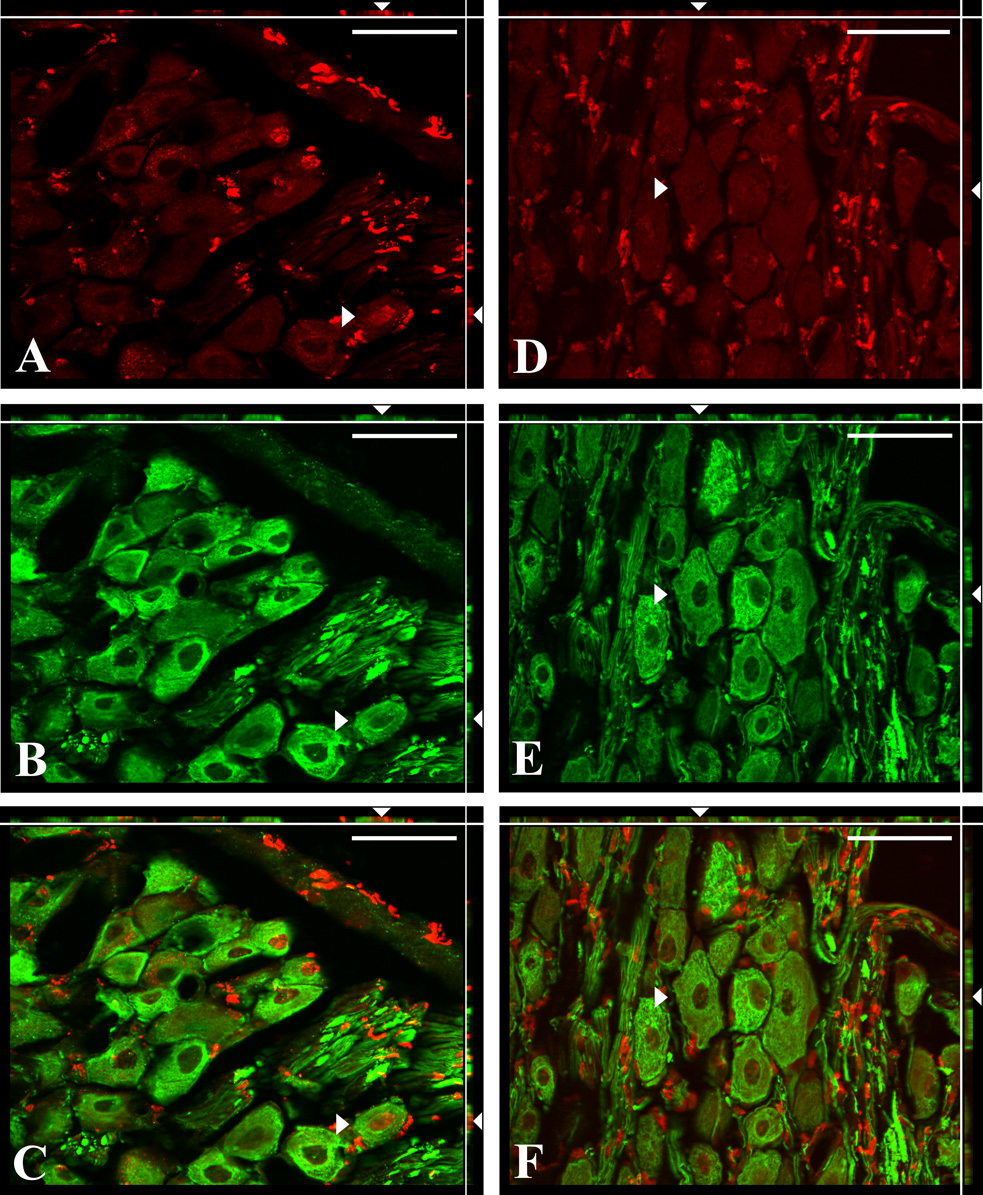
Double immunofluorescence staining for TUNEL (red) and NfM (green) in the NG sections from capsaicin (A–C) and vehicle (D–F) treated rats. TUNEL positive glia cells were present in both the capsaicin (A) and vehicle (D) treated rats indicating physiological turnover previously reported in dorsal root ganglia after axotomy (McKay et al., 2002). ApoTome images showing examples of TUNEL labeled nuclei (A, C) from NfM immunoreactive neuron (B, C) in the plane X–Y (arrowhead). Optical planes, X–Z (top margin) and Y–Z (right margin), show the depth of this neuron (arrowheads). TUNEL-labeled nuclei were surrounded by NfM-immunoreactive cytoplasm in all three dimensions what indicates that dual labeled perikarya (arrowheads) of NG neurons were undergoing cell death (C, merged image). Images D–F show the example of TUNEL-negative (D) and NfM-immunoreactive (E) neuron that is not dying (arrowhead) from vehicle treated rat in all three planes (F, merged image). Scale bars = 50 µm.
Fig. 4.
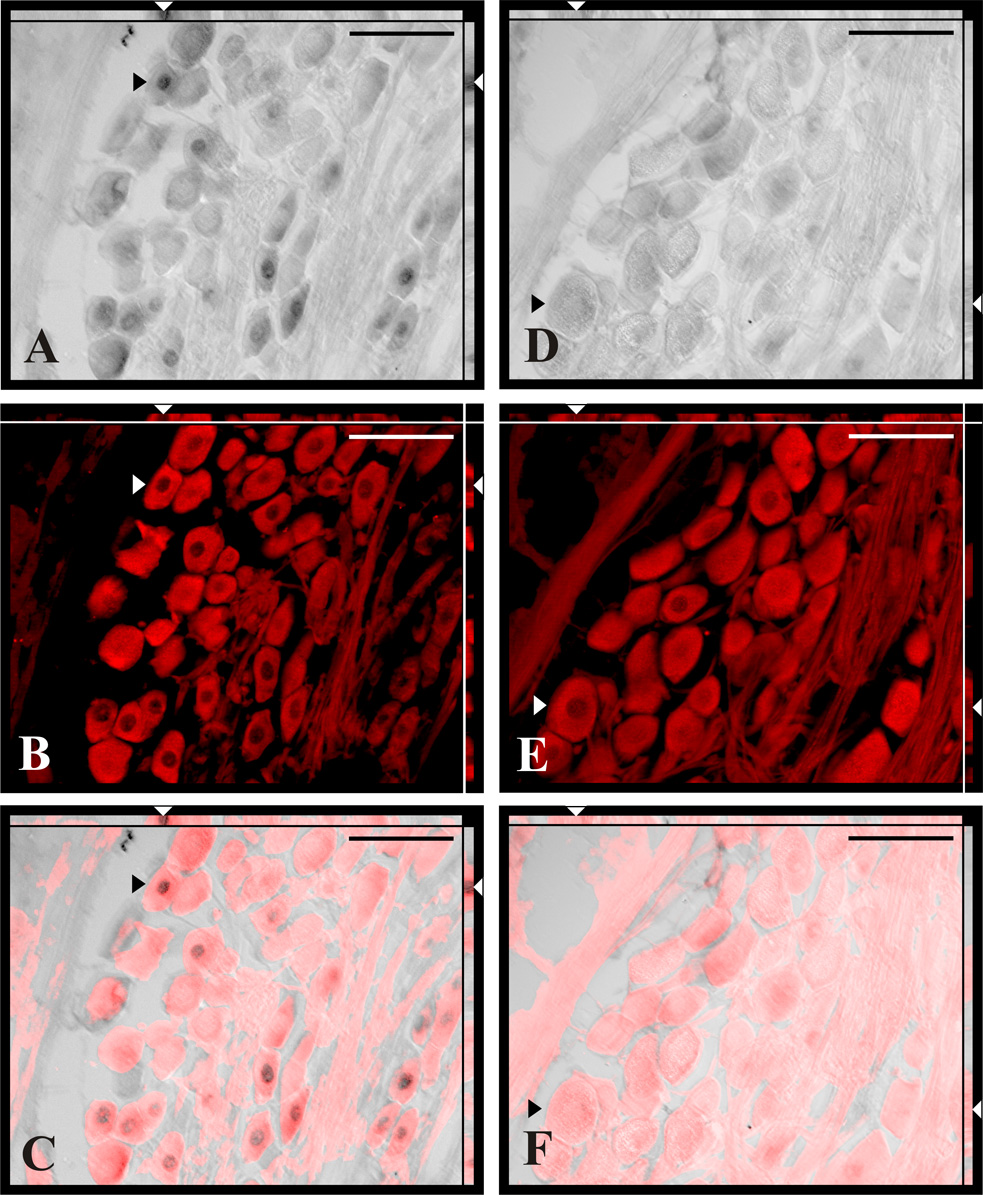
Double labeled sections of the NG for BrdU incorporation (A, D) and neuron-selective marker PGP-9.5 (B, E) from capsaicin (A–C) and vehicle (D–F) treated rats. Note that all BrdU-labeled neurons (A, arrowheads) were simultaneously PGP-9.5 immunoreactive (B, arrowheads) in capsaicin treated rats. Double labeling was confirmed on merged image analyzed in X–Y, X–Z and Y–Z optical planes revealing BrdU labeled nucleus surrounded by PGP-9.5 positive cytoplasm (C, arrowheads). Scale bars = 50 µm.
The total number of DAPI-stained neuronal nuclei in the left and right NG of vehicle-treated rats did not differ at 3, 30 and 60 days post injection (Fig. 5). However, NG from capsaicin-treated rats collected 3, 11 and 30 days post treatment contained significantly fewer DAPI-labeled neuronal nuclei than those of vehicle-treated rats collected post injection at comparable time points. The nadir of the decrease in the number of neuronal nuclei occurred at 30 days post-capsaicin treatment (Fig. 5). However, by 60 days post-injection there was no difference in neuronal numbers of the NG between the vehicle- and capsaicin-treated rats.
Fig. 5.
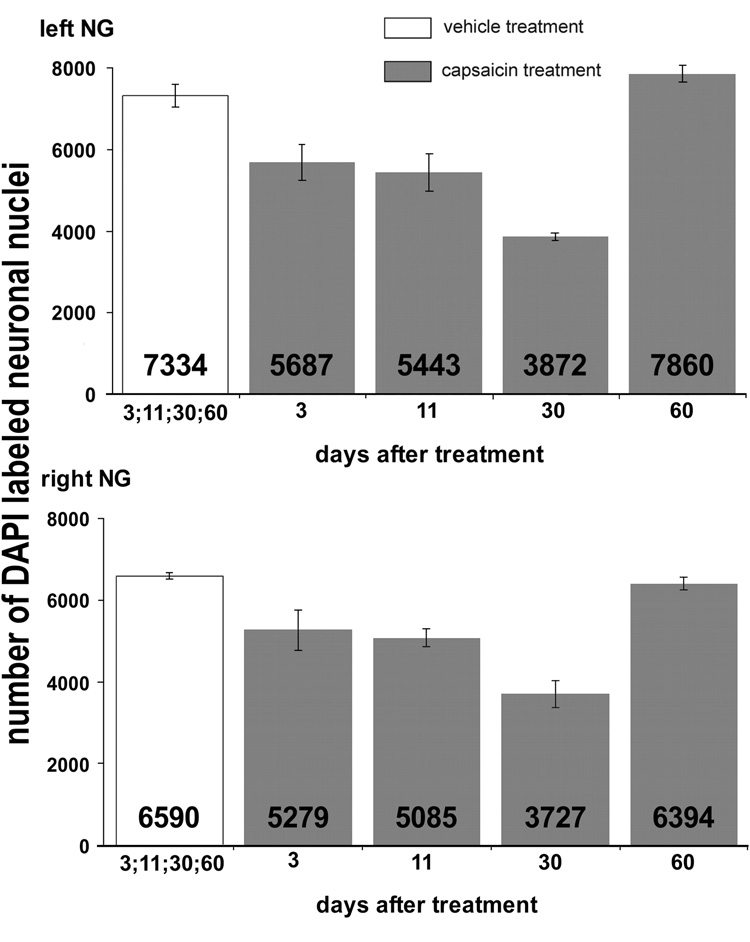
Total numbers of DAPI-labeled neuronal nuclei in the left (upper graph) and the right (lower graph) NG from vehicle- and capsaicin-treated rats collected 3, 11, 30 and 60 days after treatment (n = 4 per time point per treatment). Bars represent average neuronal number ± SEM.
We have not yet attempted extensive quantitative analysis of transmission electron micrographs of cervical vagi. Nevertheless, our electron microscopic examination of cross sections of cervical vagi collected 180 days after either capsaicin or vehicle administration (Fig. 6) revealed a striking change in axon morphology in capsaicin-treated rats. Specifically, the diameters of unmyelinated axons in vagi from capsaicin-treated rats typically were much smaller than in vehicle treated rats. This difference was due to the appearance of large numbers of very fine neurite profiles in capsaicin-treated vagi, which were never observed in the vagi of vehicle-treated rats. While vagi from both vehicle- and capsaicin-treated rats contained typical unmyelinated axon profiles with diameters of 0.6 – 0.85 microns, the vagi from capsaicin-treated rats also contained large numbers of much smaller profiles ranging in diameter from approximately 0.04 – 0.1 micron. Unmyelinated axon profiles of both vehicle- and capsaicin-treated rats were surrounded by Schwan cell processes. However, while contacts between distinct axon profiles in vehicle-treated vagi were rare, in capsaicin-treated vagi there appeared to be many instances of adjacent axons in apparent contact with each other. Contacts between adjacent axons seemed especially prevalent between very small diameter profiles, suggesting that in capsaicin treated vagi Schwan cells may not envelope each axon individually, as they do in vehicle-treated vagi. There was no apparent difference between vehicle and capsaicin-treated rats with regard to the number and diameter of myelinated axons.
Fig. 6.
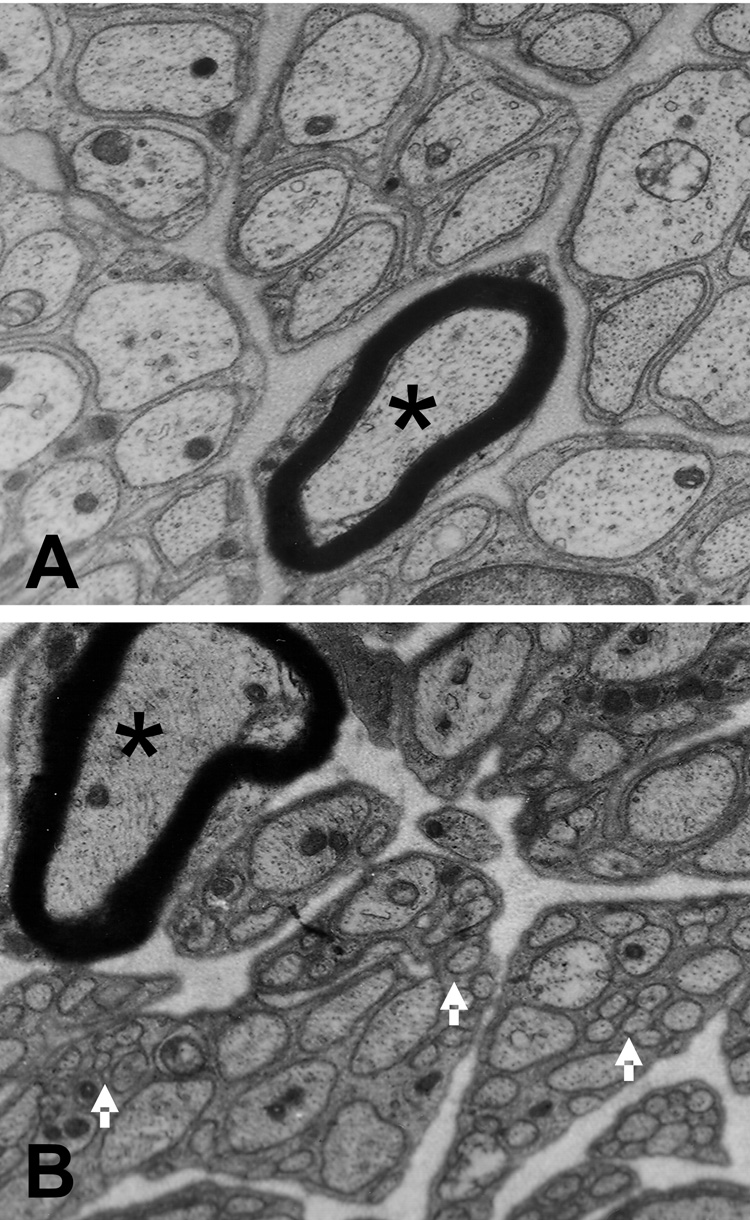
Transmission electron micrographs transverse sections of cervical vagus, 180 days after systemic vehicle (A) or capsaicin injections (B). The majority of profiles are of medium and small unmyelinated fibers. Note the presence of very small axon profiles (arrows) in the tissue from the capsaicin-treated rat. These small profiles may be neurites extending from new neurons. Such small profiles never were detected in vehicle injected rats. Asterisk indicates myelinated fiber.
Discussion
Degeneration of primary afferent terminals has been reported in the central nervous system following capsaicin treatment of neonatal and adult rats (Ritter and Dinh, 1992). Additionally, capsaicin induces degeneration of peripheral afferents in skin, cornea, urinary bladder and gut (Holzer et al., 1982; Fujita et al., 1987; Hiura et al., 1999). Although there are numerous reports of capsaicin-induced primary afferent terminal degeneration, there are only a few reports of effects of capsaicin treatment on primary afferent perikarya, and without exception these have involved capsaicin administration in neonates (Carobi, 1996; Szoke et al., 2002; Hiura et al., 2002). We found that capsaicin administration to adult rats results in strong expression of cleaved cas-3 immunoreactivity by NG neurons within 24h after treatment. Cleaved cas-3 is expressed by neurons undergoing necrosis or apoptosis (Harwood et al., 2005). Additionally capsaicin significantly increased TUNEL labeling in NG neuronal nuclei 24h after treatment. The similar time course for the cas-3- and TUNEL-positive primary sensory neurons located in dorsal root ganglia was previously reported in rats after neonatal capsaicin treatment (Jin et al., 2005). Expression of cas-3 immunoreactivity and TUNEL staining were followed by a reduction in the number of NG neuronal nuclei, confirming cell loss following capsaicin treatment. Capsaicin treatment also reduced the number of NG neurons expressing VR1-IR. VR1, known now as TRPV1, is a non-selective cation channel, that comprises the receptor for capsaicin (Caterina et al., 1997). Capsaicin’s selective neurotoxicity is mediated by TRPV1 allowing excessive influx of calcium, which destroys mitochondria and results in neuronal damage or death (Hiura, 2000). Hence, the decrease in VR1-IR we observed is consistent with destruction of capsaicin-sensitive NG neurons. These observations reveal for the first time that systemic capsaicin treatment in adult rats leads, not just to degeneration of vagal afferent axons and terminals, but to destruction of NG neuronal perikarya as well.
The most intriguing outcome of our study was that decreases in numbers of NG neuronal nuclei we observed were not persistent. In fact, by 60 days after capsaicin the numbers of neuronal nuclei in NG of capsaicin-treated rats equaled or exceeded the numbers in control rats. These results suggest that neurons that died following capsaicin treatment were rapidly replaced by new neurons. Since capsaicin treatment decreased neuronal number by at least half and produced an initial decrease in the number of VR1-IR neurons from 60% to 10%, our results suggested that more than half of NG neurons, approximately 3500 neurons/ganglion, ultimately were replaced by neurogenesis after capsaicin treatment.
Neurogenesis was confirmed by quantifying the incorporation of BrdU into neuronal nuclei. In NG from control rats BrdU-IR was virtually absent. However, at time points between 3 and 60 days post capsaicin, BrdU-IR was detected in more than 10% of neuronal nuclei, indicating that cells within the NG had reentered the S phase of the cell cycle and had proceeded to divide. The fact that virtually all BrdU-IR cells were immunopositive for PGP-9.5, a neuron-specific marker (Thompson et al., 1983) suggests that the newly generated NG cells were indeed neuronal. There have been reports that BrdU sometimes is incorporated during DNA repair prior to cell death (Palmer et al., 2000; Taupin, 2007). Incorporation of BrdU into dying neurons is not consistent with our results because cas-3-IR and TUNEL-labeled neurons were not observed 30 and 60 days after capsaicin treatment while the number of neuronal nuclei actually increased over a 60-day post-capsaicin period, and the proportion of BrdU-IR neurons significantly increased in parallel to the increase in the number of neuronal nuclei. Only 10% of NG neurons exhibited BrdU-IR, while the total number of ‘new’ neurons was estimated to be about 50%. The apparent discrepancy between the increase in neuronal number and BrdU incorporation is explained by the fact that BrdU incorporation is not stoichiometric. BrdU is incorporated into DNA only during a brief period during cell division. Even though we administered BrdU every other day, it would only be present for incorporation during a fraction of each 48h period, and thus would be incorporated into DAN of just a fraction of cells dividing (Nowakowski and Hayes, 2000; Abrous et al., 2005).
Like neuronal nuclear number, the number of neurons expressing VR1-IR decreased following capsaicin treatment. However, both the decrease in VR1-IR and its return to near control levels developed more rapidly than the decrease and subsequent increase of neuronal nuclear numbers. Therefore, we suspect that expression of VR1-IR might have been suppressed prior to cell death in capsaicin-sensitive NG neurons. In this regard it is interesting that Michael and Priestley (Michael and Priestley, 1999) reported that VR1 mRNA expression was markedly reduced in dorsal root ganglia between 4 and 7 days after axotomy. Capsaicin-induced degeneration of primary afferent terminals and axons occurs within 12 to 24h of capsaicin treatment (Ritter and Dinh, 1988), while our data suggests that death of the neuronal cell bodies in the NG occurs over several weeks. Therefore, it is likely that capsaicin-induced axonal degeneration suppresses VR1 expression prior to the cell death in a manner similar to that of axotomy in DRG neurons.
We also observed that the proportion of NG neurons expressing VR1-IR increased between three and thirty days post capsaicin and that both the percentage and total number of VR1-IR NG neurons was near control levels by 60 days after injection. It is possible that this increase reflects VR1 expression by new neurons being produced in the NG. However, an additional explanation is that the increase in VR1 expression may have been induced in capsaicin-resistant neurons that were VR1-immunonegative prior to capsaicin treatment. In support of this interpretation, it is interesting that Rashid and colleagues (Rashid et al., 2003) found that while VR1-IR was not expressed in myelinated dorsal root ganglion neurons of control rats, expression of VR1 was induced in myelinated DRG neurons two weeks after capsaicin treatment. Our present data does not allow us to determine the degree to which these two mechanisms may contribute to the expression of VR1 in our system.
The only previous quantification of capsaicin-induced destruction of NG neurons (Carobi, 1996) reported that adult rats injected with capsaicin as neonates sustained a 70% reduction in total NG neuronal number compared to untreated controls. Taken together with our results, these data suggest that the adult NG reveal quantitatively comparable capsaicin-induced damage to the NG of neonates. In contrast to our results, however, Carobi (1996) found no restoration of neuronal number between the time of neonatal capsaicin treatment (2 days postnatal) and adulthood. Our finding that NG neuronal number is restored to control levels within 60 days after capsaicin treatment of adult rats suggests that the adult rat retains precursor cells capable of replacing lost neurons by a process involving DNA synthesis and cell division. On the other hand Carobi’s results suggest that neonatal capsaicin treatment not only destroys capsaicin-sensitive neurons, but also destroys the precursor cells that are capable of replacing lost neurons. The source and phenotype of cells that restore NG neuronal number is not known. Precursor cells might be present in the adult NG or differentiate from resident NG glia following capsaicin treatment. Population of neurons expressing nestin, marker related to maturation of neuronal precursors, was previously reported in trigeminal ganglion of adult rats (Lagares et al., 2007). Additionally precursor cells with similar gene expression profiles to those, reported in trigeminal ganglion, have also been shown in dorsal root ganglia in the adult rat (Li et al., 2007). Alternatively, it is conceivable that precursors migrate to the NG from other locations and then differentiate into NG neurons. A third possibility is that some neurons of the adult NG retain the potential to divide and generate new neurons. This potential might be enhanced following capsaicin-induced damage. Induced division of neurons derived from the stem cells in vitro has been previously reported (Lin et al., 2004).
Although NG neuronal number clearly is restored following capsaicin treatment in adults, there is as yet no evidence that the new NG neurons establish connections with previously innervated tissues. For example, Dinh and Ritter (Ritter and Dinh, 1988) demonstrated that adult rats treated with capsaicin did not exhibit additional capsaicin-induced terminal degeneration in the NTS when capsaicin was readministered 4.5 months after the first capsaicin treatment. Their data suggest that new VR1-expressing neurons, which should have been present by this time, had not reinnervated the hindbrain. Results of functional experiments also suggest that reinnervation following systemic capsaicin treatment does not occur over a period of several months following capsaicin treatment. For example, reduction of food intake and inhibition of gastric emptying in response to injections of cholecystokinin are attenuated in rats treated with capsaicin as adults, and these responses remain attenuated for at least 6 months following capsaicin treatment (South and Ritter, 1988). Nevertheless, our ultrastructural examination of vagal trunks reveal a subpopulation of very fine diameter neurites, present exclusively in vagi of rats treated with capsaicin. We have not yet examined the time course over which these neurites appear or the distance they travel from the NG. It is possible that these fine fibers represent axonal sprouting from capsaicin-resistant afferents that survived capsaicin treatment. However, it also is possible that they are processes from new neurons beginning to seek their innervation targets. Determining the origin and developmental time course for these fibers will require evaluating vagal samples over a more extended time period.
In summary, we find that systemic capsaicin treatment of adult rat results in the death of at least 50% of vagal afferent neurons in NG. However, over a period of 30 to 60 days these neurons are replaced through a process of neurogenesis, involving DNA replication and cell division. To the best of our knowledge this is the first demonstration of induced postnatal neurogenesis on such a large scale in a sensory ganglion, and therefore represents a new model for studying adult neurogenesis. In addition, the recognition of high neurogenic capacity in a primary afferent ganglion raises the possibility that appropriately stimulated cells might be useful as autologous grafts to replace neurons lost through damage or disease.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-52849 and National Institute of Neurological Disorders and Stroke Grant NS-20561 and PONCIN Fellowship 2006–2007. We thank Shayne Andrew for expert technical assistance with the microscope imaging and Tricia Duffy for general technical support.
List of Abbreviations
- NG
nodose ganglion
- BrdU
5'-bromo-2'-deoxyuridine
- VR1
vanilloid receptor
- -IR
-immunoreactive
- Cas-3
cleaved caspase 3
- PGP
protein gene product
- LSAB
Labeled Streptavidin Biotin
- IF
immunofluorescence
- NfM
neurofilament M
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Bordey A. Adult neurogenesis: basic concepts of signaling. Cell Cycle. 2006;5:722–728. doi: 10.4161/cc.5.7.2614. [DOI] [PubMed] [Google Scholar]
- Carobi C. A quantitative investigation of the effects of neonatal capsaicin treatment on vagal afferent neurons in the rat. Cell Tissue Res. 1996;283:305–311. doi: 10.1007/s004410050540. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chapouton P, Jagasia R, Bally-Cuif L. Adult neurogenesis in non-mammalian vertebrates. Bioessays. 2007;29:745–757. doi: 10.1002/bies.20615. [DOI] [PubMed] [Google Scholar]
- Clancy B, Cauller LJ. Reduction of background autofluorescence in brain sections following immersion in sodium borohydride. J Neurosci Methods. 1998;83:97–102. doi: 10.1016/s0165-0270(98)00066-1. [DOI] [PubMed] [Google Scholar]
- Czaja K, Ritter RC, Burns GA. N-methyl-D-aspartate receptor subunit phenotypes of vagal afferent neurons in nodose ganglia of the rat. J Comp Neurol. 2006;496:877–885. doi: 10.1002/cne.20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita S, Miyazono Y, Ohba N. Capsaicin-induced corneal changes associated with sensory denervation in neonatal rat. Jpn J Ophthalmol. 1987;31:412–424. [PubMed] [Google Scholar]
- Gamse R, Leeman SE, Holzer P, Lembeck F. Differential effects of capsaicin on the content of somatostatin, substance P, and neurotensin in the nervous system of the rat. Naunyn Schmiedebergs Arch Pharmacol. 1981;317:140–148. doi: 10.1007/BF00500070. [DOI] [PubMed] [Google Scholar]
- Groves MJ, Schanzer A, Simpson AJ, An SF, Kuo LT, Scaravilli F. Profile of adult rat sensory neuron loss, apoptosis and replacement after sciatic nerve crush. J Neurocytol. 2003;32:113–122. doi: 10.1023/b:neur.0000005596.88385.ec. [DOI] [PubMed] [Google Scholar]
- Harwood SM, Yaqoob MM, Allen DA. Caspase and calpain function in cell death: bridging the gap between apoptosis and necrosis. Ann Clin Biochem. 2005;42:415–431. doi: 10.1258/000456305774538238. [DOI] [PubMed] [Google Scholar]
- Hiura A. Neuroanatomical effects of capsaicin on the primary afferent neurons. Arch Histol Cytol. 2000;63:199–215. doi: 10.1679/aohc.63.199. [DOI] [PubMed] [Google Scholar]
- Hiura A, Nakae Y, Nakagawa H. Cell death of primary afferent nerve cells in neonatal mice treated with capsaicin. Anat Sci Int. 2002;77:47–50. doi: 10.1046/j.0022-7722.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- Hiura A, Nakagawa H, Koshigae Y, Yoshizako A, Kubo Y, Ishizuka H. Age-related changes in the response to thermal noxious heat and reduction of C-fibers by neonatal treatment with capsaicin. Somatosens Mot Res. 1999;16:115–121. doi: 10.1080/08990229970555. [DOI] [PubMed] [Google Scholar]
- Holzer P, Bucsics A, Lembeck F. Distribution of capsaicin-sensitive nerve fibres containing immunoreactive substance P in cutaneous and visceral tissues of the rat. Neurosci Lett. 1982;31:253–257. doi: 10.1016/0304-3940(82)90029-5. [DOI] [PubMed] [Google Scholar]
- Huang GJ, Herbert J. Stimulation of neurogenesis in the hippocampus of the adult rat by fluoxetine requires rhythmic change in corticosterone. Biol Psychiatry. 2006;59:619–624. doi: 10.1016/j.biopsych.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE, Coggeshall RE. An analysis of the axon populations in the nerves to the pelvic viscera in the rat. J Comp Neurol. 1982;211:1–10. doi: 10.1002/cne.902110102. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Sugimoto T. The co-expression of VR1 and VRL-1 in the rat vagal sensory ganglia. Brain Res. 2003;980:293–296. doi: 10.1016/s0006-8993(03)02998-6. [DOI] [PubMed] [Google Scholar]
- Jin HW, Ichikawa H, Fujita M, Yamaai T, Mukae K, Nomura K, Sugimoto T. Involvement of caspase cascade in capsaicin-induced apoptosis of dorsal root ganglion neurons. Brain Res. 2005;1056:139–144. doi: 10.1016/j.brainres.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Lindvall O. Neurogenesis after ischaemic brain insults. Curr Opin Neurobiol. 2003;13:127–132. doi: 10.1016/s0959-4388(03)00017-5. [DOI] [PubMed] [Google Scholar]
- Lagares A, Li HY, Zhou XF, Avendano C. Primary sensory neuron addition in the adult rat trigeminal ganglion: evidence for neural crest glio-neuronal precursor maturation. J Neurosci. 2007;27:7939–7953. doi: 10.1523/JNEUROSCI.1203-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HY, Say EH, Zhou XF. Isolation and characterization of neural crest progenitors from adult dorsal root ganglia. Stem Cells. 2007;25:2053–2065. doi: 10.1634/stemcells.2007-0080. [DOI] [PubMed] [Google Scholar]
- Lin QX, Que HP, Lu SH, Liu SJ. Induced-division of neurons derived from neural stem cells. Sheng Li Xue Bao. 2004;56:130–136. [PubMed] [Google Scholar]
- McKay HA, Brannstrom T, Wiberg M, Terenghi G. Primary sensory neurons and satellite cells after peripheral axotomy in the adult rat: timecourse of cell death and elimination. Exp Brain Res. 2002;142:308–318. doi: 10.1007/s00221-001-0929-0. [DOI] [PubMed] [Google Scholar]
- Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci. 1999;19:1844–1854. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- Nowakowski RS, Hayes NL. New neurons: extraordinary evidence or extraordinary conclusion? Science. 2000;288:771. doi: 10.1126/science.288.5467.771a. [DOI] [PubMed] [Google Scholar]
- Nowakowski RS, Lewin SB, Miller MW. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J Neurocytol. 1989;18:311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, Spitzer G, Santarelli L, Scharf B, Hen R, Rosoklija G, Sackeim HA, Dwork AJ. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27:4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid MH, Inoue M, Bakoshi S, Ueda H. Increased expression of vanilloid receptor 1 on myelinated primary afferent neurons contributes to the antihyperalgesic effect of capsaicin cream in diabetic neuropathic pain in mice. J Pharmacol Exp Ther. 2003;306:709–717. doi: 10.1124/jpet.103.050948. [DOI] [PubMed] [Google Scholar]
- Ritter S, Dinh TT. Capsaicin-induced neuronal degeneration: silver impregnation of cell bodies, axons, and terminals in the central nervous system of the adult rat. J Comp Neurol. 1988;271:79–90. doi: 10.1002/cne.902710109. [DOI] [PubMed] [Google Scholar]
- Ritter S, Dinh TT. Age-related changes in capsaicin-induced degeneration in rat brain. J Comp Neurol. 1992;318:103–116. doi: 10.1002/cne.903180108. [DOI] [PubMed] [Google Scholar]
- Simasko SM, Ritter RC. Cholecystokinin activates both A- and C-type vagal afferent neurons. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1204–G1213. doi: 10.1152/ajpgi.00132.2003. [DOI] [PubMed] [Google Scholar]
- South EH, Ritter RC. Capsaicin application to central or peripheral vagal fibers attenuates CCK satiety. Peptides. 1988;9:601–612. doi: 10.1016/0196-9781(88)90171-4. [DOI] [PubMed] [Google Scholar]
- Szoke E, Seress L, Szolcsanyi J. Neonatal capsaicin treatment results in prolonged mitochondrial damage and delayed cell death of B cells in the rat trigeminal ganglia. Neuroscience. 2002;113:925–937. doi: 10.1016/s0306-4522(02)00208-7. [DOI] [PubMed] [Google Scholar]
- Tang X, Falls DL, Li X, Lane T, Luskin MB. Antigen-retrieval procedure for bromodeoxyuridine immunolabeling with concurrent labeling of nuclear DNA and antigens damaged by HCl pretreatment. J Neurosci. 2007;27:5837–5844. doi: 10.1523/JNEUROSCI.5048-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Doran JF, Jackson P, Dhillon AP, Rode J. PGP 9.5--a new marker for vertebrate neurons and neuroendocrine cells. Brain Res. 1983;278:224–228. doi: 10.1016/0006-8993(83)90241-x. [DOI] [PubMed] [Google Scholar]
- Zhang YL, Qiu SD, Zhang PB, Shi W. BrdU-labelled neurons regeneration after cerebral cortex injury in rats. Chin Med J (Engl) 2006;119:1026–1029. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


