Abstract
Objective
The endocannabinoid system (ECS) promotes weight gain and obesity-associated metabolic changes. Weight loss interventions may influence obesity-associated risk indirectly through modulation of the peripheral ECS. We investigated the effect of acute and chronic treatment with sibutramine on components of the peripheral ECS.
Methods and Procedures
Twenty obese otherwise healthy patients received randomized, double-blind, crossover treatment with placebo and 15 mg/day sibutramine for 5 days each, followed by 12 weeks open-label sibutramine treatment. We determined circulating anandamide and 2-arachidonoylglycerol and expression levels of endocannabinoid genes in subcutaneous abdominal adipose tissue biopsies.
Results
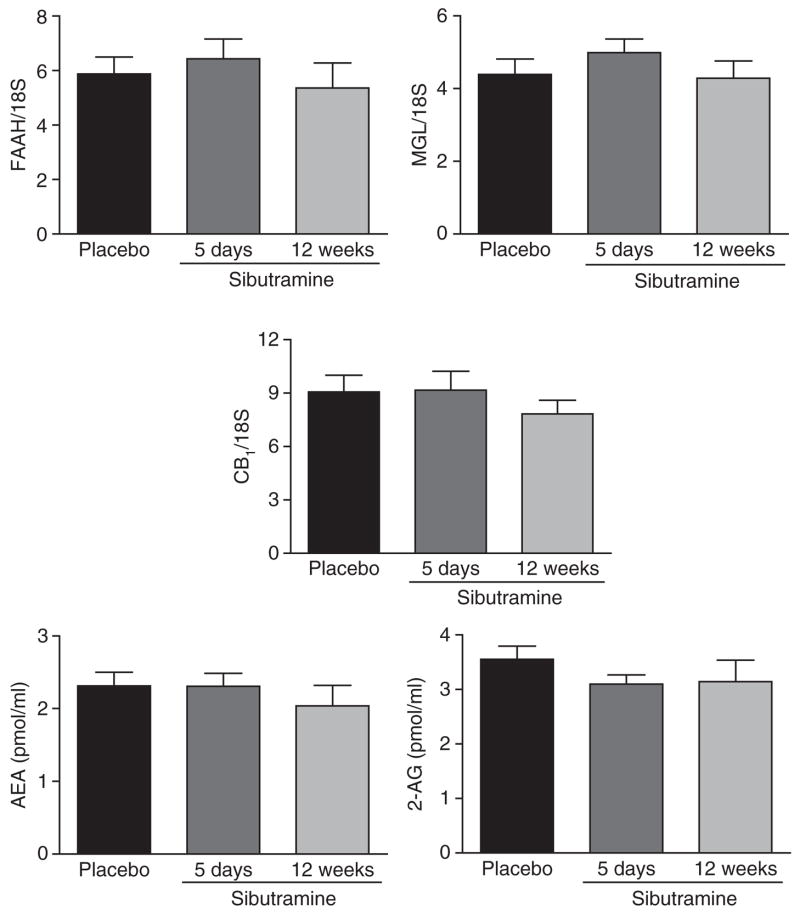
Body weight was stable during the acute treatment period and decreased by 6.0 ± 0.8 kg in those patients completing 3 months of sibutramine treatment (P < 0.05). Circulating endocannabinoids and the expression of ECS genes did not change with acute or chronic sibutramine treatment.
Discussion
The ECS is activated in obesity. We did not find any influence of 5% body weight loss induced by sibutramine on circulating levels of endocannabinoids and adipose-tissue expression of endocannabinoid genes in obese subjects. These data confirm our previous findings on dietary weight loss and suggest that the dysregulation of the ECS may be a cause rather than a consequence of obesity.
Endocannabinoids are arachidonic acid derivatives that modulate energy homeostasis, pain and inflammation, neurotransmission, and gastrointestinal function (1). In animals, the endocannabinoid system (ECS) promotes weight gain, dyslipidemia, hepatic steatosis, and insulin resistance through cannabinoid type 1 (CB1) receptor activation in brain and peripheral organs (2–6). Peripheral ECS activation was described in human obesity (6,7). Circulating 2-arachidonoyl-glycerol concentrations were correlated with deterioration of metabolic variables particularly in patients with abdominal adiposity (8,9). Furthermore, the improvement in metabolic and cardiovascular risk factors with CB1-receptor blockade suggests peripheral actions (10,11). Weight loss interventions may modulate obesity-associated risk through interaction with the peripheral ECS. To test this hypothesis, we determined circulating endocannabinoid levels and adipose-tissue gene expression of ECS genes before and after 3 months of sibutramine-assisted weight loss. Sibutramine reduces body weight by increasing the levels of norepinephrine and serotonine levels in the brain by reuptake inhibition. ese actions reduce meal size and increase thermogenesis (12).
RESEARCH METHODS AND PROCEDURES
Twenty obese subjects were randomized (age between 18 and 60 years; body weight 103.4 ± 13.2 kg; waist circumference 109 ± 11 cm (mean ± s.d.)). Subjects with blood pressure >160/100 mm Hg, with secondary causes of obesity, diabetes mellitus, or other serious conditions were excluded. Concomitant medication was stopped five elimination half-life times before study entry. Written informed consent was obtained before screening. e study was approved by our institutional review board. First, we compared acute responses during 5 days treatment with 15 mg/day sibutramine or placebo in a randomized, double-blind, crossover fashion (3 weeks washout phase). en, patients were treated for 12 weeks with open-label 15 mg/day sibutramine. Patients with blood pressure increase ≥15 mm Hg or heart rate increase ≥15 bpm on two consecutive visits were excluded. Patients were advised to maintain food intake during the acute study period and to decrease caloric intake by 600 kcal/day during open-label treatment by a dietitian. On the 5th day of each double-blind treatment and again after 12 weeks of open-label sibutramine treatment, we obtained venous blood samples and abdominal subcutaneous adipose-tissue biopsies after an overnight fast. Anandamide and 2-arachidonoylglycerol were measured by liquid chromatography/in-line mass spectrometry. CB1-receptor, fatty acid amide hydrolase (FAAH), monoglyceride lipase (MGL), leptin, and adiponectin gene expression were measured by real-time reverse transcriptase-polymerase chain reaction and normalized by 18S ribosomal RNA expression (7,8). Data as mean ± s.e.m. (Figures) or mean ± s.d. (text) were analyzed by paired t-tests. Relationship between variables was assessed by Pearson’s correlation. A P value <0.05 indicated statistical significance.
RESULTS
Whereas sibutramine was well tolerated acutely, six patients were excluded during chronic treatment because blood pressure and/or heart rate increased. One subject was excluded during the crossover period due to protocol violations. Body weight and waist circumference were stable during the acute treatment period (placebo vs. sibutramine: 103.4 ± 13.2 kg vs. 103.0 ± 13.1 kg; 109 ± 11 cm vs. 109 ± 11 cm). Body weight was decreased by 6.0 ± 0.8 kg (corresponding to 5.6% body weight loss) and waist circumference by 4 ± 0.5 cm in patients completing 3 months of sibutramine treatment (P < 0.05). Endocannabinoid levels in blood and adipose-tissue expression of ECS genes did not change with acute or chronic sibutramine treatment (Figure 1). No correlations were found between body weight and waist circumference changes with the components of the ECS measured here. At the end of the placebo treatment, ECS genes correlated with leptin and adiponectin genes independently of body weight (leptin/CB1-receptor: r = 0.54, P = 0.02; leptin/FAAH: r = 0.56, P = 0.01; leptin/MGL: r = 0.67, P = 0.001; adiponectin/CB1-receptor: r = 0.58, P = 0.01; adiponectin/FAAH: r = 0.63, P = 0.004 and adiponectin/MGL: r = 0.74, P < 0.001). Changes in leptin gene expression correlated strongly with changes in all ECS genes during chronic sibutramine treatment (Figure 2), whereas for adiponectin this correlation was only found with the MGL gene (r = 0.88, P < 0.001).
Figure 1.
Endocannabinoid system gene expression (CB1-receptor, FAAH, MGL) in subcutaneous abdominal adipose tissue and circulating AEA and 2-AG levels were determined after 5 days placebo treatment, 5 days sibutramine treatment, and 12 weeks sibutramine treatment. Paired t-test for placebo vs. 5 days (n = 19) and placebo vs. 12 weeks (n = 13). Data are mean ± s.e.m. 2-AG, 2-arachidonoylglycerol; AEA, anandamide; CB1, cannabinoid type 1; FAAH, fatty acid amide hydrolase; MGL, monoglyceride lipase.
Figure 2.
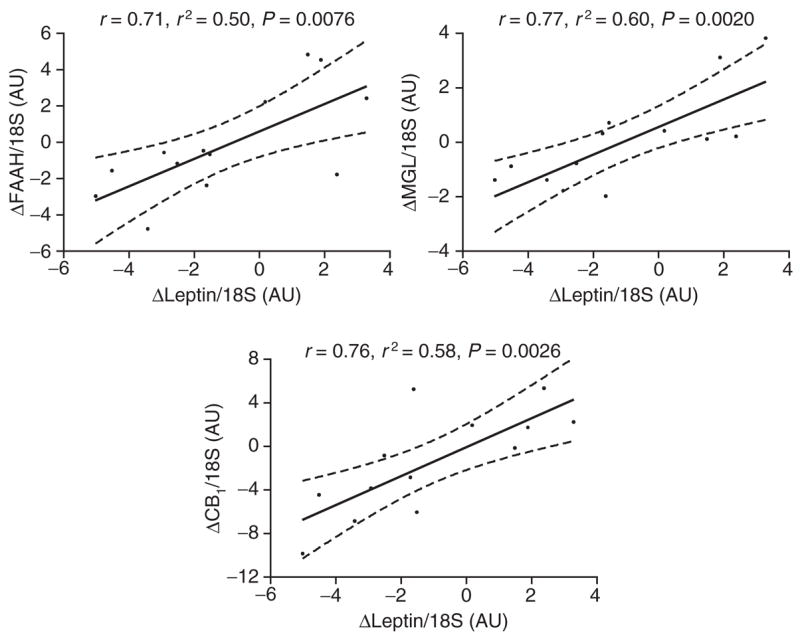
Linear regression analysis between changes in leptin gene expression and changes in endocannabinoid system gene expression (CB1-receptor, FAAH, MGL) in subcutaneous abdominal adipose tissue after 12 weeks sibutramine treatment (n = 13). AU, arbitrary unit; CB1, cannabinoid type 1; FAAH, fatty acid amide hydrolase; MGL, monoglyceride lipase.
DISCUSSION
Weight loss achieved through caloric restriction plus sibutramine treatment did not change peripheral ECS activity. Similarly, 5% weight loss through caloric restriction had no effect on peripheral ECS activity (7). The finding is surprising as high-fat food increased endocannabinoid bioavailability in mice even before the onset of obesity (3). Beside possible species differences in ECS regulation, 5–6% weight loss may not be sufficient to elicit a change in ECS activity. Peripheral ECS activity may also be more sensitive to food composition than to body weight itself. In our studies, we changed caloric intake, but food composition remained relatively stable. Finally, ECS activation may be a cause rather than a consequence of obesity, but genetic data for this notion are not conclusive at present.
The mechanisms regulating peripheral ECS activity are not understood. We observed a correlation between adipose leptin gene expression and CB1-receptor, FAAH and MGL expression. Furthermore, changes in leptin expression with sibutramine-induced weight loss were correlated with changes in CB1-receptor, FAAH and MGL gene expression, suggesting an interaction between leptin and peripheral ECS activity. Indeed, leptin has previously been shown to upregulate FAAH expression and activity in human lymphoma cells (13). Functional interaction between endocannabinoids and leptin has also been demonstrated in the hypothalamus (14). Correlation between adipose adiponectin and ECS gene expression further supports the idea that endocannabinoids regulate adiponectin production (5,6,10,11).
A limitation of our study is that we only determined circulating endocannabinoids. Endocannabinoids are produced locally and act in an autocrine or paracrine fashion. An unknown proportion spills over into the systemic circulation, where concentrations are much lower as in tissues. Changes in venous endocannabinoid concentrations could be related to changes in spill over into or clearance from the circulation. Furthermore, venous measurements do not identify the tissue sources of endocannabinoids. However, endocannabinoids are produced by human adipose tissue (6,15). Given the correlation between circulating endo-cannabinoids and CB1-receptor and FAAH gene expression in subcutaneous and visceral adipose tissue (7,8), venous measurements may provide physiologically relevant information.
We suggest that beneficial metabolic responses to sibutramine (12) cannot be explained through interactions with the ECS (16) and that sibutramine may not attenuate the clinical efficacy of CB1-receptor antagonists. We propose to evaluate combinations of sibutramine and CB1-receptor antagonists in high-risk obese patients not responding to monotherapy. Rimonabant treatment is associated with depressive symptoms in a small number of patients whereas sibutramine may have a mild antidepressant and stimulatory effect. Therefore, patients will have to be closely monitored by quantitative scales and experienced clinicians to detect early psychiatric side effects of that combination treatment.
Acknowledgments
This work was supported in part by research grants from Abbott Laboratories and by the Deutsche Forschungsgemeinschaft. Abbott Laboratories provided the study medication, but did not contribute to the design, interpretation, or writing of the study.
Footnotes
DISCLOSURE
J.J. and S.E. received lecture fees from Abbott Pharmaceuticals and Sanofi-Aventis, as well as research support from Sanofi-Aventis (S.E. and J.J.).
References
- 1.Pacher P, Batkai S, Kunos G. The endocannabionoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cota D, Marsicano G, Tschöp M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osei-Hyiaman D, Depetrillo M, Pacher P, et al. Endocannabinoid activation at hepatic CB(1) receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu YL, Connoley IP, Wilson CA, Stock MJ. Effects of the cannabinoid CB1 receptor antagonist SR141716 on oxygen consumption and soleus muscle glucose uptake in Lep(ob)/Lep(ob) mice. Int J Obes (Lond) 2005;29:183–187. doi: 10.1038/sj.ijo.0802847. [DOI] [PubMed] [Google Scholar]
- 5.Bensaid M, Gary-Bobo M, Esclangon A, et al. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol. 2003;63:908–914. doi: 10.1124/mol.63.4.908. [DOI] [PubMed] [Google Scholar]
- 6.Matias I, Gonthier MP, Orlando P, et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab. 2006;91:3171–3180. doi: 10.1210/jc.2005-2679. [DOI] [PubMed] [Google Scholar]
- 7.Engeli S, Böhnke J, Feldpausch M, et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54:2838–2843. doi: 10.2337/diabetes.54.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blüher M, Engeli S, Klöting N, et al. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes. 2006;55:3053–3060. doi: 10.2337/db06-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cote M, Matias I, Lemieux I, et al. Circulating endocannabinoid levels, abdominal obesity and related cardiometabolic risk factors in obese men. Int J Obes (Lond) 2007;31:692–699. doi: 10.1038/sj.ijo.0803539. [DOI] [PubMed] [Google Scholar]
- 10.Despres JP, Golay A, Sjöström L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- 11.Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet. 2006;368:1660–1672. doi: 10.1016/S0140-6736(06)69571-8. [DOI] [PubMed] [Google Scholar]
- 12.Hainer V, Kabrnova K, Aldhoon B, Kunesova M, Wagenknecht M. Serotonine and norepinephrine reuptake inhibition and eating behaviour. Ann NY Acad Sci. 2006;1083:252–269. doi: 10.1196/annals.1367.017. [DOI] [PubMed] [Google Scholar]
- 13.Gasperi V, Fezza F, Spagnuolo P, Pasquariello N, Maccarrone M. Further insight into the regulation of human FAAH by progesterone and leptin. NeuroToxicol. 2005;26:811–817. doi: 10.1016/j.neuro.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Di Marzo V, Goparaju SK, Wang L, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 15.Spoto B, Fezza F, Parlongo G, et al. Human adipose tissue binds and metabolizes the endocannabinoids anandamide and 2-arachidonoylglycerol. Biochimie. 2006;88:1889–1897. doi: 10.1016/j.biochi.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Filippatos TD, Kiortsis DN, Liberopoulos EN, Mikhailidis DP, Elisaf MS. A review of the metabolic effects of sibutramine. Curr Med Res Opin. 2005;21:457–468. doi: 10.1185/030079905X38132. [DOI] [PubMed] [Google Scholar]