Abstract
Increased production of reactive oxygen and nitrogen species has recently been implicated in the pathogenesis of cardiac and endothelial dysfunction associated with atherosclerosis, hypertension, and aging. Oxidant-induced cell injury triggers the activation of nuclear enzyme poly(ADP-ribose) polymerase (PARP), which in turn contributes to cardiac and vascular dysfunction in various pathophysiological conditions including diabetes, reperfusion injury, circulatory shock, and aging. Here, we investigated the effect of a new PARP inhibitor, INO-1001, on cardiac and endothelial dysfunction associated with advanced aging using Millar's new Aria pressure-volume conductance system and isolated aortic rings. Young adult (3 months old) and aging (24 months old) Fischer rats were treated for 2 months with vehicle, or the potent PARP inhibitor INO-1001. In the vehicle-treated aging animals, there was a marked reduction of both systolic and diastolic cardiac function and loss of endothelial relaxant responsiveness of aortic rings to acetylcholine. Treatment with INO-1001 improved cardiac performance in aging animals and also acetylcholine-induced, nitric oxide-mediated vascular relaxation. Thus, pharmacological inhibition of PARP may represent a novel approach to improve cardiac and vascular dysfunction associated with aging.
Poly(ADP-ribose) polymerase (PARP) is the most abundant nuclear enzyme of eukaryotic cells. When activated by DNA single-strand breaks, PARP initiates an energy-consuming cycle by transferring ADP ribose units from NAD+ to nuclear proteins. This process results in rapid depletion of the intracellular NAD+ and ATP pools, slowing the rate of glycolysis and mitochondrial respiration, eventually leading to cellular dysfunction and death (reviewed in Virág and Szabó 2002). PARP can also regulate the expression of various inflammatory mediators such as inducible nitric-oxide synthase, tumor necrosis factor-α, and intracellular adhesion molecule-1. Suppression of expression of inducible nitric-oxide synthase, tumor necrosis factor-α, and intracellular adhesion molecule-1 has been reported in PARP-deficient mice and in the presence of pharmacological inhibitors of the enzyme (reviewed in Virág and Szabó 2002).
Overactivation of PARP represents an important mechanism of tissue damage in pathological conditions associated with oxidative/nitrosative stress, including myocardial reperfusion injury, stroke, circulatory shock, autoimmune β-cell destruction, diabetic complications, and various forms of heart failure (Szabó et al., 1997; Thiemermann et al., 1997; Zingarelli et al., 1998; Soriano et al., 2001a,b; Pacher et al., 2002a–f; Virág and Szabó 2002; Szabó 2004a). Activation of PARP also contributes to the development of vascular dysfunction associated with diabetes, chronic heart failure, and aging (Soriano et al., 2001a,b; Pacher et al., 2002c–e).
Peroxynitrite, a reactive oxidant species produced from the reaction of nitric oxide and superoxide, has been established as a pathophysiological relevant endogenous trigger of DNA single-strand breakage and PARP activation (reviewed in Virág and Szabó 2002). Additional endogenous triggers of DNA single-strand breakage and PARP activation include hydrogen peroxide, hydroxyl radical, and nitroxyl anion (reviewed in Virág and Szabó 2002).
There is emerging evidence that the cardiovascular dysfunction associated with aging is related to the local formation of reactive oxygen and nitrogen species in the myocardium and vasculature (Inoue et al., 1996; Rodriguez-Martinez et al., 1998; Bejma et al., 2000; van der Loo et al., 2000; Hamilton et al., 2001; Suh et al., 2001; Csiszar et al., 2002; Lakatta, 2003; Navarro et al., 2004). The beneficial effect of a phenanthridinone derivative PARP inhibitor, PJ34, on endothelial dysfunction in aging Wistar rats (Pacher et al., 2002e) has previously been demonstrated, suggesting that oxygen and nitrogen species-induced intravascular PARP activation plays an important role in endothelial dysfunction associated with aging (Pacher et al., 2002e).
Here, we studied the effect of a new potent PARP inhibitor, INO-1001, on the baseline cardiac performance and systolic and diastolic function at different preloads in aging Fischer-344 male rats with severely impaired cardiac function, using Millar pressure-volume conductance catheter system. We have also investigated the changes in vascular reactivity by measuring acetylcholine-induced, nitric oxide-mediated vascular relaxation in isolated aortic rings.
Materials and Methods
The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication 85-23 revised 1996) and was performed with the approval of the local Institutional Animal Care and Use Committee.
Animals, Treatment Protocols
Young adult (3 months old) and aging (24 months old) Fischer rats obtained from the National Institute on Aging were treated for 2 months with vehicle, or the potent isoindolinone-based PARP inhibitor INO-1001, 30 mg/kg/orally (Inotek Pharmaceuticals, Beverly, MA) (Du et al., 2003; Khan et al., 2003; Shimoda et al., 2003; Murakami et al., 2004; Szabó et al., 2004b). In cell-free PARP assay using NAD+ and purified PARP enzyme, INO-1001 inhibited PARP activity in a dose-dependent manner with an EC50 of 3 nM. The EC50 of the prototypical PARP inhibitor 3-aminobenzamide was 200 μM. Peroxynitrite- and hydrogen peroxide-induced oxidation of dihydrorhodamine-123 were unaffected by INO-1001 in the concentration range up to 10 mM, indicating that the compound does not act as an antioxidant. The chosen dose of INO-1001 (30 mg/kg/orally) was found to effectively inhibit PARP activation in previous studies in various tissues including myocardium and vasculature (Du et al., 2003; Khan et al., 2003; Shimoda et al., 2003; Murakami et al., 2004; Szabó et al., 2004b). Rats were housed two to three per cage, fed a standard laboratory diet and water ad libitum, and maintained on a 12-h light/dark cycle.
Cardiac Remodeling
Following the hemodynamic measurements, the animals were killed by exsanguination (transection of the inferior vena cava). The heart was removed, and the left and right ventricles and atria were dissected and weighed. The weight of the ventricles and atria was then normalized to the body weight. The lungs were also removed and weighed, and wet lung weights were normalized to body weight and were used as an index of extravascular water lung accumulation.
Hemodynamic Measurements
Rats were anesthetized with thiopentone sodium (60 mg/kg i.p.) and tracheotomized to facilitate breathing (Pacher et al., 2002c,d). Animals were placed on controlled heating pads, and core temperature measured via a rectal probe was maintained at 37°C. A microtip pressure-volume catheter (SPR-838; Millar Instruments, Inc., Houston, TX) was inserted into the right carotid artery and advanced into the left ventricle (LV) under pressure control as described (Pacher et al., 2002c,d, 2003). Polyethylene cannulae (P50) were inserted into the right femoral artery and vein for measurement of mean arterial pressure. After stabilization for 20 min, the signals were continuously recorded using an ARIA pressure-volume conductance system (Millar Instruments Inc.) coupled to a Powerlab/4SP A/D converter (AD Instruments, Mountain View, CA) at a sampling rate of 1000/s, stored, and displayed on a computer.
The heart rate, maximal left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP), mean arterial pressure (MAP), maximal slope of systolic pressure increment (+dP/dt) and diastolic decrement (−dP/dt), time constant of left ventricular pressure decay, ejection fraction (EF), stroke volume (SV), cardiac output, and stroke work (SW) were computed using a cardiac pressure-volume analysis program (PVAN3.2; Millar Instruments, Inc.) as previously described (Pacher et al., 2003). Stroke volume and cardiac output were calculated and corrected according to in vitro and in vivo volume calibrations using PVAN3.2 (Pacher et al., 2003), and cardiac output was normalized to body weight [cardiac index (CI)]. Stroke work was also normalized to body weight (SWI). Total peripheral resistance index (TPRI) was calculated by the equation: TPRI = MAP/CI.
Left ventricular pressure-volume relations were measured by transiently compressing the inferior vena cava. Indexes of contractility and LV stiffness (preload recruitable stroke work (PRSW), +dP/dt end-diastolic volume relation (+dP/dt-EDV), and slope of end-systolic and end-diastolic pressure-volume relations [ESPVR (Emax) and EDPVR] were calculated using PVAN3.2. Efficiency of left ventricular work (SW/pressure volume area) was also calculated using PVAN3.2.
The volume calibration of the conductance system was performed as previously described (Pacher et al., 2003). Briefly, nine cylindrical holes in a block 1-cm deep and with known diameter ranging from 2 to 11 mm were filled with fresh heparinized whole rat blood. In this calibration the linear volume-conductance regression of the absolute volume in each cylinder versus the raw signal acquired by the conductance catheter was used as the volume calibration formula (Pacher et al., 2003). At the end of each experiment, 50 μl of 15% saline was injected i.v., and, from the shift of PV relations, parallel conductance volume was calculated by PVAN3.2 and used for correction for the cardiac mass volume as previously described (Pacher et al., 2003).
Vascular Function in Isolated Aortic Rings
Thoracic aorta were removed and cleared from periadventitial fat and cut into 3- to 4-mm width rings using an operation microscope mounted in organ baths filled with warmed (37°C) and oxygenated (95% O2, 5% CO2) Krebs' solution (CaCl2, 1.6 mM; MgSO4, 1.17 mM; EDTA, 0.026 mM; NaCl, 130 mM; NaHCO3, 14.9 mM; KCl, 4.7 mM; KH2PO4, 1.18 mM; glucose, 11 mM). Special attention was paid during the preparation to avoid damaging the endothelium. Isometric tension was measured with isometric transducers (Kent Scientific Corporation, Litchfield, CT), digitized using a MacLab A/D converter, stored, and displayed on a Macintosh computer. A tension of 1.5 g was applied, the rings were equilibrated for 60 min, and following precontraction with epinephrine (10-6 M), relaxation to acetylcholine (10−9 to 3 × 10−4 M) was measured as previously described (Pacher et al., 2002c–e).
Statistical Analyses
The results are presented as means ± S.E.M. The data were analyzed using a two-factor (age and treatment) analysis of variance. When the relevant F values of the treatment × age interaction were significant at the 5% level, pairwise comparisons were made using Dunnett's test for the effect of treatment in specific age groups and for the effect of age in specific treatment groups. Statistical significance was ascribed to p values <0.05.
Results
Cardiac Remodeling
Aging was characterized by a significant increase in the weight of the left ventricle and atria, but not the right ventricle. In addition, lung weight was also significantly increased (Fig. 1). Treatment with INO-1001 had no significant effect on atrial, left ventricular, and lung weights (Fig. 1.).
Fig. 1.
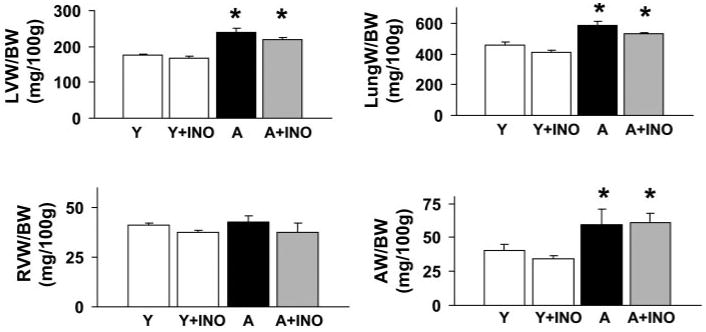
Pharmacological inhibition of PARP (for 2 months) does not affect the cardiac remodeling and lung edema associated with advanced aging. Left ventricular (LVW), right ventricular (RVW), atrial (AW) and lung (LungW) weights in young adult (Y), young adult treated with INO-1001 (Y+INO), aging (A), and aging treated with INO-1001 (A+INO) male Fischer rats were normalized to body weight (BW). Values are mean ± S.E.M. of 6 to 10 experiments in each group. *, P < 0.05 versus young adult controls.
Cardiac Function
As shown in Figs. 2 and 3, aging (A) was characterized by decreased MAP, left ventricular end-systolic pressure, +dP/dt, −dP/dt, EF, SV, CI, SWI, and heart rate. In contrast, LVEDP and time constant of left ventricular pressure decay were increased in aging animals, indicative of diastolic dysfunction. TPRI was also increased in aging rats (Fig. 3). Treatment with INO-1001 significantly improved SV, CI, and SWI and also decreased LVEDP and the time constant of left ventricular pressure decay. There was a tendency for the reduction of TPRI by the drug in aging animals and also improvement of LVSP, mean blood pressure, +dP/dt, and −dP/dt (Fig. 3.), but it did not reach statistical significance with two-factor analysis of variance followed by Dunnett's test. In contrast, in young adult control rats, INO-1001 had no effect on all hemodynamic parameters studied (Fig. 3).
Fig. 2.
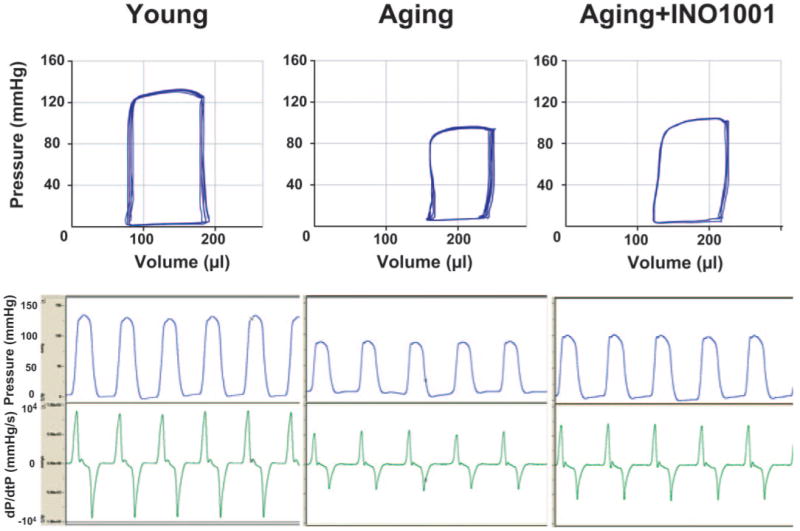
Representative PV loops (top part) and left ventricular pressure signal (bottom part) from young adult, aging, and aging treated with INO-1001 rats. Please note that the rightward shift of PV loops in aging animals, the decrease of maximal left ventricular pressure, and +dP/dt indicate depressed cardiac contractility and heart failure. INO-1001 moderately improves baseline contractile function.
Fig. 3.
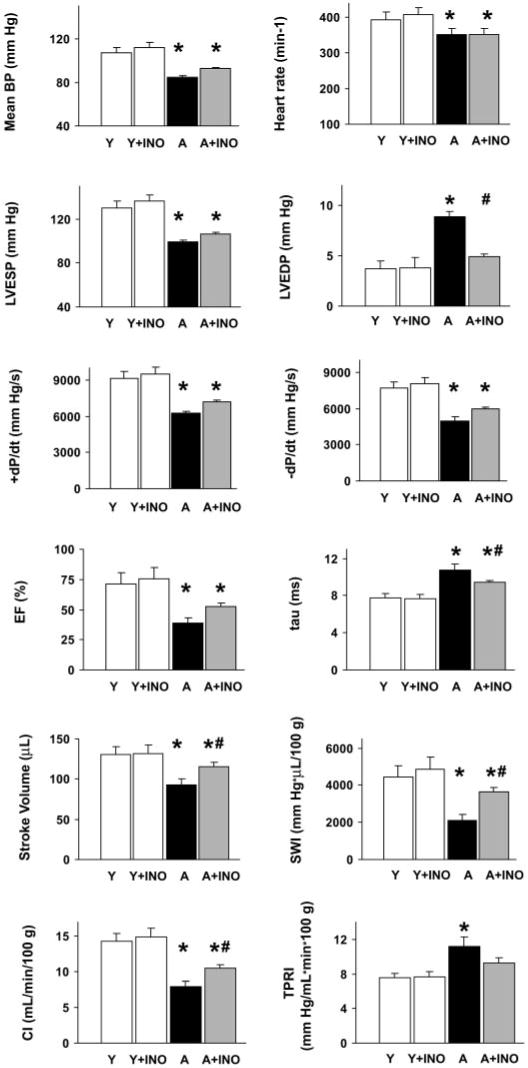
Pharmacological inhibition of PARP (for 2 months) improves cardiac dysfunction associated with advanced aging. Mean blood pressure (mean BP), LVSP, LVEDP, heart rate, +dP/dt and -dP/dt, EF, time constant of left ventricular pressure decay, stroke volume, SWI, CI, and TPRI in young adult (Y), young adult treated with INO-1001 (Y+INO), aging (A), and aging treated with INO-1001 (A+INO) male Fischer rats. Values are mean ± S.E.M. of 6 to 10 experiments in each group. *, P < 0.05 versus young controls; #, P < 0.05 versus aging.
Figure 2 shows representative PV loops from young adult, aging, and aging treated with INO-1001 rats. The rightward shift of PV loops in aging animals, the decrease in maximal left ventricular pressure, and +dP/dt indicate depressed cardiac contractility and heart failure. INO-1001 moderately improved baseline contractile function (Figs. 2 and 3).
Figure 4 shows typical PV loops obtained after inferior vena cava occlusions in aging (middle panel), aging treated with INO-1001 (lower panel), and young (upper panel) Fischer rats. Overall results of ESPVR (Emax), PRSW, +dP/dt-EDV, efficiency, and EDPVR are shown in Fig. 5.
Fig. 4.
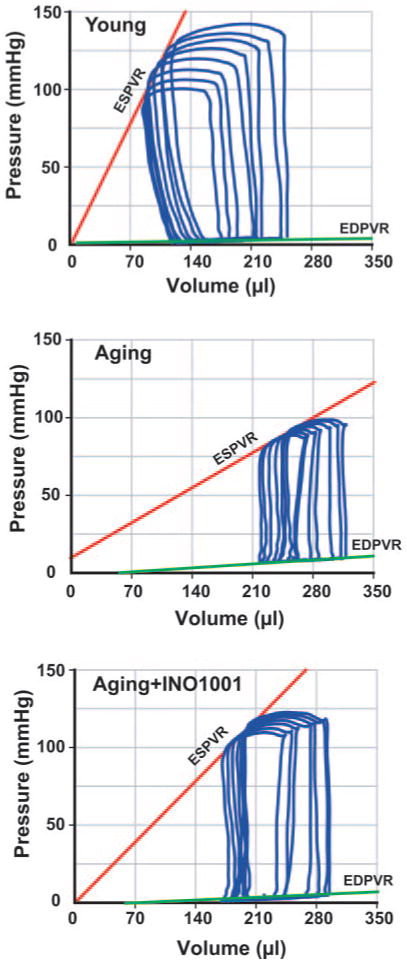
Pharmacological inhibition of PARP (for 2 months) improves ESPVR and EDPVR in advanced aging-associated heart failure. Representative PV loops obtained with PV conductance catheter system at different preloads, showing difference in ESPVR and EDPVR between young adult (Y), aging (A), and aging treated with INO-1001 (A+INO-1001) rats. The less steep ESPVR in aging animals (middle panel) compared with control young adult rats (upper panel) indicates severely decreased contractile function in aging, which is improved by INO-1001 treatment (lower panel). The steeper EDPVR in aging rats indicates increased end-diastolic stiffness, which is also improved by INO-1001 treatment.
Fig. 5.
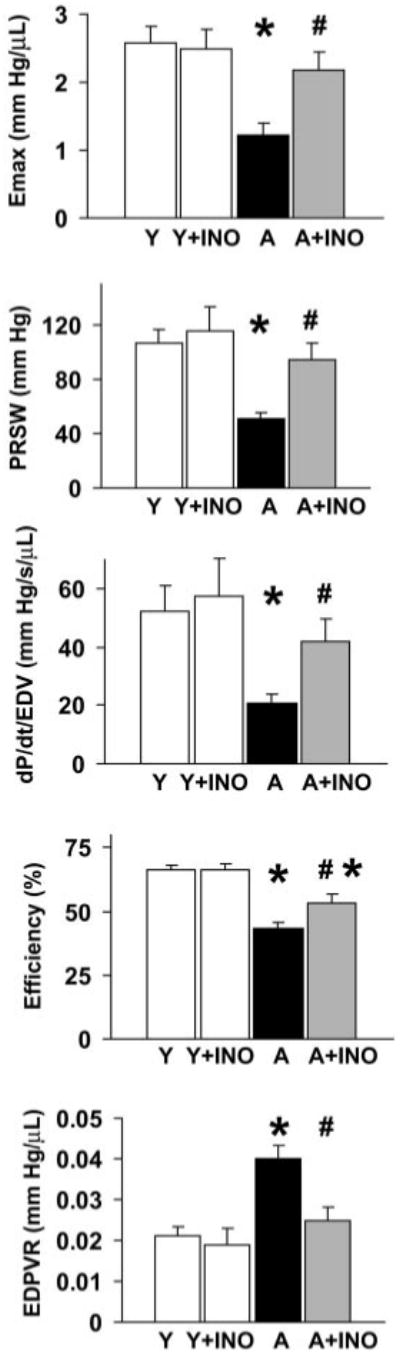
Pharmacological inhibition of PARP (for 2 months) improves ESPVR (Emax), PRSW, +dP/dt-EDV, EDPVR, and efficiency in advanced aging-associated heart failure. Overall values of Emax, PRSW, dP/dt-EDV, EDPV relationships, and efficiency. Values as mean ± S.E.M. of 6 to 10 experiments in each group. *, P < 0.05 versus young adult; #, P < 0.05 versus aging.
As shown in Figs. 4 and 5, ESPVR was steeper in young animals, suggesting decreased systolic performance in the aging rats. In contrast, EDPVR was increased in aging rats (Figs. 4 and 5), indicating increased end-diastolic stiffness. Treatment with the PARP inhibitor markedly improved both Emax and EDPVR.
In addition to the above parameters, PV loop recorded at different preloads can be used to derive other useful systolic function indexes that may be influenced less by loading conditions and cardiac mass, such as PRSW and +dP/dt-EDV relation (dP/dt-EDV) (Little, 1985; Kass et al., 1987) (Fig. 5). As shown in Fig. 5, these load-independent indexes of contractility were largely attenuated in aging animals and markedly improved by PARP inhibition. Efficiency of left ventricular work was also decreased in aging rats as compared with the young adult ones and was significantly improved by PARP inhibition (Fig. 5).
Vascular Function
Similarly, to numerous previous studies, our ex vivo experiments demonstrated the loss of endothelial function in rats with advanced aging indicated by decrease of maximal relaxation of aortic rings to ACh and rightward shift of the dose-response curve as compared with the young adult rats (Fig. 6). Pharmacological inhibition of PARP with INO-1001 (2 months) significantly improved maximal relaxation to ACh in aging animals, however, it had no effect in young adult rats where relaxation was not impaired (Fig. 6).
Fig. 6.
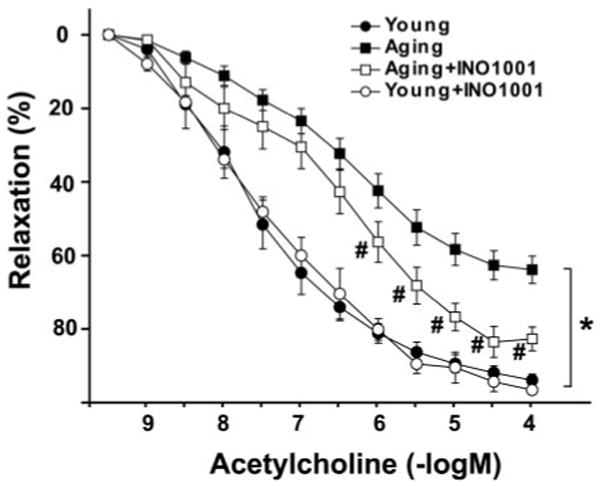
Improvement of advanced aging-induced endothelial dysfunction by pharmacological inhibition of PARP in rats. Acetylcholine-induced endothelium-dependent relaxation. Each point of the curve represent mean ± S.E.M. of 7 to 10 experiments in vascular rings. *, P < 0.05 versus young adult; #, P < 0.05 versus aging.
Discussion
In this study, we characterized the effects of pharmacological inhibition of PARP with INO-1001 on cardiac and endothelial dysfunction associated with advanced aging-associated heart failure using a pressure-volume conductance catheter system and isolated aortic rings in Fischer rats. The advantage of PV analysis is that it allows the examination of the intact chamber function independently of loading conditions (Little, 1985; Kass et al., 1987; Nakano et al., 1990). Using this approach here, we show that treatment with the potent PARP inhibitor, INO-1001, markedly improves decreased cardiac performance associated with aging.
There is increasing evidence demonstrating an aging-associated development of cardiovascular dysfunction (Anversa et al., 1989; Capasso et al., 1990; Hatake et al., 1990; Taddei et al., 1995; Higashi et al., 1997; van der Loo et al., 2000; Hamilton et al., 2001; Boluyt et al., 2004). The mechanisms responsible for this age-related cardiovascular dysfunction have not yet been clearly established. This impairment is, at least in part, related to the increased local formation of reactive oxygen and nitrogen species in the myocardium and vasculature (Inoue and Inoue, 1996; Rodriguez-Martinez et al., 1998; van der Loo et al., 2000). Superoxide anion interacts with nitric oxide forming the oxidant peroxynitrite, ONOO, which attacks various biomolecules, leading—among others—the production of a modified amino acid (nitrotyrosine) (Beckman and Koppenol, 1996). Although nitrotyrosine was initially considered a specific marker of peroxynitrite generation, other pathways can also induce tyrosine nitration (Eiserich et al., 1998). Thus, nitrotyrosine is now generally considered a collective index of reactive nitrogen species, rather than a specific indicator of peroxynitrite formation (Halliwell, 1997; Eiserich et al., 1998). Indeed, increased nitrotyrosine formation was reported in the vasculature of aging animals (van der Loo et al., 2000; Csiszar et al., 2002).
Oxidative stress accompanied by increased formation of hydrogen peroxide, superoxide anion, and peroxynitrite are endogenous inducers of DNA single-strand breakage, and DNA single-strand breakage is the obligatory trigger of PARP activation (reviewed in Virág and Szabó, 2002), which, in turn, may result in rapid depletion of the intracellular NAD+ and ATP pools, slowing the rate of glycolysis and mitochondrial respiration, and eventually leading to cellular dysfunction and necrosis. The protective effect of pharmacological inhibition of PARP or lack of the PARP gene in preventing cardiovascular dysfunction has been demonstrated in experimental models of shock, reperfusion injury, diabetes, and heart failure (another condition where oxidative stress plays a key pathogenetic role) (Szabó et al., 1997; Thiemermann et al., 1997; Zingarelli et al., 1998; Burkart et al., 1999; Soriano et al., 2001a,b; Pacher et al., 2002a–f).
The Fischer rat has served as a model to study the mechanisms of the aging-induced changes in the cardiovascular system (Anversa et al., 1989; Capasso et al., 1990; Boluyt et al., 2004). In this model, decreased cardiac performance and the development of progressive heart failure were reported after the age of 20 months (Anversa et al., 1989). Consistently with these previous results, aging in Fischer rats was associated with decreased +dP/dt, EF, and increased pulmonary edema. Although +dP/dt has been widely used as a cardiac contractile parameter, it is well recognized that it is load dependent, especially on changes in preload (Little, 1985; Kass et al., 1987). EF is also known to be influenced by both preload and afterload and therefore cannot reliably be used to assess contractile function in models where preload and afterload are altered. That is why we also determined other PV loop-derived indexes of ventricular contractility, which are less dependent on loading conditions including Emax, PRSW, and dP/dt-EDV. All these above-mentioned loading-independent indexes of left ventricular contractility, similarly to baseline indexes of contractility (+dP/dt, LVSP, and EF), were markedly decreased indicating severe systolic contractility dysfunction in aging rats. Pharmacological inhibition of PARP with INO-1001 only moderately improved load-dependent indexes of contractility (+dP/dt, LVSP, and EF) (Figs. 2 and 3). In contrast, the treatment remarkably improved all load-independent parameters of left ventricular contractility (Emax, PRSW, and dP/dt-EDV) indicating improved contractile function.
Impaired cardiac relaxation and increased end-diastolic stiffness observed in aging rats, as reflected by prolonged time constant of left ventricular pressure decay and increased EDPVR, was also attenuated by INO-1001 treatment (Fig. 5). Furthermore, INO-1001 also significantly improved acetylcholine-induced, nitric-oxide mediated, vascular relaxation of isolated aortic rings. These data are similar to the effect of a phenanthridinone-based PARP inhibitor PJ34, which also improves endothelium-dependent relaxations in the thoracic aorta of aging animals (Pacher et al., 2002e).
The current findings are in line with previous studies demonstrating that PARP inhibition improves cardiac and vascular function in various models of myocardial injury, including acute ischemia/reoxygenation, as well as various forms of chronic heart failure (diabetes-, drug- and ischemia-induced) (overviewed in Virág and Szabó, 2002; Szabó et al., 2004a).
In conclusion, our data demonstrate that pharmacological inhibition of PARP with a new, potent PARP inhibitor, INO-1001, remarkably improves both systolic and diastolic left ventricular function in an advanced aging-associated heart failure model. In addition, INO-1001 also improves endothelium-dependent ACh-evoked vascular relaxation of aortic rings. It is conceivable that the endothelial PARP pathway and the aging-associated heart failure are interrelated; an impairment of the endothelial function may lead to global or regional myocardial ischemia, which may secondarily impair cardiac performance. These data indicate that pharmacological inhibition of PARP may represent a novel approach to improve cardiovascular dysfunction associated with aging.
Acknowledgments
We are indebted to Millar Instruments, Inc. for the excellent technical support during the course of the studies.
This work was supported by Grant 1R03 AG21206-01 from the National Institutes of Health.
Abbreviations
- PARP
poly(ADP-ribose) polymerase
- LV
left ventricle
- LVSP
left ventricular systolic pressure
- LVEDP
left ventricular end-diastolic pressure
- MAP
mean arterial pressure
- +dP/dt
maximal slope of systolic pressure increment
- −dP/dt
maximal slope of diastolic decrement
- EF
ejection fraction
- SV
stroke volume
- SW
stroke work
- CI
cardiac index
- SWI
SW index
- TPRI
total peripheral resistance index
- PRSW
preload recruitable SW
- EDV
end-diastolic volume
- PV
pressure volume
- ESPVR
end-systolic pressure-volume relation
- EDPVR
end-diastolic pressure-volume relation
- A
aging
- ACh
acetylcholine
References
- Anversa P, Puntillo E, Nikitin P, Olivetti G, Capasso JM, Sonnenblick EH. Effects of age on mechanical and structural properties of myocardium of Fischer 344 rats. Am J Physiol. 1989;256:H1440–H1449. doi: 10.1152/ajpheart.1989.256.5.H1440. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Bejma J, Ramires P, Ji LL. Free radical generation and oxidative stress with ageing and exercise: differential effects in the myocardium and liver. Acta Physiol Scand. 2000;169:343–351. doi: 10.1046/j.1365-201x.2000.00745.x. [DOI] [PubMed] [Google Scholar]
- Boluyt MO, Converso K, Hwang HS, Mikkor A, Russell MW. Echocardiographic assessment of age-associated changes in systolic and diastolic function of the female F344 rat heart. J Appl Physiol. 2004;96:822–828. doi: 10.1152/japplphysiol.01026.2003. [DOI] [PubMed] [Google Scholar]
- Burkart V, Wang ZQ, Radons J, Heller B, Herceg Z, Stingl L, Wagner EF, Kolb H. Mice lacking the poly(ADP-ribose) polymerase gene are resistant to pancreatic beta-cell destruction and diabetes development induced by streptozotocin. Nat Med. 1999;5:314–319. doi: 10.1038/6535. [DOI] [PubMed] [Google Scholar]
- Capasso JM, Palackal T, Olivetti G, Anversa P. Severe myocardial dysfunction induced by ventricular remodeling in aging rat hearts. Am J Physiol. 1990;259:H1086–H1096. doi: 10.1152/ajpheart.1990.259.4.H1086. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- Du X, Matsumura T, Edelstein D, Rossetti L, Zsengeller Z, Szabó C, Brownlee M. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Investig. 2003;112:1049–1057. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, van der Vliet A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature (Lond) 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- Halliwell B. What nitrates tyrosine? Is nitrotyrosine specific as a biomarker of peroxynitrite formation in vivo? FEBS Lett. 1997;411:157–160. doi: 10.1016/s0014-5793(97)00469-9. [DOI] [PubMed] [Google Scholar]
- Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, Walter CA, Richardson A. Does oxidative damage to DNA increase with age? Proc Natl Acad Sci USA. 2001;98:10469–10474. doi: 10.1073/pnas.171202698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatake K, Kakishita E, Wakabayashi I, Sakiyama N, Hishida S. Effect of aging on endothelium-dependent vascular relaxation of isolated human basilar artery to thrombin and bradykinin. Stroke. 1990;21:1039–1043. doi: 10.1161/01.str.21.7.1039. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Oshima T, Ozono R, Matsuura H, Kajiyama G. Aging and severity of hypertension attenuate endothelium-dependent renal vascular relaxation in humans. Hypertension. 1997;30:252–258. doi: 10.1161/01.hyp.30.2.252. [DOI] [PubMed] [Google Scholar]
- Inoue M, Inoue K. Role of free radicals in and around vascular endothelial cells in the mechanism of aging. Ann NY Acad Sci. 1996;786:224–232. doi: 10.1111/j.1749-6632.1996.tb39065.x. [DOI] [PubMed] [Google Scholar]
- Kass DA, Maughan WL, Guo ZM, Kono A, Sunagawa K, Sagawa K. Comparative influence of load versus inotropic states on indexes of ventricular contractility: experimental and theoretical analysis based on pressure-volume relationships. Circulation. 1987;76:1422–1436. doi: 10.1161/01.cir.76.6.1422. [DOI] [PubMed] [Google Scholar]
- Khan TA, Ruel M, Bianchi C, Voisine P, Komjati K, Szabó C, Sellke FW. Poly(ADP-ribose) polymerase inhibition improves postischemic myocardial function after cardioplegia-cardiopulmonary bypass. J Am Coll Surg. 2003;197:270–277. doi: 10.1016/S1072-7515(03)00538-6. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- Little WC. The left ventricular dP/dtmax-end-diastolic volume relation in closed-chest dogs. Circ Res. 1985;56:808–815. doi: 10.1161/01.res.56.6.808. [DOI] [PubMed] [Google Scholar]
- Murakami K, Enkhbaatar P, Shimoda K, Cox RA, Burke AS, Hawkins HK, Traber LD, Schmalstieg FC, Salzman AL, Mabley JG, et al. Inhibition of poly (ADP-ribose) polymerase attenuates acute lung injury in an ovine model of sepsis. Shock. 2004;21:126–133. doi: 10.1097/01.shk.0000108397.56565.4a. [DOI] [PubMed] [Google Scholar]
- Nakano K, Sugawara M, Ishihara K, Kanazawa S, Corin WJ, Denslow S, Biederman RW, Carabello BA. Myocardial stiffness derived from end-systolic wall stress and logarithm of reciprocal of wall thickness. Contractility index independent of ventricular size. Circulation. 1990;82:1352–1361. doi: 10.1161/01.cir.82.4.1352. [DOI] [PubMed] [Google Scholar]
- Navarro A, Gomez C, Lopez-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol Regul Integr Comp Physiol. 2004;286:R505–R511. doi: 10.1152/ajpregu.00208.2003. [DOI] [PubMed] [Google Scholar]
- Pacher P, Cziraki A, Mabley JG, Liaudet L, Papp L, Szabó C. Role of poly(ADP-ribose) polymerase activation in endotoxin-induced cardiac collapse in rodents. Biochem Pharmacol. 2002a;64:1785–1791. doi: 10.1016/s0006-2952(02)01421-1. [DOI] [PubMed] [Google Scholar]
- Pacher P, Liaudet L, Bai P, Mabley JG, Kaminski PM, Virág L, Deb A, Szabó E, Ungvári Z, Wolin MS, et al. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation. 2003;107:896–904. doi: 10.1161/01.cir.0000048192.52098.dd. [DOI] [PubMed] [Google Scholar]
- Pacher P, Liaudet L, Bai P, Virág L, Mabley JG, Haskó G, Szabó C. Activation of poly(ADP-ribose) polymerase contributes to development of doxorubicin-induced heart failure. J Pharmacol Exp Ther. 2002b;300:862–867. doi: 10.1124/jpet.300.3.862. [DOI] [PubMed] [Google Scholar]
- Pacher P, Liaudet L, Mabley J, Komjati K, Szabó C. Pharmacologic inhibition of poly(adenosine diphosphate-ribose) polymerase may represent a novel therapeutic approach in chronic heart failure. J Am Coll Cardiol. 2002c;40:1006–1016. doi: 10.1016/s0735-1097(02)02062-4. [DOI] [PubMed] [Google Scholar]
- Pacher P, Liaudet L, Soriano FG, Mabley JG, Szabó E, Szabó C. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes. 2002d;51:514–521. doi: 10.2337/diabetes.51.2.514. [DOI] [PubMed] [Google Scholar]
- Pacher P, Mabley JG, Soriano FG, Liaudet L, Komjati K, Szabó C. Endothelial dysfunction in aging animals: the role of poly(ADP-ribose) polymerase activation. Br J Pharmacol. 2002e;135:1347–1350. doi: 10.1038/sj.bjp.0704627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Martinez MA, Alonso MJ, Redondo J, Salaices M, Marin J. Role of lipid peroxidation and the glutathione-dependent antioxidant system in the impairment of endothelium-dependent relaxations with age. Br J Pharmacol. 1998;123:113–121. doi: 10.1038/sj.bjp.0701595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda K, Murakami K, Enkhbaatar P, Traber LD, Cox RA, Hawkins HK, Schmalstieg FC, Komjati K, Mabley JG, Szabó C, et al. Effect of poly(ADP ribose) synthetase inhibition on burn and smoke inhalation injury in sheep. Am J Physiol Lung Cell Mol Physiol. 2003;285:L240–L249. doi: 10.1152/ajplung.00319.2002. [DOI] [PubMed] [Google Scholar]
- Soriano FG, Pacher P, Mabley J, Liaudet L, Szabó C. Rapid reversal of the diabetic endothelial dysfunction by pharmacological inhibition of poly(ADP-Ribose) polymerase. Circ Res. 2001a;89:684–691. doi: 10.1161/hh2001.097797. [DOI] [PubMed] [Google Scholar]
- Soriano FG, Virág L, Jagtap P, Szabó E, Mabley JG, Liaudet L, Márton A, Hoyt DG, Murthy KG, Salzman AL, et al. Diabetic endothelial dysfunction: the role of poly (ADP-ribose) polymerase activation. Nat Med. 2001b;7:108–113. doi: 10.1038/83241. [DOI] [PubMed] [Google Scholar]
- Suh JH, Shigeno ET, Morrow JD, Cox B, Rocha AE, Frei B, Hagen TM. Oxidative stress in the aging rat heart is reversed by dietary supplementation with (R)-(alpha)-lipoic acid. FASEB J. 2001;15:700–706. doi: 10.1096/fj.00-0176com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó C, Cuzzocrea S, Zingarelli B, O'Connor M, Salzman AL. Endothelial dysfunction in a rat model of endotoxic shock. Importance of the activation of poly (ADP-ribose) synthetase by peroxynitrite. J Clin Investig. 1997;100:723–735. doi: 10.1172/JCI119585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó G, Liaudet L, Hagl S, Szabó C. Poly(ADP-ribose) polymerase activation in the reperfused myocardium. Cardiovasc Res. 2004a;61:471–480. doi: 10.1016/j.cardiores.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Szabó G, Soos P, Mandera S, Heger U, Flechtenmacher C, Bahrle S, Seres L, Cziráki A, Gries A, Zsengellér Z, et al. INO-1001 a novel poly(ADP-ribose) polymerase (PARP) inhibitor improves cardiac and pulmonary function after crystalloid cardioplegia and extracorporal circulation. Shock. 2004b;21:426–432. doi: 10.1097/00024382-200405000-00005. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- Thiemermann C, Bowes J, Myint FP, Vane JR. Inhibition of the activity of poly(ADP ribose) synthetase reduces ischemia-reperfusion injury in the heart and skeletal muscle. Proc Natl Acad Sci USA. 1997;94:679–683. doi: 10.1073/pnas.94.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virág L, Szabó C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- Zingarelli B, Salzman AL, Szabó C. Genetic disruption of poly (ADP-ribose) synthetase inhibits the expression of P-selectin and intercellular adhesion molecule-1 in myocardial ischemia/reperfusion injury. Circ Res. 1998;83:85–94. doi: 10.1161/01.res.83.1.85. [DOI] [PubMed] [Google Scholar]


