Abstract
Allergic and parasitic helminth immunity is characterized by infiltration of tissues with IL-4- and IL-13-expressing cells, including Th2 cells, eosinophils and basophils1. Tissue macrophages assume a distinct phenotype, designated alternatively activated macrophages2. Relatively little is known regarding factors that trigger these host responses. Chitin, a widespread environmental biopolymer of N-acetyl-β-D-glucosamine, confers structural rigidity to fungi, crustaceans, helminths and insects3. Here, we show that chitin induces the tissue accumulation of IL-4-expressing innate immune cells, including eosinophils and basophils, when given to mice. Tissue infiltration was unaffected by the absence of Toll-like receptor-mediated LPS recognition and was abolished by treatment of chitin with the IL-4- and IL-13-inducible mammalian chitinase, AMCase4, or by injection into mice that over-expressed AMCase. Chitin mediated alternative macrophage activation in vivo and production of leukotriene B4, which was required for optimal immune cell recruitment. Chitin is a recognition element for tissue infiltration by innate cells implicated in allergic and helminth immunity and this process can be negatively regulated by a vertebrate chitinase.
Using infection with the migrating helminth, Nippostrongylus brasiliensis, to examine lung tissue responses, we confirmed prior findings that highly Stat6-dependent genes induced during infection included acidic mammalian chitinase (AMCase) and Ym1 and/or Ym2 (in human, chitinase-3-like protein 3 (CHI3L3) and CHI3L4, which could not be distinguished on the microarray)5. These proteins belong to the nine-member family of mammalian chitinase-like proteins. As compared to other family members, which were not regulated by Stat6 (Supplementary Fig. 1), we established that AMCase and Ym2 were expressed in the lung as mRNA by day 3 after infection and as protein at high levels by day 9 (Fig. 1a). N. brasiliensis larvae migrate within hours from the subcutaneous inoculation site to the lung, where organisms molt and ascend the trachea by day 2 and are swallowed to reach the intestines. In mice, adult worms are expelled by day 10 by an immune response dependent upon IL-4 and IL-136.
Figure 1. Chitin induces accumulation of innate effector cells.

a, RT-PCR and Western blot analysis of AMCase and Ym2 in the lung following N. brasiliensis infection. b, FACS analysis 1 day after 2 intranasal chitin doses to 4get mice. Eosinophil (GFP+27 Siglec F+30) and basophil (GFP+27, IgE+ cKit−27) gates shown. c, Kinetics of chitin-induced lung eosinophils. d, FACS of peritoneal cells 2 days after i.p. chitin. Eosinophils and mast cells (GFP+IgE+cKit+) gated. e, Kinetics of chitin-induced peritoneal cells. Data representative of 2–3 independent experiments.
The finding that expression of chitinase-like proteins was Stat6-dependent in the lung raised the possibility that chitin, which constitutes a structural constituent remodelled during molting in worms7, might serve as a recognition element for eliciting tissue infiltration by IL-4- and IL-13-producing immune cells. To test this, we administered chitin to the lungs of 4get mice, which contain a knock-in IL-4 reporter that allows the detection of cells competent to produce IL-48. Chitin challenge led to recruitment of eosinophils and basophils to the lungs that peaked by days 2–3 and returned to basal levels by day 9 (Fig. 1b, c). Intraperitoneal injection of chitin induced eosinophil accumulation as early as 6 hours, demonstrating that the response was not tissue-specific (Fig. 1d, e). Neutrophils were also recruited to the peritoneum early in response to chitin, although there was no influx of neutrophils to the lung (Fig. 1e, Supplemental Fig. 2). Unlike in the lung, mast cells (eGFP+, cKit+, IgE+) represent a substantial proportion of constitutively eGFP-positive cells in the peritoneum and basophils were not recruited by chitin. Compared to LPS, which resulted in neutrophil recruitment but no eosinophil recruitment to either the lung or peritoneum, chitin induced eosinophil influx in both tissues. Neither challenge altered peritoneal mast cell numbers (Supplementary Fig. 2).
Although LPS can modulate allergic immunity in some models, chitin mediated eosinophil and basophil recruitment in TLR4-deficient (LPS unresponsive) and MyD88-deficient mice (Fig. 2a, and data not shown). Next, we produced recombinant enzymatically active mouse AMCase, which binds and degrades chitin9; mutant AMCase with alterations of the active site aspartate-154 and glutamate-158 necessary for the catalytic activity10; and Ym2, a non-chitinolytic member of the chitinase-like family which is also Stat6-induced but lacks chitin-binding and chitinolytic functions11. Pre-treatment of chitin with enzymatically active AMCase, but not with mutant enzymatically-inactive AMCase or with Ym2, led to loss of eosinophil and basophil recruitment to the lung, consistent with a role for intact chitin in these cellular events (Fig. 2b, c). Similar results were seen after injection of these preparations intraperitoneally to assess eosinophil recruitment (data not shown). We generated transgenic mice that over-expressed AMCase constitutively in the lung resulting in the production of enzymatically active AMCase that approximated the levels seen at day 9 after infection with N. brasiliensis (Fig. 2d). Analysis of 2 independent transgenic lines revealed that lung-specific expression of AMCase led to no apparent abnormalities in the mice and the lungs were histologically normal (data not shown). These mice were crossed to 4get mice, thus allowing analysis of the recruitment of IL-4-competent cells to the lung. When challenged with chitin, mice over-expressing AMCase (SPAMx4get) had attenuated inflammatory responses (Fig. 2e). These experiments suggest that chitin itself robustly elicits infiltration by IL-4-competent innate cells following introduction into tissues and that the Stat6-inducible chitinase, AMCase, abrogates this activity.
Figure 2. AMCase prevents chitin-induced eosinophil and basophil recruitment.
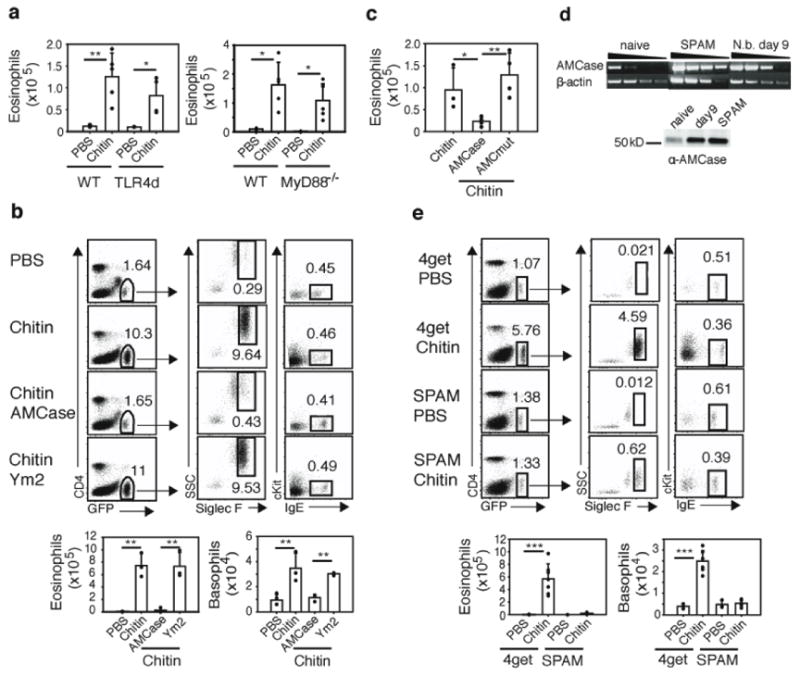
a, Designated mice received intranasal chitin and lung eosinophils were quantitated by flow cytometry. b, Chitin or chitin preincubated with recombinant AMCase or Ym2 was administered to 4get mice and recruitment of lung eosinophils and basophils was determined. c, Chitin was preincubated with PBS, recombinant AMCase, or recombinant mutant enzymatically-inactive AMCase (AMCmut) and given to 4get mice. d, AMCase expression in SPAM transgenic mice assessed by RT-PCR and Western blot analysis of lung tissues from naïve, day 9 N. brasiliensis-infected wild-type and naïve SPAM mice. e, SPAMx4get transgenic mice (SPAM) or wild-type 4get mice were given intranasal chitin. * p<0.05, ** p<0.01, and *** p<0.001 P-values were determined using Student’s t-test. Error bars represent standard deviations. In FACS plots, numbers represent percentage of gated cells from total live cells. Shown is representative of three independent experiments, with n=3–4 per group.
Although AMCase expression was Stat6- and Rag-dependent, eosinophil recruitment mediated by chitin in vivo was independent of Stat6 and Rag (Fig. 3a, b), suggesting an early innate response. Basophil recruitment to the lung was also independent of Stat6 and Rag (data not shown). Additionally, we could consistently document eosinophil chemoattractant activity in supernatants of bone marrow-derived macrophages and the RAW267.4 macrophage cell line after incubation with chitin that was unaffected by the addition of neutralizing antibody against eotaxin (data not shown). However, eosinophil chemoattraction by macrophages was inhibited if the chitin was pre-treated with enzymatically active AMCase, consistent with our in vivo results, and was also inhibited when the macrophages were pre-treated with an inhibitor of leukotriene production, MK886 (Fig. 3c). Leukotriene B4 (LTB4) is a potent chemoattractant for eosinophils12 and is induced from canine phagocytes by chitin13. Additionally, the high-affinity receptor for LTB4, BLT1, is an important mediator of early effector T cell recruitment to the lung in an allergic asthma model14. To establish a role for LTB4 in the chitin-mediated effects we observed, we challenged mice deficient in BLT1 with chitin. As compared to controls, chitin-induced eosinophil and basophil recruitment to the lung was significantly attenuated in the BLT1 knockout mice (Fig. 3d, and data not shown), despite the equivalent percentages of eosinophils present in the blood of the knockout mice as compared to wild-type mice (Supplementary Fig. 3). To determine whether macrophages play a role in vivo, we used clodronate liposomes to deplete peritoneal macrophages prior to the administration of chitin. Treatment with clodronate markedly curtailed eosinophil recruitment to the peritoneum by chitin (Fig. 3e). Mast cells are also potent producers of leukotrienes. Following administration of chitin to mast cell-deficient Kit W-sash mice15, eosinophil recruitment to the lungs was unaffected, suggesting that mast cells are not required for a chitin-mediated response (Fig. 3f).
Figure 3. Chitin-induced eosinophil recruitment is independent of Stat6 and Rag, but is dependent on BLT1.

a, Stat6−/ −×4get and 4get mice received chitin or PBS, and the lungs were analyzed for eosinophils. b, Rag−/ − and wild-type mice received chitin or PBS, and lungs were analyzed for eosinophils. Eosinophils were identified as DAPI−, FSCIo, CD4−, Siglec F+, SSChi. c, Transwell analysis of eosinophil recruitment by RAW267.4 macrophages incubated with vehicle, chitin, or AMCase-treated chitin. Where indicated, macrophages were pretreated with vehicle or MK886 (10 μM) for 10 min at 37°C prior to addition of chitin. d, BLT1−/ − mice and WT C57BL/6 mice received intranasal chitin. e, 4get mice were treated intraperitoneally with clodronate liposomes (CLL) or PBS on days 0 and 3. On day 4 mice received chitin i.p. and were analyzed 6 hrs later. f, Kit W-sh or wild-type controls received chitin intranasally. P-values were determined using Student’s t-test. Error bars represent standard deviations. * p<0.05, ** p<0.01 and *** p<0.001 Numbers in FACS plots are percentages of gated cells from total live cells. Experiments represent 2–3 independent experiments with n=3–4 per group.
The capacity of chitin to mediate effects on macrophages suggested that these cells might represent sensors for chitin in tissues. A signature gene induced in alternatively activated macrophages is arginase I2, 5. Arginase I enzymatically cleaves arginine to generate L-ornithine, a precursor for polyamines and proline, which have been implicated in cell proliferation and collagen production, respectively. To assess whether chitin induces alternative macrophage activation in vivo, we generated mice containing an IRES-driven fluorescent eYFP reporter introduced at the 3′-end of the arginase I gene (H.E.L. and R.M.L, manuscript in progress). Under resting conditions, no eYFP-positive macrophages were identified in the lungs or peritoneum of arginase I reporter mice, designated YARG (Fig. 4a, data not shown). On day 9 after N. brasiliensis infection, however, large numbers of eYFP-positive macrophages (CD11b+, CD11c−, Gr1−) were present in both tissues (Fig. 4a, b, data not shown). Thus, parasitic infection elicits accumulation of arginase I-positive macrophages, in agreement with prior studies2, 5. Next, we injected chitin into YARG mice. As early as 6 hours, and sustained up to 7 days, arginase I-positive cells with surface markers consistent with macrophages appeared in the lungs and peritoneum (Fig. 4c, f, data not shown). Further, when sorted into transwell dishes, arginase I-positive macrophages induced by chitin attracted eosinophils in a manner inhibited by MK886, consistent with a role for these cells in mediating cell recruitment by LTB4 in vivo (Fig. 4d). Additionally, we crossed YARG and SPAM mice, and challenged the lungs of these mice with chitin. In contrast to YARG mice, which developed alternatively activated macrophages by day 2, YARG x SPAM mice developed greatly attenuated numbers of these cells (Fig. 4e). Similar results occurred if chitin was digested with AMCase before challenging animals (Supplementary Fig. 4). Arginase I was induced initially in the peritoneum in macrophages with the characteristic phenotype of resident cells (CD11bhi, F4/80hi, Ly6C−, CD62L−). Macrophages with the surface phenotype of inflammatory macrophages (CD11b+, F4/80+, Ly6C+, CD62L+) did not acquire arginase I expression until later in the response, indicating that resident macrophages respond early to chitin (Fig. 4f).
Figure 4. Chitin induces alternatively activated macrophages.
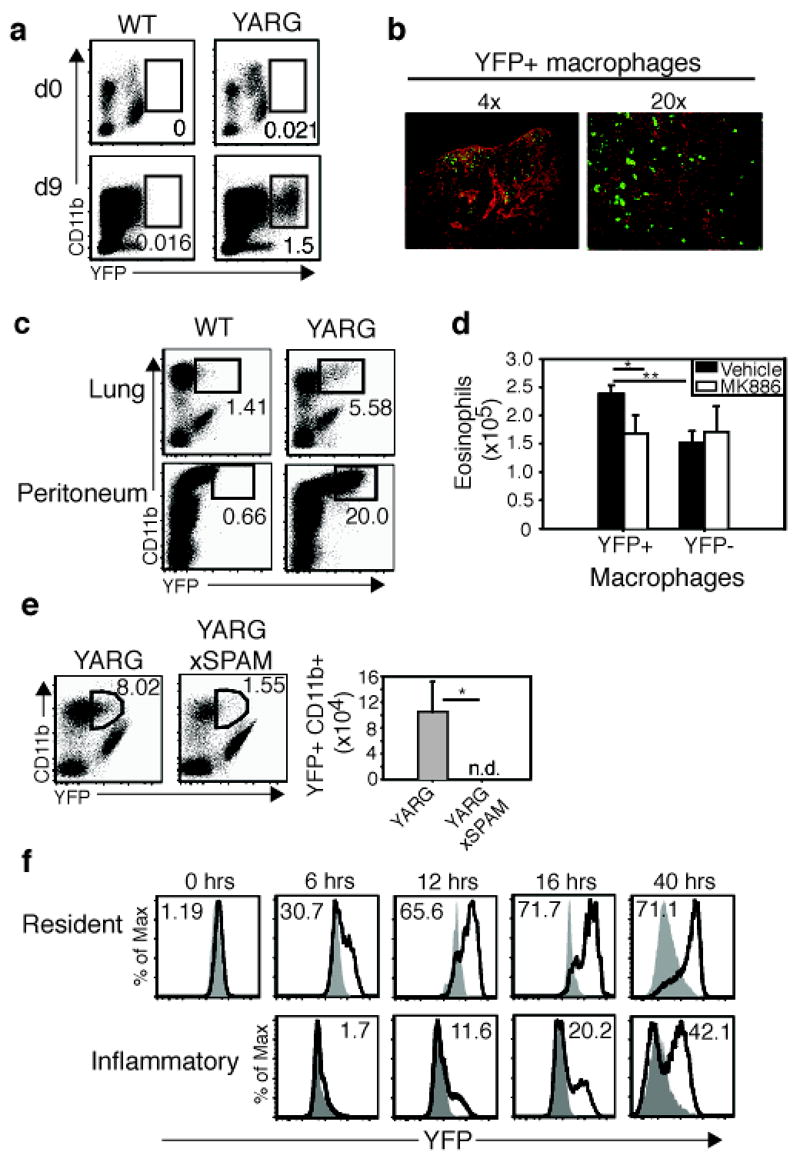
a, YARG or wild-type littermates were infected with N. brasiliensis. CD11b+ lung macrophages were analyzed for YFP expression on days 0 and 9. b, Day 9 N. brasiliensis-infected lung from YARG mice was stained with anti-GFP (green) and nuclei were counterstained with DAPI (red). Lens magnification indicated. c, Chitin administered to lung or peritoneum of YARG or wild-type mice. d, Sorted YFP+ and YFP− macrophages were treated with vehicle or 10 μm MK886 for 10 min/37°C before analysis for eosinophil chemotaxis using a transwell assay. Results average of three independent experiments. e, YARGxSPAM transgenic mice were compared with YARG littermates following intranasal chitin. f, YARG mice or negative littermates received chitin and were analyzed for YFP expression. Filled histograms represent negative littermates. Solid line represents YFP in the YARG littermate. Numbers in the histogram are the percentage YFP+. Resident and inflammatory macrophages were subset using surface markers described in the text. * p<0.05, ** p<0.01 P-values determined using Student’s t-test. Error bars represent standard deviations. In FACS plots, numbers represent percentage of gated cell from total live cells. n.d. = none detected. Each experiment represents two independent experiments with n=3 per group.
Whereas previous reports implicated AMCase as a proinflammatory mediator of allergic inflammation4, transgenic mice over-expressing AMCase showed no signs of spontaneous inflammation. Rather, our data support a role for AMCase in the feedback attenuation of chitin-induced allergic innate immune responses by enzymatically degrading chitin, thus removing the stimulus for further eosinophil and basophil recruitment. Although inhibition of LTB4 rendered mice unable to control the helminth Strongyloides venezuelensis16, Nippostrongylus was expelled from BLT1-deficient mice (data not shown), suggesting that additional pathways, such as activation of cytokine secretion by helminth proteases17, may contribute to the immune response against these complex pathogens. Prior studies have suggested that chitin may skew immunity away from Th2-mediated allergic responses, although differences in animal models, chitin preparations and delivery, and the potential for contamination by LPS and other microbial activating ligands makes direct comparisons with our studies difficult18.
Chitin is the second most abundant biopolymer in nature, with estimates of billions of metric tons produced annually in oceans alone. Despite the prodigious production by phytoplankton and crustaceans, marine sediments contain only trace amounts due to degradation by chitinolytic marine bacteria19. Chitin provides osmotic stability and tensile strength to fungal cell walls3 and scaffolds the rigid exoskeleton in insects20. Nematode chitins are important for eggshell integrity and for structure of the rigid pharynx, including the buccal cavity and grinder, a specialized cuticle that is shed and resynthesized during molting7. The use of chitin by fungi, worms and insects may have driven evolutionary pressure to maintain chitin-recognition molecules in vertebrates akin to those in plants and protochordates21, 22. Intriguingly, these data support a role for chitin as a molecular pattern recognized in tissues and linked with the accumulation of innate cells implicated in helminth and allergic immunity, including alternatively activated macrophages, eosinophils and basophils2, 23. Occupations predicted to have high environmental chitin levels, such as shellfish processors, are marked by high incidence of asthma, suggesting that this pathway may play a role in human allergic disease24.
METHODS SUMMARY
Mice
BALB/c mice, C57BL/6 mice, TLR4-deficient mice (C.C3-Tlr4Lps-d/J) were purchased from Jackson Laboratories (Bar Harbor, ME). MyD88-deficient mice25, mast cell-deficient Kit (W-sh) mice15, IL-4 reporter mice (4get mice)8, and BLT1−/ − mice have been described26. Stat6−/ − mice, Rag−/− mice and IL-5 transgenic mice were backcrossed 10 generations to 4get/BALB/c23. For information on the generation of SPAM and YARG mice see Supplementary Methods. Mice were maintained in the specific pathogen-free animal facility at UCSF according to institutional guidelines.
Chitin administration
Chitin (New England Biolabs) was washed three times in PBS and large aggregates settled for 2 min. Suspended chitin was collected and diluted 1:4 in PBS. For intranasal administration anesthetized animals aspirated 50 μl of chitin on consecutive days, and were analyzed on day 2. Where designated, 200 μl of chitin was injected once i.p. In designated experiments, chitin was preincubated with 100 μg recombinant AMCase, Ym2, or mutant AMCase for 1 hr/37°C. Additional information on recombinant protein production is available in Supplementary Methods.
Flow cytometry and cell purification
Flow cytometry was performed as described23, 27. For additional information and macrophage purification see Supplementary Methods.
Transwell Assay
RAW264.7 macrophage cell line or bone marrow-derived macrophages were distributed at 1×106 cells/well in 24-well plates in serum-free DMEM media. The following morning adherent cells were stimulated with chitin (0.8 μg) or PBS (vehicle control). Optimal chitin concentration was determined separately in a dose response curve. For macrophages sorted from YARG mice, 5×105 cells/well were distributed to 24-well plates in RPMI/10%FCS and used immediately in transwell assays. 1×106 splenocytes from IL5 transgenic x 4get mice were placed in the top well of the transwell chamber (Costar, 5.0 μm pore size, Corning Inc.) at 37°C/5% CO2 for 3 hrs. Cells that migrated to the bottom well of the transwell chamber were enumerated using counting beads (Caltag) and stained for Siglec-F-PE. MK886 was purchased from Cayman Chemical. Chemotactic index is the number of cells that migrate to the stimulus divided by the number of cells that migrate to vehicle. Experiments with less than 120,000 migrated eosinophils were excluded due to viability concerns.
Immunohistochemistry
Lungs were isolated from YARG mice and processed as described28 using rabbit anti-GFP polyclonal Ab (ab 6556; Novus Biologicals).
In vivo depletion of macrophages
Cl2MDP (clodronate) was a gift from Roche Diagnostics GmbH, Mannheim, Germany. Clodronate liposomes were prepared as described29 and 200 μl of liposomes or PBS was injected i.p. on days 0 and 3. Chitin was administered on the next day and peritoneal cell recruitment was analyzed 6 hrs later.
Supplementary Material
Supplementary Information accompanies the paper at www.nature.com/nature.
Acknowledgments
We thank D. Stetson for assistance with recombinant protein production, G. Caughey, X. Xu, and S. Akira for mice and reagents, N. Flores, C. McArthur and L. Stowring for expert technical assistance, and A. DeFranco and J. Bluestone for helpful discussion. This work was supported by NIH AI30663 and AI26918, HHMI and the Sandler Asthma Basic Research Centre at UCSF.
Footnotes
Author Information Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
References
- 1.Ramalingam TR, Reiman RM, Wynn TA. Exploiting worm and allergy models to understand Th2 cytokine biology. Curr Opin Allergy Clin Immunol. 2005;5:392–8. doi: 10.1097/01.all.0000182542.30100.6f. [DOI] [PubMed] [Google Scholar]
- 2.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 3.Bowman SM, Free SJ. The structure and synthesis of the fungal cell wall. Bioessays. 2006;28:799–808. doi: 10.1002/bies.20441. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Z, et al. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004;304:1678–82. doi: 10.1126/science.1095336. [DOI] [PubMed] [Google Scholar]
- 5.Nair MG, Guild KJ, Artis D. Novel effector molecules in type 2 inflammation: lessons drawn from helminth infection and allergy. J Immunol. 2006;177:1393–9. doi: 10.4049/jimmunol.177.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finkelman FD, et al. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev. 2004;201:139–55. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Foster JM, Nelson LS, Ma D, Carlow CK. The chitin synthase genes chs-1 and chs-2 are essential for C. elegans development and responsible for chitin deposition in the eggshell and pharynx, respectively. Dev Biol. 2005;285:330–9. doi: 10.1016/j.ydbio.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 8.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–11. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 9.Boot RG, et al. Identification of a novel acidic mammalian chitinase distinct from chitotriosidase. J Biol Chem. 2001;276:6770–8. doi: 10.1074/jbc.M009886200. [DOI] [PubMed] [Google Scholar]
- 10.Boot RG, Renkema GH, Strijland A, van Zonneveld AJ, Aerts JM. Cloning of a cDNA encoding chitotriosidase, a human chitinase produced by macrophages. J Biol Chem. 1995;270:26252–6. doi: 10.1074/jbc.270.44.26252. [DOI] [PubMed] [Google Scholar]
- 11.Jin HM, et al. Genetic characterization of the murine Ym1 gene and identification of a cluster of highly homologous genes. Genomics. 1998;54:316–22. doi: 10.1006/geno.1998.5593. [DOI] [PubMed] [Google Scholar]
- 12.Huang WW, et al. Molecular and biological characterization of the murine leukotriene B4 receptor expressed on eosinophils. J Exp Med. 1998;188:1063–74. doi: 10.1084/jem.188.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Usami Y, Okamoto Y, Takayama T, Shigemasa Y, Minami S. Chitin and chitosan stimulate canine polymorphonuclear cells to release leukotriene B4 and prostaglandin E2. J Biomed Mater Res. 1998;42:517–22. doi: 10.1002/(sici)1097-4636(19981215)42:4<517::aid-jbm6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 14.Tager AM, et al. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat Immunol. 2003;4:982–90. doi: 10.1038/ni970. [DOI] [PubMed] [Google Scholar]
- 15.Wolters PJ, et al. Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient Kit(W-sh)/Kit(W-sh) sash mice. Clin Exp Allergy. 2005;35:82–8. doi: 10.1111/j.1365-2222.2005.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Machado ER, et al. Leukotrienes play a role in the control of parasite burden in murine strongyloidiasis. J Immunol. 2005;175:3892–9. doi: 10.4049/jimmunol.175.6.3892. [DOI] [PubMed] [Google Scholar]
- 17.Phillips C, Coward WR, Pritchard DI, Hewitt CR. Basophils express a type 2 cytokine profile on exposure to proteases from helminths and house dust mites. J Leukoc Biol. 2003;73:165–71. doi: 10.1189/jlb.0702356. [DOI] [PubMed] [Google Scholar]
- 18.Shibata Y, Foster LA, Bradfield JF, Myrvik QN. Oral administration of chitin down-regulates serum IgE levels and lung eosinophilia in the allergic mouse. J Immunol. 2000;164:1314–21. doi: 10.4049/jimmunol.164.3.1314. [DOI] [PubMed] [Google Scholar]
- 19.Keyhani NO, Roseman S. Physiological aspects of chitin catabolism in marine bacteria. Biochim Biophys Acta. 1999;1473:108–22. doi: 10.1016/s0304-4165(99)00172-5. [DOI] [PubMed] [Google Scholar]
- 20.Merzendorfer H, Zimoch L. Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. J Exp Biol. 2003;206:4393–412. doi: 10.1242/jeb.00709. [DOI] [PubMed] [Google Scholar]
- 21.Kaku H, et al. Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc Natl Acad Sci U S A. 2006;103:11086–91. doi: 10.1073/pnas.0508882103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez Prada JA, et al. Ancient evolutionary origin of diversified variable regions demonstrated by crystal structures of an immune-type receptor in amphioxus. Nat Immunol. 2006;7:875–82. doi: 10.1038/ni1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006 doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cartier A, et al. Prevalence of crab asthma in crab plant workers in Newfoundland and Labrador. Int J Circumpolar Health. 2004;63(Suppl 2):333–6. doi: 10.3402/ijch.v63i0.17930. [DOI] [PubMed] [Google Scholar]
- 25.Adachi O, et al. Targeted disruption of the MyD88 gene results in loss of IL-1-and IL-18-mediated function. Immunity. 1998;9:143–50. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 26.Tager AM, et al. BLTR mediates leukotriene B(4)-induced chemotaxis and adhesion and plays a dominant role in eosinophil accumulation in a murine model of peritonitis. J Exp Med. 2000;192:439–46. doi: 10.1084/jem.192.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;2:267–77. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 28.Reinhardt RL, Hong S, Kang SJ, Wang ZE, Locksley RM. Visualization of IL-12/23p40 in vivo reveals immunostimulatory dendritic cell migrants that promote Th1 differentiation. J Immunol. 2006;177:1618–27. doi: 10.4049/jimmunol.177.3.1618. [DOI] [PubMed] [Google Scholar]
- 29.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 30.Zhang JQ, Biedermann B, Nitschke L, Crocker PR. The murine inhibitory receptor mSiglec-E is expressed broadly on cells of the innate immune system whereas mSiglec-F is restricted to eosinophils. Eur J Immunol. 2004;3:1175–84. doi: 10.1002/eji.200324723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information accompanies the paper at www.nature.com/nature.


