Abstract
Objective
Reductions in retinal blood flow are observed early in diabetes. Venules may influence arteriolar constriction and flow; therefore, we hypothesized that diabetes would induce the constriction of arterioles that are in close proximity to venules, with the constriction mediated by thromboxane and angiotensin II.
Methods
Using non-obese diabetic (NOD) mice, retinal measurements were performed 3 weeks following the age at which glucose levels exceeded 200 mg/dl, with accompanying experiments on age-matched normoglycemic NOD mice. The measurements included retinal arteriolar diameters and red blood cell velocities, and were repeated following an injection of the thromboxane synthase inhibitor Ozagrel. Mice were subdivided into equal groups given drinking water with or without the angiotensin II receptor antagonist Losartan.
Results
Retinal arterioles were constricted in hyperglycemic mice, with a significant reduction in flow. However, not all arterioles were equally affected; the vasoconstriction was limited to arterioles that were in closer proximity to venules. The arteriolar vasoconstriction (mean arteriolar diameters = 51 ± 1 μm vs 61 ± 1 μm in controls; p<0.01) was eliminated by both Ozagrel (61 ± 2 μm) and Losartan (63 ± 2 μm).
Conclusion
Venule-dependent arteriolar vasoconstriction in NOD mice is mediated by thromboxane and/or angiotensin II.
Keywords: retina, arteriole, perfusion, diabetes
Introduction
Diabetic retinopathy is a major complication of diabetes and a leading cause of blindness in Western countries [42]. Alterations in the retinal circulation caused by diabetes include ischemia, which could initiate angiogenesis and macular edema that commonly result in vision loss [26]. The induction of ischemia could be multi-factorial, with vasoconstrictive mediators potentially playing a significant role. Decreased retinal arteriolar diameters have been reported in diabetic patients [28,37,47,48], and the constriction may decrease blood flow and slow oxygen delivery to regions of the retina. The origin of the vasoactive mediators, and the delivery to arteriolar smooth muscle, have not been determined. However, recent studies in various tissues suggest that inflammation-induced constriction and endothelial dysfunction may be more severe in arterioles that are closely paired with postcapillary venules.
Arterio-venular pairing, an arrangement comprising a feeding arteriole and a closely paired counter-current venule draining the capillary bed, is a common microvascular structure found in most tissues of the body. This vascular pairing provides counter-current transport of heat, oxygen, and carbon dioxide, as well as, more recently noted, venular control of arteriolar tone [21]. Although most of the investigations into venular control of arteriolar tone have focused on the ability of venules to dilate the closely paired arterioles [3,9,10,15,33], the reverse is found in the microvascular dysfunction that accompanies inflammation. For example, Zamboni et al. performed a series of studies [32,50,51] demonstrating that ischemia-reperfusion of skeletal muscle induced constriction of arterioles, but only in arterioles that were closely paired with venules. A significant portion of the vasoconstriction could be attenuated by blocking thromboxane [32]. A study from our own lab similarly implicates thromboxane in the venule-dependent constriction of arterioles in the submucosa of the inflamed intestine [18]. Although its role in venule-dependent constriction of arterioles has not been studied, another vasoconstrictor that has been implicated in the vascular complications of diabetes is angiotensin II. All components of the renin-angiotensin system are found in the eye [29,43], with angiotensin II levels higher in the eye than in plasma [43].
Due to the lack of autonomic innervation in the retina, local mechanisms of microvascular perfusion, such as venulo-arteriolar communication, may play a significant role. Local autoregulation modulates retinal blood flow in response to pressure and metabolic demands [12,44]; however, this autoregulation could be altered in the inflamed retina by venule-dependent arteriolar dysfunction. Whether this phenomenon occurs in the diabetic retina is the primary purpose of the current study. We hypothesize that arteriolar constriction and reduced vascular flow in the diabetic retina are mediated by vasoconstrictors (such as thromboxane and/or angiotensin II). Moreover, we hypothesize that more severe constriction occurs in arterioles that are more closely paired with venules. We have addressed these questions in non-obese diabetic (NOD) mice, with the retinal microcirculation examined via intravital microscopy.
Material and Methods
Animals
Female NOD mice were purchased at an age of 7 weeks (Jackson, Bar Harbor, ME, USA). Upon arrival, none of the mice were hyperglycemic; however, elevated blood glucose levels were observed in more than one-half of the mice by 30 weeks of age. Non-fasting blood glucose levels were determined using a One Touch Ultra blood glucose monitoring system (Lifescan, Milpitas, CA, USA) and animals with levels greater than 200 mg/dl were considered diabetic. Experiments were performed on the mice following 3 weeks of hyperglycemia, at which time glucose levels typically exceeded 400 mg/dl. One-half of the diabetic mice were given Losartan, an angiotensin II receptor type 2 antagonist, via their drinking water (25 mg/kg/day). All mice were provided chow (LabDiet® JL Rat and Mouse/Auto 6F, PMI Nutrition International, LLC, Brentwood, MO, USA) and water ad libitum.
On the day of the experiment, the mice were anesthetized with intraperitoneal injections of pentobarbital (50 mg/kg, Nembutal®, Abbott Laboratories, North Chicago, IL, USA) and ketamine (50 mg/kg, Ketaset®, Animal Health, Fort Dodge, IA, USA). The eyes under examination were kept moist with a drop of Gonak® (Akorn Inc., Buffalo Grove, IL, USA) after the pupils were dilated using two drops each of tropicamide (1%; Alcon Laboratories Inc., Fort Worth, TX, USA) and phenylephrine (2.5%; Bausch & Lomb, Tampa, FL, USA). Body temperature was maintained near 37°C at all times with an infrared heat lamp over the mouse. Procedures complied with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. At the end of the experiments, animals were euthanized using an overdose of sodium pentobarbital (120 mg/kg) as approved by the Institutional Animal Care and Use Committee.
Pressure Measurements
Intraocular pressure (IOP) of each animal used for intravital microscopy was measured using TonoPen XL® (Mentor, Norwell, MA, USA). Tonopen has been reported to be an acceptable non-invasive device to measure IOP in mice [35,41]. The measurement was made using a modified method previously reported by Reitsamer et al [41]. Once the mouse was put under anesthesia, 10 measurements were made on the right eye with the Tonopen calibrated according to the manufacturer’s instructions. Any measurement below 6 mmHg was excluded from analysis, as these readings could represent insufficiently light touches of the cornea with the tip of the Tonopen [35]. The Tonopen was cleaned between each measurement by a cotton pad and alcohol, and the eye was kept moist with saline solution. Mean arterial pressure was measured in separate and age-matched mice of each group. The animal was anesthetized as described above and the carotid artery was cannulated with a catheter which was connected to a Pressure Monitor BP-1 (World Precision Instruments, Sarasota, FL, USA). Body temperature was maintained near 37°C during the procedure using an infrared heat lamp.
Intravital Microscopy
The animal was placed on a microscope stage with a cover slip over the eye, which was viewed with a Nikon 10× objective (N.A. 0.25; 1.05 cm working distance) on a Nikon fluorescence microscope (Eclipse E600FN, Nikon Instruments Inc., Melville, NY, USA). An autofluorescent image of the retina was used to locate and focus on the optic nerve head through a fluorescein filter. Arterioles extending from the optic disk generally are found between alternating venules that drain the retinal capillary bed. The alternating arrangement of arterioles and venules diverges at various angles from the optic disk, with some arterio-venular pairs close and nearly parallel with each other, while other pairs are more distantly spaced with a wider angle. The angles between each arteriole and the two neighboring venules were measured and averaged.
Fluorescently labeled red blood cells (RBCs) were injected through a femoral vein catheter to determine retinal blood velocity. The fluorescent images of the circulating RBCs in the retina were recorded on DVD (DMR-ES10 recorder, Panasonic, Osaka, Japan) through a Sony color camera (DXC-390, Sony, Tokyo, Japan) and a time-date generator (Panasonic WJ-810). The movement of a given RBC appeared as a fluorescent streak whose length was proportional to velocity and camera exposure time (in our case, 16.7 ms) in a frame of video. The recorded image was analyzed later with an image grabber (Studio Plus, Pinnacle Systems Inc., Mountain View, CA, USA) and image processor (SigmaScan Pro v 5.0.0, Systat Software Inc., Point Richmond, CA, USA).
To measure the diameter of arterioles, fluorescein isothio-cyanate (FITC)-dextran (MW = 2,000,000; 25mg/mL in saline) was infused (25 μL) through the femoral vein catheter to visualize the arterioles. An average of 15 to 20 RBC velocities (mean velocity, V) was used to estimate flow rate according to the calculation πVD2/4, assuming a uniform cylindrical diameter D for each vessel. Baseline velocity measurements were made approximately 5 minutes after the start of the labeled RBC infusion.
RBC Labeling
To obtain RBCs, approximately 1 mL of blood was collected from a C57BL/6 donor mouse. The blood was run through a column of packed sterile cotton with 10 mL of phosphate-buffered saline (PBS, pH 7.4) and then centrifuged at 3000 rpm for 10 minutes and then again for 5 minutes to separate platelets and leukocytes from RBCs. A preparation of 100 μL of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbo-cyanine perchlorate (DiI; Invitrogen Molecular Probes; D3911) stock solution (5 mg/mL) was diluted with 9900 μL PBS as a DiI labeling solution. Then 100 μL of packed RBCs were dispersed in the DiI solution, where they incubated for 30 min. The labeled RBCs were centrifuged at 3000 rpm and washed in 1 mL PBS 4-5 times and resuspended slowly in 1 mL PBS. One donor rat supplied enough RBCs for approximately 3 recipients.
Ozagrel Injection
The thromboxane synthase inhibitor Ozagrel (Sigma; [E]-3-[4-(1H-imidazol-1-ylmethyl) phenyl]-2-propenoic acid hydrochloride) was administered to all mice after the velocity and diameter of arterioles were measured. Ozagrel was injected intravenously (100 mg/kg [13,14]), and after 30 minutes, the measurements of diameter and velocity were repeated.
Statistics
Statistical differences between groups were analyzed with ANOVA using Student-Newman-Keuls post-hoc corrections (GraphPad InStat software v 3.05; GraphPad Software, San Diego, CA). Error bars are presented as ± standard error (SE), and statistical significance was set at p<0.05.
Results
NOD mice became hyperglycemic beginning at 13 weeks of age, and approximately 52% became diabetic by 30 weeks of age (Fig 1). Three weeks after a mouse became hyperglycemic, the intravital microscopic experiment was performed on that mouse, and also on an age-matched NOD mouse that did not have elevated glucose (serving as a non-diabetic control).
Figure 1.
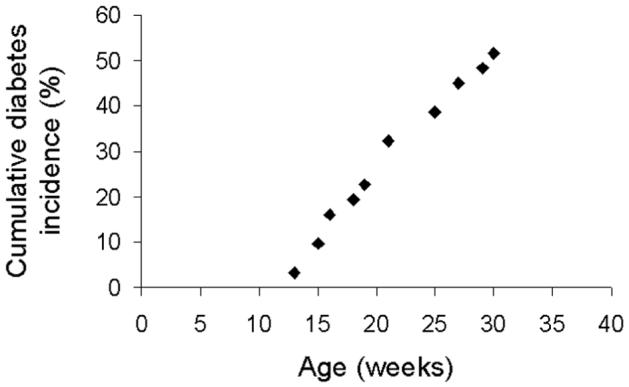
Cumulative diabetes incidence curve for the NOD mice (all female). Approximately 52% of the mice became diabetic by 30 weeks of age.
Table 1 lists the total number of mice and arterioles examined in each group, as well as blood glucose concentrations, body weights, and intraocular pressures. There was no statistically significant difference in IOP and body weight between groups. Mean arteriolar pressure (MAP) of each group was measured in separate animals, and in the four groups (N= 3 each; control, control + losartan, diabetes, and diabetes + losartan) MAP averaged 96 ± 3, 92 ± 3, 96 ± 4, and 93 ± 3 mmHg, respectively, and were not significantly different from each other.
Table 1.
Selected statistical data from the four main groups of mice
| # of mice | # of arterioles | Blood glucose level (mg/dl) | Body weight (g) | Intraocular pressure (mmHg) | |
|---|---|---|---|---|---|
| Control | 5 | 25 | 105 ± 4 | 24.0 ± 0.7 | 11.3 ± 0.1 |
| Control + Losartan | 4 | 18 | 102 ± 3 | 23.8 ± 0.5 | 11.1 ± 0.2 |
| 3 week diabetes | 5 | 22 | 452 ± 11 | 22.6 ± 0.9 | 11.5 ± 0.2 |
| 3 week diabetes +Losartan | 5 | 25 | 480 ± 16 | 23.1 ± 0.7 | 11.2 ± 0.1 |
From each mouse’s left eye, an average of ∼5 arterioles extending from the optic disk was observed, and were found in a radial arrangement around the optic disk with alternate venules (Fig 2). The average angle between a retinal arteriole and the two closest venules was used to quantify the proximity of arterio-venular pairing. Averaged arterio-venular angles ranged from 16° to 65° with a median of 32° (Fig 3). Thereafter, the arterioles were categorized into two groups: closely venule-paired arterioles (<32° angle) and more distantly venule-paired arterioles (≥ 32° angle).
Figure 2.
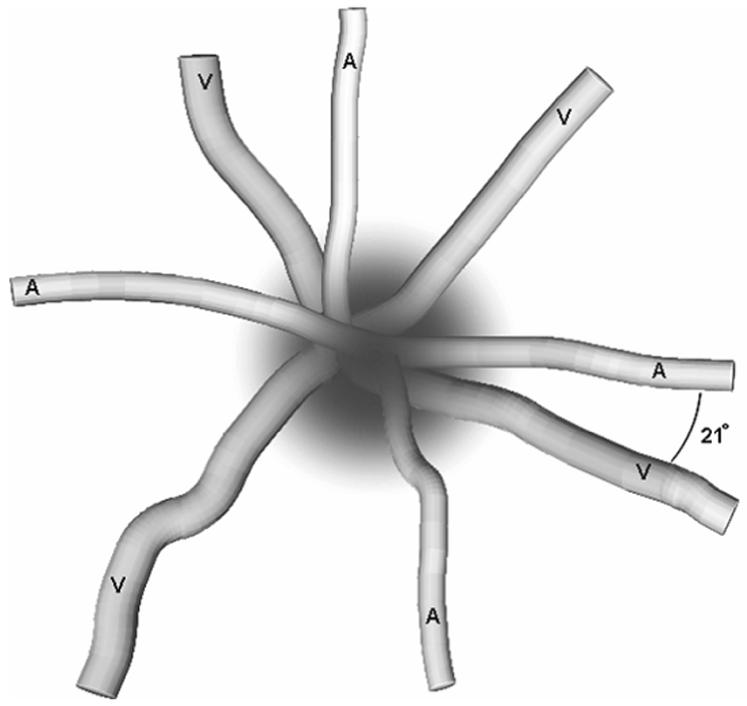
Depiction of the angles between alternating retinal arterioles (A) and venules (V) near the optic disk. One angle of 21° is indicated on the drawing.
Figure 3.
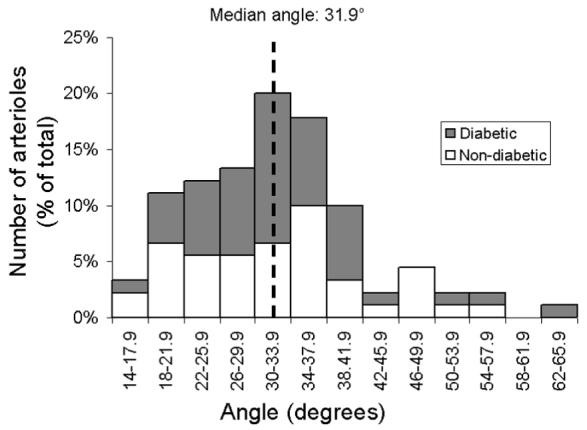
Distribution of the average pairing angle between each arteriole (N= 90 total arterioles) and the two neighboring venules in the non-diabetic (white) and diabetic (dark grey) NOD mouse retina.
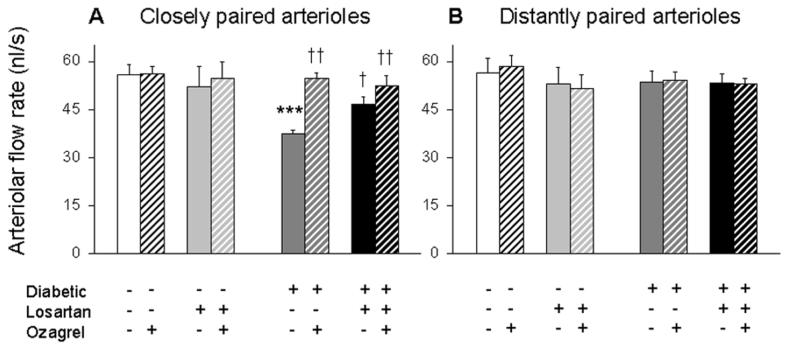
Figure 4 presents arteriolar flows in the hyperglycemic and control NOD mice. In more distantly paired arterioles (Fig 4B), flow rates were not statistically different among the four groups of mice, before and after injection of the thromboxane synthase inhibitor Ozagrel. In contrast, flow rates among the four groups were not equivalent in the more closely paired arterioles (Fig 4A). Specifically, flow was reduced significantly by ∼33% in the untreated diabetic mice, with flow rates equal to 37.5 ± 1.2 nl/s (compared with 56.0 ± 3.0 nl/s in age-matched controls). Additionally, in the closely paired arterioles, administration of Losartan in drinking water significantly increased flow in the diabetics to 46.6 ± 2.4 nl/s. Injection of Ozagrel was able to acutely restore flow rates in the diabetic mice to 54.9 ± 1.5 nl/s and 52.5 ± 3.1 nl/s, with and without Losartan, respectively.
Figure 4.
Retinal flow of closely paired (A; average venular pairing angle < 32°) and more distantly paired (B; ≥ 32°) arterioles in NOD mice. As indicated, data are separated depending on diabetic status as well as the administration of Losartan and/or Ozagrel. Data are paired into pre- (solid bar) and post- (cross-hatched bar) injection of Ozagrel in the same mice; N=9-13 arterioles per group. *** p<0.001 vs untreated Control; † p<0.05 and †† p<0.01 vs untreated Diabetic.
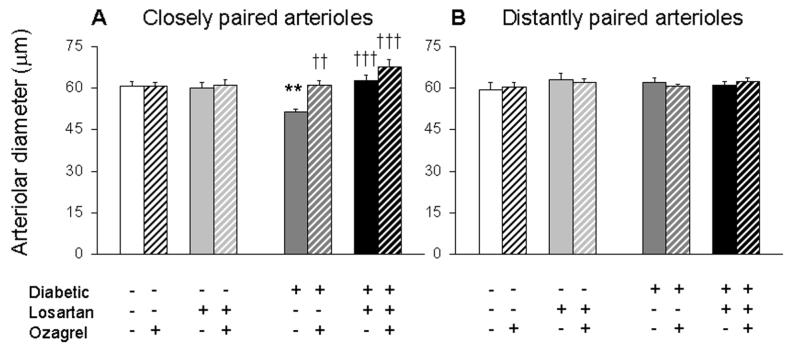
Figure 5 presents arteriolar diameters in the hyperglycemic and control NOD mice. In more distantly paired arterioles (Fig 5B), diameters were roughly equivalent among the four groups of mice, before and after injection of the thromboxane synthase inhibitor Ozagrel. As with flow rates, a contrast in diameters was observed in the more closely paired arterioles (Fig 5A). Arterioles were constricted significantly by ∼16% in the untreated diabetic mice, with diameters equal to 51.4 ± 1.2 μm (compared with 61.0 ± 1.5 μm in age-matched controls). Additionally, in the closely paired arterioles, administration of Losartan in drinking water significantly dilated diameters in the diabetics to 62.9 ± 2.0 μm. Injection of Ozagrel was able to acutely dilate diameters in the diabetic mice to 61.1 ± 1.7 μm and 67.7 ± 2.9 μm, with and without Losartan, respectively.
Figure 5.
Retinal diameters of closely paired (A; average venular pairing angle < 32°) and more distantly paired (B; ≥ 32°) arterioles in NOD mice. As indicated, data are separated depending on diabetic status as well as the administration of Losartan and/or Ozagrel. Data are paired into pre- (solid bar) and post- (cross-hatched bar) injection of Ozagrel in the same mice; N=9-13 arterioles per group. ** p<0.01 vs untreated Control; †† p<0.01 and ††† p<0.001 vs untreated Diabetic.
Discussion
Vascular complications are a major affliction for diabetic patients, especially in the eye [22,42]. It has been reported that almost all patients develop background retinopathy within 25 years of diabetes onset [27]. Though the mechanism of diabetic retinopathy is not clear, the alterations in the retinal circulation include breakdown of the blood-retinal barrier, loss of retinal pericytes, hypoxia, angiogenesis, and neural apoptosis [2,22,26]. It has been suggested that hypoxia could initiate the retinal angiogenesis and macular edema cascade. Decreased retinal oxygen levels in diabetic animals [1,30] and the improved visual contrast sensitivity in diabetic patients after enhanced oxygen delivery [16] appear to corroborate the retinal hypoxia hypothesis. The mechanisms inducing retinal hypoxia have not been elucidated, but could be a result of arteriolar constriction and/or a reduction in the number of perfused capillaries, two events that could be interrelated. Our study suggests that early arteriolar constriction in NOD mice may occur via the vasoactive molecules thromboxane and/or angiotensin II. Moreover, the vasoconstrictor-induced reductions in flow are found specifically in arterioles that are in close proximity to draining venules.
Arteriolar vasoreactivity plays a major role in the overall resistance of the vascular bed and its blood flow. Animal experiments have shown that the majority of the blood pressure drop occurs in the arterioles, prior to third-order and terminal arteriolar branching (diameter 8-30 μm) [21,34,45]. In most tissues of the body, first- and second-order arterioles are closely paired with the corresponding order of venules, with pairing becoming less structured in the third-order arterioles, and rarely found in terminal arterioles. Hester and Hammer have summarized the investigations proposing tissue blood flow regulation through venulo-arteriolar control [21]: venular contents reflect the metabolic activity of the tissue, and venule-released mediator(s) are hypothesized to control the adjacent feeding arteriolar diameter to supply the metabolic demands of the tissue [20].
Arteriolar regulation by a tissue metabolite has been described for the retina. Lactate, a metabolite produced mostly by the Müller glial and neuronal cells in retina, has been reported to act as an arteriolar dilator to regulate blood flow in the descending retinal tissue via stimulation of NO synthase [4,19,46]. Hein et al. claimed that this localized flow control is critical for modulation of retinal vascular tone due to the absence of autonomic innervation in the retinal circulation [8,19]. Whether this form of retinal arteriolar flow control is venule-dependent, as is the case for adenosine in mesenteric tissue [25], and for arachidonic acid metabolites in stimulated cremaster tissue [15], has not been investigated.
In several inflammatory conditions, venular control of arteriolar diameter tends toward vasoconstriction instead of vasodilation. Our current study supports the possibility that venule-derived agents can constrict retinal arterioles, with diabetes inducing more severe vasoconstriction in arterioles that were more closely paired with draining venules. The same phenomenon appears to be present in mesenteric tissue for hypercholesterolemic and diabetic rats [17,38,39], and in the intestine of mice that are either colitic or hypercholesterolemic [18,23,24]. Our current study points to thromboxane and/or angiotensin II as critical venule-dependent retinal vasoconstrictors following three weeks of hyperglycemia in NOD mice.
Enhanced thromboxane synthesis has been shown to be induced by hyperglycemia [11]. De la Cruz et al. [7] reported an approximate doubling of thromboxane B2 (a stable thromboxane metabolite) in the plasma of diabetic rats 15-90 days after the onset of diabetes. In the untreated diabetic rats of their study, the number of perfused capillaries dropped substantially over the 90-day period; however, aspirin- and dipyridamole-treated diabetic rats showed improved retinal vascularity. These results could be consistent with a role for thromboxane in retinal lesions of early diabetic retinopathy.
Inhibition of the thromboxane receptor or synthase has been shown to be beneficial in animal models of inflammation. In a model of streptozotocin-induced diabetes, inhibition of the thromboxane receptor eliminated the impaired functional dilation of spinotrapezius arterioles [49]. Decreased platelet aggregation has been reported after administration of a thromboxane synthase inhibitor, impeding the progression of diabetic nephropathy [40]. In a study from our lab, we demonstrated that the thromboxane synthase inhibitor Ozagrel reduced the venule-dependent arteriolar constriction induced by dextran sodium sulfate ingestion [18].
Angiotensin II, a critical element of the renin-angiotensin system (RAS) is involved in the regulation of blood pressure and electrolyte homeostasis. Angiotensin II also has been implicated in the inflammation and oxidative stress associated with diabetic vascular complications and atherosclerosis. Enhanced angiotensin II production has been reported in rat vascular smooth muscle cells maintained in high glucose conditions for 48 hours [29]. The RAS has been found in a variety of local tissues, including the reproductive tract, adrenal glands, thymus, and eye. All components of the system are expressed in ocular tissue of humans, rats, and other mammals [29,43]. Moreover, angiotensin II levels in the eye are higher than in plasma, indicating ocular production of the hormone [43]. There are two major receptor types for angiotensin II, type 1 (AT1) and type 2 (AT2), with contrasting functions. AT1 receptors mediate vasoconstriction and inflammation while AT2 receptors mediate anti-inflammatory vasodilation mediated by the release of nitric oxide (NO) [5].
The AT1 receptor antagonist Losartan has shown therapeutic efficacy in preventing diabetic complications. Leukostasis in the retina of diabetic rats has been inhibited with Losartan [36], and Losartan also reduces platelet activation in hypertensive rats [31]. However, the mechanism of Losartan’s anti-inflammatory effect is not clear, and may extend beyond the blockade of AT1 -induced constriction. Dandona et al. [5] proposed that NO-producing AT2 receptors become dominant during AT1 receptor blockade; additionally, De La Cruz et al. [6] concluded that Losartan acts as an antagonist of both AT1 and TxA2/PGH2 (TP) receptors. By one or more of these mechanisms (inhibition of AT1-induced vasoconstriction; enhanced influence of AT2-induced vasodilation; inhibition of TP receptors), Losartan helped reverse the vasoconstrictive consequences of hyperglycemia in the NOD diabetic mice of our study.
In summary, the results of this study demonstrate that the retinal vascular changes in diabetic mice were observed in arterioles closely paired with venules. The constricted diameter and reduced flow seen in these arterioles could have been caused by thromboxane and/or angiotensin II, since inhibition with Ozagrel and/or Losartan administration reversed the changes in closely venule-paired arterioles.
Acknowledgments
The authors wish to thank Patsy Carter for helping with several technical aspects of the study. This work was supported by the National Institutes of Health (EY017599; NRH).
REFERENCES
- 1.Alder VA, Yu DY, Cringle SJ, Su EN. Changes in vitreal oxygen tension distribution in the streptozotocin diabetic rat. Diabetologia. 1991;34:469–476. doi: 10.1007/BF00403282. [DOI] [PubMed] [Google Scholar]
- 2.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes - Early onset and effect of insulin. Journal of Clinical Investigation. 1998;102:783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boegehold MA. Shear-dependent release of venular nitric oxide: effect on arteriolar tone in rat striated muscle. Am J Physiol. 1996;271:H387–H395. doi: 10.1152/ajpheart.1996.271.2.H387. [DOI] [PubMed] [Google Scholar]
- 4.Brazitikos PD, Pournaras CJ, Munoz JL, Tsacopoulos M. Microinjection of L-lactate in the preretinal vitreous induces segmental vasodilation in the inner retina of miniature pigs. Invest Ophthalmol Vis Sci. 1993;34:1744–1752. [PubMed] [Google Scholar]
- 5.Dandona P, Dhindsa S, Ghanim H, Chaudhuri A. Angiotensin II and inflammation: the effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade. Journal of Human Hypertension. 2007;21:20–27. doi: 10.1038/sj.jhh.1002101. [DOI] [PubMed] [Google Scholar]
- 6.De La Cruz JP, Gonzalez-Correa JA, Guerrero A, de la Cuesta FS. Pharmacological approach to diabetic retinopathy. Diabetes Metab Res Rev. 2004;20:91–113. doi: 10.1002/dmrr.432. [DOI] [PubMed] [Google Scholar]
- 7.De La Cruz JP, Moreno A, Munoz M, Garcia Campos JM, Sanchez De La Cuesta F. Effect of aspirin plus dipyridamole on the retinal vascular pattern in experimental diabetes mellitus. J Pharmacol Exp Ther. 1997;280:454–459. [PubMed] [Google Scholar]
- 8.Delaey C, Van d V. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res. 2000;32:249–256. doi: 10.1159/000055622. [DOI] [PubMed] [Google Scholar]
- 9.Falcone JC, Bohlen HG. EDRF from rat intestine and skeletal muscle venules causes dilation of arterioles. Am J Physiol. 1990;258:H1515–H1523. doi: 10.1152/ajpheart.1990.258.5.H1515. [DOI] [PubMed] [Google Scholar]
- 10.Falcone JC, Meininger GA. Arteriolar dilation produced by venule endothelium-derived nitric oxide. Microcirculation. 1997;4:303–310. doi: 10.3109/10739689709146793. [DOI] [PubMed] [Google Scholar]
- 11.Ferroni P, Basili S, Falco A, Davi G. Platelet activation in type 2 diabetes mellitus. J Thromb Haemost. 2004;2:1282–1291. doi: 10.1111/j.1538-7836.2004.00836.x. [DOI] [PubMed] [Google Scholar]
- 12.Gardiner TA, Archer DB, Curtis TM, Stitt AW. Arteriolar involvement in the microvascular lesions of diabetic retinopathy: implications for pathogenesis. Microcirculation. 2007;14:25–38. doi: 10.1080/10739680601072123. [DOI] [PubMed] [Google Scholar]
- 13.Gomi T, Ikeda T, Ishimitsu T, Uehara Y. Effects of OKY-046, a selective thromboxane synthetase inhibitor, on blood pressure and thromboxane synthesis in spontaneously hypertensive rats. Prostaglandins Leukot Essent Fatty Acids. 1989;37:139–144. doi: 10.1016/0952-3278(89)90076-8. [DOI] [PubMed] [Google Scholar]
- 14.Gomi T, Ikeda T, Sasaki Y, Kosugi T, Shibuya Y, Sakurai J. Protective effect of thromboxane synthetase inhibitor on hypertensive renal damage in Dahl salt-sensitive rats. Clin Exp Pharmacol Physiol Suppl. 1995;22:S371–S373. doi: 10.1111/j.1440-1681.1995.tb02959.x. [DOI] [PubMed] [Google Scholar]
- 15.Hammer LW, Ligon AL, Hester RL. ATP-mediated release of arachidonic acid metabolites from venular endothelium causes arteriolar dilation. Am J Physiol Heart Circ Physiol. 2001;280:H2616–H2622. doi: 10.1152/ajpheart.2001.280.6.H2616. [DOI] [PubMed] [Google Scholar]
- 16.Harris A, Arend O, Danis RP, Evans D, Wolf S, Martin BJ. Hyperoxia improves contrast sensitivity in early diabetic retinopathy. Br J Ophthalmol. 1996;80:209–213. doi: 10.1136/bjo.80.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris NR. Reperfusion-induced changes in capillary perfusion and filtration: effects of hypercholesterolemia. Am J Physiol. 1999;277:H669–H675. doi: 10.1152/ajpheart.1999.277.2.H669. [DOI] [PubMed] [Google Scholar]
- 18.Harris NR, Whatley JR, Carter PR, Specian RD. Venular constriction of submucosal arterioles induced by dextran sodium sulfate. Inflammatory Bowel Diseases. 2005;11:806–813. doi: 10.1097/01.mib.0000178262.95980.65. [DOI] [PubMed] [Google Scholar]
- 19.Hein TW, Xu WJ, Kuo L. Dilation of retinal arterioles in response to lactate: Role of nitric oxide, guanylyl cyclase, and ATP-sensitive potassium channels. Investigative Ophthalmology & Visual Science. 2006;47:693–699. doi: 10.1167/iovs.05-1224. [DOI] [PubMed] [Google Scholar]
- 20.Hester RL, Choi J. Blood flow control during exercise: Role for the venular endothelium? Exercise and Sport Sciences Reviews. 2002;30:147–151. doi: 10.1097/00003677-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Hester RL, Hammer LW. Venular-arteriolar communication in the regulation of blood flow. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2002;282:R1280–R1285. doi: 10.1152/ajpregu.00744.2001. [DOI] [PubMed] [Google Scholar]
- 22.Kassab E, McFarlane SI, Sowers JR. Vascular complications in diabetes and their prevention. Vascular Medicine. 2001;6:249–255. doi: 10.1177/1358836x0100600409. [DOI] [PubMed] [Google Scholar]
- 23.Kim MH, Carter PR, Harris NR. P-selectin-mediated adhesion impairs endothelium-dependent arteriolar dilation in hypercholesterolemic mice. Am J Physiol Heart Circ Physiol. 2007;292:H632–H638. doi: 10.1152/ajpheart.00780.2006. [DOI] [PubMed] [Google Scholar]
- 24.Kim MH, Granger DN, Harris NR. Mediators of CD18/P-selectin-dependent constriction of venule-paired arterioles in hypercholesterolemia. Microvasc Res. 2007;73:150–155. doi: 10.1016/j.mvr.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim MH, Harris NR. Leukocyte adherence inhibits adenosine-dependent venular control of arteriolar diameter and nitric oxide. Am J Physiol Heart Circ Physiol. 2006;291:H724–H731. doi: 10.1152/ajpheart.01215.2005. [DOI] [PubMed] [Google Scholar]
- 26.Kim SY, Johnson MA, Mcleod DS, Alexander T, Otsuji T, Steidl SM, Hansen BC, Lutty GA. Retinopathy in monkeys with spontaneous type 2 diabetes. Investigative Ophthalmology & Visual Science. 2004;45:4543–4553. doi: 10.1167/iovs.04-0519. [DOI] [PubMed] [Google Scholar]
- 27.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102:520–526. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- 28.Klein R, Klein BE, Moss SE, Wong TY, Hubbard L, Cruickshanks KJ, Palta M. Retinal vascular abnormalities in persons with type 1 diabetes: the Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVIII. Ophthalmology. 2003;110:2118–2125. doi: 10.1016/S0161-6420(03)00863-7. [DOI] [PubMed] [Google Scholar]
- 29.Lavrentyev EN, Estes AM, Malik KU. Mechanism of High Glucose-Induced Angiotensin II Production in Rat Vascular Smooth Muscle Cells. Circ Res. 2007 doi: 10.1161/CIRCRESAHA.107.151852. [DOI] [PubMed] [Google Scholar]
- 30.Linsenmeier RA, Braun RD, McRipley MA, Padnick LB, Ahmed J, Hatchell DL, Mcleod DS, Lutty GA. Retinal hypoxia in long-term diabetic cats. Invest Ophthalmol Vis Sci. 1998;39:1647–1657. [PubMed] [Google Scholar]
- 31.Lopez-Farre A, Sanchez dM, Monton M, Jimenez A, Lopez-Bloya A, Gomez J, Nunez A, Rico L, Casado S. Angiotensin II AT(1) receptor antagonists and platelet activation. Nephrol Dial Transplant. 2001;16(Suppl 1):45–49. doi: 10.1093/ndt/16.suppl_1.45. [DOI] [PubMed] [Google Scholar]
- 32.Mazolewski PJ, Roth AC, Suchy H, Stephenson LL, Zamboni WA. Role of the thromboxane A2 receptor in the vasoactive response to ischemia-reperfusion injury. Plast Reconstr Surg. 1999;104:1393–1396. doi: 10.1097/00006534-199910000-00023. [DOI] [PubMed] [Google Scholar]
- 33.McKay MK, Gardner AL, Boyd D, Hester RL. Influence of venular prostaglandin release on arteriolar diameter during functional hyperemia. Hypertension. 1998;31:213–217. doi: 10.1161/01.hyp.31.1.213. [DOI] [PubMed] [Google Scholar]
- 34.Meininger GA, Fehr KL, Yates MB. Anatomic and hemodynamic characteristics of the blood vessels feeding the cremaster skeletal muscle in the rat. Microvasc Res. 1987;33:81–97. doi: 10.1016/0026-2862(87)90009-4. [DOI] [PubMed] [Google Scholar]
- 35.Moore CG, Milne ST, Morrison JC. Noninvasive measurement of rat intraocular pressure with the Tono-Pen. Invest Ophthalmol Vis Sci. 1993;34:363–369. [PubMed] [Google Scholar]
- 36.Mori F, Hikichi T, Nagaoka T, Takahashi J, Kitaya N, Yoshida A. Inhibitory effect of losartan, an AT1 angiotensin II receptor antagonist, on increased leucocyte entrapment in retinal microcirculation of diabetic rats. Br J Ophthalmol. 2002;86:1172–1174. doi: 10.1136/bjo.86.10.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moss SE, Klein R, Klein BE, Wong TY. Retinal vascular changes and 20-year incidence of lower extremity amputations in a cohort with diabetes. Arch Intern Med. 2003;163:2505–2510. doi: 10.1001/archinte.163.20.2505. [DOI] [PubMed] [Google Scholar]
- 38.Nellore K, Harris NR. L-arginine and antineutrophil serum enable venular control of capillary perfusion in hypercholesterolemic rats. Microcirculation. 2002;9:477–485. doi: 10.1038/sj.mn.7800162. [DOI] [PubMed] [Google Scholar]
- 39.Nellore K, Harris NR. Inhibition of leukocyte adherence enables venular control of capillary perfusion in streptozotocin-induced diabetic rats. Microcirculation. 2004;11:645–654. doi: 10.1080/10739680490517668. [DOI] [PubMed] [Google Scholar]
- 40.Okumura M, Imanishi M, Okamura M, Hosoi M, Okada N, Konishi Y, Morikawa T, Miura K, Nakatani T, Fujii S. Role for thromboxane A2 from glomerular thrombi in nephropathy with type 2 diabetic rats. Life Sci. 2003;72:2695–2705. doi: 10.1016/s0024-3205(03)00180-2. [DOI] [PubMed] [Google Scholar]
- 41.Reitsamer HA, Kiel JW, Harrison JM, Ransom NL, McKinnon SJ. Tonopen measurement of intraocular pressure in mice. Exp Eye Res. 2004;78:799–804. doi: 10.1016/j.exer.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 42.Rossing P. The changing epidemiology of diabetic microangiopathy in type 1 diabetes. Diabetologia. 2005;48:1439–1444. doi: 10.1007/s00125-005-1836-x. [DOI] [PubMed] [Google Scholar]
- 43.Sarlos S, Wilkinson-Berka JL. The renin-angiotensin system and the developing retinal vasculature. Invest Ophthalmol Vis Sci. 2005;46:1069–1077. doi: 10.1167/iovs.04-0885. [DOI] [PubMed] [Google Scholar]
- 44.Scholfield CN, McGeown JG, Curtis TM. Cellular physiology of retinal and choroidal arteriolar smooth muscle cells. Microcirculation. 2007;14:11–24. doi: 10.1080/10739680601072115. [DOI] [PubMed] [Google Scholar]
- 45.Williams DA, Segal SS. Feed artery role in blood flow control to rat hindlimb skeletal muscles. J Physiol. 1993;463:631–646. doi: 10.1113/jphysiol.1993.sp019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winkler BS, Starnes CA, Sauer MW, Firouzgan Z, Chen SC. Cultured retinal neuronal cells and Muller cells both show net production of lactate. Neurochem Int. 2004;45:311–320. doi: 10.1016/j.neuint.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 47.Wong TY, Klein R, Sharrett AR, Schmidt MI, Pankow JS, Couper DJ, Klein BE, Hubbard LD, Duncan BB. Retinal arteriolar narrowing and risk of diabetes mellitus in middle-aged persons. JAMA. 2002;287:2528–2533. doi: 10.1001/jama.287.19.2528. [DOI] [PubMed] [Google Scholar]
- 48.Wong TY, Shankar A, Klein R, Klein BE, Hubbard LD. Retinal arteriolar narrowing, hypertension, and subsequent risk of diabetes mellitus. Arch Intern Med. 2005;165:1060–1065. doi: 10.1001/archinte.165.9.1060. [DOI] [PubMed] [Google Scholar]
- 49.Xiang L, Naik JS, Hodnett BL, Hester RL. Altered arachidonic acid metabolism impairs functional vasodilation in metabolic syndrome. Am J Physiol Regul Integr Comp Physiol. 2006;290:R134–R138. doi: 10.1152/ajpregu.00295.2005. [DOI] [PubMed] [Google Scholar]
- 50.Zamboni WA, Roth AC, Russell RC, Graham B, Suchy H, Kucan JO. Morphologic analysis of the microcirculation during reperfusion of ischemic skeletal muscle and the effect of hyperbaric oxygen. Plast Reconstr Surg. 1993;91:1110–1123. doi: 10.1097/00006534-199305000-00022. [DOI] [PubMed] [Google Scholar]
- 51.Zamboni WA, Stephenson LL, Roth AC, Suchy H, Russell RC. Ischemia-reperfusion injury in skeletal muscle: CD 18-dependent neutrophil-endothelial adhesion and arteriolar vasoconstriction. Plast Reconstr Surg. 1997;99:2002–2007. doi: 10.1097/00006534-199706000-00028. [DOI] [PubMed] [Google Scholar]