Abstract
Background
There is a paucity of published data on the pattern of pulmonary tuberculosis among migrant workers entering Middle Eastern countries particularly Kuwait. The objectives of this study were to use routine health surveillance data i) to estimate the prevalence of pulmonary tuberculosis among migrant workers at entry in Kuwait and ii) to determine the occurrence of any time trends in the proportions of pulmonary tuberculosis positive workers over the study period.
Methods
The monthly aggregates of daily number of migrants tested and the number of pulmonary tuberculosis cases detected during routine health examinations of migrant workers from tuberculosis high-prevalence countries were used to generate the monthly series of proportions (per 100,000) of pulmonary tuberculosis cases over 120 months between January 1, 1997 and December 31, 2006 and analysed using time series methods.
Results
The overall prevalence (per 100,000) of documented pulmonary tuberculosis cases among screened migrants was 198 (4608/2328582). Year-specific prevalence (per 100,000) of tuberculosis cases consistently declined from 456 (95% CI: 424 – 490) in 1997 to 124 (95% CI: 110 – 140) in 2002 before showing a steady increase up to 183 (95% CI: 169–197) in 2006. The second-order polynomial regression model revealed significant (P < 0.001) initial decline, followed by a significant (P < 0.001) increasing trend thereafter in monthly proportions of tuberculosis cases among migrant workers.
Conclusion
The proportions of documented tuberculosis cases among migrant workers showed a significant nonlinear pattern, with an initial decline followed by a significant increasing trend towards the end of the study period. These findings underscore the need to maintain the current policy of migrants' screening for tuberculosis at entry. The public health authorities in Kuwait and perhaps other countries in the region may consider complementing the current screening protocol with interferon-γ assays to detect migrants with latent Mycobacterium tuberculosis infection. An appropriate curative or preventive chemotherapy of detected tuberculosis cases may help in further minimizing the risk of local transmission of M. tuberculosis, while contributing in global efforts to control this public health menace.
Background
Tuberculosis remains one of the leading infectious causes of death globally, killing nearly 2 million people a year [1]. Sub-Saharan Africa has the highest incidence (290 per 100000), but the most populous countries of Asia have the largest numbers of cases and together account for more than half of the global burden [2]. Tuberculosis control programmes can achieve a high level of treatment success and have been shown to be associated with a decline in reported burden of disease [3-6]. However, for the past two decades, a levelling off or a reverse trend in tuberculosis notifications has been reported from many developed countries [7,8]. This disturbed declining trend has been attributed, in part, to the spread of human immunodeficiency virus, multidrug-resistant tuberculosis, homelessness, deterioration of living conditions and health care delivery, increased drug abuse, immigration from tuberculosis high to low prevalence countries [7,9,10]. Nonetheless, reasons for this phenomenon are complex, differ from one country to another, and have not been entirely elucidated [11].
Kuwait is a small oil-rich Arabian country in the Persian Gulf region of the Middle East, having a total population of 2.5 million (Kuwaiti: 42%; Non-Kuwaiti 58%), with a gender ratio (male/female) of 1.04 at birth among nationals. Kuwait has a relatively low incidence of tuberculosis with annual notification rate of 24 active tuberculosis cases per 100,000 of population [12]. Resident non-nationals account for about 75% of these active tuberculosis cases per year [12,13], and nearly 1% of these are identified as multidrug-resistant tuberculosis cases [14]. Illegal immigration to Kuwait is almost negligible therefore, seems to play little role in tuberculosis epidemiology. Tuberculosis incidence in Kuwait showed a steady decline from 1965 to 1989. Subsequently, however, there was a rise of 2.3% per year from 1989 to 1999, both among nationals and non-nationals suggestive of Mycobacterium tuberculosis transmission from non-nationals to nationals, since a large proportion of migrants from tuberculosis high-burden countries live and work in Kuwaiti homes as domestic workers [12]. Notwithstanding the possibility of M. tuberculosis transmission from migrants to Kuwaiti nationals, there is a lack of empirical evidence for such local transmission [15].
The epidemiological importance of migration from tuberculosis high to low incidence countries has been recognized for several years; the main countermeasure has been implementation of screening programs for immigrants at the time of arrival [16,17]. But it not clear that to what an extent the increased immigration from high-incidence countries contributes to an increased risk of tuberculosis in host community of low-incidence countries [18]. Elsewhere immigrants from high-incidence countries to developed and Middle Eastern countries reportedly have high prevalence of tuberculosis [19,20], but there is a paucity of published data on the prevalence of tuberculosis in migrant workers entering Kuwait. Here, we take advantage of the routine screening of migrants for tuberculosis, upon arrival in Kuwait from tuberculosis-endemic regions, to do a first large-scale quantification of the tuberculosis status of this work population. Specifically, the cumulated data on the results of tuberculosis screening of these workers over the past ten years gave us an opportunity in this study not only 1) to estimate the prevalence of tuberculosis in this population of workers, but also 2) to ascertain if any significant time trend or changes had occurred in the prevalence of tuberculosis among these workers during the recent past.
Methods
Setting and study population
Migrants constitute about 80% of the labour force in Kuwait, and majority of them usually have a low educational attainment. These migrants originate from tuberculosis high-burden countries predominantly from Southeast Asia, Eastern Mediterranean and African regions wherein prevalence (per 100,000) of tuberculosis ranges from 152 to 547 [21]. There is large turn over of these workers; every year thousands of them leave and new ones arrive in Kuwait. Of the migrants, 46% are 20 to 44 year old and predominantly live as single, mainly because of their inability to fulfil a legal requirement of minimum wages to be able to bring their families [22,23]. Health services are free for all citizens and residents in Kuwait. In public sector, health-care system is made up of six administratively independent health-care sites; each comprises a general hospital, a health center, specialized clinics and dispensaries [24]. In Kuwait, a single tuberculosis control unit and the Kuwait National Central Laboratory under the Ministry of Health are responsible for prevention, diagnosis, treatment, case recording/reporting, contact tracing and treatment supervision under DOTS (Directly Observed Therapy, Short-course) strategy. On diagnosis of tuberculosis, all patients are offered treatment using first-line anti-tuberculosis drugs including isoniazid, rifampicin, ethambutol, and streptomycin based on drug sensitivity pattern [15].
Data source
Monthly aggregates of test results for diagnosis of pulmonary tuberculosis among migrants entered in Kuwait between January 1, 1997 and December 31, 2006 were available for this study. These migrants predominantly come from India (31%), Bangladesh (14%), Sri-Lanka (14%), Egypt (12%), Indonesia (9%), Philippine (5%), Pakistan (5%) and 10% from other countries including those from African counties such as Tanzania, Mali, Gambia, Sudan (12%) [25,26]. Routine consensual medical examination procedures are conducted on these workers upon their arrival by the Ports and Borders Health Division of the Ministry of Health, Kuwait. For the diagnosis of tuberculosis, migrants were screened by the serial application of various tests. For each migrant chest radiograph was taken. In the presence of any suspicious lesion in the lungs, confirmatory tuberculosis diagnosis was made by sputum smear examination for acid fast bacilli (AFB) using Ziehl Neelsen technique and bacterial culture. Subsequently, migrant worker was classified as a case of tuberculosis if sputum smear and/or bacterial culture were positive for AFB [27].
Ethics
As noted above, on arrival in Kuwait, migrants were screened for various infections including M. tuberculosis before issuance of residency permit. Verbal consent was solicited after fully informing each migrant about the purpose of screening. These procedures were performed according to a stated governmental policy. The study protocol was approved by the Ethics Review Committee of Faculty of Medicine, Kuwait University.
Statistical methods
The monthly aggregates of daily number of migrants tested and the number of pulmonary tuberculosis cases detected were used to generate the monthly series of proportions of pulmonary tuberculosis cases (per 100,000) over 120 months from January 1, 1997 to December 31, 2006. These monthly proportions (per 100,000) of pulmonary tuberculosis cases among migrants were used for all further analyses unless stated otherwise. Overall and year-specific prevalences (per 100,000) of tuberculosis cases along with their 95% confidence intervals (CI) were calculated.
Time series analysis
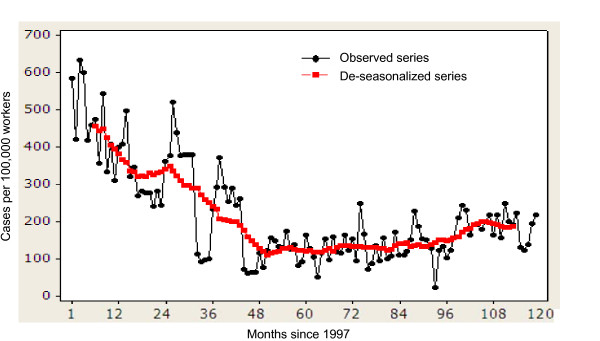
We employed standard time series methods to assess and model long term trends in the data [28]. Specifically, the purpose of this time series model was to describe any potential temporal trend in the proportions of tuberculosis cases among migrants at entry in Kuwait. We previously demonstrated a significant seasonality in the proportions of tuberculosis cases among migrants [29], therefore, trend estimation was done by first deseasonalizing the series using the moving average smoothing method. We smoothed the data by taking a 13-point (months) moving average filter (Figure 1). The modeling of the trend was then performed following the removal of seasonal effects by initially fitting a locally weighted (Lowess) scatterplot smoother (with bandwidth = 0.3) to explore the form of the long-term trend in the relationship between time (months) and monthly proportions of pulmonary tuberculosis cases [30]. Examination of the results from this exercise suggested the existence of a possible nonlinear temporal trend, and therefore a polynomial regression model was fitted to the deseasonalized data to model the observed monthly proportions of tuberculosis cases with respect to "time", and a quadratic term of time (i.e. time2). The goodness-of-fit of the final model was evaluated via residual analysis by plotting residuals against fitted values and also versus the time variable [31].
Figure 1.
Distribution of proportions of pulmonary tuberculosis cases (per 100, 000) among migrants at entry in Kuwait: 1997–2006.
Results
Descriptive statistics
During 120 months from January 1, 1997 to December 31, 2006, 2328582 migrant workers from pulmonary tuberculosis high-prevalence countries entered Kuwait and were eligible for tuberculosis screening. The mean (± standard deviation) number of migrants screened for tuberculosis each month were 19405 ± 7253. The overall prevalence (per 100,000) of documented pulmonary tuberculosis cases among migrants was 198 (4608/2328582). Total yearly pulmonary tuberculosis cases (per 100,000) consistently declined from 456 (95% CI: 424 – 490) in 1997 to 124 (95% CI: 110 – 140) in 2002 before it showed a yearly increase up to 184 (95% CI: 171–199) and 183 (95% CI: 169–197) cases in 2005 and 2006 respectively (Table 1).
Table 1.
Distribution of proportions (per 100,000) of pulmonary tuberculosis cases among migrants at entry screening in Kuwait: 1997–2006.
| Year | Total tested | No. positive | No. positive (per 100,000) | 95% confidence limits |
| 1997 | 161682 | 737 | 456 | 424 – 490 |
| 1998 | 163326 | 508 | 311 | 285 – 339 |
| 1999 | 177129 | 523 | 295 | 271 – 322 |
| 2000 | 130984 | 261 | 199 | 176 – 225 |
| 2001 | 178472 | 225 | 126 | 111 – 144 |
| 2002 | 221566 | 275 | 124 | 110 – 140 |
| 2003 | 254608 | 334 | 131 | 118 – 146 |
| 2004 | 327216 | 436 | 133 | 123 – 144 |
| 2005 | 356983 | 657 | 184 | 171 – 199 |
| 2006 | 356616 | 652 | 183 | 169 – 197 |
| Total | 2328582 | 4608 | 198 | 192 – 204 |
Polynomial regression model
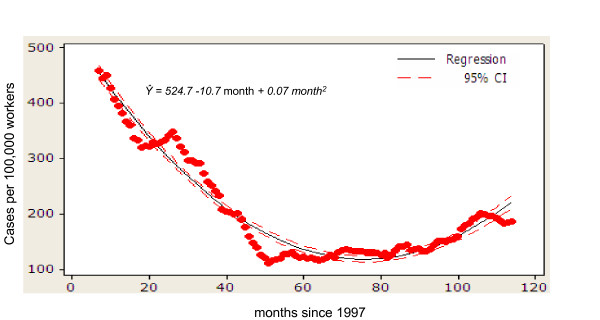
Overall second-order polynomial regression model with time as the single predictor was significant (F-statistic = 961; p < 0.001) (Table 2). The polynomial terms in the model were also statistically significant (p < 0.001), and the point estimates (± standard errors) were = 524.684 (± 7.853), = -10.657 (± 0.294), = 0.070 (± 0.002). The monthly series of proportions of pulmonary tuberculosis cases among migrants revealed a significant (P < 0.001) initial decline, followed by a significant (P < 0.001) increasing trend thereafter during 120 months of the study period (Figure 2). The two terms in the model together explained about 95% variation in the monthly proportions of tuberculosis cases among migrants (coefficient of determination: R2 = 0.948). The plot of observed verses predicted monthly proportions of tuberculosis cases showed adequate fit of the model. Residual analysis to evaluate the aptness of the model suggested that quadratic response function is a good-fit.
Table 2.
Polynomial regression model of the deseasonalized monthly proportions (per 100,000) of pulmonary tuberculosis cases among migrants at entry screening in Kuwait, 1997–2006.
| Linear and quadratic terms | Un-standardized partial regression coefficients | t-statistic | p | |
| Estimate | SE | |||
| Time () | -10.657 | 0.294 | 36.25 | < 0.001 |
| Time2 () | 0.070 | 0.002 | 29.78 | < 0.001 |
| Constant () | 524.684 | 7.853 | ||
SE = standard error
Coefficient of determination (R2) = 0.948
F-statistic for overall significance of the model = 961; P < 0.001
Figure 2.
Polynomial regression model fitted to deseasonalized data on the proportions of pulmonary tuberculosis cases among migrants at entry in Kuwait: 1997–2006.
Discussion
To our knowledge, this study constitutes one of the largest ever investigations conducted any where in the world for estimating the tuberculosis burden in migrants at entry to tuberculosis low-incidence regions. There is a limited evidence to suggest that migrants from tuberculosis high-burden countries pose a threat to low-incidence host communities [32,33]. However, it has been argued that migrants with latent M. tuberculosis infection may remain undetected thus posing a threat at least within their migrant communities, since many migrants are socially isolated and live in overcrowded conditions known to enhance the spread of infection [34,35]. Moreover, this topic is of particular relevance to the countries in Middle East. Kuwait like other countries in the region has a major influx of migrants from tuberculosis high-burden areas. Many of these migrants serve as domestic workers and live in Kuwaiti homes – a pattern of social mixing with host communities perhaps unreported from other tuberculosis low-incidence countries. These migrants thus, may serve as sources of new M. tuberculosis infection not only to Kuwaiti nationals but also to migrant community in Kuwait [12]. Also, it has been suggested that screening for tuberculosis and infection with M. tuberculosis among migrants has the potential to yield a large number of persons who can benefit from curative or preventive interventions [36].
In Kuwait, we found that the overall prevalence (per 100,000) of tuberculosis cases among migrants was 198 (4608/2328582) or 0.198% during the entire 10-year study period. Almost a similar magnitude of prevalence of tuberculosis has been reported in high-burden counties in South Asia [37,38]. The longitudinal data series based on a 10 year period of observations also uniquely allowed an investigation of the temporal epidemiology of tuberculosis in workers migrating to the country. This has also specifically enabled us to establish that the proportions of migrants with pulmonary tuberculosis at entry in the country have reduced dramatically over the past decade, such that the tuberculosis prevalence in the cohort of workers recruited in 2002 was only around 0.124% (275/221566) in contrast to a peak of 0.456% (737/161682) observed in counterparts in 1997. However, in the following years, a small but significant reversal in the prevalence of tuberculosis cases among migrants occurred, which needs to be monitored.
Trend analysis of these data revealed the occurrence of a nonlinear pattern in the prevalence of tuberculosis in migrants over the 10-year study period. Proportions of tuberculosis cases among workers showed an initial decline between 1997 and 2002 and a subsequent steady increase till the end of the study. The observed initial downward trend in the proportions of tuberculosis cases appears to corroborate previous findings of decreasing prevalence of tuberculosis during the same period in migrants from India, other Asian countries and sub-Saharan Africa to Canada [39]. Also decreasing prevalence of tuberculosis among migrants in our study tended to mimic decreasing tuberculosis burden in Southeast Asia and Eastern Mediterranean region during the same period [40]. This observed decline in the prevalence of tuberculosis over 1997 to 2002 may be the result of an effective implementation of WHO-recommended DOTS strategy during the same period by the public health authorities in the respective countries of origin of these migrants [40,41]. If found to be true, this suggests that sustained DOTS intervention in affected areas over several years could by reducing transmission in those areas contributed significantly in minimizing the risk of exporting M. tuberculosis infection into Kuwait and perhaps to other countries in the region. Alternatively, this decline may simply indicate that more workers from a different socio-economic background with lower prevalence of tuberculosis were enlisted during that period. We do not have pertinent data at present to investigate this likely change in migrants' demographic characteristics which might have been associated with the observed decline and this aspect merits further investigations.
The slight but significant increase in proportions of tuberculosis cases among migrants towards the end of the time series (2005–2006) was consistent with contemporary reports of increased global tuberculosis caseloads [21,42], and with the projected increase in the tuberculosis burden during the same period for the countries of origins of the migrants from Southeast Asia, Eastern Mediterranean and African regions [21,40]. A longer period of observation however is required to confirm this small but significantly increasing trend in the prevalence of tuberculosis at the end of time series. This upward trend towards the end of the study period may be an outcome of a shift in the health priority of public health authorities in the endemic countries resulting in the slow down of tuberculosis control efforts. Alternatively, this increasing trend may be a mirror image of increasing burden of multidrug-resistant tuberculosis in countries of origins of the migrants as suggested previously [21,43,44]. We do not have relevant data to corroborate this contention. However, as noted earlier, about 75% of 500 tuberculosis cases in Kuwait each year occur among migrants. Of these tuberculosis cases, 1% are multidrug-resistant tuberculosis cases and nearly all of them occur in resident migrants [12-14].
Limitations of the study
Some limitations of this study should be considered while interpreting the results. First, as only few variables of interest were available for longitudinal analysis, we are unable to evaluate the roles of demographic factors, e.g. age, gender, for their potential associations with the observed changes in the prevalence of tuberculosis among migrants. Second, the non-availability of information on exact locations within their countries of origins precluded any spatial or location-based analysis in this study. Finally, some workers might have been incubating M. tuberculosis infection and/or at early stage of the disease and remained undetected with current screening protocol. It is therefore, likely that the proportions of migrants with pulmonary tuberculosis may have been some what underestimated in this study.
Conclusion
Analysis of the longitudinal screening data on pulmonary tuberculosis has shown not only that the prevalence of pumonary tuberculosis may be declining in the migrants thus reducing the risk that they may pose to the nationals and resident migrants' community in the host country but also that tuberculosis control in endemic countries may be a contributory factor and indeed should be maintained to keep the incidence of M. tuberculosis infection declining. The final conclusion of specific significance to public health authorities in Kuwaiti and other Gulf countries' is that the data, particularly either the levelling off or slight rise in tuberculosis in these migrants towards the end of the study period, suggest that there is a need to maintain the current policy of entry screening, which has facilitated the control of tuberculosis so far. However, this strategy appears to be inadequate for detection of migrants with latent M. tuberculosis infection, since, as noted earlier each year 75% of about 500 new cases of tuberculosis are notified among the resident migrants in Kuwait [12,13]. Therefore, to detect migrants with latent infection the current screening protocol may be complemented with more sensitive techniques such as interferon-γ assays reportedly having estimated sensitivity: 80–95% and specificity: 95–100% to detect latent M. tuberculosis infection [45]. The more sensitive screening protocol combined with treatment of detected cases may ensure the maintenance of minimum risk of local transmission of M. tuberculosis and contribute in global efforts to control this public health menace.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SA conceived, designed, analyzed, interpreted the data and drafted the manuscript. HGHHM supervised data collection and reviewed the manuscript. Both the authors have read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
The cooperation of the staff of the Ports and Borders Health Division, Ministry of Health, Kuwait in data compilation is gratefully acknowledged. We are grateful to the referees of the journal for their thoughtful comments that have improved the presentation of manuscript. The study was funded by Kuwait University Research Administration grant no. MC 01/05.
Contributor Information
Saeed Akhtar, Email: saeed.akhtar@hsc.edu.kw.
Hameed GHH Mohammad, Email: hameed@kma.org.kw.
References
- World Health Organization . WHO Report 2005 (WHO/HTM/TB/2005349) Geneva: WHO; 2005. Global Tuberculosis Control: Surveillance, Planning, Financing. [Google Scholar]
- Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet. 2003;362:887–899. doi: 10.1016/S0140-6736(03)14333-4. [DOI] [PubMed] [Google Scholar]
- Gledovic Z, Jovanovic M, Pekmezovic T. Tuberculosis trends in Central Serbia in the period 1956–1996. Int J Tuberc Lung Dis. 2000;4:32–35. [PubMed] [Google Scholar]
- Marrerio A, Caminero JA, Rodriguez R, Billow NE. Towards elimination of tuberculosis in a low income country: the experience of Cuba, 1962–97. Thorax. 2000;55:39–45. doi: 10.1136/thorax.55.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YP, Kim SJ, Lew WJ, Lee EK, Han YC. The seventh nationwide tuberculosis prevalence survey in Korea, 1995. Int J Tuberc Lung Dis. 1998;2:27–36. [PubMed] [Google Scholar]
- Styblo K, Dankova D, Drapela J, Galliova J, Jezek Z, Krivanek J, Kubik A, Langerova M, Radkovsky J. Epidemiological and clinical study of tuberculosis in the district of Kolfn, Czechoslovakia: Report for the first 4-years of the study (1961–1964) Bull World Health Organ. 1967;37:8I9–874. [PMC free article] [PubMed] [Google Scholar]
- Snider DE, Jr, Roper WI. The new tuberculosis. N Engl J Med. 1992;326:703–705. doi: 10.1056/NEJM199203053261011. [DOI] [PubMed] [Google Scholar]
- Zuber PLF, McKenna MT, Binkin NJ, Onarato IM, Castro KG. Long term risk of tuberculosis among foreign-born persons in the United States. JAMA. 1997;278:304–307. doi: 10.1001/jama.278.4.304. [DOI] [PubMed] [Google Scholar]
- Brudeny K, Dobkins J. Resurgent tuberculosis in New York City: Human Immunodeficiency virus, homelessness and decline of tuberculosis control programs. Am Rev Resp Dis. 1991;144:745–749. doi: 10.1164/ajrccm/144.4.745. [DOI] [PubMed] [Google Scholar]
- Story A, Murad S, Roberts W, Verheyen M, Hayward AC, London Tuberculosis Nurses Network Tuberculosis in London: the importance of homelessness, problem drug use and prison. Thorax. 2007;62:667–671. doi: 10.1136/thx.2006.065409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder HL. Misbehaviour of a dying epidemic: a call for less speculation and better surveillance. Tuberc Lung Dis. 1992;73:181–183. doi: 10.1016/0962-8479(92)90082-U. [DOI] [PubMed] [Google Scholar]
- Behbehani N, Abal A, Al-Shami A, Enarson DA. Epidemiology of tuberculosis in Kuwait from 1965 to 1999. Int J Tuberc Lung Dis. 2002;6:465–469. doi: 10.5588/09640569512940. [DOI] [PubMed] [Google Scholar]
- Abal AT, Ahmad S, Mokaddas E. Variations in the occurrence of the S315T mutation within the katG gene in isoniazid-resistant clinical Mycobacterium tuberculosis isolates from Kuwait. Microb Drug Resist. 2002;8:99–105. doi: 10.1089/107662902760190644. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Mokaddas E. The occurrence of rare rpoB Tmutations in rifampicin-resistent Mycobacterium tuberculosis isolates from Kuwait. Int J Antimicrob Agents. 2005;26:205–212. doi: 10.1016/j.ijantimicag.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Mokaddas E, Ahmad S, Samir I. Secular trends in susceptibility patterns of Mycobacterium tuberculosis isolates in Kuwait, 1996–2005. Int J Tuberc Lung Dis. 2008;12:319–325. [PubMed] [Google Scholar]
- Ormerod LP. Tuberculosis screening and prevention in new immigrants 1983–88. Respir Med. 1990;84:269–271. doi: 10.1016/S0954-6111(08)80051-0. [DOI] [PubMed] [Google Scholar]
- Rieder HL, Zellweger JP, Keizer ST, Migliori GB. Tuberculosis control in Europe and international migration. Eur Respir J. 1994;7:1545–1553. doi: 10.1183/09031936.94.07081545. [DOI] [PubMed] [Google Scholar]
- Borgdorff MW, Nagelkerke N, van Soolingen D, de Haas PE, Veen J, van Embden JD. Analysis of tuberculosis transmission between nationalities in the Netherlands in the period 1993–1995 using DNA fingerprinting. Am J Epidemiol. 1998;147:187–195. doi: 10.1093/oxfordjournals.aje.a009433. [DOI] [PubMed] [Google Scholar]
- Al-Marri MR. Childhood tuberculosis in the State of Qatar: the effect of a limited expatriate screening programme on the incidence of tuberculosis. Int J Tuberc Lung Dis. 2001;5:831–837. [PubMed] [Google Scholar]
- Lillebaek T, Andersen AB, Dirksen A, Smith E, Skovgaard LT, Kok-Jensen A. Persistent high incidence of tuberculosis in immigrants in a low-incidence country. Emerg Infect Dis. 2002;8:679–684. doi: 10.3201/eid0807.010482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO report 2008 (WHO/HTM/TB/2008393) Geneva: WHO; 2008. Global Tuberculosis Control: Surveillance, Planning, Financing. [Google Scholar]
- Anonymous . The annual report. Public Health Authority for Civil Information, Kuwait; 1989. [Google Scholar]
- Akhtar S, Mohammad HGHH. Spectral analysis of HIV seropositivity among migrant workers entering Kuwait. BMC Infect Dis. 2008;8:37. doi: 10.1186/1471-2334-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous . Kuwait: Facts and Figures. 8. Ministry of Information, State of Kuwait; 2004. [Google Scholar]
- Al-Mufti S, Al-Owaish R, Mendkar YI, Pacsa A. Screening work force for HIV, HBV and HCV infections in Kuwait. Kuwait M J. 2002;34:24–27. [Google Scholar]
- Iqbal J, Sher A. Determination of prevalence of lymphatic filariasis among migrant workers in Kuwait detecting circulating filarial antigen. J Med Microbiol. 2006;55:401–405. doi: 10.1099/jmm.0.46376-0. [DOI] [PubMed] [Google Scholar]
- Sher A, Mohammad HGHH, Al-Owish R. Infectious Diseases detected among immigrants in Kuwait. Kuwait M J. 2004;36:124–127. [Google Scholar]
- Brockwell PJ, Davis RA. Introduction to Time Series and Forecasting. 2. Spring-Verlag, New York, USA; 2002. [Google Scholar]
- Akhtar S, Mohammad HGHH. Seasonality in pulmonary tuberculosis among migrant workers entering Kuwait. BMC Infect Dis. 2008;8:3. doi: 10.1186/1471-2334-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevland WS, Devlin SJ. Robust locally-weighted regression and smoothing scatter plots. J Am Stat Assoc. 1988;74:829–838. doi: 10.2307/2286407. [DOI] [Google Scholar]
- Neter J, Wasserman W, Kutner MH. In: Applied Linear Statistical Models. 4. Richard D, editor. Irwin, Inc. Homewood Illinois, USA; 1996. [Google Scholar]
- Bwire R, Nagelkerke N, Keizer ST, Année-van Bavel J, Sijbrant J, van Burg JL, Borgdorff MW. Tuberculosis screening among immigrants in The Netherlands: what is its contribution to public health? Neth J Med. 2000;56:63–71. doi: 10.1016/S0300-2977(99)00118-7. [DOI] [PubMed] [Google Scholar]
- Dahle UR, Sandven P, Heldal E, Caugant DA. Continued low rates of transmission of Mycobacterium tuberculosis in Norway. J Clin Microbiol. 2003;41:2968–2973. doi: 10.1128/JCM.41.7.2968-2973.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire H, Dale JW, McHugh TD, Butcher PD, Gillespie SH, Costetsos A, Al-Ghusein H, Holland R, Dickens A, Marston L, Wilson P, Pitman R, Strachan D, Drobniewski FA, Banerjee DK. Molecular epidemiology of tuberculosis in London 1995–7 showing low rate of active transmission. Thorax. 2002;57:617–622. doi: 10.1136/thorax.57.7.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker R, Bell A, Pitman R, Zellweger JP, Heldal E, Hayward A, Skulberg A, Bothamley G, Whitfield R, de Vries G, Watson JM. Tuberculosis screening in migrants in selected European countries shows wide disparities. Eur Respir J. 2006;27:801–807. doi: 10.1183/09031936.06.00104305. [DOI] [PubMed] [Google Scholar]
- Rieder HL, Zellweger JP, Raviglione MC, Keizer ST, Migliori GB. Tuberculosis control in Europe and international migration. Eur Respir J. 1994;7:1395–1396. doi: 10.1183/09031936.94.07081545. [DOI] [PubMed] [Google Scholar]
- Dye C. Global epidemiology of tuberculosis. Lancet. 2006;367:938–940. doi: 10.1016/S0140-6736(06)68384-0. [DOI] [PubMed] [Google Scholar]
- Akhtar S, White F, Hasan R, Akhtar S, White F, Hasan R, Rozi S, Younus M, Ahmed F, Husain S, Khan BS. Hyperendemic pulmonary tuberculosis in peri-urban areas of Karachi, Pakistan. BMC Public Health. 2007;37:70. doi: 10.1186/1471-2458-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creatore MI, Lam M, Wobeser WL. Patterns of tuberculosis risk over time among recent immigrants to Ontario, Canada. Int J Tuberc Lung Dis. 2005;9:667–672. [PubMed] [Google Scholar]
- Dye C, Watt CJ, Bleed DM, Hosseini SM, Raviglione MC. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. JAMA. 2005;293:2767–2775. doi: 10.1001/jama.293.22.2767. [DOI] [PubMed] [Google Scholar]
- Subramani R, Santha T, Frieden TR, Radhakrishna S, Gopi PG, Selvakumar N, Sadacharam K, Narayanan PR. Active community surveillance of the impact of different tuberculosis control measures, Tiruvallur, South India, 1968–2001. Int J Epidemiol. 2007;36:387–393. doi: 10.1093/ije/dyl216. [DOI] [PubMed] [Google Scholar]
- Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet. 2003;362:887–899. doi: 10.1016/S0140-6736(03)14333-4. [DOI] [PubMed] [Google Scholar]
- Wells CD, Cegielski JP, Nelson LJ, Laserson KF, Holtz TH, Finlay A, Castro KG, Weyer K. HIV infection and multidrug-resistant tuberculosis: the perfect storm. J Infect Dis. 2007;196:S86–S107. doi: 10.1086/518665. [DOI] [PubMed] [Google Scholar]
- Kruijshaar ME, Watson JM, Drobniewski F, Anderson C, Brown TJ, Magee JG, Smith EG, Story A, Abubakar I. Increasing antituberculosis drug resistance in the United Kingdom: analysis of National Surveillance Data. BMJ. 2008;336:1231–1234. doi: 10.1136/bmj.39546.573067.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahid P, Pai M, Hopewell PC. Advances in the diagnosis and treatment of tuberculosis. Proc Am Thorax Soc. 2006;3:103–110. doi: 10.1513/pats.200511-119JH. [DOI] [PMC free article] [PubMed] [Google Scholar]