Abstract
In the present review we discuss the potential use of two long-lived mice of the genus Peromyscus—the white-footed mouse (P. leucopus) and the deer mouse (P. maniculatus) maximum lifespan potential ∼8 years for both—to test predictions of theories about aging from the oxidative stress theory, mitochondrial theory and inflammatory theory. Previous studies have shown that P. leucopus cells exhibit superior antioxidant defense mechanisms and lower cellular production of reactive oxygen species (ROS) than do cells of the house mouse, Mus musculus (maximum lifespan ∼3.5 years). We present new data showing that mitochondria in P. leucopus cells produce substantially less ROS than mitochondria in M. musculus cells, and that P. leucopus mitochondria exhibit superior stress resistance to those of M. musculus. We also provide evidence that components of the DNA repair system (e.g., pathways involved in repair of DNA damage induced by γ-irradiation) are likely to be more efficient in P. leucopus than in M. musculus. We propose that mitochondrial stress resistance, ROS detoxification pathways and more efficient DNA repair contribute to the previously documented resistance of P. leucopus cells toward oxidative stress-induced apoptosis. The link between these three pathways and species longevity is discussed.
Keywords: Aging, Oxidative stress, Mitochondrial stress, DNA repair, Peromyscus, Longevity
Long-lived mice of the genus Peromyscus: a useful model for successful aging in small, mouse-like rodents
Comparative studies of species with unusually long lifespans may provide useful insights into the mechanisms determining successful aging, and identify genetically encoded mechanisms responsible for differences in mammalian longevity. The practical problem is to identify species pairs that are reasonably closely related taxonomically but that differ significantly in longevity, have genomic information available, are relatively easy to obtain, and can be bred and maintained in the laboratory at low cost. In this review we focus on the potential use of the long-lived mice of the genus Peromyscus [including the white-footed mouse (P. leucopus) and the deer mouse (P. maniculatus)] to test predictions of the oxidative stress theory, mitochondrial theory and inflammatory theory of aging.
Sacher and Hart (1978) originally proposed P. leucopus and Mus musculus (house mouse) as a longevity contrast pair. Both species belong to the superfamily of mouse-like rodents (Muroidea) and share a common ancestor 20–25 million years ago (Steppan et al. 2004). However, mice of the genus Peromyscus, despite close physical resemblance to the house mouse, have unusually long lifespans for their size: the maximum recorded longevities for P. leucopus and P. maniculatus in captivity are 8.2 years (Sacher and Hart 1978), and 8.33 years (Nowak and Paradiso 1983), respectively, approximately twice as long as the maximum lifespan potential of M. musculus. Previous data showed that, in aged P. leucopus, the hypothalamic-pituitary-ovarian axis remains intact and fertility is maintained at least until 5.5 years (Steger et al. 1980; Burger and Gochfeld 1992), the rate of accumulation of DNA damage (in liver and kidney cells) is delayed (Su et al. 1984), and cells and tissues exhibit a number of anti-aging adaptations to combat oxidative damage (Csiszar et al. 2007c). Given these considerations, P. leucopus is a useful model of longevity in small mouse-like rodents. There are also practical advantages to using rodents of the Peromyscus genus in aging studies (Burger and Gochfeld 1992; Guo et al. 1993), including the availability of information on husbandry, toxicology, and pathology, and the fact that the genome of at least four Peromyscus species will be sequenced in the foreseeable future (P. leucopus and P. maniculatus are scheduled for 2× and 7× coverage, respectively; for current status see http://www.genome.gov/10002154). Since the selection of animal models in biogerontology is often driven by feasibility considerations, it is important to note that mice of the genus Peromyscus are readily available from the Peromyscus Genetic Stock Center at the University of South Carolina (http://stkctr.biol.sc.edu/). These mice are the descendants of 38 ancestors captured between 1982 and 1985 in North Carolina.
Cellular oxidative stress resistance
Harman originally proposed the free radical theory of aging half a century ago (Harman 1956), yet the relationship between oxidative stress and aging is still much debated (Sohal et al. 1990, 1993; Sohal and Brunk 1992; Sohal and Orr 1992). There is strong evidence that aging in mammals is associated with an increased production of reactive oxygen species (ROS), and oxidative macromolecular damage accrues with age in virtually every tissue studied (Van Remmen and Richardson 2001; Van Remmen et al. 2003a; Csiszar et al. 2005). However, genetic knockout mice for major cellular antioxidant enzymes often fail to exhibit significant changes in longevity (Van Remmen et al. 2003b; Mansouri et al. 2006; Sentman et al. 2006). In contrast, the general concept that oxidative stress is involved in many age-related diseases, including atherosclerosis, hypertension, diabetic vasculopathy and Alzheimer’s disease, appears robust.
We are currently testing predictions of the oxidative stress hypothesis of aging in long-lived mice of the genus Peromyscus. Because oxidative stress clearly plays a central role in cardiovascular aging (Csiszar et al. 2002, 2005; Ungvari et al. 2004; Labinskyy et al. 2006b), we have compared vascular ROS homeostasis and oxidative stress resistance in P. leucopus and M. musculus. Our recent studies have demonstrated that in vascular tissues of P. leucopus there is attenuated production of ROS (O2−; H2O2) from NAD(P)H oxidase (Csiszar et al. 2007c). The differences in NAD(P)H oxidase activity in short- and long-lived species is significant, because upregulation of NAD(P)H oxidase has been shown to contribute to age-related oxidative stress in the cardiovascular system of rats (Hamilton et al. 2001; Csiszar et al. 2002; Adler et al. 2003). NAD(P)H oxidase also plays a major role in vascular pathophysiological alterations in metabolic diseases (e.g., diabetes), which many investigators consider “accelerated vascular aging” (based on similarities of the gene expression profile in senescent vessels to those observed in metabolic diseases). It is unknown how NAD(P)H oxidase activity differs among other (e.g., brain) tissues of P. leucopus and M. musculus. Future studies should also elucidate whether P. leucopus is protected from age-related increases in NAD(P)H oxidase activity.
We have recently reported a higher glutathione peroxidase and catalase content in P. leucopus, and a more abundant expression of eNOS (endothelial nitric oxide synthase) associated with increased endothelial NO production in large arteries (Csiszar et al. 2007c). Previous studies have also shown that brain and heart of P. leucopus have higher activities of catalase and glutathione peroxidase (Sohal et al. 1993) and lower levels of protein oxidative damage, as well as lower susceptibility to oxidative damage in response to experimental oxidative stress (Sohal et al. 1993) than those of M. musculus. We also showed that endothelial cells of P. leucopus are substantially more tolerant of oxidative stress (induced by oxidized low-density lipoprotein or H2O2 treatment) than those of M. musculus (Csiszar et al. 2007c). Previous studies demonstrated that inhibition of glutathione peroxidase in various cell types enhances oxidative stress-induced apoptosis (Ran et al. 2004; Van Remmen et al. 2004; Ungvari et al. 2007a). Also, there is a positive correlation between glutathione peroxidase activity and oxidative stress resistance in human cell lines (Marklund et al. 1984). These findings suggest that higher cellular glutathione peroxidase content may contribute to the superior oxidative stress resistance in P. leucopus. Inhibition of hemeoxygenase-1 (HO-1) also can enhance oxidative stress-induced apoptosis (Abraham and Kappas 2005; Kruger et al. 2006; Ungvari et al. 2007a). Because expression of HO-1 is also greater in the vascular tissue of P. leucopus than in M. musculus vessels (Csiszar et al. 2007c), it is possible that HO-1 contributes to cellular resistance to oxidative damage in P. leucopus cells.
There is an emerging view that ROS, in addition to inactivating NO (which acts as a cellular survival factor) and causing oxidative damage, play important signaling roles in the cell. Oxidative/nitrosative stress and consequent activation of numerous downstream effector pathways are thought to be implicated in the inflammatory process in most organs (Pacher et al. 2007). Many age-related degenerative diseases, including atherosclerosis (Ross 1993), are, in part, inflammatory diseases, and recent studies have shown that even in “healthy aging” there is a pro-inflammatory shift in skeletal muscle (Pedersen et al. 2003; Roubenoff et al. 2003; Phillips and Leeuwenburgh 2005), vascular (Csiszar et al. 2002, 2003, 2004) and cardiac (Lee et al. 2002) gene expression profiles (including upregulation of TNFα, IL-6 and iNOS). There is growing evidence that high levels of inflammatory cytokines contribute to a pro-inflammatory microenvironment that facilitates the development of vascular disease and sarcopenia in aging. In particular, it has become established that both vascular and skeletal muscle aging are associated with dysregulation of TNFα expression (Csiszar et al. 2002, 2003, 2004, 2007b; Pedersen et al. 2003; Roubenoff et al. 2003; Bátkai et al. 2007). TNFα is a master regulator of NAD(P)H oxidase activity, leukocyte infiltration of tissues, and apoptosis. Despite the increasing evidence for a role of inflammation in aging, there are no current studies comparing inflammatory processes in short- and long-lived species. We propose that the M. musculus–Peromyscus longevity contrast pair can be exploited to test for species differences in the link between age-related inflammation, oxidative stress and longevity.
Recent studies have uncovered important cross-talk between inflammatory cytokines, ROS and pro-inflammatory genes in the pathogenesis of age-related diseases. Of particular note are studies demonstrating that aging-induced oxidative stress activates NF-κB in the vascular endothelium (Helenius et al. 1996; Donato et al. 2007; Ungvari et al. 2007b), which is likely a major factor contributing to increased expression of adhesion molecules and iNOS (Cernadas et al. 1998; Csiszar et al. 2002; Ungvari et al. 2007b). NF-κB activation and chronic inflammation seem to be a generalized phenomenon in aging, because increases in NF-κB activity have been observed in aged rat skeletal muscle, liver, brain and also cardiac muscle (Helenius et al. 1996; Korhonen et al. 1997; Radak et al. 2004; Zhang et al. 2004). The finding that scavenging of H2O2 attenuates NF-κB activation in aged vessels (Ungvari et al. 2007b) suggests a role for mitochondria-derived H2O2 in regulation of endothelial NF-κB activity in aging. Local leukocyte recruitment is an early step in atherogenesis and many other degenerative diseases, and is controlled by the expression of cell adhesion molecules. It is significant that inhibition of mitochondrial ROS production has been shown in vitro to decrease expression of the adhesion molecule ICAM-1 and attenuate monocyte adhesiveness to the endothelium in aged rat arteries (Ungvari et al. 2007b). In aging mice that overexpress human catalase in mitochondria, cardiac pathology has been shown to be delayed, oxidative damage reduced, H2O2 production and H2O2-induced aconitase inactivation attenuated, and mitochondrial deletions reduced (Schriner et al. 2005). On the basis of these findings, future studies should test the hypotheses that (1) cells of long-lived mice of the Peromyscus genus are protected against oxidative stress induced by inflammatory stimuli; and (2) oxidative stress elicits a blunted inflammatory response in Peromyscus as compared to the shorter-lived M. musculus.
Mitochondrial oxidative stress resistance
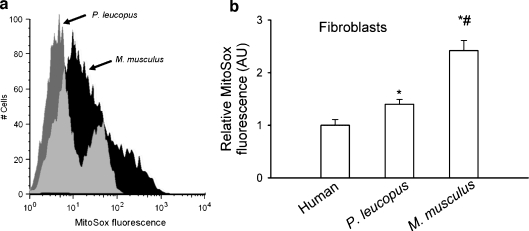
The mitochondrial theory of aging, put forward by Harman (Harman 1972), postulates that mitochondria are the main source of ROS in aged cells. According to this theory, mitochondria-derived H2O2 diffuses readily through cell membranes and contributes to a variety of macromolecular oxidative modifications. The original working version of this theory invoked accumulation of oxidative damage to proteins, lipids and DNA as the primary causal factor in the aging process (Harman 1972). It was proposed that, over time, oxidative macromolecular damage accumulates because the anti-oxidant and repair systems that prevent or remove the damage are imperfect. The long-term effect of this accumulation of macromolecular damage is the decline in cell function characteristic of aging (Sohal and Brunk 1992; Sohal and Orr 1992, 1998; Ku and Sohal 1993; Sohal et al. 1993; Sohal and Weindruch 1996; Lass et al. 1998; Bayne et al. 2005). As discussed above, mitochondria-derived ROS (mtROS; most importantly, H2O2) also act as pro-inflammatory signaling molecules, which have important implications for the aging process. In addition to being a source of ROS, mitochondria also determine cellular energetics, regulate NAD(P)/NAD(P)H levels— and consequently, the activity of several enzymes—and contribute to the induction of apoptosis. Comparative studies addressing mtROS homeostasis, mitochondrial function and mitochondrial stress resistance are especially useful to address predictions of the mitochondrial theory of aging. In this context, previous studies by us and others have revealed that heart, liver, smooth muscle and fibroblast mitochondria of P. leucopus produce less O2− and H2O2 than shorter-living species (Sohal et al. 1993; Brunet-Rossinni 2004; Csiszar et al. 2007c) (Fig. 1). These findings agree with the predictions of the oxidative stress and mitochondrial hypotheses of aging. Our recent studies using the mitochondrial O2− specific dye MitoSox have demonstrated that mitochondria in P. leucopus fibroblasts produce low amounts of ROS even when stimulated with high glucose (Z. Ungvari and P. Pacher, unpublished observation). The underlying mechanisms for differences in mtROS generation and mitochondrial oxidative stress resistance are likely multifaceted. mtROS generation is affected by mitochondrial number per se, as well as the respiratory substrate, presence or absence of inhibitors of electron transport, mitochondrial membrane potential (ΔΨ) and ΔpH. In addition, differential expression of antioxidant enzymes, which neutralize ROS (e.g., glutathione peroxidase, Mn-SOD, catalase, peroxyredoxin), is likely to contribute to the lower mtROS generation and/or oxidative stress resistance of P. leucopus mitochondria.
Fig. 1.
a Representative histograms of flow cytometry experiments demonstrating that the mean fluorescent intensity of oxidized MitoSox is greater in cultured Mus musculus fibroblasts than in Peromyscus leucopus cells. MitoSox staining was used to measure mitochondrial O2− generation (for methods, see Mukhopadhyay et al. 2007a, b). Cell debris (low forward and side scatter), dead (Sytox Green and annexin V-positive) and apoptotic cells (annexin V-positive) were excluded from the analysis (Mukhopadhyay et al. 2007a, b). b Summary data for MitoSox fluorescence intensities in cultured human, P. leucopus and M. musculus fibroblasts. Cells were grown in 96-well plates and buildup of MitoSox fluorescence was assessed using a Tecan Infinite M200 fluorescent plate reader as described in Csiszar et al. (2007c). Hoechst 33258 fluorescence representing DNA content/cell mass was used for normalization. Data are means ± SEM (n = 8 in each group), * P < 0.05. vs human, # P < 0.05 vs P. leucopus
The critical role played by mitochondria in programmed cell death is attributed to the opening of the mitochondrial permeability transition (MPT) pore (Zorov et al. 1997; Pacher et al. 2001; Pacher and Hajnoczky 2001; Zorov et al. 2007). There is growing evidence that cytoplasmic Ca2+ signaling and mitochondrial Ca2+ homeostasis are coupled through highly regulated Ca2+ uptake and release processes driven by ΔΨ (Akerman 1978; Pacher et al. 2001, 2002; Pacher and Hajnoczky 2001; Zorov et al. 2007). Cross-talk between cytoplasmic Ca2+ signals and mitochondrial Ca2+ waves (resulting in MPT) is believed to be important in cellular commitment to apoptosis (Pacher et al. 2001; Pacher and Hajnoczky 2001). It is thought that the capability of mitochondria to withstand stimuli that elicit MPT and thus to maintain ΔΨ and to retain calcium is very important for cellular survival. Recent studies by us and by others have demonstrated that cells from longer-living, relatively small mammalian species (including naked mole rats, bats and P. leucopus; Labinskyy et al. 2006a; Csiszar et al. 2007a, 2007c; Harper et al. 2007; Ungvari et al. 2008) are resistant to the pro-apoptotic effects of multiple stressors. Thus far, however, the link between mitochondrial stress resistance and longevity has not been investigated.
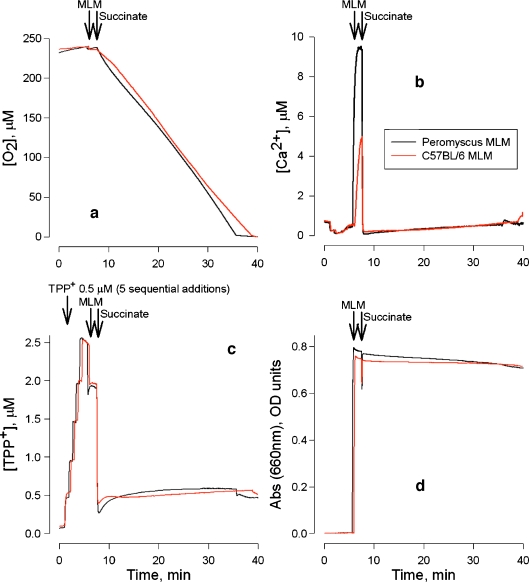
We are currently testing the hypothesis that long-lived animals not only have lower cellular mtROS production, but also exhibit superior mitochondrial stress resistance. Here we report the first results of recent studies comparing stress resistance of mitochondria isolated from the livers of M. musculus and P. leucopus. The comparison was based on physiological responses of mitochondria of these species to sequential loads of Ca2+ (in vitro model of Ca2+ oscillations). A standard experimental model of supplementing isolated mouse liver mitochondria with optimal (5 mM) amounts of succinate (the respiratory substrate for complex II) in the presence of rotenone (respiratory complex I inhibitor) was used (Krasnikov et al. 1997, 2005; Kuzminova et al. 1998). Our results showed no difference in basal oxygen uptake, Ca2+ retention, ΔΨ maintenance or volume change between P. leucopus and M. musculus liver mitochondria (Fig. 2). After mitochondria were added to the buffer and endogenous respiratory substrates were consumed (Fig. 2a), mitochondria were energized with succinate plus rotenone. Under these conditions complete mitochondrial energization occurs almost immediately (Fig. 2c). Oxygen uptake was linear and the three other measured parameters were stable during the experiment (∼40 min). Ca2+ retention capacity was tested for both mitochondrial samples using an in vitro model of Ca2+ oscillations (sequential additions of Ca2+ in the presence of succinate and rotenone). Under these conditions complete mitochondrial energization took ∼3 min (Fig. 3c).
Fig. 2a–d.
Simultaneous measurement of four parameters in isolated mouse liver mitochondria (MLM). Black tracesP. leucopus, red tracesM. musculus (strain C57BL/6). The incubation medium contained 300 mM sucrose, 5 mM HEPES, 1 mM KH2PO4, pH 7.4. MLM (1 mg/ml) were energized by addition of 5 mM succinate plus 1 μM rotenone (final concentrations) to the medium according to published methods (Krasnikov et al. 1997, 2005; Kuzminova et al. 1998). a–d O2 uptake, Ca2+ flux, ΔΨ, and swelling (as reflected by changes in optical density), respectively. Decrease in O2 concentration reflects O2 uptake by mitochondria. Note stable baseline levels of the measured parameters after addition of succinate in b– d
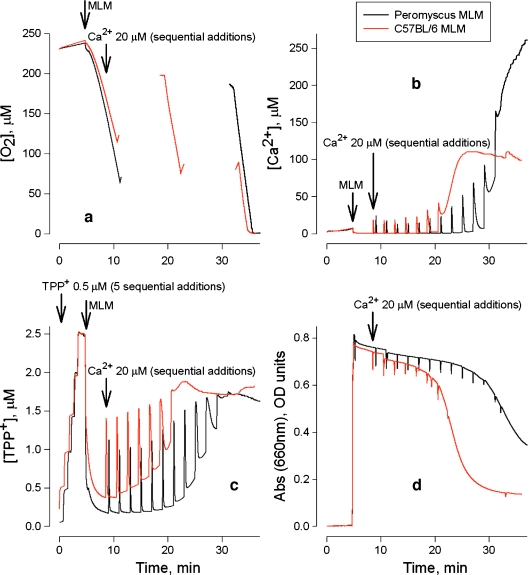
Fig. 3a–d.
Effects of sequential additions of Ca2+ on four parameters measured simultaneously in isolated MLM. Black tracesP. leucopus, red tracesM. musculus (strain C57BL/6). The incubation medium contained 300 mM sucrose, 5 mM Hepes, 1 mM KH2PO4, and 5 mM succinate, pH 7.4 according to previously described methods (Krasnikov et al. 1997, 2005; Kuzminova et al. 1998). Addition of MLM (1 mg/ml) was followed by addition of rotenone (1 μM). The initial rate of oxygen uptake (a) was measured in a closed cuvette. To prevent anoxic conditions during the experiment, the cuvette was periodically opened to allow equilibration of the incubation medium with ambient oxygen. At the end of the experiment the cuvette was sealed and respiration rate for uncoupled mitochondrial state recorded. Additions of Ca2+ were 20 μM each (final concentration). a–d O2 uptake, Ca2+ flux, ΔΨ, and swelling, respectively. The decrease in O2 concentration reflects O2 uptake by mitochondria (a); increase in Ca2+ concentration is due to Ca2+ efflux from mitochondria (b); increase in TPP+ concentration reflects ΔΨ dissipation (c); decrease in absorbance reflects mitochondrial swelling (d)
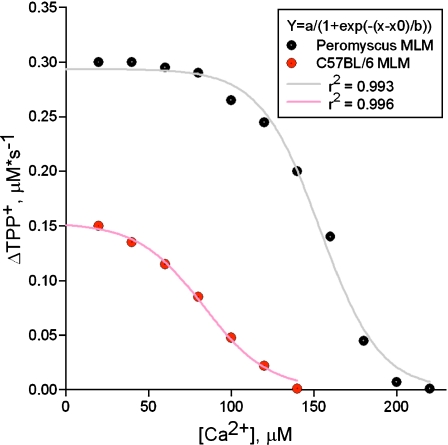
After mitochondria were maximally energized, sequential additions of Ca2+ were introduced to mitochondria every 2 min. There was no difference in the rate of oxygen uptake between P. leucopus and M. musculus mitochondria throughout the experiments (Fig. 3a). However, during pulses of loading with Ca2+, substantial differences in Ca2+ retention capacity (Fig. 3b), ΔΨ maintenance (Fig. 3c), and mitochondrial volume (Fig. 3d) were observed. As predicted, mitochondria isolated from longer-living P. leucopus exhibited greater resistance to Ca2+ stress compared to M. musculus. We also compared ΔΨ changes induced by Ca2+ loading for both P. leucopus and M. musculus. Data were collected for each time Ca2+ was introduced into the experimental buffer. Because Ca2+ uptake into mitochondria is ΔΨ-dependent, Ca2+ uptake is accompanied by an initial partial, transient depolarization followed by ΔΨ restoration (Fig. 3c). In our system, ΔΨ is measured by means of a TPP+-selective electrode. Redistribution of TPP+ across the mitochondrial membrane is directly proportional to ΔΨ. Thus, the initial rate of TPP+ uptake by mitochondria reflects ΔΨ restoration. Values of ΔTPP+ in μM s−1 plotted against representative amounts of Ca2+ loaded into mitochondria are shown in Fig. 4.
Fig. 4.
Dependence of ΔΨ restoration on [Ca2+] loading in P. leucopus and M. musculus liver mitochondria. For both species the dependence of ΔΨ restoration on [Ca2+] loaded is sigmoidal. Both data sets fit the equation 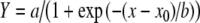 . Coefficients a, b and x0 for P. leucopus and M. musculus are a = 0.29 and 0.15, b= −18.0 and −19.7, x0 = 154 and 83.5, respectively
. Coefficients a, b and x0 for P. leucopus and M. musculus are a = 0.29 and 0.15, b= −18.0 and −19.7, x0 = 154 and 83.5, respectively
The dependence of ΔΨ restoration during increased Ca2+ loading for both species can be described by similar equations containing three independent variables (a, b and x0; Fig. 4). The proposed model predicts that maintenance of ΔΨ by the respiratory chain (Y) depends on at least three parameters affecting Ca2+ transition through mitochondrial transport systems (coefficients a, b and x0). An implication of this model is that the effects on Ca2+ transition caused by differences in the intensity of H+ leaks should be considered. Previous studies have suggested that, during the initial stages of MPT induction (when high-amplitude swelling of mitochondria is not yet detectable), the pore can exist in the H+-selective state (Novgorodov and Gudz 1996). This could increase H+ leaks, which in turn could lead to reversal of activity of the ΔΨ-dependent Ca2+ uniporter and, finally, to collapse of ΔΨ, concomitant high-amplitude swelling, and subsequent mitochondrial failure. In our model, coefficient ‘a’ reflects the rate of ΔΨ restoration after the first addition of Ca2+. Coefficient ‘b’ corresponds to the median rate of ΔΨ restoration (below this rate dissipation of ΔΨ begins). Coefficient ‘x0’ is the median value of the Ca2+ corresponding to the maximal amount of Ca2+ that mitochondria are capable of retaining before dissipation of ΔΨ starts.
Taken together, our findings suggest that P. leucopus mitochondria exhibit superior resistance to Ca2+ loading compared to M. musculus mitochondria. We propose that mitochondria that possess greater Ca2+ retention capacity could significantly delay induction of apoptosis under conditions of cellular stress. We propose two working hypotheses to explain our results: (1) P. leucopus mitochondria have more effective Ca2+ buffering systems in the matrix; or (2) the MPT of P. leucopus exhibits different functional/structural characteristics from those of M. musculus mitochondria [e.g., relative deficiency of cyclophilin D (CypD)]. In support of the second hypothesis, neurons from CypD-knockout mice are indeed resistant to ROS. Moreover, brain mitochondria, which lack CypD, are capable of retaining considerably more Ca2+ compared to mitochondria obtained from wild-type mice (Forte et al. 2007). However, it is also possible that the respiratory chains for both M. musculus and P. leucopus mitochondria possess similar efficiencies and levels of H+ leaks, and that the earlier onset of the MPT in M. musculus is due to inferior efficiency of ROS detoxification systems.
In conclusion, our studies indicate that mitochondria from young P. leucopus produce substantially less ROS than young M. musculus mitochondria and exhibit superior resistance to stress. We hypothesize that mitochondrial stress resistance in P. leucopus contributes to the remarkable resistance of P. leucopus cells to oxidative stress-induced apoptosis (Csiszar et al. 2007c). Further studies should address this hypothesis in detail. There is little data available regarding age-related alterations in mitochondrial function and phenotype in P. leucopus. We are aware of only one electron microscopic study, which revealed no age-related structural alterations in P. leucopus mitochondria (King et al. 1982). Thus, further studies are definitely needed to elucidate whether cells of long-lived mice of the genus Peromyscus (especially postmitotic cells) are protected against age-related increases in mtROS generation, impairment of mitochondrial biogenesis, or altered mitochondrial gene expression and mitochondrial functional decline. Also, it should be determined whether mitochondrial stress resistance or cellular resistance to apoptotic stimuli in long-lived mice of the genus Peromyscus is altered in aging.
Repair of oxidative DNA damage
It has been estimated that eukaryotic cells must repair thousands of DNA lesions per day to counteract endogenous sources of DNA damage, such as DNA damage generated by cellular production of ROS/RNS (Lindahl 1993). It is assumed that failure to repair DNA lesions can lead to deleterious mutations, genomic instability, or cell death, thereby accelerating the aging process or causing life-threatening diseases such as cancer. Investigators in earlier studies have proposed a strong correlation between DNA repair efficiency and maximum species lifespan (Hart et al. 1979a). However, most of these earlier studies used relatively simple experimental approaches, and the role of DNA damage accumulation in the aging process and its contribution to species longevity are still a matter of debate. The mechanisms by which DNA is repaired vary greatly depending on the type of DNA lesion, and include repair of double-strand breaks, nucleotide excision repair, and base excision repair (Peterson and Cote 2004). DNA double-strand breaks arise in DNA due to ionizing radiation, chemical exposure or stalled or collapsed DNA replication forks.
Two highly conserved major pathways exist for repairing double-strand breaks: nonhomologous end joining and homologous recombination. The nucleotide excision repair pathway is used for the removal of a variety of DNA-distorting lesions, including UV-induced pyrimidine dimers (Peterson and Cote 2004). The base-excision repair pathway is responsible for repair of oxidized and alkylated DNA bases, as well as abasic sites generated by spontaneous depurination (Peterson and Cote 2004). The pathophysiological consequences of each type of damage are different (e.g., DNA lesions that are substrates for base excision repair are highly mutagenic). Therefore, interspecies differences in efficiencies of repair systems for individual damage components likely contribute to different aging and pathophysiological phenotypes (e.g., cancer, apoptotic cell loss, cellular dysfunction). In-depth comparative studies could elucidate interspecies differences in the various steps of repair pathways and the enzymatic machinery that facilitates access to chromatin. Below, we summarize the available information on DNA repair mechanisms in P. leucopus and provide a perspective on this field.
Previous data from the Hart laboratory suggested that the rate of accumulation of age-related DNA damage (single-strand breaks) in liver and kidney of P. leucopus is slower than in house mice (Su et al. 1984). Hart and coworkers selectively assessed the efficiency of repair for individual DNA damage components. In one set of experiments fibroblasts from various short- and long-lived species (from mice to Homo sapiens) were exposed to UV irradiation. The ability of fibroblasts to perform unscheduled DNA synthesis after UV irradiation (a measure of global nucleotide excision-repair) was measured autoradiographically (Hart and Setlow 1974). Using this method, a positive correlation between life-span and efficiency of repair of UV-induced DNA damage was proposed. Subsequent studies using the same methods to test this hypothesis using the M. musculus–P. leucopus longevity contrast pair yielded results in full agreement with this hypothesis (Hart et al. 1979b).
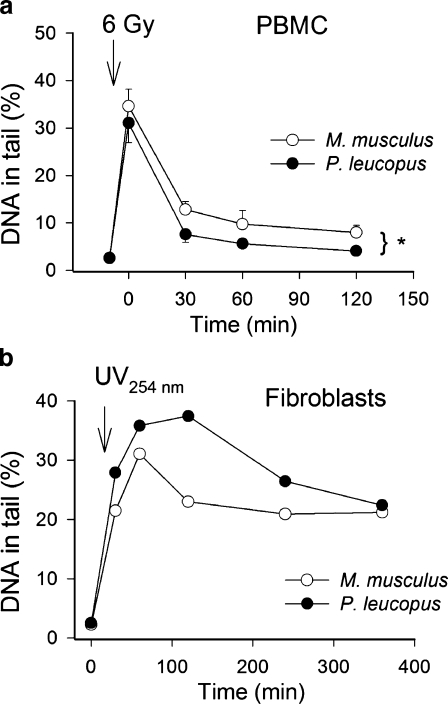
We are currently comparing relative rates of DNA repair in fibroblast cell lines from various long- and short-lived mammalian species after UV treatment, γ-irradiation or oxidant challenge using the single-cell gel electrophoresis ("comet") assay, a new, simple and sensitive method of evaluating DNA damage and repair in individual cells. We recently revisited the question of nucleotide excision-repair in P. leucopus using this technique. Interestingly, P. leucopus fibroblasts exhibited a greater extent of DNA damage immediately post-UV254 nm treatment, and nucleotide excision-repair seemed to be less effective (Fig. 5b) than in M. musculus cells. In contrast, our results so far indicate that cells of P. leucopus exhibit less H2O2-induced DNA damage than cells of shorter-lived species, including M. musculus (Csiszar et al. 2007c). A substantial portion of H2O2-induced DNA damage is thought to be due to oxidants generated from iron-mediated Fenton reactions, and there appear to be at least two distinguishable classes of iron-mediated Fenton oxidants of DNA. However, some current studies argue against the importance of the Fenton reaction in this context (Ischiropoulos and Beckman 2003). Differences in the ability of H2O2 to cause DNA damage in M. musculus and P. leucopus therefore may reflect differences in free-radical detoxification systems or chromatin structure differences rendering it less accessible to H2O2. At present it is not clear how DNA repair efficiency compares in M. musculus and P. leucopus cells after H2O2 treatment. It is also unknown how repair of peroxynitrite (ONOO−)-induced DNA damage differs between the two species.
Fig. 5a,b.
DNA repair in P. leucopus. a Animals were γ-irradiated (6 Gy) and DNA damage assessed in peripheral blood mononuclear cells (PBMC) by single-cell electrophoresis (“comet assay”) (Ungvari et al. 2007a) at 30 min, 1 and 2 h post-irradiation. Damaged DNA migrates during electrophoresis from the nucleus towards the anode, forming the shape of a “comet” with a head (cell nucleus with intact DNA) and a tail (relaxed and broken DNA). The frequency distribution of tail DNA content was obtained (median values shown; P < 0.05). b Primary mouse and P. leucopus fibroblasts were treated with UV254 nm (10 mJ/m2) and the extent of DNA damage assessed by comet assay at 0.5, 1, 2, and 3 h post-irradiation (Csiszar et al. 2007c). Median values of tail DNA content at each time point are shown
It is thought that the spectrum of damages due to H2O2 is similar to (but not congruent with) that caused by ionizing radiation—that is, mainly base-excision repair. With this in mind, the second objective of our ongoing studies is to investigate the effect of in vivo γ-irradiation on DNA damage in M. musculus and P. leucopus peripheral blood leucocytes. P. leucopus cells exhibited less DNA damage and greater efficiency of repair in response to the same dose of γ irradiation (6 Gy; Fig. 5). Most of the DNA-damaging effects of ionizing radiation are induced by •OH radicals (indirect effects) and by one-electron oxidation (direct effects). It is unknown which of these mechanisms is more important for the observed interspecific differences in cellular sensitivity to γ radiation. We suggest that the differences in the extent of DNA damage immediately post-irradiation likely reflect differences in the nuclear DNA organization or antioxidant defenses, since irradiation was performed at 0°C, which effectively slows enzymatic repair processes.
In conclusion, DNA repair is an important mechanism by which cells maintain genomic integrity, and the efficiency of DNA repair pathways may contribute to interspecies differences in both the aging process and longevity. Because ROS have been implicated in cancer and age-related degenerative diseases, the link between decline in DNA repair capacity and/or defects in repair factors, late-life diseases and accelerated aging in mammals is an active area of interest. The M. musculus–Peromyscus longevity contrast pair is an exceptionally good model for studying association between longevity and DNA repair efficiency, mitochondrial stress resistance and ROS-detoxification pathways. We hope that this review will stimulate interest among biologists in long-lived mice of the genus Peromyscus as study organisms and in a comparative approach to aging research.
Acknowledgments
This work was supported by grants from the American Heart Association (0435140N) San Antonio Area, the NIH (HL077256, HL43023, AG022873 and AG025063), Philip Morris International and Philip Morris USA and the San Antonio Area Foundation and by the Intramural Research Program of the NIH. Apologies are extended to all those whose findings or opinions pertinent to this topic were not referenced or discussed due to limitations of space or inadvertent omissions.
Abbreviations
- CR
Caloric restriction
- AGE
Advanced glycosylation end-product
- TNFα
Tumor necrosis factor-alpha
- IL-6
Interleukin-6
- iNOS
Inducible nitric oxide synthase
- ROS
Reactive oxygen species
- mtROS
Mitochondrial reactive oxygen species
- RNS
Reactive nitrogen species
- NF-κB
Nuclear factor-kappa B
- Mn-SOD
Manganese superoxide dismutase
- TPP
Tetraphenyl phosphonium
- CypD
Cyclophilin D
- ONOO-
Peroxynitrite
- •OH
Hydroxyl radical
- SEM
Standard error of the mean
- MLM
Mouse liver mitochondria
- MPT
Mitochondrial permeability transition
Contributor Information
Zoltan Ungvari, Phone: +1-914-3165727, FAX: +1-914-5944018, Email: zoltan_ungvari@nymc.edu.
Andrej Podlutsky, Phone: +1-210-5626013, FAX: +1-210-5625093, Email: podlutsky@uthscsa.edu.
References
- Abraham NG, Kappas A (2005) Heme oxygenase and the cardiovascular-renal system. Free Radic Biol Med 39(1):1–25 [DOI] [PubMed]
- Adler A, Messina E, Sherman B et al (2003) NAD(P)H oxidase-generated superoxide anion accounts for reduced control of myocardial O2 consumption by NO in old Fischer 344 rats. Am J Physiol Heart Circ Physiol 285(3):H1015–H1022 [DOI] [PubMed]
- Akerman KE (1978) Changes in membrane potential during calcium ion influx and efflux across the mitochondrial membrane. Biochim Biophys Acta 502(2):359–366 [DOI] [PubMed]
- Bátkai S, Rajesh M, Mukhopadhyay P et al (2007) Decreased age-related cardiac dysfunction, myocardial nitrative stress, inflammatory gene expression and apoptosis in mice lacking fatty acid amide hydrolase. Am J Physiol Heart Circ Physiol 293(2):H909–H918 [DOI] [PMC free article] [PubMed]
- Bayne AC, Mockett RJ, Orr WC et al (2005) Enhanced catabolism of mitochondrial superoxide/hydrogen peroxide and aging in transgenic Drosophila. Biochem J 391(2):277–284 [DOI] [PMC free article] [PubMed]
- Brunet-Rossinni AK (2004) Reduced free-radical production and extreme longevity in the little brown bat (Myotis lucifugus) versus two non-flying mammals. Mech Ageing Dev 125(1):11–20 [DOI] [PubMed]
- Burger J, Gochfeld M (1992) Survival and reproduction in Peromyscus leucopus in the laboratory: viable model for aging studies. Growth Dev Aging 56(1):17–22 [PubMed]
- Cernadas MR, Sánchez de Miguel L, García-Durán M et al (1998) Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res 83(3):279–286 [DOI] [PubMed]
- Csiszar A, Ungvari Z, Edwards JG et al (2002) Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90(11):1159–1166 [DOI] [PubMed]
- Csiszar A, Ungvari Z, Koller A et al (2003) Aging-induced proinflammatory shift in cytokine expression profile in rat coronary arteries. FASEB J 17(9):1183–1185 [DOI] [PubMed]
- Csiszar A, Ungvari Z, Koller A et al (2004) Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics 17(1):21–30 [DOI] [PubMed]
- Csiszar A, Pacher P, Kaley G et al (2005) Role of oxidative and nitrosative stress, longevity genes and poly(ADP-ribose) polymerase in cardiovascular dysfunction associated with aging. Curr Vasc Pharmacol 3(3):285–291 [DOI] [PMC free article] [PubMed]
- Csiszar A, Labinskyy N, Orosz Z et al (2007a) Vascular aging in the longest-living rodent, the naked mole rat. Am J Physiol 293(2):H919–H927 [DOI] [PubMed]
- Csiszar A, Labinskyy N, Smith K et al (2007b) Vasculoprotective effects of anti-tumor necrosis factor-{alpha} treatment in aging. Am J Pathol 170(1):388–698 [DOI] [PMC free article] [PubMed]
- Csiszar A, Labinskyy N, Zhao X et al (2007c) Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell 6(6):783–797 [DOI] [PubMed]
- Donato AJ, Eskurza I, Silver AE et al (2007) Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 100(11):1659–1666 [DOI] [PubMed]
- Forte M, Gold BG, Marracci G et al (2007) Cyclophilin D inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proc Natl Acad Sci USA 104(18):7558–7563 [DOI] [PMC free article] [PubMed]
- Guo Z, Wang M, Tian G et al (1993) Age- and gender-related variations in the activities of drug-metabolizing and antioxidant enzymes in the white-footed mouse (Peromyscus leucopus). Growth Dev Aging 57(2):85–100 [PubMed]
- Hamilton CA, Brosnan MJ, McIntyre M et al (2001) Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension 37(2):529–534 [DOI] [PubMed]
- Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol (11):298–300 [DOI] [PubMed]
- Harman D (1972) The biologic clock: the mitochondria. J Am Geriatr Soc 20(4):145–147 [DOI] [PubMed]
- Harper JM, Salmon AB, Leiser SF et al (2007) Skin-derived fibroblasts from long-lived species are resistant to some, but not all, lethal stresses and to the mitochondrial inhibitor rotenone. Aging Cell 6(1):1–13 [DOI] [PMC free article] [PubMed]
- Hart RW, Setlow RB (1974) Correlation between deoxyribonucleic acid excision-repair and life-span in a number of mammalian species. Proc Natl Acad Sci USA 71(6):2169–2173 [DOI] [PMC free article] [PubMed]
- Hart RW, D’Ambrosio SM, Ng KJ et al (1979a) Longevity, stability and DNA repair. Mech Ageing Dev 9(3–4):203–223 [DOI] [PubMed]
- Hart RW, Sacher GA, Hoskins TL (1979b) DNA repair in a short- and a long-lived rodent species. J Gerontol 34(6):808–817 [DOI] [PubMed]
- Helenius M, Hänninen M, Lehtinen SK et al (1996) Aging-induced up-regulation of nuclear binding activities of oxidative stress responsive NF-kB transcription factor in mouse cardiac muscle. J Mol Cell Cardiol 28(3):487–498 [DOI] [PubMed]
- Ischiropoulos H, Beckman JS (2003) Oxidative stress and nitration in neurodegeneration: cause, effect, or association. J Clin Invest 111(2):163–169 [DOI] [PMC free article] [PubMed]
- King TS, Karasek M, Petterborg LJ et al (1982) Effects of advancing age on the ultrastructure of pinealocytes in the male white-footed mouse (Peromyscus leucopus). J Exp Zool 224(2):127–134 [DOI] [PubMed]
- Korhonen P, Helenius M, Salminen A (1997) Age-related changes in the regulation of transcription factor NF-kappa B in rat brain. Neurosci Lett 225(1):61–64 [DOI] [PubMed]
- Krasnikov BF, Kuzminova AE, Zorov DB (1997) The Ca2+ -induced pore opening in mitochondria energized by succinate-ferricyanide electron transport. FEBS Lett 419(1):137–140 [DOI] [PubMed]
- Krasnikov BF, Zorov DB, Antonenko YN et al (2005) Comparative kinetic analysis reveals that inducer-specific ion release precedes the mitochondrial permeability transition. Biochim Biophys Acta 1708(3):375–392 [DOI] [PubMed]
- Kruger AL, Peterson SJ, Schwartzman ML et al (2006) Up-regulation of heme oxygenase provides vascular protection in an animal model of diabetes through its antioxidant and antiapoptotic effects. J Pharmacol Exp Ther 319(3):1144–1152 [DOI] [PubMed]
- Ku HH, Sohal RS (1993) Comparison of mitochondrial pro-oxidant generation and anti-oxidant defenses between rat and pigeon: possible basis of variation in longevity and metabolic potential. Mech Ageing Dev 72(1):67–76 [DOI] [PubMed]
- Kuzminova AE, Zhuravlyova AV, Vyssokikh MYU et al (1998) The permeability transition pore induced under anaerobic conditions in mitochondria energized with ATP. FEBS Lett 434(3):313–316 [DOI] [PubMed]
- Labinskyy N, Csiszar A, Orosz Z et al (2006a) Comparison of endothelial function, O2.- and H2O2 production, and vascular oxidative stress resistance between the longest-living rodent, the naked mole rat, and mice. Am J Physiol 291(6):H2698–H2704 [DOI] [PubMed]
- Labinskyy N, Csiszar A, Veress G et al (2006b) Vascular dysfunction in aging: potential effects of resveratrol, an anti-inflammatory phytoestrogen. Curr Med Chem 13(9):989–996 [DOI] [PMC free article] [PubMed]
- Lass A, Sohal BH, Weindruch R et al (1998) Caloric restriction prevents age-associated accrual of oxidative damage to mouse skeletal muscle mitochondria. Free Radic Biol Med 25(9):1089–1097 [DOI] [PMC free article] [PubMed]
- Lee CK, Allison DB, Brand J et al (2002) Transcriptional profiles associated with aging and middle age-onset caloric restriction in mouse hearts. Proc Natl Acad Sci USA 99(23):14988–14993 [DOI] [PMC free article] [PubMed]
- Lindahl T (1993) Instability and decay of the primary structure of DNA. Nature 362(6422):709–715 [DOI] [PubMed]
- Mansouri A, Muller FL, Liu Y et al (2006) Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech Ageing Dev 127(3):298–306 [DOI] [PubMed]
- Marklund SL, Westman NG, Roos G et al (1984) Radiation resistance and the CuZn superoxide dismutase, Mn superoxide dismutase, catalase, and glutathione peroxidase activities of seven human cell lines. Radiat Res 100(1):115–123 [DOI] [PubMed]
- Mukhopadhyay P, Rajesh M, Haskó G et al (2007a) Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Protocol 2(9):2295–2301 [DOI] [PMC free article] [PubMed]
- Mukhopadhyay P, Rajesh M, Yoshihiro K et al (2007b) Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem Biophys Res Commun 358(1):203–208 [DOI] [PMC free article] [PubMed]
- Novgorodov SA, Gudz TI (1996) Permeability transition pore of the inner mitochondrial membrane can operate in two open states with different selectivities. J Bioenerg Biomembr 28(2):139–146 [DOI] [PubMed]
- Nowak RM, Paradiso JL (1983) Walker’s mammals of the world. The Johns Hopkins University Press, Baltimore
- Pacher P, Hajnoczky G (2001) Propagation of the apoptotic signal by mitochondrial waves. EMBO J 20(15):4107–4121 [DOI] [PMC free article] [PubMed]
- Pacher P, Csordás G, Hajnóczky G et al (2001) Mitochondrial ca(2+) signaling and cardiac apoptosis. Biol Signals Recept 10(3–4):200–223 [DOI] [PubMed]
- Pacher P, Thomas AP, Hajnóczky G (2002) Ca2+ marks: miniature calcium signals in single mitochondria driven by ryanodine receptors. Proc Natl Acad Sci USA 99(4):2380–2385 [DOI] [PMC free article] [PubMed]
- Pacher P, Beckman JS, Liaudet L et al (2007) Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87(1):315–424 [DOI] [PMC free article] [PubMed]
- Pedersen M, Bruunsgaard H, Weis N et al (2003) Circulating levels of TNF-alpha and IL-6-relation to truncal fat mass and muscle mass in healthy elderly individuals and in patients with type-2 diabetes. Mech Ageing Dev 124(4):495–502 [DOI] [PubMed]
- Peterson CL, Cote J (2004) Cellular machineries for chromosomal DNA repair. Genes Dev 18(6):602–616 [DOI] [PubMed]
- Phillips T, Leeuwenburgh C (2005) Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J 19(6):668–670 [DOI] [PubMed]
- Radák Z, Chung HY, Naito H et al (2004) Age-associated increase in oxidative stress and nuclear factor kappaB activation are attenuated in rat liver by regular exercise. FASEB J 18(6):749–750 [DOI] [PubMed]
- Ran Q, Liang H, Gu M et al (2004) Transgenic mice overexpressing glutathione peroxidase 4 are protected against oxidative stress-induced apoptosis. J Biol Chem 279(53):55137–55146 [DOI] [PubMed]
- Ross R (1993) The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362(6423):801–809 [DOI] [PubMed]
- Roubenoff R, Parise H, Payette HA et al (2003) Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med 115(6):429–435 [DOI] [PubMed]
- Sacher GA, Hart RW (1978) Longevity, aging and comparative cellular and molecular biology of the house mouse, Mus musculus, and the white-footed mouse, Peromyscus leucopus. Birth Defects Orig Art Ser 14(1):71–96 [PubMed]
- Schriner SE, Linford NJ, Martin GM et al (2005) Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308(5730):1909–1911 [DOI] [PubMed]
- Sentman ML, Granström M, Jakobson H et al (2006) Phenotypes of mice lacking extracellular superoxide dismutase and copper- and zinc-containing superoxide dismutase. J Biol Chem 281(11):6904–6909 [DOI] [PubMed]
- Sohal RS, Brunk UT (1992) Mitochondrial production of pro-oxidants and cellular senescence. Mutat Res 275(3–6):295–304 [DOI] [PubMed]
- Sohal RS, Orr WC (1992) Relationship between antioxidants, prooxidants, and the aging process. Ann N Y Acad Sci 663:74–84 [DOI] [PubMed]
- Sohal RS, Orr WC (1998) Role of oxidative stress in senescence. Aging (Milano) 10(2):149–151 [PubMed]
- Sohal RS, Weindruch R (1996) Oxidative stress, caloric restriction, and aging. Science 273(5271):59–63 [DOI] [PMC free article] [PubMed]
- Sohal RS, Sohal BH, Brunk UT (1990) Relationship between antioxidant defenses and longevity in different mammalian species. Mech Ageing Dev 53(3):217–227 [DOI] [PubMed]
- Sohal RS, Ku HH, Agarwal S (1993) Biochemical correlates of longevity in two closely related rodent species. Biochem Biophys Res Commun 196(1):7–11 [DOI] [PubMed]
- Steger RW, Peluso JJ, Huang HH et al (1980) Effects of advancing age on the hypothalamic-pituitary-ovarian axis of the female white-footed mouse (Peromyscus leucopus). Exp Aging Res 6(4):329–339 [DOI] [PubMed]
- Steppan S, Adkins R, Anderson J (2004) Phylogeny and divergence-date estimates of rapid radiations in muroid rodents based on multiple nuclear genes. Syst Biol 53(4):533–553 [DOI] [PubMed]
- Su CM, Brash DE, Turturro A et al (1984) Longevity-dependent organ-specific accumulation of DNA damage in two closely related murine species. Mech Ageing Dev 27(2):239–247 [DOI] [PubMed]
- Ungvari Z, Csiszar A, Kaley G (2004) Vascular inflammation in aging. Herz 29(8):733–740 [DOI] [PubMed]
- Ungvari Z, Orosz Z, Rivera A et al (2007a) Resveratrol increases vascular oxidative stress resistance. Am J Physiol 292(5):H2417–H2424 [DOI] [PubMed]
- Ungvari Z, Orosz Z, Labinskyy N et al (2007b) Increased mitochondrial H2O2 production promotes endothelial NF-kB activation in aged rat arteries. Am J Physiol Heart Circ Physiol 293(1):H37–H47 [DOI] [PubMed]
- Ungvari Z, Csiszar A, Buffenstein R et al (2008) Oxidative stress in vascular senescence: lessons from successfully aging species. Front Biosci (in press) [DOI] [PubMed]
- Van Remmen H, Richardson A (2001) Oxidative damage to mitochondria and aging. Exp Gerontol 36(7):957–968 [DOI] [PubMed]
- Van Remmen H, Hamilton ML, Richardson A (2003a) Oxidative damage to DNA and aging. Exerc Sport Sci Rev 31(3):149–153 [DOI] [PubMed]
- Van Remmen H, Ikeno Y, Hamilton M et al (2003b) Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics 16(1):29–37 [DOI] [PubMed]
- Van Remmen H, Qi W, Sabia M et al (2004) Multiple deficiencies in antioxidant enzymes in mice result in a compound increase in sensitivity to oxidative stress. Free Radic Biol Med 36(12):1625–1634 [DOI] [PubMed]
- Zhang J, Dai J, Lu Y et al (2004) In vivo visualization of aging-associated gene transcription: evidence for free radical theory of aging. Exp Gerontol 39(2):239–247 [DOI] [PubMed]
- Zorov DB, Krasnikov BF, Kuzminova AE et al (1997) Mitochondria revisited. Alternative functions of mitochondria. Biosci Rep 17(6):507–520 [DOI] [PubMed]
- Zorov DB, Isaev NK, Plotnikov EY et al (2007) The mitochondrion as janus bifrons. Biochemistry (Moscow) 72(10):1115–1126 [DOI] [PubMed]