Abstract
Although less dramatic than in females, male mammals experience decreasing reproductive function during aging. In primates, multiple facets of the hypothalamic-pituitary-gonadal axis show evidence of gradual age-related decline, including behavioral, neuroendocrine and endocrine alterations such as decreased testosterone levels, reduced circulating dehydroepiandrosterone sulfate (DHEAS) levels, increased numbers of sperm abnormalities, and a general decline in physiological responses. In this review we consider a range of age-related changes in males. These measures, including more subtle aging characteristics, are interesting additional indices for detecting the timing of age-related changes in behavioral, neuroendocrine, and endocrine responses. Evidence of potential effects of calorie restriction as an intervention in reproductive aging is also discussed. A discernable decline occurs in both metabolic and reproductive endocrine processes during male aging. This cascade of events includes neuroendocrine and behavioral changes; biomarkers such as circulating DHEAS also show clear age-related decline. The varied changes that occur during male aging are considered in the context of primate aging in general.
Keywords: Calorie restriction, Male aging, Neuroendocrine systems, Primate, Reproduction
Introduction
The past two decades have seen an upward trend in the average age of couples having children (Buwe et al. 2005), with the average age of motherhood in the United States increasing from 21.4 years in 1971 to an all-time high of 25.1 years (Thacker 2004). Although some of this demographic shift can be attributed to lower teen birth rates, much of it is due to an increase in older women having children. Women between the ages of 35 and 45 years now bear more children than in the previous three decades (Thacker 2004). Paralleling this rise, since 1980 there has been a 16–24% increase in the birth rate for U.S. fathers over the age of 35 years (Kidd et al. 2001; Eskenazi et al. 2003; Buwe et al. 2005). The increase in older adults having children can be traced to a number of factors. The two biggest contributors, however, are socioeconomic pressures and the implementation of assisted reproductive technologies (ART) (Plas et al. 2000). These developments have driven a subsequent interest in understanding the consequences of normal reproductive aging in both genders.
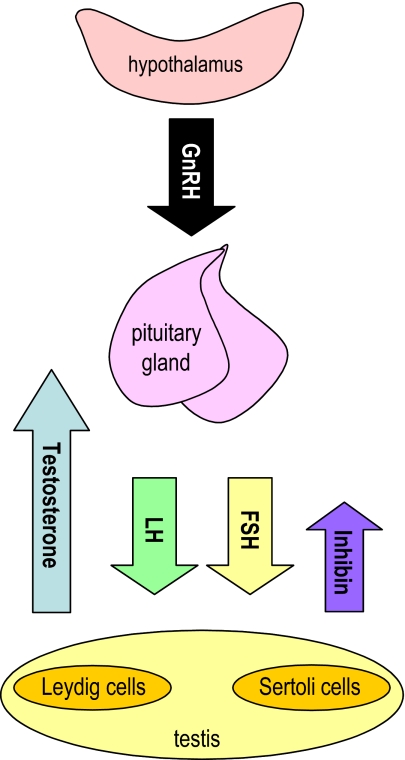
The hypothalamic-pituitary-gonadal (HPG) axis is central to reproductive function and retains remarkable similarity across vertebrate taxa (Everett 1994). After a period of latency early in life, the HPG axis becomes activated at puberty. A period of reproductive maturity and activity ensues, followed by a period (variable in length) of reproductive senescence and loss of function. Reproductive decline during aging occurs in a wide variety of species and shows variable patterns depending on species and gender (Packer et al. 1998; Kirkwood and Austad 2000; Austad 2001). In male primates, the HPG axis (Fig. 1) appears to be relatively resistant to change and overt reproductive decline is almost negligible compared with females. Subtle alterations do occur in the male system, however, resulting in decreased circulating testosterone and dehydroepiandrosterone sulfate (DHEAS) levels, increased numbers of sperm abnormalities, decreased bone density and progressively weakened muscle function. The present review examines some of the documented changes that occur during aging in primates and considers the potential of experimental interventions, such as calorie restriction (CR), in delaying the process of reproductive endocrine aging.
Fig. 1.
Schematic representation of the male hypothalamic-pituitary-gonadal axis and the major hormones involved. Hypothalamic production of gonadotropin-releasing hormone (GnRH) stimulates anterior pituitary gland production of gonadotropins, including luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Testicular response to LH induces synthesis and release of testosterone from Leydig cells; FSH acts on Sertoli cells to stimulate inhibin production and support spermatogenesis
An overview of aging
Aging is a complex multifactorial process involving molecular, cellular and systemic level changes (Weindruch and Walford 1988; Weinert and Timiras 2003). When various physiological systems go awry, the result is an age-related disarray of systemic function leading to homeostatic imbalance, pathology and ultimately death (Weindruch and Walford 1988; Kirkwood and Austad 2000). Some examples of age-related homeostatic imbalance are impaired stress response, increased pathology, decline in memory function and alterations in circadian organization (Asai et al. 2001; Kenyon 2001; Yamazaki et al. 2002; Kolker et al. 2003; Oster et al. 2003; Weinert and Timiras 2003; Huang and Manton 2004; Hofman and Swaab 2006; Kunieda et al. 2006). Numerous theories have been proposed to explain the process of normal aging, including the Disposable Soma Theory, Gene Regulation Theory, Free Radical Theory and Neuroendocrine Theory, which address aging processes alone or in combination with other models (Kirkwood and Austad 2000; Miller et al. 2002; Weinert and Timiras 2003). For additional details about theories of aging the reader is referred to reviews by Austad (2001), Roth et al. (2004), and Weindruch and Sohal (1997).
Neuroendocrine aging
The focus of this review is on endocrine and neuroendocrine changes during aging, and so the Neuroendocrine Theory will be discussed in more detail. In essence, this theory proposes that aging is a result of neural and endocrine functional changes that are crucial for (1) coordinating communication and response of an organism to its environment, (2) programming physiological responses to that environment, and (3) maintaining an optimal functional state to balance reproduction and survival while responding to environmental needs (Weindruch and Walford 1988; Weinert and Timiras 2003). The hypothalamus and anterior pituitary gland are critical pacemakers and act as the critical liaison between reproductive (via the HPG axis) and stress responses [via the hypothalamic-pituitary-adrenal axis (HPA)]. In this regard, the neuroendocrine theory has a holistic view that takes into account complex, homeostatic inter-relationships between hormonal and neural signals. There is clearly wide variation among vertebrate groups in patterns of neuroendocrine aging (Holmes et al. 2003; Ottinger et al. 2003, 2004). Thus, it becomes difficult to determine causal or physiological relationships between reproductive aging and other aspects of organismal aging.
Components of reproductive decline
In humans, women experience reproductive quiescence at approximately 50 years of age (Kidd et al. 2001; Buwe et al. 2005; Wu et al. 2005). In men, aging follows a more gradual time course with functional deterioration at several sites within the HPG axis, including central neuroendocrine regulators, hypothalamic gonadotropin-releasing hormone, pituitary gland gonadotropins, and testicular testosterone (Ottinger 1998; Harman et al. 2001; Moffat et al. 2002). Still, men generally do not experience complete reproductive senescence and maintain spermatogenesis well into old age (Plas et al. 2000; Kidd et al. 2001; Buwe et al. 2005; Henkel et al. 2005). One consequence of aging-related reproductive decline in men and women is clinical infertility, which has a major impact on public health (Kidd et al. 2001; Eskenazi et al. 2003). The proportion of infertility that is attributable to male factors ranges from 25% to 50% (Kidd et al. 2001; Agarwal and Said 2003; Eskenazi et al. 2003; Larson-Cook et al. 2003). There are also findings that link paternal aging to reduced number of live births and increased genetic disorders in offspring (Thacker 2004; Buwe et al. 2005; Slama et al. 2005; Zubkova and Robaire 2006). The question of how paternal age influences fertility and reproductive outcome is not easily defined nor easily answered since there are no longitudinal studies. Instead, the alterations of semen parameters in the aging male are almost always described on the basis of cross-sectional sampling (Plas et al. 2000). Several recent studies, however, have shown that many aspects of male fertility are affected by aging (Kidd et al. 2001; Henkel et al. 2005; Zubkova and Robaire 2006).
In addition to age, lifestyle factors such as nutrition can have a substantial impact on the HPG axis of both genders. Increased average body mass index (BMI) typically increases with age, hence reproductive capacity is not only impacted negatively by age but also by the pathologies often associated with obesity (Jensen et al. 2004; Fejes et al. 2005; Kort et al. 2006). Men with BMI>25 kg/m2 have been shown to have lower sperm concentrations and total sperm counts compared with men with non-obese BMI (Jensen et al. 2004). Another study examined 520 normal, healthy men, aged 26–45 years, and found an inverse relationship between BMI and number of motile sperm cells, and an association between BMI and sperm DNA fragmentation per subject (Kort et al. 2006).
Male reproductive aging
Declining circulating testosterone, behavioral changes, and DHEAS
In men, one of the most the most salient age-related reproductive changes is a decline in testosterone production beginning after 30 years of age and continuing gradually for the remainder of life (Ottinger 1998; Zirkin and Chen 2000; Hardy and Schlegel 2004; Henkel et al. 2005; Stocco and Wang 2006). The Massachusetts Male Aging Study showed a 0.4–0.8% annual decline in total testosterone, while biologically active free testosterone serum levels decreased by 1.2–1.7% per year after 50 years of age (Plas et al. 2000; Henkel et al. 2005). This testosterone decline may reflect a reduced testicular response to gonadotropins and/or declining response to reduced hypothalamic response in spite of lower testosterone feedback. Nonetheless, the testosterone decline is associated with weakening muscle function, reduced bone density and other age-related metabolic endocrine changes (Harman et al. 2001; Moffat et al. 2002).
There is an interesting observation that appears to be consistent across species. As circulating testosterone levels decline one would also predict an increase in GnRH production and release, which in turn, would stimulate production and release of pituitary gland luteinizing hormone (LH). Yet despite the loss of negative feedback, pituitary and circulating levels of LH show little age-related change (Ottinger 1998; Zirkin and Chen 2000; Hardy and Schlegel 2004). In contrast, follicle-stimulating hormone (FSH) increases with age in association with decreased inhibin from the Sertoli cells (Ottinger 1998; Zirkin and Chen 2000). As might be expected, there is great individual variation among males in the chronological timing and rate of decrease (Ottinger 1998; Henkel et al. 2005). While more difficult to quantify, male sexual behavior appears to be a sensitive, predictive measure of declining reproductive function in humans, as well as male nonhuman primates (for review, see Ottinger 1998). Interestingly, behavioral changes appear to precede detectable declines in circulating testosterone both in male primates and in other male animal models of aging (Black and Lane 2002; Ottinger et al. 2004; Ottinger 2007). Therefore, monitoring circulating testosterone alone may not be the best measure of general male aging and certainly must be considered in the context of other measurements.
Circulating levels of the adrenal steroid DHEAS clearly decrease with aging in male rhesus macaques, prompting consideration of this hormone as a biomarker of aging (Roth et al. 2004; Urbanski et al. 2004). In addition, both cortisol and DHEAS have clear 24-hour rhythms, with peak levels in late morning. A study of 24-hour rhythms of cortisol and DHEAS in young adult and aging rhesus males revealed that normal cortisol rhythms persisted in aged males, whereas DHEAS rhythms were damped (Downs et al. 2008). Together, these data suggest that aging impacts testosterone and DHEAS levels differentially. Further, the reliable age-related decline in the amplitude of DHEAS peaks may provide a useful biomarker for individual rates of reproductive aging.
Markers of male gonadal aging
Other research on reproductive function and fertility in males has traditionally focused on semen analysis. Spermiograms provide a composite clinical measure of semen quality (Plas et al. 2000). A significant inverse relationship was found between semen volume and age, with a clear decrease (3–30%) between men ≤30 years of age and men ≥50 years of age (Kidd et al. 2001; Eskenazi et al. 2003; Henkel et al. 2005; Zubkova and Robaire 2006). In contrast, much of the literature suggests that sperm concentration remains constant or even increases with age (Kidd et al. 2001; Henkel et al. 2005). These apparently conflicting observations of decreased semen volume and increased sperm concentration with age may be due to reduced accessory gland function, especially the seminal vesicle in elderly men (Henkel et al. 2005).
There are very consistent reports of significantly lower sperm motility in the range of 3–37% when comparing men ≥50 years to men ≤30 years of age (Kidd et al. 2001; Zubkova and Robaire 2006). Overall, sperm motility can decline 0.7–4.7% per year, depending on whether motility, progressive motility, or total progressive motility is measured (Eskenazi et al. 2003). The decrease in overall sperm motility may correlate with the observed decline in plasma testosterone since sperm maturation and induction of motility in the epididymis both require bioactive testosterone (Henkel et al. 2005). Similarly, a negative correlation has been found in normal sperm morphology and age (Zubkova and Robaire 2006). In men ≥50 years, morphologically abnormal sperm increased 4–22% compared with men ≤30 years old (Kidd et al. 2001). In another study of 1,655 men between the ages of 17 and 66, normal sperm morphology declined 0.47% annually (Henkel et al. 2005).
Sperm count, a standard measure in most spermiograms, does not provide a good measure of aging compared with concentration or motility because sperm are produced in excess of amounts needed for fertility. Additionally, there is considerable intra-individual variation in spermiogram measures. Consequently, cross-sectional studies on a single semen parameter are difficult to interpret (Plas et al. 2000; Eskenazi et al. 2003). Measurement of DNA integrity considers differences in male and female gamete meiotic potential. Since spermiogenesis is a continuous process starting during puberty, spermatogonia undergo many replications and DNA duplications, reaching roughly 150 divisions by the age of 20 years with a linear increase of ∼23 divisions per year thereafter. Thus, spermatogonia of a 28-year-old father may have already undergone ∼380 mitoses and DNA replications; a 35-year-old male, up to 540. In contrast, oogenesis in female primates is completed prior to birth in 22 cell divisions (Plas et al. 2000; Buwe et al. 2005; Zubkova and Robaire 2006).
The risk of sperm abnormalities can be assessed using the sperm chromatin structure assay (SCSA), a flow cytometric technique performed on semen to determine the susceptibility of sperm nuclear DNA to acid-induced DNA denaturation in situ. The resulting percentage of denatured single-stranded DNA, relative to total DNA, provides a DNA fragmentation index (DFI) (Agarwal and Said 2003; Evenson and Wixon 2006). In a number of studies, a DFI level ≥30% was found to predict low success of in vivo fertility and in vitro ART procedures (Larson-Cook et al. 2003; Bungum et al. 2004). Because sperm susceptibility to DNA fragmentation increases with age, this measure can provide valuable information on DNA integrity for in vitro technologies.
Models for the study of reproductive aging in males
Rodent models
There are numerous justifications for using rodent models in reproductive aging research. Rodents are relatively short-lived, inexpensive to maintain with controlled genetic variability, health and reproductive parameters are well characterized, transgenic strains allow study of specific alterations of aging processes and their physiological decline parallels many of the normal reproductive deficits observed in aged men. The Brown Norway rat has decreased serum and intratesticular testosterone levels resulting from impaired Leydig cell steroidogenesis with aging (Zirkin and Chen 2000; Syntin et al. 2001; Chen et al. 2002). A decline in specific intermediary steroids along the steroidogenic pathway within these cells appears to be due to malfunctions of the pathway/enzyme systems rather than a loss of Leydig cell numbers (Chen et al. 1996; Chen and Zirkin 1999; Zirkin 2006). Although serum LH levels did not differ, there were measurable changes in the LH pulse and interval (Chen et al. 2002). In humans, rodents, and other vertebrate classes, age-related loss in the numbers of spermatogenic cells within the seminiferous tubules begins focally, with atrophic tubules often located adjacent to tubules exhibiting normal spermatogenesis (Ottinger 1998). In the rat, the loss of germ cells eventually spreads throughout the testis, causing a progressive loss of testis weight (Syntin et al. 2001). With increasing age, rodent epididymal epithelium acquires morphological hallmarks of decay (Jervis and Robaire 2003), including significantly greater levels of reactive oxygen species production by Leydig cell mitochondria compared with that of younger males (Chen et al. 2001, 2004). Finally, spermatozoa from older rats display altered chromatin packaging and integrity (Zubkova and Robaire 2006).
Also important is the neurological impact of male reproductive decline, and specifically of testosterone loss. Recently it has become clear that the male brain is androgen-responsive and experiences an age-related testosterone depletion, making it vulnerable to senescence potentially associated with androgen loss. Men with Alzheimer’s disease (AD) have significantly lower testosterone levels than aged men without AD (Moffat et al. 2002; Rosario et al. 2006). However, testosterone depletion appears to occur well before clinical and pathological diagnosis of AD, suggesting that low testosterone contributes to AD pathogenesis rather than results from it. This has been verified in 3xTg-AD mice (a triple transgenic mouse model of AD) in which androgen depletion accelerated the development of AD-like neuropathology. Both the deposition of β-amyloid plaques and behavioral impairment increased with testosterone loss but were mitigated by androgen treatment (Rosario et al. 2006).
Nonhuman primate models
Studies in mammals must consider how late-life diseases may alter normal genetic and physiological mechanisms (Miller et al. 2002). Findings in rodent and other models provide insight into reproductive aging and form the basis for the study of mechanisms of aging revealed in other species. Given the complexity of human physiology, however, it is important to study nonhuman primates (NHP) as basic research findings are translated into clinical applications to human health. This literature provides the underpinning to understand the normal progression of reproductive decline in humans. In this regard, the nonhuman primate, in particular the rhesus macaque (Macaca mulatta), is an excellent model for understanding fundamental tenants and characteristics of primate aging.
Rhesus macaques adapt well to laboratory settings and have been used for the better part of a century in biomedical research. A great deal is known about their general husbandry, nutritional requirements, breeding practices and veterinary care. As healthy rhesus macaques age, values for routine hematological and blood chemistry variables exhibit notable, biologically relevant changes that reflect normal patterns of the aging process (Smucny et al. 2001). The rhesus macaque shares 97.5% genetic homology to humans (Gibbs et al. 2007) and a maximum lifespan of 40 years (Ingram et al. 1990; Roth et al. 2004). This close evolutionary relationship produces a highly similar aging phenotype to humans, as reviewed previously by Roth et al. (2004). Important parallels between humans and rhesus exist with regard to metabolism, body composition, cardiopulmonary system, reproduction and behavior.
Studies of semen characteristics in aging nonhuman primates have been cross-sectional, often with single or short time-point measures and small sample sizes (Platz et al. 1980; Tollner et al. 1990; Schaffer et al. 1992; VandeVoort et al. 1993; Morrell 1997; Yeoman et al. 1997; Ramesh et al. 1998; Ji et al. 2001). Some studies have focused on testosterone levels in male rhesus macaques, with somewhat contradictory results, similar to the human data (Mattison et al. 2001; Black and Lane 2002; Mattison et al. 2003; Roth et al. 2004). This difference in data is likely due to individual variability in testosterone levels and the pulsatile nature of testosterone release. Use of remote sampling and determination of circadian rhythms, for example, has revealed a clear age-related testosterone decline in male rhesus macaques (Urbanski et al. 2006).
Evaluating reproductive aging in males: sperm and semen quality
As with other aging biomarkers, there is no single measure of male reproductive potential which correlates perfectly with age. Although some measures, such as the sperm chromatin structure assay, can be highly predictive of fertility, they do not necessarily relate to aging. Other measures, such as for testosterone, require multiple samples and long-term monitoring to produce meaningful results. There is a need for a comprehensive battery of tests which, taken as a whole, can provide a complete assessment of reproductive health and fertilizing capability. These measures, presented in Table 1 include sperm count, concentration, weight, volume, pH, motility and morphology—all typical spermiogram measures of semen production that are part of standard fertility clinic or research protocols. More extensive tests may include hypo-osmotic swelling (HOS) assay, SCSA, zona pellucida binding and acrosome reaction assay (ZP binding and AR), and seminal plasma constituent analysis, but are not usually performed due to cost and time constraints (Jeyendran 2003).
Table 1.
Summary of semen measurements
| Measure | Rationale |
|---|---|
| Ejaculate appearance | Indicative of cell (sperm) numbers |
| Ejaculate weight | Indicative of accessory sex gland production and secretion |
| Ejaculate color | Abnormal color may indicate accessory sex gland or other clinical pathology |
| Ejaculate volume | Low volume may indicate retrograde semen flow into the bladder or accessory sex gland pathology |
| Osmolarity/osmolality | Indicative of ionic composition |
| pH | Indicative of ratio of alkaline seminal vesicle secretions and acidic prostatic secretions |
| Count | Indicative of overall spermatogenesis success |
| Concentration | Indicative of successful spermatogenesis and accessory sex gland production |
| Motility | Indicative of sperm ability to reach the ova |
| Morphology | Indicative of cell maturation status |
| Activation (capacitation) | Indicative of sperm ability to reach the ova and then penetrate the zona pellucida |
| Agglutination | May indicate possible surface antigen problems |
| ZP binding | Sperm binding to the ZP outer surface is a prerequisite for oocyte vitelline membrane binding and penetration |
| Acrosome reaction | Prior to fertilization the outer acrosomal membrane fuses with the surrounding plasma membrane |
| Hypo-osmotic swelling assay | Indicative of intact sperm membrane. Membrane integrity can influence motility, activation, acrosome reaction and is required for successful fusion with the ova |
| Seminal plasma composition | Overall measure of accessory sex gland contribution |
| SCSA | Indicative of DNA packaging and sperm development capabilities |
Summary of some measures of semen and sperm quality
Hypo-osmotic swelling assay The capacity of sperm to swell in the presence of a hypo-osmotic solution is indicative of membrane integrity and normal function, which is crucial for fertilization. Whereas histological techniques and ‘live-dead’ morphology stains only measure whether the membrane is morphologically intact, this assay evaluates the functional integrity of the sperm membrane and fertilizing potential (Jeyendran et al. 1984). This assay has been used in research with nonhuman primates as well as applied clinically (Kholkute et al. 2000; Jeyendran 2003; Rutllant et al. 2003).
Sperm chromatin structure assay The SCSA is the most statistically robust tool used to measure sperm nuclear DNA fragmentation and has proven highly effective in predicting fertility outcome both in vivo and in vitro (Agarwal and Said 2003; Larson-Cook et al. 2003). Alterations in genomic organization of the sperm nuclei appear to be negatively correlated with the fertility potential of sperm (Evenson and Wixon 2006). This suggests that pathologically increased sperm DNA fragmentation may be a significant paternal factor in assisted reproductive failures (Tesarik et al. 2006).
ZP binding and acrosome reaction assay Eutherian spermatozoa require capacitation to penetrate the zona pellucida, or glycoprotein membrane surrounding the oocyte (Mortimer 1994; Yanagimachi 1994). The sequence of events leading to in vivo fertilization is: ejaculation → capacitation → ZP binding → acrosome reaction → oocyte penetration → fertilization. An in vitro assay quantifies the ability of collected sperm to complete this sequence (VandeVoort et al. 1992; Jeyendran 2003). In rhesus macaques, in vitro capacitation of sperm is facilitated by exposure to two activators: caffeine and dbcAMP (VandeVoort et al. 1994). The capacitated sperm can then bind to ZP membranes. Following ZP binding but prior to actual fertilization, the outer acrosomal membrane fuses with the surrounding sperm plasma membrane. The hydrolytic contents of the acrosome are then released between the fused membranes and allow the underlying plasma membrane to fuse with the inner vitelline membrane of the oocyte and fertilization occurs. The ZP-induced acrosome reaction occurs rapidly (VandeVoort et al. 1994, 1997). Because ZP binding and acrosomal reaction occur on the external surface of the oocyte and activated sperm head, these processes can be observed microscopically and quantified using dual fluorescence staining.
Seminal plasma constituent analysis While much effort has been directed at measuring sperm integrity, sperm only make up 1–5% of the total volume of ejaculate (Owen and Katz 2005). The remaining constituents are produced by several accessory sex glands, including the bulbourethral gland, seminal vesicles and prostate. These constituents include potassium, semenogelin, bicarbonate, fructose, magnesium, prostaglandins, ascorbic acid, calcium, zinc, citric acid, seminal vesicle proteins, prostate-specific antigen and albumin (Harrison and Lewis 1986; Mortimer 1994; Lewis-Jones et al. 1996; Gonzales 2001; Jeyendran 2003; Owen and Katz 2005). Fructose in human semen provides energy for the motile sperm (Lewis-Jones et al. 1996; Elzanaty et al. 2002). Accordingly, measurement of seminal fructose has been used in fertility labs and by the World Health Organization as a marker of seminal vesicle function (Harrison and Lewis 1986; Gonzales 2001). In the rhesus macaque, secretions from the cranial lobe of the prostate mix with fluid from the seminal vesicles to bring about coagulation of the seminal plasma following ejaculation (Harrison and Lewis 1986). Prostate-derived citrate in human semen may contribute to the high buffering capacity of semen, which allows sperm to survive in the acidic vaginal environment (Owen and Katz 2005).
Microarrays The application of microarray technology has great potential as a tool for evaluating reproductive capacity and associated changes in gene expression. High-density oligonucleotide expression arrays allow a quick screen of thousands of transcripts to determine genes of interest within the HPG axis, including the anterior pituitary gland, epididymis, Leydig cells and spermatogenic cells (Syntin et al. 2001; Jervis and Robaire 2003; Chen 2004; Chen et al. 2004; Wrobel and Primig 2005; He et al. 2006). Microarrays in combination with further verification by semi-quantitative reverse transcriptase polymerase chain reaction (PCR), quantitative real-time PCR, in situ hybridization, immunohistochemistry, or other techniques provide powerful tools to evaluate fundamental molecular alterations associated with age, chemical exposure, disease pathology or nutrition.
Effects of calorie restriction
Calorie restriction (CR) has demonstrated benefits for age-related maladies (McCay et al. 1935; Weindruch and Walford 1988; Lane et al. 1999a, b; Black et al. 2001; Hursting et al. 2003; Koubova and Guarente 2003; Dhahbi et al. 2004). Essential nutrients and vitamins are provided while limiting total energy (calorie) intake, allowing for investigation of effects of energy restriction without the potential confound of insufficient critical dietary elements (Thompson et al. 2002). Studies have shown that moderate CR extends lifespan by slowing the rate of physiological decline and retarding age-related chronic diseases in a variety of species. To date, this nutritional paradigm has been found to be effective in protozoa, yeast, rotifers, fleas, nematodes, spiders, flies, mollusks, fish, mice, rats, dogs and possibly nonhuman primates (squirrel monkeys, cynomolgus and rhesus macaques) (Weindruch and Walford 1988; Ingram et al. 1990; Lane et al. 1999a, b; Roth et al. 1999). Some of the beneficial health effects of CR include reduced adiposity, lower body temperature, lower blood pressure, reduction of glucose, fasting plasma insulin levels, and high density lipoproteins while increasing insulin sensitivity. CR affects neuroendocrine systems, reducing levels of growth hormone, thyroid-stimulating hormone and thyroid hormones, insulin-like growth factor 1, gonadotropins, and oxidative stress (Lane et al. 1997; Gresl et al. 2001; Heilbronn and Ravussin 2003; Koubova and Guarente 2003; Gredilla and Barja 2005). The degree and duration of CR influences the benefits of CR on rates of survival and aging (Merry 2002). Although the exact mechanisms are unclear, CR improves function of a variety of physiological systems (Hursting et al. 2003).
The relevance of calorie restriction to primates has been tested in a long term study of CR and aging in non-human primates at the National Institute on Aging (NIA) (Ingram et al. 1990). Definitive evidence showing that CR extends life span in these animals is not yet available since they have a maximum life expectancy of 40 years; however, emerging data from the NIA study as well as ongoing studies of rhesus and cynomolgus macaques at the University of Wisconsin-Madison, University of Maryland, and Wake Forest University School of Medicine support the beneficial effects of CR.
Animals in the NIA study were gradually calorically restricted until a total restriction of 30% had been reached. Consistent with the CR paradigm, the diet was supplemented with additional vitamins and minerals to guard against malnutrition (Ingram et al. 1990; Mattison et al. 2005). A variety of endpoints have been measured and results are consistent with CR-induced attenuation of age-related changes in plasma triglycerides, oxidative damage, and glucose regulation (Roth et al. 2002). CR animals weigh less, have less body fat, and lower body temperatures than their matched control counterparts, which are maintained at their ideal ad libitum weight. These data provide early evidence of the potential benefit of CR for lowering the risk for diabetes, cardiovascular disease, and tumor incidence (Lane et al. 1999a, b; Roth et al. 2002; Mattison et al. 2003). The Wisconsin Regional Primate Research Center study is reporting similar findings, despite some experimental differences (Gresl et al. 2001).
Impact of moderate calorie restriction on reproduction
The effects of moderate CR on the HPG axis have been mixed, depending on the level of CR and species (Weindruch and Walford 1988). CR delayed sexual maturation in rats, but also extended reproductive function (Holehan and Merry 1985; McShane and Wise 1996; Gredilla and Barja 2005). Female rhesus macaques, aged 7–27 years, showed no adverse effects of long-term 30% CR (Black et al. 2001; Lane et al. 2001; Mattison et al. 2003; Wu 2006). Moderate CR begun in prepubertal male rhesus macaques caused delayed sexual maturation and an associated slower increase in circulating testosterone (Lane et al. 1997; Roth et al. 2000). Concomitantly, skeletal growth was also limited in these animals (Roth et al. 2000; Mattison et al. 2003). These investigations into the effects of CR on male rhesus macaque reproduction, especially during aging (Downs et al. 2004), provide interesting insight on the consequences of CR. The NIA study of the impact of CR on semen characteristics and sperm function will provide additional insight on the impact of CR on reproductive fitness in male primates.
Summary
In male primates, including humans, neural and gonadal components of the reproductive axis show evidence of age-related demise, resulting in gradual loss of reproductive function. These changes include declining circulating testosterone and DHEAS levels, reduced male sexual behavior, and evidence of increased sperm abnormalities with reduced semen quality. Calorie restriction provides beneficial effects to a variety of physiological systems and appears to have potential benefits to specific aspects of male reproduction. Further studies will provide more detailed insight into the genetic and molecular mechanisms affected by aging, which may also respond to interventions such as calorie restriction.
Acknowledgements
The authors wish to acknowledge support from the Department of Animal and Avian Sciences, University of Maryland; NIH Grants RR-00163, AG-019914, AG-029612, and the intramural program of the National Institute on Aging, National Institutes of Health.
References
- Agarwal A, Said TM (2003) Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update 9:331–345 [DOI] [PubMed]
- Asai M, Yoshinobu Y, Kaneko S, Mori A, Nikaido T, Moriya T, Akiyama M, Shibata S (2001) Circadian profile of Per gene mRNA expression in the suprachiasmatic nucleus, paraventricular nucleus, and pineal body of aged rats. J Neurosci Res 66:1133–1139 [DOI] [PubMed]
- Austad SN (2001) An experimental paradigm for the study of slowly aging organisms. Exper Gerontol 36:599–605 [DOI] [PubMed]
- Black A, Lane MA (2002) Nonhuman primate models of skeletal and reproductive aging. Gerontology 48:72–80 [DOI] [PubMed]
- Black A, Allison DB, Shapses SA, Tilmont EM, Handy AM, Ingram DK, Roth GS, Lane MA (2001) Calorie restriction and skeletal mass in rhesus monkeys (Macaca mulatta): evidence for an effect mediated through changes in body size. J Gerontol A Biol Sci Med Sci 56:B98–B107 [DOI] [PubMed]
- Bungum M, Humaidan P, Spano M, Jepson K, Bungum L, Giwercman A (2004) The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF, and ICSI. Hum Reprod 19:1401–1408 [DOI] [PubMed]
- Buwe A, Guttenbach M, Schmid M (2005) Effect of paternal age on the frequency of cytogenetic abnormalities in human spermatozoa. Cytogenet Genome Res 111:213–228 [DOI] [PubMed]
- Chen HL (2004) Gene expression by the anterior pituitary gland: effects of age and caloric restriction. Mol Cell Endocrinol 222:21–31 [DOI] [PubMed]
- Chen HL, Zirkin BR (1999) Long-term suppression of Leydig cell steroidogenesis prevents Leydig cell aging. Proc Natl Acad Sci USA 96:14877–14881 [DOI] [PMC free article] [PubMed]
- Chen HL, Huhtaniemi I, Zirkin BR (1996) Depletion and repopulation of Leydig cells in the testes of aging Brown Norway rats. Endocrinology 137:3447–3452 [DOI] [PubMed]
- Chen HL, Cangello D, Benson S, Folmer J, Zhu H, Trush MA, Zirkin BR (2001) Age-related increase in mitochondrial superoxide generation in the testosterone-producing cells of Brown Norway rat testes: relationship to reduced steroidogenic function. Exp Gerontol 36:1361–1373 [DOI] [PubMed]
- Chen HL, Hardy MP, Zirkin BR (2002) Age-related decreases in Leydig cell testosterone production are not restored by exposure to LH in vitro. Endocrinology 143:1637–1642 [DOI] [PubMed]
- Chen HL, Irizarry RA, Luo LD, Zirkin BR (2004) Leydig cell gene expression: effects of age and caloric restriction. Exp Gerontol 39:31–43 [DOI] [PubMed]
- Dhahbi JM, Kim H-J, Mote PL, Beaver RJ, Spindler SR (2004) Temporal linkage between the phenotypic and genomic responses to caloric restriction. Proc Natl Acad Sci USA 101:5524–5529 [DOI] [PMC free article] [PubMed]
- Downs JL, Garyfallou VT, Aghazadeh-Sanai N, Urbanski HF (2004) Effect of aging and caloric restriction on circadian hormone release in male rhesus macaques. Annual Meeting of the Society for Neuroscience, San Diego, California, pp 23–27, October 2004
- Downs JL, Mattison JA, Ingram DK, Urbanski HF (2008) Effect of age and caloric restriction on circadian adrenal steroid rhythms in rhesus macaques. Neurobiol Aging (in press). doi:10.1016/j.neurobiolaging.2007.03.011 [DOI] [PMC free article] [PubMed]
- Elzanaty S, Richthoff J, Malm J, Giwercman A (2002) The impact of epididymal and accessory sex gland function on sperm motility. Hum Reprod 17:2904–2911 [DOI] [PubMed]
- Eskenazi B, Wyrobek AJ, Sloter E, Kidd SA, Moore L, Young S, Moore D (2003) The association of age and semen quality in healthy men. Hum Reprod 18:447–454 [DOI] [PubMed]
- Evenson DP, Wixon RL (2006) Use of the sperm chromatin structure assay (SCSA) as a diagnostic tool in the human infertility clinic. Fertility Magazine 4:15–18
- Everett JW (1994) Pituitary and hypothalamus: perspectives and overview. In: Knobil E, Neill JD, Greenwald GS, Markert CL, Pfaff DW (eds) The physiology of reproduction, 2nd edn. Raven Press, New York
- Fejes I, Koloszar S, Szollodsi J, Zavaczki Z, Pal A (2005) Is semen quality affected by male body fat distribution. Andrologia 37:155–159 [DOI] [PubMed]
- Gibbs RA, Rogers J, Katze MG, Bumgarner R, Gibbs RA, Weinstock GM (2007) Evolutionary and biomedical insights from the rhesus macaque genome. Science 316:222–234 [DOI] [PubMed]
- Gonzales GF (2001) Function of seminal vesicles and their role on male fertility. Asian J Androl 3:251–258 [PubMed]
- Gredilla R, Barja G (2005) Minireview: the role of oxidative stress in relation to caloric restriction and longevity. Endocrinology 146:3713–3717 [DOI] [PubMed]
- Gresl TA, Colman RJ, Roecker EB, Havighurst TC, Huang Z, Allison DB, Bergman RN, Kemnitz JW (2001) Dietary restriction and glucose regulation in aging rhesus monkeys: a follow-up report at 8.5 yr. Am J Physiol Endocrinol Metab 281:E757–E765 [DOI] [PubMed]
- Hardy MP, Schlegel PN (2004) Testosterone production in the aging male: where does the slowdown occur. Endocrinology 145:4439–4440 [DOI] [PubMed]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR (2001) Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab 86:724–731 [DOI] [PubMed]
- Harrison RM, Lewis RW (1986) The male reproductive tract and its fluids. In: Dukelow WR, Erwin J (eds) Comparative primate biology: reproduction and development. Liss, New York
- He ZP, Chan WY, Dym M (2006) Microarray technology offers a novel tool for the diagnosis and identification of therapeutic targets for male infertility. Reproduction 132:11–19 [DOI] [PubMed]
- Heilbronn LK, Ravussin E (2003) Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr 78:361–369 [DOI] [PubMed]
- Henkel R, Maass G, Schuppe HC, Jung A, Schubert J, Schill WB (2005) Molecular aspects of declining sperm motility in older men. Fertil Steril 84:1430–1437 [DOI] [PubMed]
- Hofman MA, Swaab DF (2006) Living by the clock: the circadian pacemaker in older people. Ageing Res Rev 5:33–51 [DOI] [PubMed]
- Holehan AM, Merry BJ (1985) The control of puberty in the dietary restricted female rat. Mech Ageing Dev 32:179–191 [DOI] [PubMed]
- Holmes DJ, Thomson SL, Wu J, Ottinger MA (2003) Reproductive aging in female birds. Exp Gerontol 38:751–756 [DOI] [PubMed]
- Huang H, Manton KG (2004) The role of oxidative damage in mitochondria during aging: a review. Front Biosci 9:1100–1117 [DOI] [PubMed]
- Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC (2003) Calorie restriction, aging, and cancer prevention: mechanisms of action and a applicability to humans. Annu Rev Med 54:131–152 [DOI] [PubMed]
- Ingram DK, Cutler RG, Weindruch R, Renquist DM, Knapka JJ, April M, Belcher CT, Clark MA, Hatcherson CD, Marriott BM, Roth GS (1990) Dietary restriction and aging—the initiation of a primate study. J Gerontol 45:B148–B163 [DOI] [PubMed]
- Jensen TK, Andersson AM, Jorgensen N, Andersen AG, Carlsen E, Petersen JH, Skakkebaek NE (2004) Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril 82:863–870 [DOI] [PubMed]
- Jervis KM, Robaire B (2003) Effects of caloric restriction on gene expression along the epididymis of the Brown Norway rat during aging. Exp Gerontol 38:549–560 [DOI] [PubMed]
- Jeyendran RS (2003) Protocols for semen analysis in clinical diagnosis. Parthenon Publishing Group, New York
- Jeyendran RS, Vanderven HH, Perezpelaez M, Crabo BG, Zaneveld LJD (1984) Development of an assay to assess the functional integrity of the human-sperm membrane and its relationship to other semen characteristics. J Reprod Fertil 70:219–228 [DOI] [PubMed]
- Ji WZ, He XC, Wang H, Bavister BD, Li XL (2001) Semen parameters and cryopreservation of spermatozoa of Assamese macaque (Macaca assamensis). Biol Reprod 64 Suppl 1:312–312
- Kenyon C (2001) A conserved regulatory system for aging. Cell 105:165–168 [DOI] [PubMed]
- Kholkute SD, Gopalkrishnan K, Puri CP (2000) Variations in seminal parameters over a 12-month period in captive bonnet monkeys. Primates 41:393–405 [DOI] [PubMed]
- Kidd SA, Eskenazi B, Wyrobek AJ (2001) Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril 75:237–248 [DOI] [PubMed]
- Kirkwood TBL, Austad SN (2000) Why do we age. Nature 408:233–238 [DOI] [PubMed]
- Kolker DE, Fukuyama H, Huang DS, Takahashi JS, Horton TH, Turek FW (2003) Aging alters circadian and light-induced expression of clock genes in golden hamsters. J Biol Rhythms 18:159–169 [DOI] [PubMed]
- Kort HI, Massey JB, Elsner CW, Mitchell-Leef D, Shapiro DB, Witt MA, Roudebush WE (2006) Impact of body mass index values on sperm quantity and quality. J Androl 27:450–452 [DOI] [PubMed]
- Koubova J, Guarente L (2003) How does calorie restriction work. Genes Dev 17:313–321 [DOI] [PubMed]
- Kunieda T, Minamino T, Katsuno T, Tateno K, Nishi J, Miyauchi H, Orimo M, Okada S, Komuro I (2006) Cellular senescence impairs circadian expression of clock genes in vitro and in vivo. Circ Res 98:532–539 [DOI] [PubMed]
- Lane MA, Ingram DK, Roth GS (1997) Beyond the rodent model: calorie restriction in rhesus monkeys. Age 20:45–56 [DOI] [PMC free article] [PubMed]
- Lane MA, Ingram DK, Roth GS (1999a) Calorie restriction in nonhuman primates: effects on diabetes and cardiovascular disease risk. Toxicol Sci 52:41–48 [DOI] [PubMed]
- Lane MA, Tilmont EM, De Angelis H, Handy A, Ingram DK, Kemnitz JW, Roth GS (1999b) Short-term calorie restriction improves disease-related markers in older male rhesus monkeys (Macaca mulatta). Mech Ageing Dev 112:185–196 [DOI] [PubMed]
- Lane MA, Black A, Handy AM, Shapses SA, Tilmont EM, Kiefer TL, Ingram DK, Roth GS (2001) Energy restriction does not alter bone mineral metabolism or reproductive cycling and hormones in female rhesus monkeys. J Nutr 131:820–827 [DOI] [PubMed]
- Larson-Cook KL, Brannian JD, Hansen KA, Kasperson KM, Aamold ET, Evenson DP (2003) Relationship between the outcomes of assisted reproductive techniques and sperm DNA fragmentation as measured by the sperm chromatin structure assay. Fertil Steril 80:895–902 [DOI] [PubMed]
- Lewis-Jones DI, Aird IA, Biljan MM, Kingsland CR (1996) Effects of sperm activity on zinc and fructose concentrations in seminal plasma. Hum Reprod 11:2465–2467 [DOI] [PubMed]
- Mattison JA, Roth GS, Ingram DK, Lane MA (2001) Endocrine effects of dietary restriction and aging: the national institute on aging study. J Anti-Aging Med 4:215–223 [DOI]
- Mattison JA, Lane MA, Roth GS, Ingram DK (2003) Calorie restriction in rhesus monkeys. Exp Gerontol 38:35–46 [DOI] [PubMed]
- Mattison JA, Black A, Huck J, Moscrip T, Handy A, Tilmont E, Roth GS, Lane MA, Ingram DK (2005) Age-related decline in caloric intake and motivation for food in rhesus monkeys. Neurobiol Aging 26:1117–1127 [DOI] [PubMed]
- McCay CM, Crowell MF, Maynard LA (1935) The effect of retarded growth upon the length of the life span and upon the ultimate body size. J Nutr 10:63–70 [PubMed]
- McShane TM, Wise PM (1996) Life-long moderate caloric restriction prolongs reproductive life span in rats without interrupting estrous cyclicity: effects on the gonadotropin-releasing hormone luteinizing hormone axis. Biol Reprod 54:70–75 [DOI] [PubMed]
- Merry BJ (2002) Molecular mechanisms linking calorie restriction and longevity. Int J Biochem Cell Biol 34:1340–1354 [DOI] [PubMed]
- Miller RA, Harper JM, Dysko RC, Durkee SJ, Austad SN (2002) Longer life spans and delayed maturation in wild-derived mice. Exp Biol Med 227:500–508 [DOI] [PubMed]
- Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM (2002) Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J Clin Endocrinol Metab 87:5001–5007 [DOI] [PubMed]
- Morrell JM (1997) Cryopreservation of marmoset sperm (Callithrix jacchus). Cryo-Lett 18:45–54
- Mortimer D (1994) Practical laboratory andrology. Oxford University Press, Inc., New York
- Oster H, Baeriswyl S, van der Horst GTJ, Albrecht U (2003) Loss of circadian rhythmicity in aging mPer1(-/-) mCry2(-/-) mutant mice. Genes Dev 17:1366–1379 [DOI] [PMC free article] [PubMed]
- Ottinger MA (1998) Male reproduction: testosterone, gonadotropins, and aging. In: Mobbs CV, Hof PR (eds) Interdisciplinary topics in gerontology: functional endocrinology of aging. Karger, New York
- Ottinger MA (2007) Neuroendocrine aging in birds: comparing lifespan differences and conserved mechanisms. Ageing Res Rev 6:46–53 [DOI] [PubMed]
- Ottinger MA, Reed E, Wu J, Thompson N, French JB (2003) Establishing appropriate measures for monitoring aging in birds: comparing short and long lived species. Exp Gerontol 38:747–750 [DOI] [PubMed]
- Ottinger MA, Abdelnabi M, Li Q, Chen K, Thompson N, Harada N, Viglietti-Panzica C, Panzica GC (2004) The Japanese quail: a model for studying reproductive aging of hypothalamic systems. Exp Gerontol 39:1679–1693 [DOI] [PubMed]
- Owen DH, Katz DF (2005) A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J Androl 26:459–469 [DOI] [PubMed]
- Packer C, Tatar M, Collins A (1998) Reproductive cessation in female mammals. Nature 392:807–811 [DOI] [PubMed]
- Plas E, Berger P, Hermann M, Pfluger H (2000) Effects of aging on male fertility. Exp Gerontol 35:543–551 [DOI] [PubMed]
- Platz CC Jr, Wildt DE, Bridges CH, Seager SW, Whitlock BS (1980) Electroejaculation and semen analysis in a male lowland gorilla, Gorilla gorilla gorilla. Primates 21:130–132 [DOI]
- Ramesh V, Ramachandra SG, Krishnamurthy HN, Rao AJ (1998) Electroejaculation and seminal parameters in bonnet monkeys (Macaca radiata). Andrologia 30:97–100 [DOI] [PubMed]
- Rosario ER, Carroll JC, Oddo S, LaFerla FM, Pike CJ (2006) Androgens regulate the development of neuropathology in a triple transgenic mouse model of Alzheimer’s disease. J Neurosci 26:13384–13389 [DOI] [PMC free article] [PubMed]
- Roth GS, Ingram DK, Lane MA (1999) Calorie restriction in primates: will it work and how will we know. J Am Geriatr Soc 47:896–903 [DOI] [PubMed]
- Roth GS, Ingram DK, Black A, Lane MA (2000) Effects of reduced energy intake on the biology of aging: the primate model. Eur J Clin Nutr 54:S15–S20 [DOI] [PubMed]
- Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D, Metter EJ (2002) Biomarkers of caloric restriction may predict longevity in humans. Science 297:811–811 [DOI] [PubMed]
- Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, Ingram DK (2004) Aging in rhesus monkeys: relevance to human health interventions. Science 305:1423–1426 [DOI] [PubMed]
- Rutllant J, Pommer AC, Meyers SA (2003) Osmotic tolerance limits and properties of rhesus monkey (Macaca mulatta) spermatozoa. J Androl 24:534–541 [DOI] [PubMed]
- Schaffer NE, McCarthy TJ, Fazleabas AT, Jeyendran RS (1992) Assessment of semen quality in a baboon (Papio anubis) breeding colony. J Med Primatol 21:47–48 [PubMed]
- Slama R, Bouyer J, Windham G, Fenster L, Werwatz A, Swan SH (2005) Influence of paternal age on the risk of spontaneous abortion. Am J Epidemiol 161:816–823 [DOI] [PubMed]
- Smucny DA, Allison DB, Ingram DK, Roth GS, Kemnitz JW, Kohama SG, Lane MA (2001) Changes in blood chemistry and hematology variables during aging in captive rhesus macaques (Macaca mulatta). J Med Primatol 30:161–173 [DOI] [PubMed]
- Stocco DM, Wang XJ (2006) The role of COX2 in steroidogenesis in the aging Leydig cell. 39th Annual Meeting of the Society for the Study of Reproduction, Omaha, NE,
- Syntin P, Chen HL, Zirkin BR, Robaire B (2001) Gene expression in brown Norway rat Leydig cells: effects of age and of age-related germ cell loss. Endocrinology 142:5277–5285 [DOI] [PubMed]
- Tesarik J, Mendoza-Tesarik R, Mendoza C (2006) Sperm nuclear DNA damage: update on the mechanism, diagnosis and treatment. Reprod Biomed Online 12:715–721 [DOI] [PubMed]
- Thacker PD (2004) Biological clock ticks for men, too: genetic defects linked to sperm of older fathers. JAMA 291:1683–1685 [DOI] [PubMed]
- Thompson HJ, Zhu ZJ, Jiang WQ (2002) Protection against cancer by energy restriction: all experimental approaches are not equal. J Nutr 132:1047–1049 [DOI] [PubMed]
- Tollner TL, Vandevoort CA, Overstreet JW, Drobnis EZ (1990) Cryopreservation of spermatozoa from cynomolgus monkeys (Macaca fascicularis). J Reprod Fertil 90:347–352 [DOI] [PubMed]
- Urbanski HF, Downs JL, Garyfallou VT, Mattison JA, Lane MA, Roth GS, Ingram DK (2004) Effect of caloric restriction on the 24-hour plasma DHEAS and cortisol profiles of young and old male rhesus macaques. Ann NY Acad Sci 1019:443–447 [DOI] [PubMed]
- Urbanski HF, Garyfallou VT, Lemos DR, Downs JL, Brown DI (2006) Expression profiling of genes in the testis of rhesus macaques during development and aging. Proceedings of the 8th European Congress of Endocrinology, Glasgow, 1–5 April 2006
- VandeVoort CA, Tollner TL, Overstreet JW (1992) Sperm zona pellucida interaction in cynomolgus and rhesus macaques. J Androl 13:428–432 [PubMed]
- VandeVoort CA, Neville LE, Tollner TL, Field LP (1993) Noninvasive semen collection from an adult orangutan. Zoo Biol 12:257–265 [DOI]
- VandeVoort CA, Tollner TL, Overstreet JW (1994) Separate effects of caffeine and dbcAMP on macaque sperm motility and interaction with the zona pellucida. Mol Reprod Dev 37:299–304 [DOI] [PubMed]
- VandeVoort CA, Yudin AI, Overstreet JW (1997) Interaction of acrosome-reacted macaque sperm with the macaque zona pellucida. Biol Reprod 56:1307–1316 [DOI] [PubMed]
- Weindruch R, Walford RL (1988) The retardation of aging and disease by dietary restriction. Charles C. Thomas, Springfield
- Weindruch R, Sohal RS (1997) Caloric intake and aging. N Engl J Med 337:986–994 [DOI] [PMC free article] [PubMed]
- Weinert BT, Timiras PS (2003) Theories of aging. J Appl Physiol 95:1706–1716 [DOI] [PubMed]
- Wrobel G, Primig M (2005) Mammalian male germ cells are fertile ground for expression profiling of sexual reproduction. Reproduction 129:1–7 [DOI] [PubMed]
- Wu JM (2006) Effects of moderate calorie restriction on ovarian function and decline in rhesus monkeys. Animal and Avian Sciences, University of Maryland:235
- Wu JM, Zelinski MB, Ingram DK, Ottinger MA (2005) Ovarian aging and menopause: current theories, hypotheses, and research models. Exp Biol Med 230:818–828 [DOI] [PubMed]
- Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD (2002) Effects of aging on central and peripheral mammalian clocks. 99:10801–10806 [DOI] [PMC free article] [PubMed]
- Yanagimachi R (1994) Mammalian fertilization. In: Knobil E, Neill JD, Greenwald GS, Markert CL, Pfaff DW (eds) The physiology of reproduction, 2nd edn. Raven Press, New York
- Yeoman RR, Ricker RB, Williams LE (1997) Vibrostimulation of ejaculation yields increased motile spermatozoa compared with electroejaculation in squirrel monkeys (Saimiri boliviensis). Contemp Topics (AALAS) 35:62–64 [PubMed]
- Zirkin BR (2006) Steroidogenic decline in aging Leydig cells: the roles of cAMP and reactive oxygen-induced damage. The 39th Annual Meeting of the Society for the Study of Reproduction, Omaha, NE
- Zirkin BR, Chen HL (2000) Regulation of Leydig cell steroidogenic function during aging. Biol Reprod 63:977–981 [DOI] [PubMed]
- Zubkova EV, Robaire B (2006) Effects of ageing on spermatozoal chromatin and its sensitivity to in vivo and in vitro oxidative challenge in the Brown Norway rat. Human Reprod 21:2901–2910 [DOI] [PubMed]