Abstract
Manganese chloride (MnCl2) is capable of stimulating luteinizing hormone releasing hormone (LHRH) secretion in adult male Sprague-Dawley rats through the activation of the hypothalamic nitric oxide/cyclic guanosine monophosphate (cGMP)/protein kinase G pathway. The present study aimed to determine the involvement of specific neurotransmitters involved in this action. Our results indicate that dopamine, but not glutamic acid and prostaglandinds, mediates the MnCl2 stimulated secretion of LHRH from medial basal hypothalami in vitro, as well as increases the activity of nitric oxide synthase. Furthermore, a biphasic response was observed in that gamma aminobutyric acid (GABA) release was also increased, which acts to attenuate the MnCl2 action to stimulate LHRH secretion. Although it is clear that manganese (Mn+2) can acutely induce LHRH secretion in adult males, we suggest that the additional action of MnCl2 to release GABA, a LHRH inhibitor, may ultimately contribute to suppressed reproductive function observed in adult animals following exposure to high chromic levels of Mn+2.
Keywords: luteinizing hormone, prolactin, dopamine, gamma aminobutyric acid, glutamic acid, prostaglandin E, nitric oxide
Manganese (Mn+2) is an essential metal which acts as a cofactor for many enzymes and therefore, plays important biological functions (Keen et al., 1984). Nevertheless, high doses of Mn+2 exert toxic effects in the brain (Yamada et al., 1986) and the accumulation of Mn+2 in the basal ganglia produces an irreversible neurological syndrome similar to Parkinson's disease (Cotzias, 1958; Mena, 1974). High levels of this metal can cause alterations in development as well as reproductive dysfunction (Grey and Laskey, 1980; Laskey et al., 1982), and Mn+2 deficiency produces impairment of growth and reproduction in rats of both sexes (Boyer et al., 1942; Smith et al., 1944). It was reported that chronic administration of Mn+2 at low doses to female rats resulted in increased serum levels of puberty-related hormones such as luteinizing hormone (LH), follicle-stimulating hormone (FSH), and estradiol, and advanced the time of vaginal opening (Pine et al., 2005). Also, it was found to accelerate daily sperm production and efficiency of spermatogenesis in prepubertal males (Lee et al., 2006). These effects were explained by a hypothalamic action of the metal that facilitates the secretion of prepubertal luteinizing hormone releasing hormone (LHRH) in both sexes (Lee et al., 2006, 2007; Pine et al., 2005).
The LHRH released from the hypothalamus into capillaries of the hypophyseal portal vessels is carried to the hypophyseal gonadotropes where it stimulates the release of LH and FSH (McCann, 1982). Glutamic acid (GA) and catecholamines are important neurotransmitters involved in LHRH release (Rettori et al., 1994; Ojeda et al., 1979). GA stimulates the noradrenergic neuronal terminals resulting in the release of norepinephrine (NE). This in turn activates the α1-adrenergic receptors located on nitridergic neurons, thus, increasing intracellular calcium (Ca+2), which activates nitric oxide synthase (NOS) and leads to production of nitric oxide (NO) (Rettori et al., 1994). NO diffuses and activates soluble guanylate cyclase, therefore, generating cyclic guanosine monophosphate (cGMP) which causes an activation of protein kinase G (PKG) and the exocytosis of LHRH (Karanth et al., 2004). We have shown that MnCl2 increases LHRH secretion from medial basal hypothalamus (MBH), at least in part, by activating the NO/cGMP/PKG dependent pathway in adult male rats (Prestifilippo et al., 2007). Another pathway that can also be activated by NO is cyclooxygenase (COX). Activation of this pathway results in the production of prostaglandin E2 (PGE2), which, through adenylate cyclase, is capable of increasing cAMP/protein kinase A (PKA) and thus, induces exocytosis of LHRH (Rettori et al., 1992, 1993). Thus, one of the aims of the present study is to discern whether MnCl2 can also affect this COX signaling pathway.
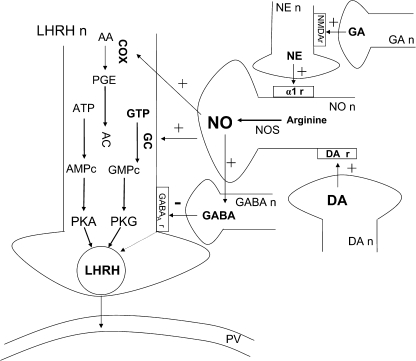
In order to better understand the mechanism of the Mn+2 action to influence the above pathway(s), it is important to analyze potential actions and interactions between Mn+2 and specific neurotransmitters. Interestingly, stimulation of dopamine (DA) type 2 receptors causes an increase in intracellular Ca+2, which activates the NOS/NO system (McCleskey et al., 1987; Melis et al., 1994), resulting in increased LHRH release. Furthermore, NO is also capable of inducing the release of gamma aminobutyric acid (GABA), a recognized inhibitory neurotransmitter of LHRH release (Seilicovich et al., 1995). Therefore, assessing potential interactions between these two neurotransmitter systems may help discern more about how this metal affects reproductive hormone secretion. (See schematic diagram of the interactions between different neurotransmitters that could take place and participate in LHRH release, Fig. 1.)
FIG. 1.
Schematic diagram of the interactions between different neurotransmitters and LHRH release. For explanation, see Introductory section. Luteinizing hormone releasing hormone neuron, LHRHn; dopamine neuron, DA n; GABAA receptor, GABAAr; glutamic acid neuron, GA n; NMDA receptor, NMDA r; norepinephrine neuron, NE n; α1-adrenergic receptors, α1-r; guanylate cyclase, GC; GTP; cGMP; adenylate cyclase, AC; ATP; cAMP; arachidonic acid, AA; portal vessel, PV; +, stimulation or increase; −, inhibition or decrease.
MATERIAL AND METHODS
Animals.
Male rats of the Sprague-Dawley strain (220–250 g) were kept in group cages in an animal room having a photoperiod of 14 h of light (05.00–19.00 h) and a temperature of 22°C–24°C. Animals had free access to laboratory chow and tap water. The experimental procedures reported here were approved by the Animal Care Committee of the Center of Pharmacology and Botanical Studies of the National Council for Research of Argentina and carried out in accord with the guidelines of the National Institutes of Health.
In vivo studies.
The rats were implanted with a cannula into the lateral cerebral ventricle, under tribromoethanol (3.5%, 1 ml/100 g animal body weight, i.p.) anesthesia, using a stereotaxic instrument and coordinates from the atlas. The correct localization of the cannula in the ventricle was confirmed at the end of the experiment. The experiments were performed a week after the implantation of the cannula. Twenty-four hours before the experiment, an indwelling catheter was placed into the right external jugular vein and advanced to the right atrium for the collection of blood samples. The day of experiment, conscious, freely moving rats were divided into two groups of 10 animals each. After the collection of the first blood sample (0.5 ml), the rats were microinjected intracerebroventricularly (i.c.v.) during 1 min with 5 μl of sterile saline (control group) or 10 μg of MnCl2/5 μl sterile saline. Every 30 min thereafter, blood samples (0.5 ml) were obtained until 120 min following the i.c.v. injections. Each blood sample was immediately replaced with 0.5 ml of saline containing 50 IU of heparin/ml. After centrifugation, the plasma was stored frozen at –20°C until the determination of plasma LH and prolactin by radioimmunoassay (RIA).
In vitro studies.
After decapitation and removal of the brains, the MBH were dissected by making frontal cuts just behind the optic chiasm, extending dorsally 1.0 mm, a horizontal cut extended from this point caudally to just behind the pituitary stalk. Longitudinal cuts were made 1.0 mm lateral to the midline bilaterally. All incubations were carried out in a Dubnoff shaker (50 cycles per min; 95% O2/5% CO2) at 37°C. The hypothalami (seven to eight for each group) were preincubated individually in glass tubes in 500ul of Krebs-Ringer bicarbonate-buffered medium (NaCl 124.40mM, KCl 4.98mM, NaHCO3 24.88mM, CaCl2 1.50mM, MgCl2 1.42mM, KH2PO4 1.25mM containing 0.1% glucose, pH: 7.4). After this preincubation (15 min) the medium was discarded and replaced with fresh medium alone or containing the substances to be tested. The incubation continued for 30 min. At the end of the incubation period the media were removed and the tissues were homogenized and submitted to appropriate extraction procedure and stored at −20°C until the respective assays were conducted.
Radioimmunoassays.
Plasma LH and prolactin levels were measured by RIA. Rat LH antiserum (NIDDK-anti-rLH-S-II), antigen (NIDDK-rLH-I-9), and reference preparation (NIDDK-rLH-RP-3); and rat PRL antiserum (NIDDK-anti-rPRL-S-9), and reference preparation (NIDDK-rPRL-RP-3) were purchased from the NIH Pituitary Hormones and Antisera Center, Harbor, UCLA, Medical Center, Torrance, CA. The interassay variations for these assays were 6.6% and the intra-assay variations were 3.6%. All samples were measured in duplicate and the results were expressed as ng of LH or prolactin per ml of plasma. LHRH release into the incubation media was measured by RIA utilizing a highly specific LHRH antiserum kindly provided by Ayala Barnea (University of Texas Southwestern Medical Center, Dallas, TX). The intra-assay coefficient of variation ranged from 7.3% and the interassay coefficient of variation was 8.9%. The sensitivity of the assay was 0.2 pg/100 μl. The data were expressed as pg LHRH released/mg protein/MBH.
To determine hypothalamic PGE content, tissues were homogenized individually in 1.5 ml of ice cold ethanol 100%, centrifuged at 10,000 × g for 15 min at 4°C and the supernatant collected and evaporated in a Speed-Vac. Medium were extracted by tree fold of ethyl acetate and evaporated in a Speed-Vac. The residues were resuspended with RIA buffer and the determination of PGE by RIA was performed by using a rabbit antiserum from Sigma Chemical Co. (St Louis, MO). The intra-assay and interassay coefficients of variation for PGE were 8.2 and 12%, respectively. The results were expressed as pg of PGE contained per MBH.
Determination of DA release.
DA was extracted from the incubation media, where the MBH were incubated, with Tris-HCl buffer (2M, pH:8,7) and 3,4-dihydroxybenzylamine hydrobromide (100 ng/ml), as internal standard and dehydrated alumina. After shaking for 10 min, samples were centrifuged at 19,000 × g for 10 min. Pellets were washed three times with deionized water, centrifuged and 200 μl of H3PO4 (0.1M) was added. After shaking for 2 min, samples were centrifuged and supernatants from the media were filtered and injected in an analytical column (Luna 5 μ C-18, 4.6 × 250 mm, Phenomenex) maintained at 37°C. DA was determined by high performance liquid chromatography with electrochemical detection and registered with an integrator. The mobile phase was prepared with NaH2PO4 (100mM), heptanesulfonic acid (1mM), ethylenediaminetetraacetic acid (0.5mM), and 6% acetonitrile, pH: 3.0. Quantification and recovery calculation was performed using the Gilson 712 System Controller Software. The data were expressed as ng of DA released per mg of protein/MBH.
Determination of NOS activity.
Determination of NOS activity was performed by a modification (Canteros et al., 1995) of the 14C-arginine method of Bredt and Snyder (1989). After the incubation period (30 min) the MBH were immediately homogenized in 0.5 ml of N-(2-hydroxyethyl)-piperazine-N′-2-ethanesulfonic acid (HEPES) (20mM, pH: 7.4) with addition of CaCl2 (1.25mM) and DL-dithiothreitol (DTT, 1mM). The reaction was started by adding NADPH (nicotinamide adenine dinucleotide phosphate, reduced) (120μM) and 200,000 dpm of 14C-arginine (360 mCi/mmol) to the homogenates. The tubes were incubated for 15 min at 37°C in a Dubnoff metabolic shaker (50 cycles per min and 95%O2/5%CO2 atmosphere). At the end of this incubation period, the tubes were immediately centrifuged at 10,000 × g for 10 min at 4°C. The supernatants were immediately applied to individual columns containing 1 ml of Dowex AG 50 W-X8 200 mesh sodium form, and washed with 2.0 ml of double distilled water. All collected fluid from each column was counted for 14C-citrulline activity in a scintillation counter. Because NOS converts arginine into equimolar quantities of citrulline and NO, the data were expressed as pmol of NO produced per MBH per min.
Measurements of radioconversion of 14C-arachidonic acid to prostanoids.
These measurements were performed by chromatography of ethyl acetate extracts and counting of the labeled prostanoids as previously reported (Franchi et al., 1994). The area of each of the radioactive peaks corresponding to authentic prostanoids was calculated and expressed as a percentage of the total radioactivity of the plates per MBH.
Determination of GABA and GA release.
The concentration of GABA and GA were determined after derivatization with phenylisothiocyanate by high performance liquid chromatography and UV detection at 254 nm, as previously described (Jarry et al., 1992). The drugs used did not interfere with the derivatization process. The mobile phase was prepared with sodium acetate buffer (0.57M) and 10% acetonitrile, pH: 6.5. The data were expressed as pmol per mg of protein/MBH.
Chemicals.
LHRH for iodination and standards were purchased from Peninsula Laboratories, Inc., Division of Bachem (San Carlos, CA). Iodine-125 for iodination was purchased from New England Nuclear Life Science Product (Boston, MA). Manganese chloride (MnCl2) was purchased from Anedra (San Fernando, Buenos Aires, Argentina). HEPES, DTT, NADPH, Haloperidol, and GABA were purchased from Sigma Aldrich (St Louis, MO). Dowex AG 50 W-X8 200-400 mesh sodium form was obtained from Bio-Rad (Hercules, CA), and the 14C-arginine-monohydrochloride 360 mCi/mmol and 14C-arachidonic acid 56 μCi/mmol were from Amersham Pharmacia (Buckinghamshire, HP, UK).
Statistical analysis.
Experiments were repeated at least twice employing seven to eight animals per group in each experiment. All data are expressed as the mean ± SEM. Comparisons between groups were performed by using a one-way ANOVA followed by the Student-Newman-Keuls multiple comparison test for unequal replicates. Student's t-test was used when comparing two groups. Differences with p values < 0.05 were considered significant.
RESULTS
Effect of MnCl2 on Plasma LH Levels
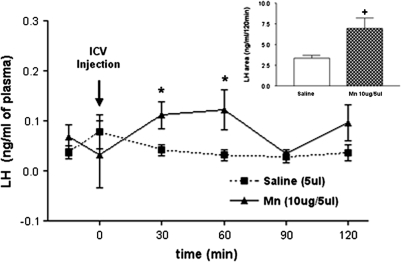
We have previously reported that MnCl2 can cause LHRH release from the MBH in adult male rats (Prestifilippo et al., 2007) and this peptide controls the pituitary secretion of LH and FSH. In order to determine the in vivo effect of MnCl2 on plasma LH levels, rats were stereotaxically implanted with a cannula into the lateral cerebral ventricle and received a single i.c.v. injection of MnCl2 (10 μg/5 μl saline). Results show that MnCl2 increased the plasma LH levels at 30 min (p < 0.05) postinjection. The hormone remained elevated at 60 min (p < 0.05), then declined to preinjection levels by 90-min postinjection (Fig. 2). Control rats injected with saline did not show a change in LH secretion at any of the time points. Calculation of the areas under the respective curves of plasma LH for 120 min further demonstrated the MnCl2-induced increase (p < 0.05) in plasma LH levels (Fig. 2, inset).
FIG. 2.
Effect of saline or MnCl2 on plasma LH levels measured every 30 min for 120 min. Note the increased secretion of LH during the first 60 min in the MnCl2-treated rats. The inset depicts the overall difference between the areas under the respective curves of LH secretion. *p < 0.05 and +p < 0.05 vs. saline injection. Values represent mean ± SEM.
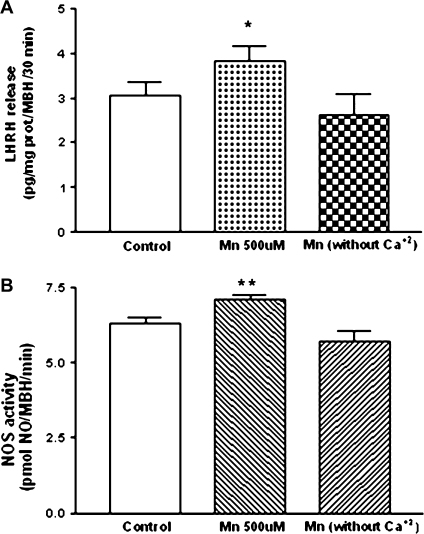
Role of CaCl2 in MnCl2-Induced LHRH Release and NOS Activity
In order to evaluate whether MnCl2 is able to replace calcium, we assessed the effect of MnCl2 in vitro on LHRH release determined by RIA and NOS activation assessed by the conversion of 14C-arginine into 14C-citrulline and NO in the presence or absence of CaCl2 in the incubation medium. MnCl2 (500μM) increased LHRH release (p < 0.05; Fig. 3A) and NOS activity (p < 0.01; Fig. 3B) in the presence of CaCl2 (1.25mM). These effects were abolished in calcium free medium (Figs. 3A and 3B).
FIG. 3.
Effect of MnCl2 on LHRH release (A) and NOS activity (B) from MBH incubated in medium containing Ca+2 (1.25mM) or incubated in Ca+2 free medium. Note that the MnCl2-stimulated increases in both LHRH and NOS activity did not occur in absence of Ca+2 in the incubation medium. *p < 0.05 and **p < 0.01 versus respective control. Values represent mean ± SEM.
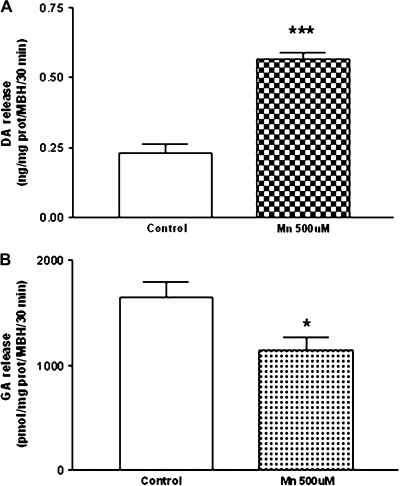
Effect of MnCl2 on DA and GA Release from MBH
The control of LHRH release is exerted by different neurotransmitters including DA and GA. Therefore, with the purpose of determining whether MnCl2 could affect their secretion, we evaluated the release of DA and GA from the MBH by high-performance liquid chromatography (HPLC). Results indicate that MnCl2 (500μM) caused a marked increase (p < 0.001) in DA release during a 30 min incubation (Fig. 4A). Conversely, at the same time we observed a decrease (p < 0.05) in the release of GA (Fig. 4B).
FIG. 4.
Effect of MnCl2 on DA (A) and GA (B) release from MBH in vitro. These results depict opposite effects of MnCl2 on DA and GA secretion. *p < 0.05 and ***p < 0.001 versus control. Values represent mean ± SEM.
Participation of DA in the MnCl2-Induced Release of LHRH
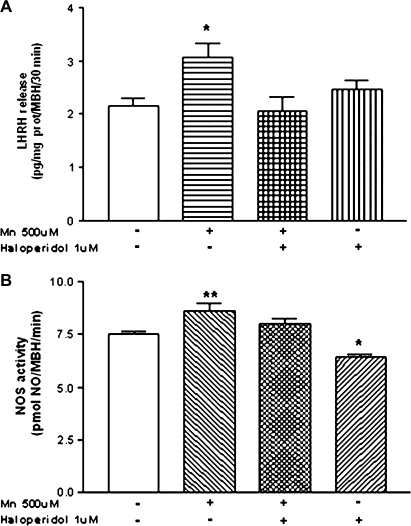
In order to demonstrate that the augmentation in DA release exerted by MnCl2 is involved in LHRH secretion, we assessed the effect of haloperidol, a well-known DA receptor antagonist. Our results indicate that haloperidol (1μM) blocked the MnCl2-stimulated LHRH release from the MBH and did not alter the basal release of LHRH (p < 0.05; Fig. 5A). In addition, the MnCl2 stimulation of NOS activity was blocked by haloperidol (p < 0.01; Fig. 5B), and there was a modest reduction in NOS activity noted in tissues incubated in haloperidol alone (p < 0.05; Fig. 5B).
FIG. 5.
Effect of haloperidol, a DA receptor antagonist, on MnCl2-induced LHRH release (A) and NOS activity (B) from MBH in vitro. Note that the haloperidol blocked both of the actions induced by MnCl2. *p < 0.05 and **p < 0.01 versus control (open bars). Values represent mean ± SEM. The − or + symbols indicate the absence or presence of MnCl2 and haloperidol in the incubation medium.
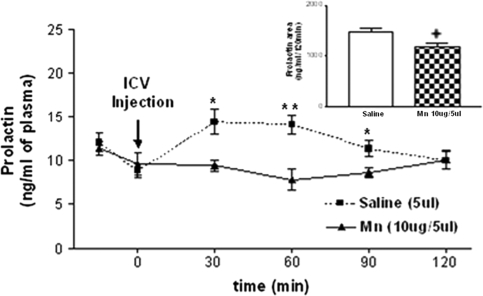
Effect of MnCl2 on Plasma Prolactin Levels
It is well documented that hypothalamic DA inhibits prolactin release from the pituitary gland. To further demonstrate the involvement of MnCl2 on DA release, we evaluated the in vivo effect of MnCl2 on plasma prolactin levels determined by RIA. One injection of MnCl2 (10 μg/5 μl saline, i.c.v.) reduced plasma prolactin levels compared with saline-injected controls at 30 (p < 0.05), 60 (p < 0.01), and 90 min (p < 0.05), returning to basal levels at 120 min. (Fig. 6). The area under the 120 min curve of prolactin secretion confirmed that MnCl2 caused a decrease in prolactin levels (p < 0.05) compared with the saline-treated group (Fig. 6, inset).
FIG. 6.
Effect of saline or MnCl2 on plasma prolactin levels measured every 30 min for 120 min. Note the decreased prolactin plasma levels between 30 and 90 min in the MnCl2-treated rats. The inset depicts the overall difference between the areas under the respective curves prolactin. *p < 0.05; +p < 0.05, and **p < 0.01 versus saline injection. Values represent mean ± SEM.
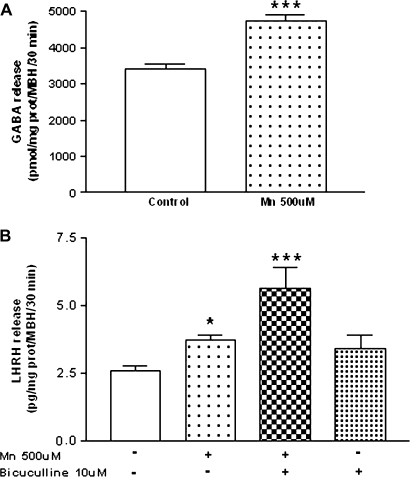
Actions and Interactions of MnCl2 and GABA on LHRH Release
The LHRH release can be inhibited by GABA. Therefore, we studied the effect of MnCl2 on GABA release from the MBH by HPLC. Results demonstrate that MnCl2 increased (p < 0.001) GABA release (Fig. 7A). Because GABA inhibits LHRH release, we next studied the effect of MnCl2 in the presence of bicuculline (10μM), a GABAA receptor antagonist, on LHRH release. Bicuculline enhanced MnCl2-induced LHRH release (p < 0.001) but had no effect on basal secretion (Fig. 7B), suggesting that GABA attenuates the stimulation of LHRH release induced by MnCl2.
FIG. 7.
Effect of MnCl2 on GABA release (A) and the action of bicuculline (B), a GABAA receptor antagonist, on MnCl2-stimulated LHRH release from MBH in vitro. Note that both GABA and LHRH release were increased by MnCl2, and that bicuculline enhanced MnCl2-induced LHRH secretion but had no effect when incubated alone. *p < 0.05, and ***p < 0.001 versus control. Values represent mean ± SEM. The − or + symbols indicate the absence or presence of MnCl2 and bicuculline in the incubation medium.
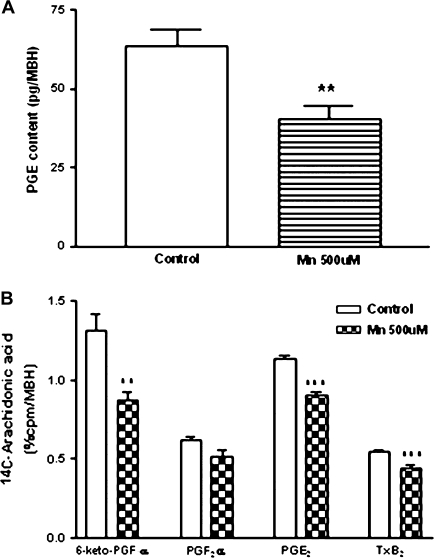
Effect of MnCl2 on Hypothalamic Prostaglandins Involved in LHRH Release
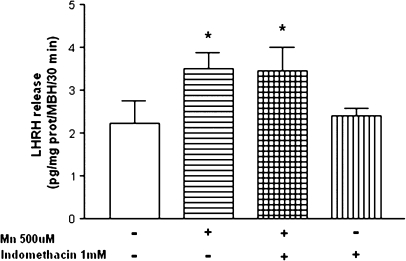
We have demonstrated that the hypothalamic content of PGE was decreased (p < 0.01) following exposure to MnCl2 in vitro (Fig. 8A). Coincidently, MnCl2 inhibited hypothalamic COX activity measured by the radioconversion assay of 14C-arachidonic acid to 14C-labeled prostanoids. MnCl2 diminished 14C-6-keto-PGF1α (p < 0.01), 14C-PGE2 (p < 0.001), 14C-tromboxane (T×B2, p < 0.001), and did not change 14C-PGF2α as compared with control groups (Fig. 8B). Furthermore, the inhibition of COX by indomethacin did not block the MnCl2-induced increase in LHRH release (p < 0.05; Fig. 9). Collectively, these results suggest that the PGE/cAMP/PKA pathway does not participate in the release of LHRH induced by MnCl2.
FIG. 8.
Effect of MnCl2 on PGE content (A) and COX activity (B) in MBH in vitro. Note that MnCl2 decreased PGE content and inhibited COX activity. **p < 0.01 and ***p < 0.001 versus respective control. Values represent mean ± SEM.
FIG. 9.
Effect of a COX inhibitor, Indomethacin, on MnCl2-induced LHRH release in MBH in vitro. Note that indomethicin did not alter MnCl2-stimulated LHRH release. *p < 0.05 versus control. Values represent mean ± SEM. The − or + symbols indicate the absence or presence of MnCl2 and indomethacin in the incubation medium.
DISCUSSION
The results of the present study demonstrate that MnCl2 stimulates LHRH release from MBH. This acute stimulatory effect on the in vitro release of LHRH was supported by the increase in plasma LH levels shown in vivo after a single i.c.v. injection of MnCl2. Also, MnCl2 is capable of stimulating DA secretion and NOS activity in the MBH. Because it was shown that DA can induce NOS/NO (Melis et al., 1994, 1996), and based on the important role of NO in the control of LHRH (Rettori et al., 1993) this suggests that DA/NO activation may mediate the MnCl2-stimulated secretion of LHRH. Previously, DA in low concentrations has been shown to enhance LHRH release from MBH of male rats in vitro (Negro-Vilar et al., 1979). Also, it was shown recently that MnCl2 stimulates LHRH release from prepubertal female (Pine et al., 2005) and male (Lee et al., 2006) rats, and that this was due to the activation of guanylyl cyclase and subsequent stimulation of the cGMP/PKG pathway (Lee et al., 2007).
In the present study, we assessed whether DA/NO interactions were involved in the MnCl2-induced release of LHRH. Support for the involvement of this pathway was revealed by the fact that the specific DA receptor antagonist, haloperidol, blocked the stimulatory effect of MnCl2 on LHRH release and NOS activity. Interestingly, it has been shown that autoxidation of DA is potentiated by Mn+2 in comparison to other biologically important divalent cations, suggesting that this autoxidation of DA is associated with increased generation of free radicals (Donaldson et al., 1981).
Further evidence for a DA involvement regarding hypothalamic actions of MnCl2 is supported by what is known about DA release and its effect on prolactin secretion from the anterior pituitary gland. DA is released from the nerve terminals of the tuberoinfundibular dopaminergic neurons of the hypothalamus and acts on D2 receptors located on pituitary lactotrophs to inhibit prolactin release (Neill and Nagy, 1994). In this regard, we showed that MnCl2 stimulated DA release from hypothalami incubated in vitro, and that plasma prolactin levels were markedly decreased following the central administration of the metal. Interestingly, with regard to DA release, it has been shown that the MnCl2-induced increase in DA release from striatal synaptosomes is due to its permeation through presynaptic voltage-dependent Ca+2 channels, suggesting that Mn+2 may be a potential substitute for Ca+2 in the exocytotic process (Drapeau and Nachshen, 1984). However, our results using hypothalamic tissue indicate that this was not the case, because without Ca+2 in the incubation medium, MnCl2 was ineffective in stimulating both LHRH release and NOS activity.
Prostaglandins can act as cellular messengers in LHRH release in response to NE (Ojeda et al., 1982; Rettori et al., 1992). In our experiments, MnCl2 decreased COX activity, as well as hypothalamic PGE content. Also, we found that MnCl2 decreased the release of GA, as described similarly in the hippocampus (Takeda et al., 2003). We have shown previously that GA stimulates LHRH release via the NE/NO pathway (Kamat et al., 1995). Thus, our results showing that MnCl2 caused significant decreases in hypothalamic GA and PGEs demonstrated that this pathway is not involved in Mn+2 control of LHRH secretion.
In addition to revealing a MnCl2 action to stimulate DA-induced LHRH release, we have also uncovered an action of the metal on the inhibitory transmitter, GABA. This is an important inhibitory neurotransmitter involved in LHRH secretion and we previously reported that NO stimulates release of GABA from MBH in vitro in order to attenuate the pulsatile secretion of LHRH driven by NO (Seilicovich et al., 1995). In the present work, we observed that MnCl2 stimulated GABA release from MBH in vitro. The evidence for attenuation of LHRH release by GABA was confirmed when hypothalamic fragments were incubated with MnCl2 in the presence or absence of bicuculline, a GABAA receptor blocker. This showed that when the GABA action was blocked, an enhanced release of LHRH occurred.
Although more research is needed, the information attained thus far suggests that MnCl2 can cause differential effects on reproductive systems depending upon the dose of the metal in relation to the sex and age of the animals at the time of exposure. We showed previously that MnCl2 stimulates LHRH release in immature female rats by a NO-independent mechanism. In this regard, the MnCl2 directly activated guanylyl cyclase at a dose which was ten fold less than that used in the adult males from the present study (Lee et al., 2007). Furthermore, chronic low dose administration of MnCl2 continued to release LHRH/LH in immature female and male rats, eventually causing precocious pubertal development (Lee et al., 2006; Pine et al., 2005). Taken together, these results prompted us to suggest that although MnCl2 may play a beneficial role to stimulate LHRH release at the time of normal puberty, it could also be harmful in that it may cause precocious pubertal development if an individual is exposed to low but elevated levels of the metal too early in life. Interestingly, it has also been shown that exposure to high levels of the metal from gestation to young adulthood can suppress growth and development of the reproductive system (Grey and Laskey, 1980; Laskey et al., 1982). In the present study, we observed that the higher dose of MnCl2 required to stimulate LHRH release in adult males was via activation of NO, and hence, it also caused a biphasic effect to release GABA. This biphasic effect of MnCl2 to stimulate LHRH release, yet has the ability to release GABA in adult male rats is of potential importance. GABA has been shown to be inhibitory on LHRH release in adult (Seilicovich et al., 1995), but not prepubertal rats (Feleder et al., 1996; Scacchi et al., 1998). Thus, although it is clear that MnCl2 acutely induces LHRH release, we suggest that the ability of the metal to induce GABA secretion in adult males may represent a neurotoxic effect to ultimately inhibit LHRH secretion, perhaps following a more prolonged exposure. This may provide an explanation for reports showing an overall suppression of reproductive function in adults following chronic exposure to high doses of MnCl2 (Boyer et al., 1942; Grey and Laskey, 1980; Laskey et al., 1982; Smith et al., 1944).
FUNDING
Agencia Nacional de Promoción Científica Tecnológica (BID 1728 OC-AR PICT 03/14264); the Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 6149); and the National Institutes of Health (ESO-13143 and ESO-9106).
Acknowledgments
We are greatly indebted to Ricardo Horacio Orzuza for technical assistance and Ana Ines Casella for her administrative assistance.
References
- Boyer PH, Shaw JH, Phillips PH. Studies on manganese deficiency in the rat. J. Biol. Chem. 1942;143:417–425. [Google Scholar]
- Bredt DS, Snyder SH. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc. Natl. Acad. Sci. U. S. A. 1989;86:9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteros G, Rettori V, Franchi A, Genaro A, Cebral E, Faletti A, Gimeno M, McCann SM. Ethanol inhibits luteinizing hormone-releasing hormone (LHRH) secretion by blocking the response of LHRH neuronal terminals to nitric oxide. Proc. Natl. Acad. Sci. U. S. A. 1995;92:3416–3420. doi: 10.1073/pnas.92.8.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotzias GC. Manganese in health and disease. Physiol. Rev. 1958;38:503–532. doi: 10.1152/physrev.1958.38.3.503. [DOI] [PubMed] [Google Scholar]
- Donaldson J, LaBella FS, Gesser D. Enhanced autoxidation of dopamine as a possible basis of manganese neurotoxicity. Neurotoxicology. 1981;2:53–64. [PubMed] [Google Scholar]
- Drapeau P, Nachshen DA. Manganese fluxes and manganese-dependent neurotransmitter release in presynaptic nerve endings isolated from rat brain. J. Physiol. 1984;348:493–510. doi: 10.1113/jphysiol.1984.sp015121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feleder C, Jarry H, Leonhardt S, Wuttke W, Moguilevsky J. The GABAergic control of gonadotropin-releasing hormone secretion in male rats during sexual maturation involves effects on hypothalamic excitatory and inhibitory amino acid systems. Neuroendocrinology. 1996;64:305–312. doi: 10.1159/000127133. [DOI] [PubMed] [Google Scholar]
- Franchi AM, Chaud M, Rettori V, Suburo A, McCann SM, Gimeno M. Role of nitric oxide in eicosanoid synthesis and uterine motility in estrogen-treated rat uteri. Proc. Natl. Acad. Sci. U. S. A. 1994;91:539–543. doi: 10.1073/pnas.91.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey LE, Laskey JW. Multivariate analysis of the effects of manganese on the reproductive physiology and behavior of the male house mouse. J. Toxicol. Environ. Health. 1980;6:861–867. doi: 10.1080/15287398009529904. [DOI] [PubMed] [Google Scholar]
- Jarry H, Hirsch B, Leonhardt S, Wuttke W. Amino acid neurotransmitter release in the preoptic area of rats during the positive feedback actions of estradiol on LH release. Neuroendocrinology. 1992;56:133–140. doi: 10.1159/000126220. [DOI] [PubMed] [Google Scholar]
- Kamat A, Yu WH, Rettori V, McCann SM. Glutamic acid induces luteinizing hormone releasing hormone release via alpha receptors. Brain Res. Bull. 1995;37:233–235. doi: 10.1016/0361-9230(94)00280-e. [DOI] [PubMed] [Google Scholar]
- Karanth S, Yu WH, Mastronardi CA, McCann SM. Inhibition of stimulated ascorbic acid and luteinizing hormone-releasing hormone release by nitric oxide synthase or guanyl cyclase inhibitors. Exp. Biol. Med. (Maywood) 2004;229:72–79. doi: 10.1177/153537020422900109. [DOI] [PubMed] [Google Scholar]
- Keen CL, Lönnerdal B, Hurley LS. Manganese. In: Frieden E, editor. Biochemistry of the Essential Ultratrace Elements. New York: Plenum Publishing Co.; 1984. pp. 89–132. [Google Scholar]
- Laskey JW, Rehnberg JF, Hein JF. Effects of chronic manganese exposure on selected reproductive parameters. J. Toxicol. Environ. Health. 1982;9:677–687. doi: 10.1080/15287398209530195. [DOI] [PubMed] [Google Scholar]
- Lee B, Hiney JK, Pine MD, Srivastava VK, Dees WL. Manganese stimulates luteinizing hormone releasing hormone secretion in prepubertal female rats: Hypothalamic site and mechanism of action. J. Physiol. 2007;578:765–772. doi: 10.1113/jphysiol.2006.123083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Pine M, Johnson L, Rettori V, Hiney JK, Dees WL. Manganese acts centrally to activate reproductive hormone secretion and pubertal development in male rats. Reprod. Toxicol. 2006;22:580–585. doi: 10.1016/j.reprotox.2006.03.011. [DOI] [PubMed] [Google Scholar]
- McCann SM. Physiology and pharmacology of LHRH and somatostatin. Annu. Rev. Pharmacol. Toxicol. 1982;22:491–515. doi: 10.1146/annurev.pa.22.040182.002423. [DOI] [PubMed] [Google Scholar]
- McCleskey EW, Fox AP, Feldman DH, Cruz LJ, Olivera BM, Tsien RW, Yoshikami D. v-Conotoxin: Direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Prot. Natl. Acad. Sci. U. S. A. 1987;84:4327–4331. doi: 10.1073/pnas.84.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis MR, Stancampiano R, Argiolas A. Prevention by NGnitro-L-arginine methyl ester of apomorphine- and oxytocin-induced penile erection and yawning: Site of action in the brain. Pharmacol. Biochem. Behav. 1994;48:799–804. doi: 10.1016/0091-3057(94)90349-2. [DOI] [PubMed] [Google Scholar]
- Melis MR, Succu S, Argiolas A. Dopamine agonists increase nitric oxide production in the paraventricular nucleus of the hypothalamus: Correlation with penile erection and yawning. Eur. J. Neurosci. 1996;8:2056–2063. doi: 10.1111/j.1460-9568.1996.tb00725.x. [DOI] [PubMed] [Google Scholar]
- Mena I. The role of manganese in human disease. Ann. Clin. Chem. 1974;214:489–495. [PubMed] [Google Scholar]
- Negro-Vilar A, Ojeda SR, McCann SM. Catecolaminergic modulate of luteinizing hormone-releasing hormone release by median eminence terminals in vitro. Endocrinology. 1979;104:1749–1757. doi: 10.1210/endo-104-6-1749. [DOI] [PubMed] [Google Scholar]
- Neill JD, Nagy GM. Prolactin secretion and its control. In: Knobil, Neill JD, editors. The Physiology of Reproduction. New York: Rave Press; 1994. pp. 1148–1453. [Google Scholar]
- Ojeda SR, Negro-Vilar A, McCann SM. Release of prostaglandin E2 by hypothalamic tissue: Evidence for their involvement in catecholamine-induced luteinizing hormone-releasing hormone release. Endocrinology. 1979;104:617–624. doi: 10.1210/endo-104-3-617. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Negro-Vilar A, McCann SM. Evidence for involvement of alpha-adrenergic receptors in norepinephrine-induced PGE2 and LHRH release from the median eminence. Endocrinology. 1982;110:409–412. doi: 10.1210/endo-110-2-409. [DOI] [PubMed] [Google Scholar]
- Pine M, Lee B, Dearth R, Hiney JK, Dees WL. Manganese acts centrally to stimulate luteinizing hormone secretion: A potential influence on female pubertal development. Toxicol. Sci. 2005;85:880–885. doi: 10.1093/toxsci/kfi134. [DOI] [PubMed] [Google Scholar]
- Prestifilippo JP, Fernandez-Solari J, Mohn C, De Laurentiis A, McCann SM, Dees W, Rettori V. Effect of manganese on luteinizing hormone-releasing hormone secretion in adult male rats. Toxicol. Sci. 2007;97:75–80. doi: 10.1093/toxsci/kfm015. [DOI] [PubMed] [Google Scholar]
- Rettori V, Belova N, Dees WL, Nyberg CL, Gimeno M, McCann SM. Role of nitric oxide in the control of luteinizing hormone-releasing hormone release in vivo and in vitro. Proc. Natl. Acad. Sci. U. S. A. 1993;90:10130–10134. doi: 10.1073/pnas.90.21.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettori V, Gimeno M, Lyson K, McCann SM. Nitric oxide mediates norepinephrine-induced prostaglandin E2 release from the hypothalamus. Proc. Natl. Acad. Sci. U. S. A. 1992;89:11543–11546. doi: 10.1073/pnas.89.23.11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettori V, Kamat A, McCann SM. Nitric oxide mediates the stimulation of luteinizing-hormone releasing hormone release induced by glutamic acid in vitro. Brain Res. Bull. 1994;33:501–503. doi: 10.1016/0361-9230(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Scacchi P, Carbone S, Szwarcfarb B, Rondina D, Wuttke W, Moguilevsky J. Interactions between GABAergic and serotoninergic systems with excitatory amino acid neurotransmission in the hypothalamic control of gonadotropin secretion in prepubertal female rats. Brain Res. Dev. Brain Res. 1998;105:51–58. [PubMed] [Google Scholar]
- Seilicovich A, Duvilanski BH, Pisera D, Theas S, Gimeno M, Rettori V, McCann SM. Nitric oxide inhibits hypothalamic luteinizing hormone-releasing hormone release by releasing gamma-aminobutyric acid. Proc. Natl. Acad. Sci. U. S. A. 1995;92:3421–3424. doi: 10.1073/pnas.92.8.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Medlicott M, Ellis GH. Manganese deficiency in the rabbit. Arch. Biochem. Biophys. 1944;4:281–289. [Google Scholar]
- Takeda A, Sotogaku N, Oku N. Influence of manganese on the release of neurotransmitters in rat striatum. Brain Res. 2003;965:279–282. doi: 10.1016/s0006-8993(02)04157-4. [DOI] [PubMed] [Google Scholar]
- Yamada M, Ohno S, Okayasu I, Hatakeyama S, Watanabe H, Ushio K, Tsukagoshi H. Chronic manganese poisoning: A neuropathological study with determination of manganese distribution in the brain. Acta Neuropathol. 1986;70:273–278. doi: 10.1007/BF00686083. [DOI] [PubMed] [Google Scholar]