Abstract
Phenotype results from interactions between genetics and environment, but for most environmental chemical exposures, such interactions are theoretical. The phenotypic response of the testis to in utero dibutyl phthalate (DBP) exposure was compared between two strains of Long-Evans (LE) rats, the orl substrain with inherited cryptorchidism and an outbred (wt) strain. orl and wt LE rats were exposed daily between gestational day (GD) 12 and GD21 to DBP dose levels ranging from 50 to 200 mg/kg by oral gavage and sensitive phthalate testicular end points examined at either GD19, GD21, or postnatal day (PND) 21. At 50 mg/kg DBP, GD19 expression of Cyp17a1, Insl3, and Scarb1 was significantly reduced in orl but not wt testis. At GD21, statistically significant differential strain effects (orl more sensitive than wt) were observed for testicular expression of Scarb1 at 50 and 200 mg/kg DBP and Star at 200 mg/kg DBP. Similarly, DBP exposure disproportionately increased GD21 seminiferous cord diameters and numbers of multinucleated germ cells in the orl strain. At PND21, body weight–corrected testis weights were lowered significantly by DBP exposure at all dose levels in the orl strain but not in wt rats. While the frequency of undescended testes after 200 mg/kg DBP exposure in the orl strain appeared increased, these data were not statistically significant. These results demonstrated enhanced sensitivity of the orl rat to phthalate exposure as compared to its parent strain, a potentially important model of the effects of gene-environment interaction on development of male reproductive malformations.
Keywords: phthalate, testis, gonocyte, fetal, gene, cryptorchidism, susceptibility
It has long been understood that both genetic and environmental factors determine phenotype (Hunter, 2005), but for male reproductive disorders such as cryptorchidism (undescended testis), the contributions of specific genetic alleles or environmental exposures to human disease are poorly understood (Foresta et al., 2008; Hotchkiss et al., 2008). In both rodents and humans, fetal testis–derived hormones [Insulin-like 3 (Insl3) and testosterone] and their action on reproductive tissues are central contributors to normal male reproductive development. Mutations in genes controlling fetal testis hormone production or hormone signaling processes in extratesticular tissues can cause reproductive maldevelopment (Yeh et al., 2002; Zimmermann et al., 1999), but in most human cases, genetic determinants have not been identified. In utero exposure of animal models to high dose levels of estrogenic or antiandrogenic toxicants produces a suite of male reproductive malformations including cryptorchidism (Hotchkiss et al., 2008). In general, human exposure to endocrine disrupting toxicants occurs at much lower levels than those employed in animal studies, but human data suggesting an association between endocrine-active environmental toxicant exposure and altered male endocrine-related reproductive end points are published (Main et al., 2006; Swan et al., 2005). Together, these data support a model in which genetic and environmental factors controlling hormone production and action synergize to produce the human male reproductive phenotype.
Animal models of human congenital cryptorchidism are rare, but one such model is the orl rat. In this Long-Evans (LE) substrain, 62–65% of males have unilateral or bilateral undescended testes (Mouhadjer et al., 1989). Like many cases of human cryptorchidism, the undescended orl testis is positioned in the superficial inguinal pouch, indicating abnormal inguinal-scrotal descent. Testicular descent requires proper development of the fetal gubernaculum, an abdominal wall ligament attaching to the testis (Wensing, 1988). In orl rats, the gubernaculum fails to enlarge in late gestation (Barthold et al., 2006), and gene expression studies during this time period suggest a defect in cytoskeleton function and muscle cell development (Barthold et al., 2008). The underlying genetic aberration in the orl rat is unknown but appears to be polygenic (our unpublished observations). Because insufficient androgen activity leads to a similar phenotype (Husmann and McPhaul, 1991; Spencer et al., 1991), these data suggest that the orl rat may have a defect in androgen action.
Environmental chemical exposure in utero can perturb fetal male endocrinology. One class of endocrine disrupting chemicals that produces a suite of male reproductive disorders after fetal exposure is the phthalates. Reproductive effects of exposure include altered seminiferous cord formation, multinucleated gonocyte formation, epididymal agenesis, nipple retention, hypospadias, and cryptorchidism (Foster, 2006; Gray et al., 2006). Phthalate exposure reduces production of fetal testis testosterone via a decrease in Leydig cell steroidogenic gene expression (Lehmann et al., 2004; Shultz et al., 2001); Leydig cell Insl3 gene expression also is compromised (Lehmann et al., 2004; McKinnell et al., 2005; Wilson et al., 2004). This altered Leydig cell gene expression explains the maldevelopment of hormone-dependent extratesticular reproductive tissues, but some fetal testis histological effects occur via a mechanism unrelated to testosterone deficiency (Gaido et al., 2007; Scott et al., 2007).
Here, the goal was to develop a gene-environment animal model for male reproductive maldevelopment. Because of genetic susceptibility to cryptorchidism and a potential perturbation of androgen action in the orl strain, we hypothesized that the orl rat would show increased susceptibility of the reproductive system to fetal phthalate exposure.
MATERIALS AND METHODS
Animals and phthalate exposure.
Breeding colonies of LE wt and orl strains were maintained within the Life Science Center at Alfred I. duPont Hospital for Children. This center is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. The Nemours Institutional Animal Care and Use Committee approved all animal protocols. Animals were housed in polycarbonate cages containing pine shavings in a room controlled for temperature (70°C ± 2°C) and a reverse light cycle (12:12; lights on at 10 P.M.) and had unrestricted access to food (LabDiet Rat Chow 5021; PMI Nutrition International, Brentwood, MO) and tap water. Timed pregnant females were generated by housing two to three females with a single male prior to the dark cycle and checking for vaginal sperm the following morning. When sperm were found, this day was designated gestational day (GD) 1. Between GD12 and GD21, animals were exposed each day to DBP (CAS number 84-74-2; Sigma Chemical Co., St Louis, MO) by oral gavage between 8 and 10 A.M. DBP dose levels of 50, 100, and 200 mg/kg body weight (DBP50, DBP100, and DBP200) were chosen to produce an effect on testicular end points but not saturate the response (Gaido et al., 2007; Lehmann et al., 2004). The exposure window covers the period of fetal testis morphogenesis and major gubernacular development (Barthold et al., 2006; Klonisch et al., 2004). DBP was diluted in corn oil and an equal gavage volume of 1 ml administered to each dam using a 1-ml syringe and gavage needle. Dams and postnatal day (PND) 21 animals were killed by carbon dioxide inhalation and fetal animals by decapitation with a sharp surgical scissor. At PND21 prior to euthanasia, testicular position was determined by palpation of the inguinal and scrotal regions; position was confirmed visually during necropsy. Testes and epididymides were dissected free of fat and weighed. For testicular position and organ weight determination, a range of 1–12 wt and 1–8 orl pups/litter were examined (average of 6.5 and 3.4 for wt and orl, respectively).
Real-time quantitative PCR.
Tissue collection was performed 6 and 4 h after the final DBP exposure on GD19 and GD21, respectively. These time points were chosen to detect significant reductions in Leydig cell gene expression (Thompson et al., 2005). Using a stereomicroscope, testes were isolated, placed into RNAlater (Applied Biosystems, Foster City, CA) and stored at 4°C until RNA extraction. Total RNA purification, reverse transcription, qPCR using Taqman chemistry, and data analysis were performed as described previously (Barthold et al., 2008). Expression levels of test genes were determined relative to glyceraldehyde-3-phosphate dehydrogenase (Gapdh) expression. Numbers of litters examined were 3 (wt and orl GD19 oil), 4 (orl GD19 DBP50; orl GD21 oil and DBP200), 5 (wt GD19 DBP50; wt GD21 oil and DBP200; orl GD21 DBP50), or 7 (wt GD21 DBP50). Numbers of testis pairs (i.e., pups) pooled per litter prior to RNA isolation ranged from 1 to 4 (average of 2.7 and 2.4 for wt and orl, respectively). Taqman gene expression assay (Applied Biosystems) identification numbers were the following: cytochrome P450, family 11, subfamily A, polypeptide 1 (Cyp11a1): Rn01421676_m1; cytochrome P450, family 17, subfamily A, polypeptide 1 (Cyp17a1): Rn01444704_g1; steroidogenic acute regulatory protein (Star): Rn00586095_m1; Scavenger receptor class B, member 1 (Scarb1): Rn00580588_m1; Insl3: Rn00586632_m1; and Gapdh: Rn99999916_s1.
Fetal testis histology.
GD19 and GD21 testes were isolated using a stereomicroscope, placed in modified Davidson's fixative overnight (Howroyd et al., 2005), dehydrated in alcohol, embedded in paraffin, and sections of 5-micron thickness stained with hematoxylin and eosin. To quantify multinucleated germ cell (MNG) formation, sections were viewed with a 40× objective using an Axiostar plus microscope (Carl Zeiss, Inc., Thornwood, NY) and the number of seminiferous cord sections with one or more MNG recorded. To quantify cord diameter, sections were viewed with a 10× objective using a BX51 microscope (Olympus America, Inc., Center Valley, PA) and cord diameters calculated using the measurement tool within Image Pro Plus software (Media Cybernetics, Inc., Bethesda, MD). The length of the longest diameter between the basement membranes along the short axis of a seminiferous cord section was recorded. For both MNG and cord diameter quantification at GD21, one section of a single testis from one to five pups/litter (average of 2.3) and a range of 31–75 cord sections/pup (average of 52 and 50 for wt and orl, respectively) were examined. For GD19 MNG and cord diameter quantification, one section or two nonadjacent sections from one or two pups/litter and a range of 18–69 cord sections/pup (average of 38 and 42 for wt and orl, respectively) were examined. Numbers of litters examined were 3 (wt and orl GD19 oil), 4 (wt and orl GD21 oil; orl GD21 DBP200), 5 (wt and orl GD19 DBP50; orl GD21 DBP50), or 6 (wt GD21 DBP50 and wt GD21 DBP200). Morphological measurements were performed with the observer blinded to treatment group.
Statistics.
For all statistical analyses, the dam was the experimental unit. For all data except organ weights, a two-way ANOVA compared results between strains (including all treatment groups), between DBP treatment groups (including both strains), and identified an interaction between these two factors. Body weight was a significant covariate of testis weight (p < 0.001) and epididymal weight (p < 0.005); therefore, effects of strain and treatment on organ weights and an interaction between strain and treatment was examined statistically using a two-way analysis of covariance (ANCOVA) with pup body weight being the covariate. For within-strain statistical analysis, an unpaired t-test was used for GD19 data and a one-way ANOVA followed by a Dunnett's posttest for GD21 and PND21 data. For strain or treatment effects in the two-way ANOVA or ANCOVA, a p value of < 0.05 was considered statistically significant. For an interaction between strain and treatment indicating DBP exposure affected one strain more than the other, a p value of < 0.2 was considered statistically significant because this cut off correlated with statistical analyses performed within strains that showed one strain was significantly affected by phthalate exposure while the other strain was not. In addition, others have used this interaction p value cut off for similar data (Wilson et al., 2007). Two-way ANCOVA was performed using SPSS version 14 (SPSS, Inc., Chicago, IL) and all other statistics with Prism 5.0 software (GraphPad Software, Inc., San Diego, CA).
RESULTS
Although orl average litter size (9.0 ± 0.7 SEM) was lower than wt (13.3 ± 0.8 SEM), DBP exposure had no consistent effect on litter sizes in either strain (data not shown) and did not influence maternal (data not shown) or PND21 pup body weights (Table 1).
TABLE 1.
Postnatal Day 21 End Points
| wt |
orl |
|||||||
| Oil (n = 7) | DBP50 (n = 5) | DBP100 (n = 4) | DBP200 (n = 3) | Oil (n = 8) | DBP50 (n = 8) | DBP100 (n = 6) | DBP200 (n = 5) | |
| Male body weight (g) | 47.6 ± 4.0 | 40.7 ± 3.0 | 45.6 ± 3.3 | 38.7 ± 0.6 | 39.1 ± 1.8 | 37.2 ± 1.5 | 39.8 ± 3.3 | 43.0 ± 3.0 |
| Epididymal weight (mg) | 18.8 ± 1.5 | 17.3 ± 1.7 | 16.5 ± 1.9 | 14.2 ± 2.1 | 16.3 ± 1.3 | 16.3 ± 1.4 | 14.1 ± 1.5 | 17.1 ± 1.7 |
| Testis weighta (mg) | 94.1 ± 2.6 | 94.2 ± 2.9 | 103.2 ± 3.3 | 97.0 ± 3.8 | 94.6 ± 2.3 | 82.4 ± 2.4 | 82.1 ± 2.7 | 86.4 ± 2.9 |
Note. Oil vehicle or DBP exposure of 50, 100, or 200 mg/kg was via gavage of the dam each day between GD12 and GD21. Group sizes equal the number of litters. Data shown are litter means (for male body weight) and least squares means (for organ weights; adjusted for male body weight) ± SEM. wt: LE rat strain; orl: orl substrain of LE rat strain.
Two-way ANCOVA strain p < 0.05 and interaction p < 0.05, using pup body weight as a covariate.
Fetal End Points
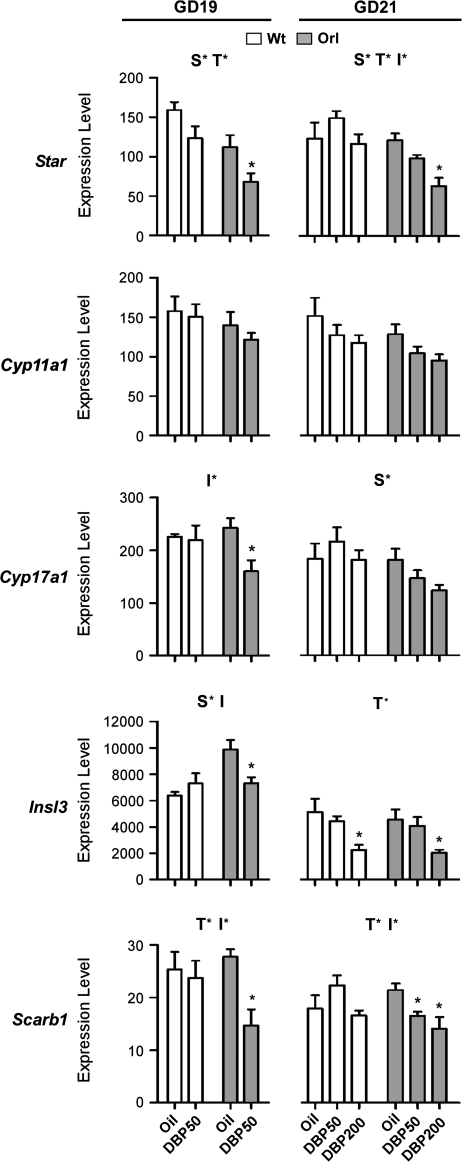
The effects of daily DBP exposure via gavage beginning on GD12 on fetal Leydig cell gene expression were compared between wt and orl strains. At GD19, DBP50 significantly reduced mRNAs of Cyp17a1, Scarb1, and Insl3 in orl, but not wt, testis (Fig. 1). This was shown by a significant interaction (p < 0.2) between strain and treatment effects using a two-way ANOVA statistical test and a significant (p < 0.05) within-strain decrease. While GD19 Star mRNA levels were significantly reduced by DBP50 exposure (indicated by a significant treatment effect), this reduction was similar in both strains; within-strain p values for DBP50 exposure were 0.07 and 0.03 for wt and orl, respectively. In both orl and wt rats, Cyp11a1 mRNA levels were not altered.
FIG. 1.
Fetal testis gene expression in wt and orl rats after DBP exposure. Fetal testis mRNA levels for each indicated gene relative to Gapdh were measured by Taqman-based qPCR. wt (open bars) and orl (filled bars) were exposed daily to vehicle (oil) or DBP from GD12 to either GD19 or GD21. Data are shown as the mean ± SEM. At each GD, a significant effect of strain, treatment, or interaction between strain and treatment was determined by two-way ANOVA. Above each graph, S* indicates a significant strain effect (p < 0.05), T* indicates a significant treatment effect (p < 0.05), and I* indicates a significant interaction between strain and treatment (p < 0.2). Significant DBP exposure effects within each strain were determined by a t-test (GD19 data) or ANOVA with Dunnett's posttest (GD21 data). Asterisks above a column indicate significant within-strain effects (p < 0.05) compared to oil treatment.
To extend these results, phthalate effects on Leydig cell gene expression were also examined at GD21 at two DBP dose levels (Fig. 1). At this age, significant strain differences were observed for reductions in Star and Scarb1 mRNA levels. Compared to vehicle-exposed animals, significant within-strain Star reductions occurred only in orl rats at DBP200 and at DBP50 and DBP200 for Scarb1. Mean mRNA levels for Cyp17a1 after DBP exposure trended lower in orl rats (within-strain Dunnett posttest p value of 0.09 for DBP200 exposure and test for linear trend p value of 0.03), but susceptibility between wt and orl rats did not achieve statistical significance by two-way ANOVA (interaction p < 0.34). Insl3 expression at GD21 was reduced significantly after DBP200 exposure in both wt and orl strains.
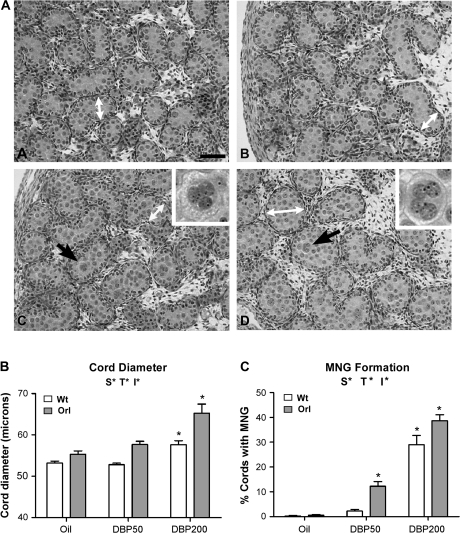
In addition to Leydig cell gene expression effects, histological changes were observed in seminiferous cords at GD21 after DBP exposure, and a quantitative comparison of wt and orl responses was performed. In vehicle-exposed animals, no overt histological differences were observed between testes from wt and orl rats (Fig. 2A). No qualitative changes in Leydig cell nuclear/cytoplasmic ratio or aggregation were noted at any age. In both strains at GD19, no MNG were seen in testes after either vehicle or DBP50 exposure (data not shown). Using Image Pro software to measure GD19 seminiferous cord diameters, these were not different among wt rats exposed to vehicle (65.3 ± 3.0 μm) or DBP50 (62.6 ± 1.2 μm) and orl rats exposed to vehicle (66.4 ± 0.9 μm) or DBP50 (65.2 ± 0.4 μm). After DBP exposure through GD21, apparent increases were found in both strains in seminiferous cord diameter and the number of seminiferous cords with at least one MNG (Fig. 2A). To determine if the orl rat was particularly susceptible to these DBP-induced effects seen at GD21, histological changes were quantified after DBP50 and DBP200 exposures. Seminiferous cord diameters were similar in vehicle-exposed wt (53.2 ± 0.5 μm) and orl (55.3 ± 0.8 μm) animals (Fig. 2B), as were the percentages of seminiferous cords with MNG (wt: 0.2 ± 0.2; orl: 0.5 ± 0.3) (Fig. 2C). Within each strain, cord diameters and the percentage of cords with at least one MNG were increased significantly following DBP exposure. In DBP50-treated rats, cord diameters were 52.8 ± 0.4 μm in wt and 57.7 ± 0.8 μm in orl, and after DPB200 exposure, measurements rose to 57.7 ± 0.9 μm in wt and 65.3 ± 2.2 μm in orl. The percentages of seminiferous cords with MNG were 2.2 ± 0.7 for wt DBP50, 29.0 ± 3.8 for wt DBP200, 12.2 ± 1.9 for orl DBP50, and 38.7 ± 2.4 for orl DBP200. By two-way ANOVA, strain and treatment both had significant effects on seminiferous cord diameter and MNG formation, and the significant interaction between strain and treatment on these two end points showed that the orl strain was more susceptible to DBP-induced histological changes. Both strains displayed significantly increased seminiferous cord diameters after DBP200 exposure, but the magnitude of increase was greater in orl rats. MNG were significantly induced in the orl strain at DBP50 and in both strains at DBP200.
FIG. 2.
Fetal testis histopathology at GD21 in wt and orl rats after GD12–GD21 DBP exposure. (A) Testis histology from oil-exposed (A) and DBP200-exposed (C) wt rats and from oil-exposed (B) and DBP200-exposed (D) orl rats. The white arrows indicate examples of areas used for seminiferous cord diameter measurements. After DBP exposure, MNG were observed (black arrows) in some seminiferous cords. Higher magnification images of MNG are shown in the insets. Bar = 50 microns. (B) Quantification of testis seminiferous cord diameter after DBP exposure. (C) Quantification of testis MNG formation. The percentage of seminiferous cords with at least one MNG was determined. Data in (B) and (C) are shown as the mean ± SEM. A significant effect of strain, treatment, or interaction between strain and treatment was determined by two-way ANOVA. Above each graph, S* indicates a significant strain effect (p < 0.05), T* indicates a significant treatment effect (p < 0.05), and I* indicates a significant interaction between strain and treatment (p < 0.2). As determined by one-way ANOVA and Dunnett's post test, asterisks above a column indicate significant within-strain effects (p < 0.05) compared to oil treatment.
Postnatal End Points
After a GD12–GD21 DBP exposure, wt and orl pups were examined at PND21 for testis histology, epididymal and testis weights, and incidence of undescended testis. No changes in testicular microscopic histology were observed in either strain at DBP dose levels up to 200 mg/kg (data not shown). Compared to wt, body weights trended lower in orl animals (two-way ANOVA strain p value of 0.12), but DBP exposure was without effect on body weight in either strain (Table 1). However, body weight was a significant covariate for both epididymal and testis weights (p < 0.05). Body weight–adjusted epididymal weights were unchanged in both strains at all DBP dose levels. In contrast, DBP exposure at all dose levels examined significantly reduced body weight–adjusted weights of both scrotal and undescended testes in the orl strain. There was no significant difference in mean testis weights based on testicular position (data not shown). By two-way ANCOVA, a significant effect of strain on testis weight was observed, and the significant interaction of strain and DBP treatment demonstrated that orl rats were uniquely susceptible to DBP-induced reductions in prepubertal testis weights.
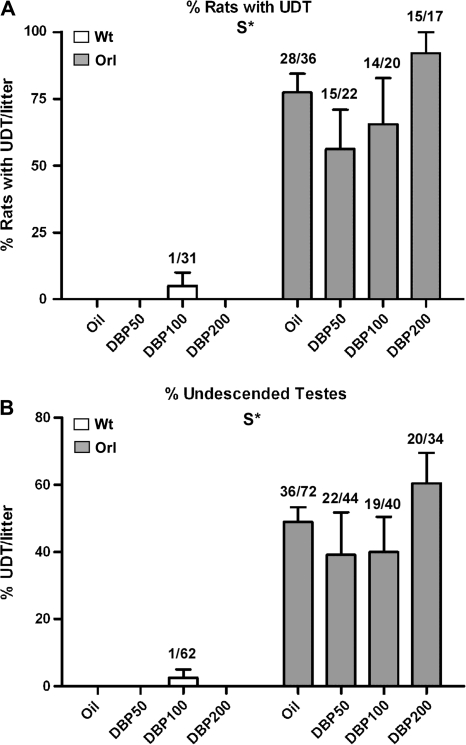
At PND21, rates of cryptorchidism after DBP exposure were examined in orl and wt strains (Fig. 3). Except for one animal with unilateral cryptorchidism in the DBP100 group, all vehicle control and treated wt rats had descended testes. In contrast, orl rats showed a high frequency of undescended testis in both vehicle control and DBP exposure groups. The percentages of rats/litter with a least one undescended testis were 77.4 ± 7.1 for oil-exposed orl and 56.3 ± 14.8, 65.6 ± 17.3, and 92.0 ± 8.0 for DBP50-, DBP100-, and DBP200-exposed orl, respectively (Fig. 3A). Percentages of undescended testes/litter in the orl strain were 48.9 ± 4.5, 39.1 ± 12.7, 40.0 ± 10.4, and 60.4 ± 9.1 for oil, DBP50, DBP100, and DBP200, respectively (Fig. 3B). Although the incidence and severity of cryptorchidism appeared higher in the orl DBP200 group, there was no statistically significant change in the number of rats/litter with undescended testis or total undescended testes/litter at any DBP dose level.
FIG. 3.
Rates of undescended testis at PND21 in wt and orl rats after GD12–GD21 DBP exposure. UDT was measured in two ways. (A) The percentage of pups with unilateral or bilateral undescended testis (UDT) per litter is shown. Numbers above each column represent the fraction of animals within the entire group with at least one undescended testis. (B) The percentage of undescended testes per litter is shown. Numbers above each column represent the fraction of undescended testes within the entire group. Data are shown as the mean ± SEM. Above each graph and determined by two-way ANOVA, S* indicates a significant strain effect (p < 0.05).
DISCUSSION
To develop a gene-environment rat model of reproductive tract malformation, a substrain of the LE rat (orl) genetically predisposed to undescended testis was exposed to the antiandrogenic endocrine disruptor DBP. Without endocrine disruptor exposure, the orl fetal gubernaculum shows reduced growth and delayed inversion, resulting in a high spontaneous rate of undescended testis (62–65%) in male offspring (Barthold et al., 2006; Mouhadjer et al., 1989). Since fetal gubernacular development is driven by hormones (Insl3 and testosterone) produced by the testis (Hutson et al., 1997; Nef and Parada, 1999; Zimmermann et al., 1999) and fetal DBP exposure inhibits testicular Insl3 and testosterone production (Shultz et al., 2001; Wilson et al., 2004), we hypothesized that the endogenous orl genetic defect responsible for gubernaculum maldevelopment would synergize with DBP exposure and lead to enhanced levels of undescended testis. Results showed that rates of undescended testis in the orl rat after DBP200 exposure moved higher but not sufficiently to achieve statistical significance.
Multiple reasons for the lack of statistical significance of the undescended testis data are possible. First, undescended testis rates in the orl rat are variable, requiring relatively large effects or large group sizes for statistically significant detection. A second reason is that the control group in the current study had higher rates of undescended testis than historical controls from our orl colony. Historically, 35–40% of testes in untreated orl litters are undescended, but rates approaching 50% were observed in the present study. The reason for this discrepancy is unknown but may be a chance observation. A final reason could be an insufficient level or duration of DBP exposure. In the Spraque-Dawley rat, a DBP dose level of 500 mg/kg from GD3 to PND20 is required to induce undescended testis (Mylchreest et al., 1998); at this dose level and exposure duration, about one-quarter of Sprague-Dawley pups had undescended testes. Therefore, even a high DBP dose level in this rat strain produces an incompletely penetrant phenotype. In our hands, a DBP500 exposure of orl rats from GD12 to GD21 produced nonviable pups (Johnson and Barthold, unpublished data), precluding the use of this higher dose level.
Although we were unable to demonstrate a significant effect of DBP on penetrance of the undescended testis phenotype, we observed that the orl rat was more susceptible than its LE parental strain to the fetal testicular effects of phthalate exposure. Increased orl sensitivity to fetal DBP exposure was evident at both fetal and prepubertal life stages at dose levels from 50 to 200 mg/kg. This increased susceptibility was observed for fetal Leydig cell hormone-related gene expression decreases, induction of seminiferous cord histological defects, and reductions in prepubertal testis weights. These effects were demonstrated by a significant interaction between strain and treatment effects using two-way ANOVA analyses. The DBP50 dose level used is the lowest observed-effect level for Sprague-Dawley rat Leydig cell steroidogenic gene expression changes in the published literature (Lehmann et al., 2004) and induction of MNG in the Fisher344 rat strain (our unpublished observations). With the exception of Cyp11a1, the orl rat displayed statistically significantly enhanced sensitivity to phthalate-induced reductions of all examined hormone-related genes. Postnatal testis weight reductions can result from lowered germ cell numbers secondary to reduced fetal and/or neonatal Sertoli cell proliferation (Orth et al., 1988). In the Wistar rat, in utero DBP exposure reduces the number of fetal Sertoli cells concomitant with low testosterone levels (Scott et al., 2007), and mice with genetic defects in androgen signaling also show lower fetal and prepubertal Sertoli cell numbers (Johnston et al., 2004; Scott et al., 2007; Tan et al., 2005). Because increased sensitivity of the orl fetal testis to steroidogenic gene expression effects was correlated with reduced prepubertal testis weights, these data suggest that the orl prepubertal testis weight phenotype may be due to relatively low fetal testis androgen levels. However, the lack of a dose-response in the postnatal testis weight data suggests that the underlying process in the orl rat has reached a plateau by DBP50 exposure. Since steroidogenic gene changes continued to decrease beyond DBP50, reduced steroidogenesis may not mediate phthalate-induced decreases in postnatal testis weight. Arguing against reductions in Sertoli cell numbers driving phthalate-induced decreases in postnatal testis weights is the observation that Sertoli cell numbers recover to normal levels by PND25 after gestational exposure of Wistar rats to DBP500 (Hutchison et al., 2008; Scott et al., 2008). Future experiments such as quantifying Sertoli cell numbers and measuring androgen-related end points are needed to examine their relationship (if any) to postnatal testis weight reductions in the orl rat.
The basis for increased sensitivity of the orl rat to phthalate exposure is unknown. Our ongoing genetic studies of the orl rat suggest that at least two loci determine susceptibility to the cryptorchidism trait and that inheritance is at least partially dominant with reduced expressivity. Our breeding data parallel the breeding studies of Ikadai et al. (1988) of the trans-scrota rat, which suggest that transmission of cryptorchidism in that phenotypically similar rat strain is multiallelic. In expression studies of the fetal gubernaculum and testis (Barthold et al., 2008), the greatest differences between orl and wt strains were noted in the gubernaculum, suggesting that the orl genetic background primarily alters signaling in the direct target tissue for testicular descent (the gubernaculum) and not the testis. However, the data reported here show that the orl genetic background also modifies the testicular response to endocrine disruptor exposure.
Several possibilities exist that could explain these strain-specific testicular effects. Potentially, phthalate pharmacokinetics are altered in the orl rat leading to increased testis tissue levels of the toxic DBP metabolite (monobutyl phthalate). Alternatively, the orl testis pharmacodynamic response to phthalate exposure could be changed. If altered pharmacodynamics is responsible for increased susceptibility, the genetic make up of the orl rat appears to affect more than one molecular pathway targeted in the testis by phthalates. Increased MNG formation and altered cord formation are observed in multiple rat strains as well as the mouse after phthalate exposure (Gaido et al., 2007; Scott et al., 2007); however, steroidogenic gene expression and testosterone levels in the mouse are not reduced by phthalate exposure (Gaido et al., 2007). Therefore, the phthalate testicular response diverges into two separate pathways, with one affecting the Leydig cell and the other the seminiferous cord. Because both of these pathways showed increased susceptibility, the orl rat genetic defect appears to modify an upstream molecular pathway in the phthalate mechanism. Expression changes in one or more testicular genes occurring early after phthalate exposure in somatic testicular cells may be responsible for reductions in Leydig cell hormone-related gene expression (Gaido et al., 2007; Johnson et al., 2007; Thompson et al., 2005). Comparing these early phthalate gene changes in the orl and LE rat strains may provide mechanistic insights into the altered expression of steroidogenic genes.
Strain differences in response to fetal phthalate exposure have also been observed between Wistar and Sprague-Dawley rats (Wilson et al., 2007). Similar to the results reported here, molecular differences were manifest as strain-dependent alterations in Insl3 and testosterone production by the fetal testis. Major species differences exist between mouse and rat regarding susceptibility to testosterone decreases after fetal phthalate exposure (Gaido et al., 2007). Together, these data provide increasing evidence for genetic control of testicular susceptibility to in utero phthalate exposure. Exploiting these strain susceptibility differences in genetic association studies may provide insights into the molecular mechanism of phthalate-induced reproductive toxicity.
FUNDING
National Institutes of Health (P20 RR020173 to J.S.B. and K.J.J.); Nemours Biomedical Research to J.S.B. and K.J.J.
Acknowledgments
The authors thank Dr Jobayer Hossain for statistical technical help and performing the two-way ANCOVA.
References
- Barthold JS, McCahan SM, Singh AV, Knudsen TB, Si X, Campion L, Akins RE. Altered expression of muscle- and cytoskeleton-related genes in a rat strain with inherited cryptorchidism. J. Androl. 2008;29:352–366. doi: 10.2164/jandrol.107.003970. [DOI] [PubMed] [Google Scholar]
- Barthold JS, Si X, Stabley D, Sol-Church K, Campion L, McCahan SM. Failure of shortening and inversion of the perinatal gubernaculum in the cryptorchid Long-Evans orl rat. J. Urol. 2006;176:1612–1617. doi: 10.1016/j.juro.2006.06.063. [DOI] [PubMed] [Google Scholar]
- Foresta C, Zuccarello D, Garolla A, Ferlin A. Role of hormones, genes and environment in human cryptorchidism. Endocr. Rev. 2008 doi: 10.1210/er.2007-0042. doi:10.1210/er.2007-0042. [DOI] [PubMed] [Google Scholar]
- Foster PM. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int. J. Androl. 2006;29:140–147. doi: 10.1111/j.1365-2605.2005.00563.x. discussion 181–185. [DOI] [PubMed] [Google Scholar]
- Gaido KW, Hensley JB, Liu D, Wallace DG, Borghoff S, Johnson KJ, Hall SJ, Boekelheide K. Fetal mouse phthalate exposure shows that gonocyte multinucleation is not associated with decreased testicular testosterone. Toxicol. Sci. 2007;97:491–503. doi: 10.1093/toxsci/kfm049. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Wilson VS, Stoker T, Lambright C, Furr J, Noriega N, Howdeshell K, Ankley GT, Guillette L. Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals. Int. J. Androl. 2006:96–104. doi: 10.1111/j.1365-2605.2005.00636.x. discussion 105–108. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Rider CV, Blystone CR, Wilson VS, Hartig PC, Ankley GT, Foster PM, Gray CL, Gray LE. Fifteen years after “Wingspread”––environmental endocrine disrupters and human and wildlife health: Where we are today and where we need to go. Toxicol. Sci. 2008 doi: 10.1093/toxsci/kfn030. doi:10.1093/toxsci/kfn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howroyd P, Hoyle-Thacker R, Lyght O, Williams D, Kleymenova E. Morphology of the fetal rat testis preserved in different fixatives. Toxicol. Pathol. 2005;33:300–304. doi: 10.1080/01926230590896145. [DOI] [PubMed] [Google Scholar]
- Hunter DJ. Gene-environment interactions in human diseases. Nat. Rev. Genet. 2005;6:287–298. doi: 10.1038/nrg1578. [DOI] [PubMed] [Google Scholar]
- Husmann DA, McPhaul MJ. Time-specific androgen blockade with flutamide inhibits testicular descent in the rat. Endocrinology. 1991;129:1409–1416. doi: 10.1210/endo-129-3-1409. [DOI] [PubMed] [Google Scholar]
- Hutchison GR, Scott HM, Walker M, McKinnell C, Ferrara D, Mahood IK, Sharpe RM. Sertoli cell development and function in an animal model of testicular dysgenesis syndrome. Biol. Reprod. 2008;78:352–360. doi: 10.1095/biolreprod.107.064006. [DOI] [PubMed] [Google Scholar]
- Hutson JM, Hasthorpe S, Heyns CF. Anatomical and functional aspects of testicular descent and cryptorchidism. Endocr. Rev. 1997;18:259–280. doi: 10.1210/edrv.18.2.0298. [DOI] [PubMed] [Google Scholar]
- Ikadai H, Ajisawa C, Taya K, Imamichi T. Suprainguinal ectopic scrota of TS inbred rats. J. Reprod. Fertil. 1988;84:701–707. doi: 10.1530/jrf.0.0840701. [DOI] [PubMed] [Google Scholar]
- Johnson KJ, Hensley JB, Kelso MD, Wallace DG, Gaido KW. Mapping gene expression changes in the fetal rat testis following acute dibutyl phthalate exposure defines a complex temporal cascade of responding cell types. Biol. Reprod. 2007;77:978–989. doi: 10.1095/biolreprod.107.062950. [DOI] [PubMed] [Google Scholar]
- Johnston H, Baker PJ, Abel M, Charlton HM, Jackson G, Fleming L, Kumar TR, O'Shaughnessy PJ. Regulation of Sertoli cell number and activity by follicle-stimulating hormone and androgen during postnatal development in the mouse. Endocrinology. 2004;145:318–329. doi: 10.1210/en.2003-1055. [DOI] [PubMed] [Google Scholar]
- Klonisch T, Fowler PA, Hombach-Klonisch S. Molecular and genetic regulation of testis descent and external genitalia development. Dev. Biol. 2004;270:1–18. doi: 10.1016/j.ydbio.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Lehmann KP, Phillips S, Sar M, Foster PM, Gaido KW. Dose-dependent alterations in gene expression and testosterone synthesis in the fetal testes of male rats exposed to di (n-butyl) phthalate. Toxicol. Sci. 2004;81:60–68. doi: 10.1093/toxsci/kfh169. [DOI] [PubMed] [Google Scholar]
- Main KM, Mortensen GK, Kaleva MM, Boisen KA, Damgaard IN, Chellakooty M, Schmidt IM, Suomi AM, Virtanen HE, Petersen DV, et al. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ. Health Perspect. 2006;114:270–276. doi: 10.1289/ehp.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnell C, Sharpe RM, Mahood K, Hallmark N, Scott H, Ivell R, Staub C, Jegou B, Haag F, Koch-Nolte F, et al. Expression of insulin-like factor 3 protein in the rat testis during fetal and postnatal development and in relation to cryptorchidism induced by in utero exposure to di(n-Butyl) phthalate. Endocrinology. 2005;146:4536–4544. doi: 10.1210/en.2005-0676. [DOI] [PubMed] [Google Scholar]
- Mouhadjer N, Pointis G, Malassine A, Bedin M. Testicular steroid sulfatase in a cryptorchid rat strain. J. Steroid. Biochem. 1989;34:555–558. doi: 10.1016/0022-4731(89)90144-1. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Cattley RC, Foster PM. Male reproductive tract malformations in rats following gestational and lactational exposure to di(n-butyl) phthalate: An antiandrogenic mechanism? Toxicol. Sci. 1998;43:47–60. doi: 10.1006/toxs.1998.2436. [DOI] [PubMed] [Google Scholar]
- Nef S, Parada LF. Cryptorchidism in mice mutant for Insl3. Nat. Genet. 1999;22:295–299. doi: 10.1038/10364. [DOI] [PubMed] [Google Scholar]
- Orth JM, Gunsalus GL, Lamperti AA. Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology. 1988;122:787–794. doi: 10.1210/endo-122-3-787. [DOI] [PubMed] [Google Scholar]
- Scott HM, Hutchison GR, Jobling MS, McKinnell C, Drake AJ, Sharpe RM. Relationship between androgen action in the ‘male programming window', fetal sertoli cell number and adult testis size in the rat. Endocrinology. 2008 doi: 10.1210/en.2008-0413. doi:10.1210/en.2008-0413. [DOI] [PubMed] [Google Scholar]
- Scott HM, Hutchison GR, Mahood IK, Hallmark N, Welsh M, De Gendt K, Verhoeven G, O'Shaughnessy P, Sharpe RM. Role of androgens in fetal testis development and dysgenesis. Endocrinology. 2007;148:2027–2036. doi: 10.1210/en.2006-1622. [DOI] [PubMed] [Google Scholar]
- Shultz VD, Phillips S, Sar M, Foster PM, Gaido KW. Altered gene profiles in fetal rat testes after in utero exposure to di(n-butyl) phthalate. Toxicol. Sci. 2001;64:233–242. doi: 10.1093/toxsci/64.2.233. [DOI] [PubMed] [Google Scholar]
- Spencer JR, Torrado T, Sanchez RS, Vaughan ED, Jr, Imperato-McGinley J. Effects of flutamide and finasteride on rat testicular descent. Endocrinology. 1991;129:741–748. doi: 10.1210/endo-129-2-741. [DOI] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ. Health Perspect. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KA, De Gendt K, Atanassova N, Walker M, Sharpe RM, Saunders PT, Denolet E, Verhoeven G. The role of androgens in Sertoli cell proliferation and functional maturation: Studies in mice with total or Sertoli cell-selective ablation of the androgen receptor. Endocrinology. 2005;146:2674–2683. doi: 10.1210/en.2004-1630. [DOI] [PubMed] [Google Scholar]
- Thompson CJ, Ross SM, Hensley J, Liu K, Heinze SC, Young SS, Gaido KW. Differential steroidogenic gene expression in the fetal adrenal gland versus the testis and rapid and dynamic response of the fetal testis to di(n-butyl) phthalate. Biol. Reprod. 2005;73:908–917. doi: 10.1095/biolreprod.105.042382. [DOI] [PubMed] [Google Scholar]
- Wensing CJ. The embryology of testicular descent. Horm. Res. 1988;30:144–152. doi: 10.1159/000181051. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Howdeshell KL, Lambright CS, Furr J, Gray LE., Jr Differential expression of the phthalate syndrome in male Sprague-Dawley and Wistar rats after in utero DEHP exposure. Toxicol. Lett. 2007;170:177–184. doi: 10.1016/j.toxlet.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Lambright C, Furr J, Ostby J, Wood C, Held G, Gray LE., Jr Phthalate ester-induced gubernacular lesions are associated with reduced insl3 gene expression in the fetal rat testis. Toxicol. Lett. 2004;146:207–215. doi: 10.1016/j.toxlet.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, et al. Generation and characterization of androgen receptor knockout (ARKO) mice: An in vivo model for the study of androgen functions in selective tissues. Proc. Natl Acad. Sci. U.S.A. 2002;99:13498–13503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S, Steding G, Emmen JM, Brinkmann AO, Nayernia K, Holstein AF, Engel W, Adham IM. Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Mol. Endocrinol. 1999;13:681–691. doi: 10.1210/mend.13.5.0272. [DOI] [PubMed] [Google Scholar]